Abstract
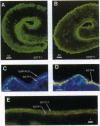
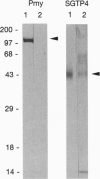
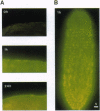
Adult Schistosoma mansoni blood flukes reside in the mesenteric veins of their vertebrate hosts, where they absorb immense quantities of glucose through their tegument by facilitated diffusion. Previously, we obtained S. mansoni cDNAs encoding facilitated-diffusion schistosome glucose transporter proteins 1 and 4 (SGTP1 and SGTP4) and localized SGTP1 to the basal membranes of the tegument and the underlying muscle. In this study, we characterize the expression and localization of SGTP4 during the schistosome life cycle. Antibodies specific to SGTP4 appear to stain only the double-bilayer, apical membranes of the adult parasite tegument, revealing an asymmetric distribution relative to the basal transporter SGTP1. On living worms, SGTP4 is available to surface biotinylation, suggesting that it is exposed at the hose-parasite interface. SGTP4 is detected shortly after the transformation of free-living, infectious cercariae into schistosomula and coincides with the appearance of the double membrane. Within 15 min after transformation, anti-SGTP4 staining produces a bright, patchy distribution at the surface of schistosomula, which becomes contiguous over the entire surface of the schistosomula by 24 hr after transformation. SGTP4 is not detected in earlier developmental stages (eggs, sporocysts, and cercariae) that do not possess the specialized double membrane. Thus, SGTP4 appears to be expressed only in the mammalian stages of the parasite's life cycle and specifically localized within the host-interactive, apical membranes of the tegument.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUEDING E. Carbohydrate metabolism of schistosoma mansoni. J Gen Physiol. 1950 May 20;33(5):475–495. doi: 10.1085/jgp.33.5.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baszczynski C. L., Fallis L. Isolation and nucleotide sequence of a genomic clone encoding a new Brassica napus napin gene. Plant Mol Biol. 1990 Apr;14(4):633–635. doi: 10.1007/BF00027511. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cattaneo R. Different types of messenger RNA editing. Annu Rev Genet. 1991;25:71–88. doi: 10.1146/annurev.ge.25.120191.000443. [DOI] [PubMed] [Google Scholar]
- Chan L. RNA editing: exploring one mode with apolipoprotein B mRNA. Bioessays. 1993 Jan;15(1):33–41. doi: 10.1002/bies.950150106. [DOI] [PubMed] [Google Scholar]
- Crouch M. L., Tenbarge K. M., Simon A. E., Ferl R. cDNA clones for Brassica napus seed storage proteins: evidence from nucleotide sequence analysis that both subunits of napin are cleaved from a precursor polypeptide. J Mol Appl Genet. 1983;2(3):273–283. [PubMed] [Google Scholar]
- Davies S. J., Pearce E. J. Surface-associated serine-threonine kinase in Schistosoma mansoni. Mol Biochem Parasitol. 1995 Mar;70(1-2):33–44. doi: 10.1016/0166-6851(95)00002-i. [DOI] [PubMed] [Google Scholar]
- Desrosiers R., Tanguay R. M. Methylation of Drosophila histones at proline, lysine, and arginine residues during heat shock. J Biol Chem. 1988 Apr 5;263(10):4686–4692. [PubMed] [Google Scholar]
- Ericson M. L., Murén E., Gustavsson H. O., Josefsson L. G., Rask L. Analysis of the promoter region of napin genes from Brassica napus demonstrates binding of nuclear protein in vitro to a conserved sequence motif. Eur J Biochem. 1991 May 8;197(3):741–746. doi: 10.1111/j.1432-1033.1991.tb15966.x. [DOI] [PubMed] [Google Scholar]
- Ericson M. L., Rödin J., Lenman M., Glimelius K., Josefsson L. G., Rask L. Structure of the rapeseed 1.7 S storage protein, napin, and its precursor. J Biol Chem. 1986 Nov 5;261(31):14576–14581. [PubMed] [Google Scholar]
