Abstract
In Southeast Asia, swamp eels (Synbranchidae: Monopterus spp.) are a common source of human gnathostomiasis, a foodborne zoonosis caused by advanced third-stage larvae (AL3) of Gnathostoma spp. nematodes. Live Asian swamp eels are imported to US ethnic food markets, and wild populations exist in several states. To determine whether these eels are infected, we examined 47 eels from markets and 67 wild-caught specimens. Nematodes were identified by morphologic features and ribosomal intergenic transcribed spacer–2 gene sequencing. Thirteen (27.7%) M. cuchia eels from markets were infected with 36 live G. spinigerum AL3: 21 (58.3%) in liver; 7 (19.4%) in muscle; 5 (13.8%) in gastrointestinal tract, and 3 (8.3%) in kidneys. Three (4.5%) wild-caught M. albus eels were infected with 5 G. turgidum AL3 in muscle, and 1 G. lamothei AL3 was found in a kidney (both North American spp.). Imported live eels are a potential source of human gnathostomiasis in the United States.
Keywords: Gnathostoma spinigerum, G. turgidum, G. lamothei, Monopterus albus, M. (Amphipnous) cuchia, Asian swamp eels, Synbranchidae, human gnathostomiasis, introduced species, Florida, Georgia, New Jersey, New York, parasites, foodborne diseases
Keywords: Suggested citation for this article: Cole RA, Choudhury A, Nico LG, Griffin KM. Gnathostoma spinigerum in live Asian swamp eels (Monopterus spp.) from food markets and wild populations, United States. Emerg Infect Dis [Internet]. 2014 Apr [date cited]. http://dx.doi.org/10.3201/eid2004.131566
In parts of Asia, wild-caught and aquaculture-reared swamp eels (Synbranchidae: Monopterus spp.) are widely consumed as food by humans (1–3) and are a common source of human gnathostomiasis, a foodborne zoonosis caused by advanced third-stage larvae (AL3) of Gnathostoma spp. nematodes. (4–8). Over the past 2 decades, many thousands of swamp eels (Synbranchidae: Monopterus spp.) have been legally shipped alive from Asia to North America, where they were distributed to numerous ethnic food markets in major cities in the United States and Canada (9; L.G. Nico, unpub. data). An earlier survey of live Asian swamp eels from ethnic markets in the United States and introduced wild populations in Florida found substantial parasite burden in both market and wild swamp eels sampled; however, the researchers did not examine eels for Gnathostoma spp. (9).
In US ethnic food markets, imported swamp eels from Asia, together with a variety of other native and nonnative fishes, are commonly displayed alive. Consumers are able to purchase the animals and have them processed on site (gutted/filleted) or they can butcher their live purchase at home (9). Most of these market fish are purchased for food, but some are introduced into the wild. For instance, in Asia and certain western countries, several live fish and other animals sold in food markets and other venues are subsequently released into open waters by groups conducting ceremonial religious practices (10–12) with some releases that apparently involved swamp eels (9,13; L.G. Nico, unpub. data). Because a large number of fishborne parasitic zoonoses are found throughout the world (14,15), the importation of live fish infected with parasites from their native waters poses a threat to humans (14,16). Moreover, releasing imported foreign fish infected with parasites into open waters may introduce and spread nonnative parasites harmful to native faunas (17,18).
Swamp eels are a group of eel-like percomorph fishes naturally distributed in tropical and temperate regions of the New and Old Worlds (19). They are not native to the United States or Canada, but at least 5 separate introduced populations of Asian swamp eels (Monopterus spp.) have been established in open waters in the continental United States. These consist of 3 populations in peninsular Florida, 1 in northern Georgia, and 1 most recently established population in southern New Jersey (9,20). The live food trade is the suspected source of all or many of these introductions (9). Genetic analysis revealed that the introduced wild populations are composed of 3 genetically distinct clades within the M. albus (Zuiew, 1793) complex, a widely-distributed group native to eastern and southeastern Asia (20,21). A separate Asian swamp eel species, M. cuchia (Hamilton-Buchanan, 1822), also referred to as Amphipnous cuchia, is native to northern and northeastern India, Bangladesh, Nepal, Myanmar, and Pakistan (22). M. cuchia and members of the M. albus complex have been documented in animals in the live food trade and in ethnic food markets in the USA, but M. cuchia has not yet been documented in the United States in wild populations (L.G. Nico, unpub. data). All swamp eel species sold in the live food trade have behavioral and physiologic adaptations that make them attractive for live import and increase the risk for their invasion success in the wild. For example, both M. albus and M. cuchia eels are air breathers and, if kept moist, they can survive for months out of water and without food (23; L.G. Nico, unpub. data). Some M. albus swamp eels are protogynous hermaphrodites and change naturally from female to male, supposedly in response to environmental cues (24).