- Farrell C. L., Pardridge W. M. Blood-brain barrier glucose transporter is asymmetrically distributed on brain capillary endothelial lumenal and ablumenal membranes: an electron microscopic immunogold study. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5779–5783. doi: 10.1073/pnas.88.13.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fripp P. J. The sites of (1-14C) glucose assimilation in Schistosoma haematobium. Comp Biochem Physiol. 1967 Dec;23(3):893–898. doi: 10.1016/0010-406x(67)90349-0. [DOI] [PubMed] [Google Scholar]
- Gerber H. P., Seipel K., Georgiev O., Höfferer M., Hug M., Rusconi S., Schaffner W. Transcriptional activation modulated by homopolymeric glutamine and proline stretches. Science. 1994 Feb 11;263(5148):808–811. doi: 10.1126/science.8303297. [DOI] [PubMed] [Google Scholar]
- Hackett F. The culture of Schistosoma mansoni and production of life cycle stages. Methods Mol Biol. 1993;21:89–99. doi: 10.1385/0-89603-239-6:89. [DOI] [PubMed] [Google Scholar]
- Hara-Nishimura I., Inoue K., Nishimura M. A unique vacuolar processing enzyme responsible for conversion of several proprotein precursors into the mature forms. FEBS Lett. 1991 Dec 2;294(1-2):89–93. doi: 10.1016/0014-5793(91)81349-d. [DOI] [PubMed] [Google Scholar]
- Herbert A., Lowenhaupt K., Spitzner J., Rich A. Chicken double-stranded RNA adenosine deaminase has apparent specificity for Z-DNA. Proc Natl Acad Sci U S A. 1995 Aug 1;92(16):7550–7554. doi: 10.1073/pnas.92.16.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiesel R., Wissinger B., Schuster W., Brennicke A. RNA editing in plant mitochondria. Science. 1989 Dec 22;246(4937):1632–1634. doi: 10.1126/science.2480644. [DOI] [PubMed] [Google Scholar]
- Hockley D. J., McLaren D. J. Schistosoma mansoni: changes in the outer membrane of the tegument during development from cercaria to adult worm. Int J Parasitol. 1973 Jan;3(1):13–25. doi: 10.1016/0020-7519(73)90004-0. [DOI] [PubMed] [Google Scholar]
- Isseroff H., Bontá C. Y., Levy M. G. Monosaccharide absorption by Schistosoma mansoni. I. Kinetic characteristics. Comp Biochem Physiol A Comp Physiol. 1972 Dec 1;43(4):849–858. doi: 10.1016/0300-9629(72)90157-0. [DOI] [PubMed] [Google Scholar]
- Josefsson L. G., Lenman M., Ericson M. L., Rask L. Structure of a gene encoding the 1.7 S storage protein, napin, from Brassica napus. J Biol Chem. 1987 Sep 5;262(25):12196–12201. [PubMed] [Google Scholar]
- Karas M., Hillenkamp F. Laser desorption ionization of proteins with molecular masses exceeding 10,000 daltons. Anal Chem. 1988 Oct 15;60(20):2299–2301. doi: 10.1021/ac00171a028. [DOI] [PubMed] [Google Scholar]
- Kaufmann R., Spengler B., Lützenkirchen F. Mass spectrometric sequencing of linear peptides by product-ion analysis in a reflectron time-of-flight mass spectrometer using matrix-assisted laser desorption ionization. Rapid Commun Mass Spectrom. 1993 Oct;7(10):902–910. doi: 10.1002/rcm.1290071010. [DOI] [PubMed] [Google Scholar]
- Laclette J. P., Shoemaker C. B., Richter D., Arcos L., Pante N., Cohen C., Bing D., Nicholson-Weller A. Paramyosin inhibits complement C1. J Immunol. 1992 Jan 1;148(1):124–128. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawrence M. C., Izard T., Beuchat M., Blagrove R. J., Colman P. M. Structure of phaseolin at 2.2 A resolution. Implications for a common vicilin/legumin structure and the genetic engineering of seed storage proteins. J Mol Biol. 1994 May 20;238(5):748–776. doi: 10.1006/jmbi.1994.1333. [DOI] [PubMed] [Google Scholar]
- Lönnerdal B., Janson J. C. Studies on Brassica seed proteins. I. The low molecular weight proteins in rapeseed. Isolation and characterization. Biochim Biophys Acta. 1972 Aug 31;278(1):175–183. [PubMed] [Google Scholar]
- Monsalve R. I., Menéndez-Arias L., López-Otín C., Rodríguez R. Beta-turns as structural motifs for the proteolytic processing of seed proteins. FEBS Lett. 1990 Apr 24;263(2):209–212. doi: 10.1016/0014-5793(90)81375-x. [DOI] [PubMed] [Google Scholar]
- Monsalve R. I., Villalba M., López-Otín C., Rodríguez R. Structural analysis of the small chain of the 2S albumin, napin nIII, from rapeseed. Chemical and spectroscopic evidence of an intramolecular bond formation. Biochim Biophys Acta. 1991 Jun 24;1078(2):265–272. doi: 10.1016/0167-4838(91)90568-k. [DOI] [PubMed] [Google Scholar]