Farmed and wild M. albus eels in Asian countries are reported to have a high prevalence of infection with G. spinigerum nematodes (4–8). This nematode is native to Asia and the most commonly reported cause of gnathostomiasis in humans in Asia (6,25). Species of Gnathostoma have a 3-host life cycle. Cyclopoid copepods act as first intermediate host and consume stage 2 larvae (L2) that develop into early L3 in the copepod’s hemocoel. The copepod infected with the early L3 is then consumed by second intermediate hosts such as freshwater or saltwater fish, amphibians, reptiles, or birds, in which it migrates from the stomach into other organs (most commonly the liver and striated muscle) where it develops to AL3. Felids and canids are typical definitive hosts (7). Humans become infected by consuming raw or undercooked meat from second intermediate hosts. Once in the human host, AL3 do not develop further, but continue to migrate through tissues, including subcutaneous spaces, visceral organs, and the central nervous system (26).
As many as 13 species of Gnathostoma are currently recognized as valid (27). Although it has been hypothesized that all species of Gnathostoma can infect humans, only 6 species have been reported to infect humans: G. binucleatum, G. doloresi, G. hispidum, G. malaysiae, G. nipponicum, and G. spinigerum (27). These zoonotic species use a variety of animals as definitive hosts: cats (G. binucleatum and G. spinigerum), pigs (G. doloresi and G. hispidum), rats (G. malaysiae), weasels (G. nipponicum) and dogs (G. spinigerum). Four species of Gnathostoma have been reported from wildlife in the United States. Among the 4, G. procyonis (raccoons) is widely distributed in the United States, whereas G. turgidum (opossums), G. miyazakii (otter), and G. socialis (mink) have patchy distributions (27).
To determine whether imported M. cuchia swamp eels were infected with Gnathostoma spp., we examined live eels obtained from various ethnic food markets in 3 major metropolitan areas in the eastern United States. We also examined individual wild M. albus eels, from populations introduced into open waters in Florida and New Jersey, for the presence of AL3 to determine their ability to host endemic or introduced Gnathostoma spp.
Materials and Methods
Fish Sampling and Examinations
Asian swamp eels examined for Gnathostoma spp. infection included 47 specimens from 5 ethnic market in 3 major metropolitan areas in the eastern United States and 67 wild-caught specimens from 4 of the 5 known introduced populations established in the continental United States (Table 1). All market specimens identified as M. (Amphipnous) cuchia eels purchased live from ethnic food markets during 2010–2012 included the following: 1) 10 specimens obtained from 3 markets in New York’s Chinatown in Manhattan; 2) 12 specimens from a single market in the Atlanta, Georgia, area; and 3) 25 specimens from a single market in the Orlando, Florida, area. On the basis of species identification and information in US Fish and Wildlife Service Law Enforcement Management Information System (USFWS-LEMIS) live-animal shipment records, we concluded that all or most of the market M. cuchia eels likely originated in Bangladesh and were shipped by air to the United States.
Table 1. Summary information on live Asian swamp eels from market and wild populations in the United States examined for larval stages of Gnathostoma spp. in 47 Monopterus cuchia swamp eels purchased from 5 ethnic food markets and 67 wild-caught M. albus (clades A, B, and C) from 4 introduced populations*.
| Sources and eel identifications (dates sampled) |
No. samples |
Total length, mm, min/max, (mean) |
Body weight, g, min/max (mean) |
No. eels (%) infected with Gnathostoma spp. |
Eel specimen, parasite species, intensity, and tissue infected |
| Market samples: all M. cuchia | |||||
| New York Chinatown, 3 markets (2011 Aug 22) | 10 | 631–850 (707) | 208–693 (359) | 3 (30): G. spinigerum | Mc 28 Gs 1K, 1M |
| Mc 30 Gs 1G | |||||
| Mc 32 Gs 1M | |||||
| Orlando, Florida, 1 market (2011 Jan 27, Oct 17, Oct 31; 2012 Jan 9) | 25 | 546–781 (669) | 173–565 (350) | 5 (20): G. spinigerum | Mc 17 Gs 4L |
| Mc 21 Gs 1G | |||||
| Mc 37 Gs 1L | |||||
| Mc 58 Gs 1G, 2M | |||||
| Mc 59 Gs 2G, 1M | |||||
| Atlanta, Georgia, 1 market (2010 Oct 25) | 12 | 663–825 (730) | 316–796 (486) | 5 (41.7): G. spinigerum | Mc 3 Gs 1G |
| Mc 9 Gs 12L, 2M | |||||
| Mc 10 Gs 1L | |||||
| Mc 11 Gs 1L | |||||
| Mc 12 Gs 2L | |||||
| All market samples (2010–2012) |
47 |
546–850 (692) |
174–796 (386) |
13 (27.7): G. spinigerum |
|
| Wild population samples | |||||
| Florida,Tampa area: M. albus clade C (2011 Nov 29–30) | 14 | 140–912 (347) | 5–693 (95) | 3 (21.4): G. turgidum; G. lamothei | Ma 48 Gt 4M |
| Ma 49 Gt 1M | |||||
| Ma 54 Gl 1K | |||||
| Florida, North Miami area: M. albus clade C (2012 Feb 6) | 11 | 292–710 (522) | 22–343 (168) | 0 | |
| Florida, Homestead area: M. albus clade B (2012 Mar 10 & 12) | 23 | 230–650 (431) | 6–309 (91) | 0 | |
| New Jersey: M. albus clade A (2012 Apr 18) | 19 | 190–630 (314) | 4–192 (35) | 0 | |
| All wild population samples (2011–2012) | 67 | 140–912 (395) | 4–693 (89) | 3 (4.5): G. turgidum; G. lamothei | |
*Min, minimum; max, maximum; Mc, M. cuchia; Gs, G. spingerum; K, kidney; M, muscle; G, gut; L, liver; ND, not determined; Ma, M. albus; Gt, G. turgidum; Gl, G. lamothei.
Wild-caught swamp eel specimens collected during 2011–2012 were members of the M. albus species complex and included 3 geographically disjunct populations in peninsular Florida and 1 in New Jersey. Each wild population is a distinct clade (20; L.G. Nico, unpub. data). Populations and sites sampled included the following: 1) Tampa area population (clade C), 14 specimens from 2 sites in the Frog Creek drainage, Tampa Bay Basin, in Manatee County (near 27°35′18′′N, 82°30′35′W and 27°35′20′′N, 82°32′28′′W); 2) North Miami population (clade C), 11 specimens from 2 sites in the Snake Creek Canal (canal C-9) drainage, Broward and Dade counties (near 25°58′36′′N, 80°13′46′′W and 25°57′36′′N, 80°12′18′′W); 3) Florida Homestead population (clade B), 23 specimens collected from canals C-111 and L-31N, Dade County, near Everglades National Park (near 25°30′19′′N, 80°33′35′′W and 25°23′14′′N, 80°33′29′′W); and 4) New Jersey population (clade A), 19 specimens from Silver Lake in Gibbsboro, Camden County (near 39°50′21′′N, 74°57′44′′W). All sampled sites were inland, freshwater systems, and eels were collected in stream, canal, and lake habitats by using electrofishing gear.
Within 1–3 days of purchase or collection, swamp eels were transported to the US Geological Survey facility in Gainesville, Florida, where groups of <10 live swamp eels from each sampled population (i.e., market source or wild population) were held in large, clean indoor fiberglass tanks (120 cm long × 60 cm wide × 60 cm high) in 15 cm of noncirculating water from a tap source. Water in holding tanks had a pH of 7, salinity of 0.2 ppt, and temperature or 24–31°C. Large eels were separated from small eels to prevent cannibalism. Captive swamp eels held for more than several days were intermittently offered live commercially-raised earthworms (Lumbricus terrestris) as food, although few individuals fed on the worms. After a holding period from 1 day to several weeks, small numbers of swamp eels (1–9 individuals) were shipped live at selected intervals from November 2010 through May 2012 by overnight courier to the US Geological Survey, National Wildlife Health Center, where they were immediately euthanized with a solution of MS-222 (475 mg/L water) (tricaine methanesulfonate; Sigma, St. Louis, MO, USA) Each eel was then assigned a unique identifier code and the specimen’s total length (TL) from tip of snout to posterior end of tail was measured to the nearest mm, and weighed to the nearest gram..
Eels were decapitated, then skinned and filleted; all muscle was removed and liver, kidney, and gastrointestinal tracts were removed and separated. All organs were examined for AL3 stages of Gnathostoma spp. by using a dissection stereomicroscope (magnification ×4–7) then placed in a commercial grade food blender in a 5% pepsin hydrochloric acid solution and macerated. Tissue digest solution was placed in a shaking hot water bath at 37°C for overnight up to 24 hours. All digested samples were centrifuged at 3,000 g; solid residue was then rinsed in phosphate-buffered solution, and residue was examined for AL3 with a dissection stereomicroscope (magnification ×4–7).
Fixation and Morphologic Identification of Nematodes
A subset of AL3 were fixed in toto in warm, 10% neutral-buffered formalin and then stored in 70% ethanol with 0.5% glycerin for morphologic identification. For all other AL3s, the posterior 2/3 of the worm was excised and fixed in cold molecular grade 100% ethanol and stored at 4°C for up to 5 months for subsequent DNA extraction and sequencing. The remaining anterior portions were fixed for whole mounts as before. Morphologic identifications were made on the basis of published keys (7,28,29).