- Ramwani J., Mishra R. K. Purification of bovine striatal dopamine D-2 receptor by affinity chromatography. J Biol Chem. 1986 Jul 5;261(19):8894–8898. [PubMed] [Google Scholar]
- Rogers S. H., Bueding E. Anatomical localization of glucose uptake by Schistosoma mansoni adults. Int J Parasitol. 1975 Jun;5(3):369–371. doi: 10.1016/0020-7519(75)90086-7. [DOI] [PubMed] [Google Scholar]
- Sato K., Asada T., Ishihara M., Kunihiro F., Kammei Y., Kubota E., Costello C. E., Martin S. A., Scoble H. A., Biemann K. High-performance tandem mass spectrometry: calibration and performance of linked scans of a four-sector instrument. Anal Chem. 1987 Jul 1;59(13):1652–1659. doi: 10.1021/ac00140a016. [DOI] [PubMed] [Google Scholar]
- Schneuwly S., Kuroiwa A., Baumgartner P., Gehring W. J. Structural organization and sequence of the homeotic gene Antennapedia of Drosophila melanogaster. EMBO J. 1986 Apr;5(4):733–739. doi: 10.1002/j.1460-2075.1986.tb04275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. A place in the world for RNA editing. Cell. 1995 Jun 16;81(6):833–836. doi: 10.1016/0092-8674(95)90002-0. [DOI] [PubMed] [Google Scholar]
- Silk M. H., Spence I. M., Gear J. H. Ultrastructural studies of the blood fluke--Schistosoma mansoni. I. The integument. S Afr J Med Sci. 1969 Apr;34(1):1–10. [PubMed] [Google Scholar]
- Skelly P. J., Kim J. W., Cunningham J., Shoemaker C. B. Cloning, characterization, and functional expression of cDNAs encoding glucose transporter proteins from the human parasite Schistosoma mansoni. J Biol Chem. 1994 Feb 11;269(6):4247–4253. [PubMed] [Google Scholar]
- Skelly P. J., Stein L. D., Shoemaker C. B. Expression of Schistosoma mansoni genes involved in anaerobic and oxidative glucose metabolism during the cercaria to adult transformation. Mol Biochem Parasitol. 1993 Jul;60(1):93–104. doi: 10.1016/0166-6851(93)90032-s. [DOI] [PubMed] [Google Scholar]
- Smith J. H., Reynolds E. S., Von Lichtenberg F. The integument of Schistosoma mansoni. Am J Trop Med Hyg. 1969 Jan;18(1):28–49. [PubMed] [Google Scholar]
- Takata K., Kasahara T., Kasahara M., Ezaki O., Hirano H. Immunolocalization of glucose transporter GLUT1 in the rat placental barrier: possible role of GLUT1 and the gap junction in the transport of glucose across the placental barrier. Cell Tissue Res. 1994 Jun;276(3):411–418. doi: 10.1007/BF00343939. [DOI] [PubMed] [Google Scholar]
- Takata K., Kasahara T., Kasahara M., Ezaki O., Hirano H. Ultracytochemical localization of the erythrocyte/HepG2-type glucose transporter (GLUT1) in the ciliary body and iris of the rat eye. Invest Ophthalmol Vis Sci. 1991 Apr;32(5):1659–1666. [PubMed] [Google Scholar]
- Thorens B., Cheng Z. Q., Brown D., Lodish H. F. Liver glucose transporter: a basolateral protein in hepatocytes and intestine and kidney cells. Am J Physiol. 1990 Dec;259(6 Pt 1):C279–C285. doi: 10.1152/ajpcell.1990.259.2.C279. [DOI] [PubMed] [Google Scholar]
- Thorens B., Lodish H. F., Brown D. Differential localization of two glucose transporter isoforms in rat kidney. Am J Physiol. 1990 Dec;259(6 Pt 1):C286–C294. doi: 10.1152/ajpcell.1990.259.2.C286. [DOI] [PubMed] [Google Scholar]
- Uglem G. L., Read C. P. Sugar transport and metabolism in Schistosoma mansoni. J Parasitol. 1975 Jun;61(3):390–397. [PubMed] [Google Scholar]
- Wilson R. A., Barnes P. E. The tegument of Schistosoma mansoni: observations on the formation, structure and composition of cytoplasmic inclusions in relation to tegument function. Parasitology. 1974 Apr;68(2):239–258. [PubMed] [Google Scholar]
- Zhong C., Skelly P. J., Leaffer D., Cohn R. G., Caulfield J. P., Shoemaker C. B. Immunolocalization of a Schistosoma mansoni facilitated diffusion glucose transporter to the basal, but not the apical, membranes of the surface syncytium. Parasitology. 1995 May;110(Pt 4):383–394. doi: 10.1017/s0031182000064726. [DOI] [PubMed] [Google Scholar]