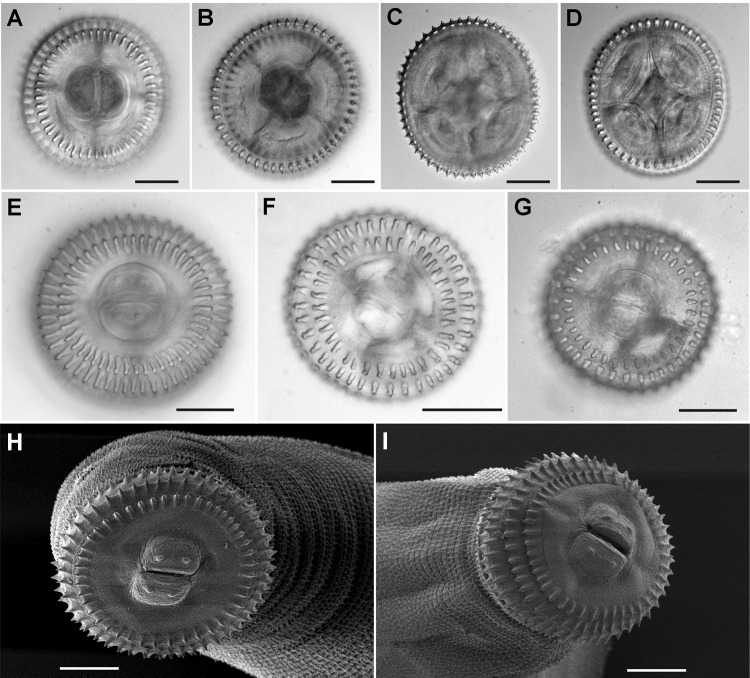
To obtain cephalic bulb hooklet counts, we placed cephalic bulbs in a 20% ethanol, 2% glycerin solution in which bulbs were severed and oriented in an en face position in a drop of the same medium and placed under a coverslip. Hooklets on the first 2 rows (Figure 1, panels A, B) were counted by using an Olympus BX 51 microscope with brightfield and Nomarski DIC optics (Olympus Corp., Center Valley, PA, USA). The cephalic bulb was reoriented with the lips facing down for counting the 3rd and 4th rows (Figure 1, panels C, D). Images were captured digitally.
Figures 1.
A–D) Views showing the technique used for hook counts of Gnathostoma spp., United States, En face (panels A, B) and posterior (panels C,D) views showing the technique used for hook counts; specimen shown here is of Gnathostoma spinigerum from eel 59 specimen b from gastrointestinal digestion. E–G) En face mounts of the cephalic bulbs of specimens identified as 3 different species on the basis of molecular data: panel E, specimen eel 59 G, a, G. spinigerum; panel F, specimen eel 48 M, c, G. turgidum, and panel G, specimen eel 54 K, a, G. lamothei. Note the difference between the hook counts in row 1 between G. spinigerum and the 2 other species (Table 2). H–I) Scanning electromicrograph of specimens from eel 9, identified as G. spinigerum on the basis of cephalic bulb hook counts. Scale bars = 50 µm.
For scanning electron microscopy, the anterior portions of roundworms were post-fixed in osmium tetroxide in phosphate buffer, dehydrated through a graded ethanol series, and infiltrated with hexamethyldisilizane, following which the hexamethyldisilizane was allowed to evaporate off the specimens that were then mounted on stubs, sputter coated with gold, and scanned by using a Philips XL-20 Scanning Electron Microscope (Philips, Andover, MA, USA). Images were captured digitally.
DNA Sequencing and Analysis
DNA was extracted following the Animal Tissue Protocol using QIAGEN’s DNeasy Blood & Tissue Kit (QIAGEN Inc. Valencia, CA, USA). Primers, NEWS2 (forward) 5′-TGTGTCGATGAAGAACGCAG-3′ and ITS2-RIXO (reverse) 5′-TTCTATGCTTAAATTCAGGGG-3′ were used to amplify a 600-bp fragment of the 5.8S rRNA gene and the intergenic transcribed spacer 2 (ITS-2) by using PCR (30) to corroborate morphologic identifications. Five microliters of the reaction mixture was examined by 1% agarose gel containing 0.0001% Gel Red (Phenix Research Products, Candler, NC, USA) by gel electrophoresis. Primers and nucleotides were removed from the PCR products by using ExoSAP-IT for PCR Product Clean-Up (Affymetrix, Santa Clara, CA, USA) as specified in manufacturer’s instructions. PCR products were sequenced at the University of Wisconsin – Madison Biotechnology Center’s DNA Sequencing Facility using the BigDye Terminator v3.1 (Applied Biosystems, Foster City, CA, USA) DNA sequencing system. Reaction products were analyzed by using an Applied Biosystems 3730xl automated DNA sequencing instrument. Sequences were examined with Finch TV 1.4.0 (Geospiza, Inc., Seattle, WA, USA; www.geospiza.com) and manually edited. A total of 23 individual worm sequences from 11 eels were available for analysis. These sequences were aligned along with 20 sequences of Gnathostoma spp. available on GenBank: G. spinigerum, G. binucleatum, G. hispidum, G. nipponicum, G. miyazakii, G. lamothei, G. doloresi, and G. turgidum from NCBI Database using ClustalW version 5.1 in MEGA (30) and manually trimmed to remove overhang. Sequences from this study were deposited in GenBank under accession nos. KF648531–KF648553.
Molecular analyses were conducted with MEGA version 5 (31). Cluster analyses were performed by using the unweighted pair group method with arithmetic mean (UPGMA) (32) and neighbor-joining algorithms. Statistical support for groupings was estimated by using bootstrap analysis.
Results
All 47 market swamp eels (M. cuchia) examined were adult-sized (Table 1). The 67 wild-caught specimens (M. albus) examined included juveniles and adults. Based on results from an age growth study conducted on M. albus swamp eels from a subtropical lake in China (33), sizes of swamp eels in current study corresponded to estimated ages ranging from <1 year (eels <200 mm TL) to ≥5 years (eels >500 mm TL).
Thirty-six AL3 of G. spinigerum roundworms were recovered from 13 (27.7%) M. cuchia swamp eels purchased from markets (5 from an Atlanta market; 5 from Orlando markets; 3 from New York markets). Five AL3 of G. turgidum roundworms and 1 AL3 of G. lamothei roundworms were collected from 2 (2.9%) and 1 (1.4%) of the M. albus eels obtained from wild populations in the Tampa, Florida, area. All AL3 were live and found in the digest residues of the following tissues: 21 in livers, 12 in muscles, 5 in gastrointestinal tracts and 4 in the kidneys. Only 1 eel had grossly visible white nodules on the liver, which contained AL3 of G. spinigerum. Among wild M. albus populations, gnathostomes were only collected from swamp eels from the Tampa population (Table 1).
All AL3 of G. spinigerum were found in imported eels from the markets, whereas G. lamothei and G. turgidum were only found in introduced wild swamp eels from open waters in the Tampa area of Florida. Matching the molecular data to the cephalic bulb hooklet counts (Table 2) corroborated that AL3 of G. spinigerum could be readily distinguished from G. turgidum and G. lamothei by the higher number of hooks in the first row (Figure 1, panels E–I). AL3 of G. turgidum and G. lamothei showed overlap of hook number in rows 1–3, but the 2 species could be distinguished by the fewer hooks in the 4th row of G. lamothei.
Table 2. Hooklet numbers from the 4 rows of the cephalic bulbs of AL3 Gnathostoma spp. and corresponding GenBank sequences collected from live Asian eels from market and wild populations in the United States*.
| Eel species, individual no., collection site,† tissue infected‡, AL3 | Row 1 | Row 2 | Row 3 | Row 4 | Species identification and accession no. |
|---|---|---|---|---|---|
| Mc 3 G | 46 | 46 | 49 | 51 | G. spinigerum KF648531 |
| Mc 9 L, a | 49 | 50 | ND | ND | G. spinigerum KF648532 |
| Mc 9 L, b | 48 | 53 | 55 | 56 | G. spinigerum KF648533 |
| Mc 9 L, c | 42 | 45 | 43 | 52 | G. spinigerum KF648534 |
| Mc 9 L, d | 43 | 44 | 47 | 49 | G. spinigerum |
| Mc 9 L, e | 43 | 49 | 50 | 52 | G. spinigerum |
| Mc 9 L, f | 45 | 49 | 50 | 52 | G. spinigerum |
| Mc 9 L, g | 43 | 44 | 49 | 51 | G. spinigerum |
| Mc 10 L | 42 | 47 | 48 | 54 | G. spinigerum |
| Mc 11 L | 40 | 44 | 50 | 44 | G. spinigerum |
| Mc 12 L | 44 | 41 | 45 | 49 | G. spinigerum |
| Mc 17 L, a | 45 | 43 | 45 | 50 | G. spinigerum |
| Mc 17 L, b | 44 | 48 | 49 | 50 | G. spinigerum |
| Mc 17 L, c | 41 | 44 | 47 | 49 | G. spinigerum |
| Mc 17 L, d | ND | ND | ND | ND | G. spinigerum KF648535 |
| Mc 17 K | 43 | 44 | 44 | 46 | G. spinigerum |
| Mc 21 G | 42 | 45 | 47 | 52 | G. spinigerum KF648536 |
| Mc 26 M | 33 | 39 | 40 | 53 | G. spinigerum |
| Mc 28 K | 43 | 47 | 50 | 54 | G. spinigerum KF648552 |
| Mc 28 M | 42 | 43 | 45 | 46 | G. spinigerum KF648551 |
| Mc 30 G | 40 | 43 | 42 | 47 | G. spinigerum KF648550 |
| Mc 32 M | 44 | 45 | 50 | – | G. spinigerum KF648553 |
| Mc 37 L | 42 | 47 | 50 | 54 | G. spinigerum KF648549 |
| Mc 58 M, a | ND | ND | 44 | 52 | G. spinigerum KF648542 |
| Mc 58 M, b | 46 | 49 | 51 | 52 | G. spinigerum KF648540 |
| Mc 58,G, c | 45 | 47 | 50 | 52 | G. spinigerum KF648541 |
| Mc 59 G, a | 47 | 48 | 47 | 52/53 | G. spinigerum KF648538 |
| Mc 59 G, b | 48 | 50 | 52 | 55 | G. spinigerum KF648539 |
| Mc 59 M, c |
42 |
46 |
47 |
52 |
G. spinigerum KF648537 |
| Mean ± SD | 41.8 ± 3.2 | 45.9 ± 3.0 | 47.6 ± 3.3 | 51 ± 2.9 | G. spinigerum (this study) |
| Mean |
42.9 ± 2.4 |
44.3 ± 2.0 |
44.9 ± 3.4 |
49.0 ± 2.9 |
G. spinigerum† |
| Ma 48 M, 2a | 35 | 37 | 37 | 44 | G. turgidum KF648547 |
| Ma 48 M,1a | 36 | 34 | 37 | 42 | G.turgidum KF648548 |
| Ma 48 M, b | 32 | 39 | 40 | 48 | G. turgidum KF648546 |
| Ma 48 M, c | 37 | 40 | 41 | 45 | G. turgidum KF648545 |
| Ma 49 M, a |
35 |
37 |
36 |
42 |
G. turgidum KF648544 |
| Mean ± SD | 35 ± 1.9 | 37.4 ± 2.3 | 38.2 ± 2.2 | 44.2 ± 2.5 | G. turgidum (this study) |
| Mean ± SD |
30.8 ± 2.8 |
34.0 ± 2.4 |
36.7 ± 3.6 |
39.6 ± 2.7 |
G. turgidum‡ |
| Ma 54 A,K | 36 | 38 | 36 | 36 | G. lamothei (this study) KF648543 |
*AL3, advanced larval stage 3; Mc, Monopterus cuchia; G, gut; L, liver; ND, not determined; K, kidney; M, muscle; Ma, M. albus. Letters after tissue type indicate that multiple larvae were found in 1 sample. †See (29). ‡See (28).
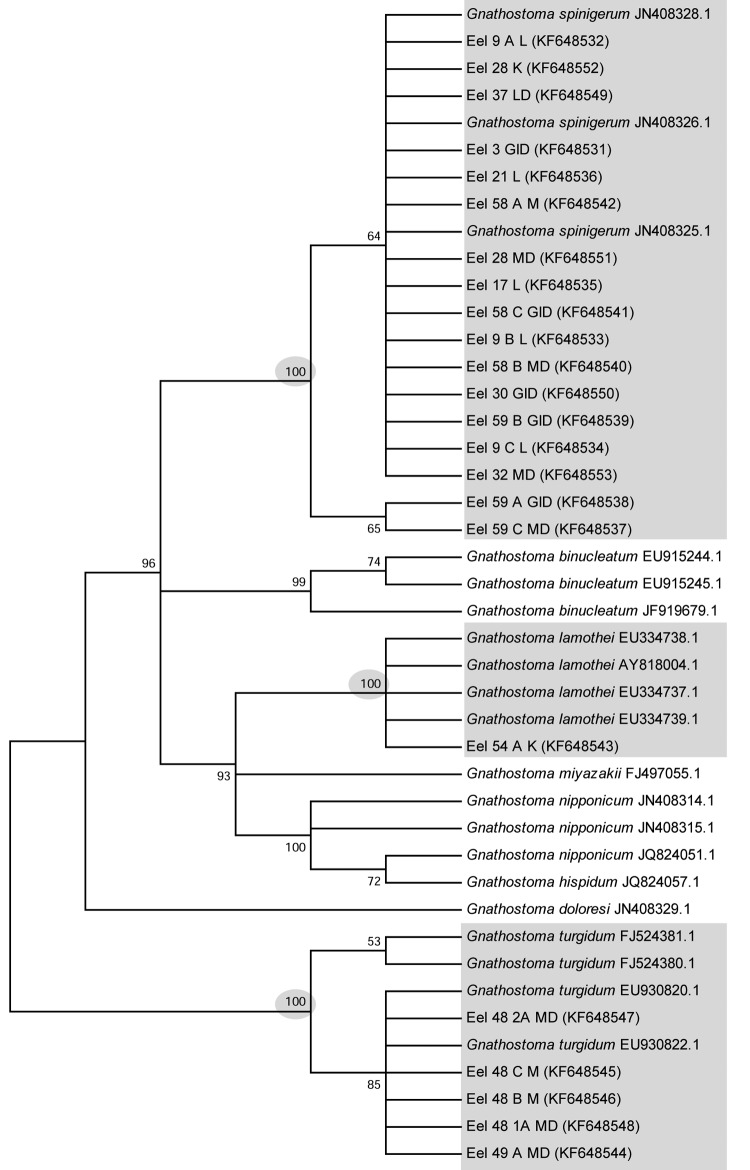
Assembled sequences of the amplicons varied from 511 to 659 bp. Mapping sequences to a reference sequence of G. spinigerum, (GenBank accession no. AB181155) comprising partial 18S, entire ITS-1, 5.8S, ITS-2, and partial 28S regions, demonstrated our sequences (see Figure 2 for accession nos.) comprised partial 5.8S, entire ITS-2 and partial 28S regions of the rRNA. After alignment and trimming, the resulting aligned database used for UPGMA and neighbor-joining analyses comprised only the ITS-2 region. Sequences from the aligned file mapped to a region between bp 1415 and 1790 on the reference sequence (AB181155), i.e., entirely within the reported ITS-2 region (Figure 2; Table 2). The UPGMA and neighbor-joining analyses resulted in the 23 isolates from this study falling into 3 distinct clusters, each representing a distinct species of Gnathostoma: G. spinigerum (17 isolates), G. lamothei (1 isolate), and G. turgidum (5 isolates) with high nodal support. Only the neighbor-joining tree is shown.
Figure 2.
Dendogram showing the condensed bootstrap consensus tree (1,000 replicates) produced by neighbor-joining analysis for Ganthostoma spp. Partitions reproduced in <50% bootstrap replicates are collapsed. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates) is shown next to the branches. The sequences from the gnathostome larvae analyzed in this study fall within 3 distinct clusters (gray shading) corresponding to 3 species, with high nodal support (100%).
Discussion
During 2005–2008, more than 1 billion live animals were legally imported into the United States for food and pet trade markets (34). Possibly beginning in the 1990s, large numbers of live Asian swamp eels were shipped from several different countries in Asia to US ethnic food markets (9). Through a series of Freedom of Information Act requests, we obtained USFWS-LEMIS shipment records for live animal imports for July1996–February 2010. Of the 815,000 imported live swamp eels, >95% were listed as originating from wild populations, not aquaculture sources. LEMIS provides records for wildlife shipments transported through 18 ports especially designated for such commerce. In their countries of origin (e.g., Thailand, Vietnam, China, Cambodia), swamp eels are commonly consumed as food by humans and widely available in food markets. In their native ranges, swamp eels and various other Asian freshwater fishes are infected with Gnathostoma spp. larvae (8,35). Increased global trade of live fish increases the risk that gnathostomes and other fish-borne parasites will be introduced into regions along with their introduced hosts.
Gnathostomiasis is a major foodborne parasitic zoonosis and a notable public health problem in areas where raw or undercooked freshwater fish are consumed by humans. Most human infections are in Southeast Asia, especially in Thailand. Infected persons can exhibit intermittent migratory subcutaneous swellings, which often recur over several years because of larval migrans. In some instances, larvae migrate into deeper tissues, causing visceral gnathostomaisis, which can be fatal if the larvae invade the central nervous system (7). Because human gnathostomiasis has been documented with increased frequency in countries where the parasite is not endemic, it is currently regarded as an emerging imported disease (26). Travel to a gnathostone-endemic area (within the past 10 years) and consuming raw or undercooked fresh water fish, frogs, poultry, or shellfish are key criteria used in diagnosing gnathostomiasis (26,36).
Our data show that live swamp eels imported to the United States from gnathostome-endemic areas could serve as a source of infection to humans in the United States. Therefore, travel outside of the United States to gnathostome-endemic areas may be limiting as a criterion in diagnosis. On rare occasions, an autochthonous infection has been reported in the United States (37,38). Several studies report a high prevalence of G. spinigerum in wild or farmed swamp eels in Southeast Asia (4–8).
Our recovery of live G. spinigerum from live swamp eels shipped from Asian sources to the United States is not surprising because the eels can survive for long periods in transport and at the market. In addition, AL3 are hardy and can remain alive for some time after the intermediate host is dead. The larvae can also survive 9–12 days at −9 to −4°C, 1 month at 4°C, and 8–9 days in 28%–35% ethanol (7). Considering food safety, evisceration of eels in this study rid the carcass of most of the worms (71.4%); however, 28.5% of larvae were found in the muscle, causing a risk for the consumer of raw or undercooked meat, or meat that was not frozen sufficiently before it was eaten raw. With respect to release of G. spinigerum roundworms into native fish and wildlife, through disposal of offal or the actual release of live swamp eels into open waters, could facilitate parasite introduction because the US environment has all the components of the parasite’s life cycle: canines and felines that serve as definitive hosts; cyclopoid copepods that serve as first intermediate hosts; fish, amphibians, and birds that serve as second intermediate hosts; and reptiles that serve as paratenic hosts (7).
Past studies have documented high prevalence of G. spinigerum larvae in live M. albus eels sampled from the wild, aquaculture settings, and markets in Thailand, Vietnam, and a few other Asian countries (8,35). However, G. spinigerum worms have not been reported in M. cuchia, a swamp eel also native to Asia but with a different natural geographic distribution than that of members of the M. albus species complex. M. cuchia eels are native to Bangladesh, the probable source of our US market specimens. In Bangladesh, although 40% of dogs surveyed were infected with G. spinigerum, human gnathostomiasis is reportedly uncommon (39,40).
The recovery of G. turgidum and G. lamothei from 2 and 1 M. albus eels, respectively, collected in open waters of Florida demonstrates that this introduced species of eel is a suitable host for North American species of Gnathostoma. Although these species have not been reported to be zoonotic, it has been suggested that all species of Gnathostoma can most likely infect humans (7). The previous record of G. turgidum infection in the United States is from the liver of a Florida black bear (Ursus americanus floridanus) in 1932 (27). Adult G. turgidum worms infect species of opossum (Didelphidae) and are prevalent in Mexico and South and Central America (27). Frogs (Rana zweifeli) and mud turtles (Kinosternum integrum) were the main second intermediate and paratenic hosts respectively of G. turgidum in Mexico (28). Adult G. lamothei infections in raccoons (Procyon lotor hernandezii) have been described in Mexico (27).
Acknowledgments
We extend a special thanks to Hannah Hubanks, who assisted with eel necropsies and parasite collection. For assistance procuring wild specimens, we are grateful to Travis Tuten, Howard Jelks, Andre Daniels, Chris Smith, Scott Collenberg, Dave Gandy, Jennifer Rehage, Jessica Lee, Amy Narducci, and David Roche. Linda S. Nico helped survey and sample markets.
This study was funded by US Geological Survey, Invasive Species Program. All procedures using fish were approved by the Animal Care and Use Committee of USGS–Southeast Ecologic Science Center.
Biography
Dr Cole is a research zoologist and the head of the Diagnostic Parasitology Laboratory at the US Geological Survey, National Wildlife Health Center in Madison, WI. Her research interests are parasites of native and introduced fish and wildlife.
References
- 1.Smith HM. The fresh-water fishes of Siam, or Thailand. Washington (DC): Smithsonian Institution. United States National Museum Bulletin. 1945;188:1–622.
- 2.Cheng QT, Zhou CW. The fishes of Shandong Province [in Chinese]. Jinan (China): Shandong Science and Technology Press; 1997. [Google Scholar]
- 3.Khanh NH, Ngan HTB. Current practices of rice field eel Monopterus albus. Aquaculture Asia. 2010;15:26–30. [Google Scholar]
- 4.Nuamtanong S, Waikagul J, Anantaphruti MT. Gnathostome infection in swamp eels, Fluta alba, in central Thailand. Southeast Asian J Trop Med Public Health. 1998;29:144–7 . [PubMed] [Google Scholar]
- 5.Le XT, Rojekittikhun W. A survey of infective larvae of Gnathostoma in eels sold in Ho Chi Minh City. Southeast Asian J Trop Med Public Health. 2000;31:133–7 . [PubMed] [Google Scholar]
- 6.Rojekittikhun W. Gnathostomiasis in Thailand. In: Arizono N, Chai JY, Nawa Y, Takahashi Y, editors. Asian parasitology, vol. 1: food-borne helminthiasis in Asia. Chiba (Japan): Federation of Asian Parasitologists; 2005. p. 241–59. [Google Scholar]
- 7.Waikagul J, Diaz Chamacho SP. Gnathostomiasis. In: Murrell KD, Fried B, editors. Food-borne parasitic zoonoses: fish and plant–borne parasites. New York: Springer; 2007. p. 235–61. [Google Scholar]
- 8.Sieu TPM, Dung TTK, Nga NTQ, Hien TV, Daisgaard A, Waikagul J, et al. Prevalence of Gnathostoma spinigerum infection in wild and cultured swamp eels in Vietnam. J Parasitol. 2009;95:246–8. 10.1645/GE-1586.1 [DOI] [PubMed] [Google Scholar]
- 9.Nico LG, Sharp P, Collins TM. Imported Asian swamp eels (Synbranchidae: Monopterus) in North American live food markets: Potential vectors on non-native parasites. Aquatic Invasions. 2011;6:69–76.
- 10.Severinghaus LL, Chi L. Prayer animal release in Taiwan. Biol Conserv. 1999;89:301–4. 10.1016/S0006-3207(98)00155-4 [DOI] [Google Scholar]
- 11.Shiu H, Stokes L. Buddhist animal release practices: historic, environmental, public health and economic concerns. Contemporary Buddism. 2008;9:181–96.
- 12.Liu X, McGarrity ME, Li Y. The influence of traditional Buddhist wildlife release on biological invasions. Conservation Letters. 2012;5:107–14.
- 13.Henry S. Buddhists release creatures into Passaic. Herald News 2007. Aug 13.
- 14.Chai J-Y, Murrell KD, Lymbery AJ. Fish-borne parasitic zoonoses: status and issues. Int J Parasitol. 2005;35:1233–54. 10.1016/j.ijpara.2005.07.013 [DOI] [PubMed] [Google Scholar]
- 15.Phan VT, Ersbøll AK, Nguyen TT, Nguyen KV, Nguyen HT, Murrell D, et al. Freshwater aquaculture nurseries and infection of fish with zoonotic trematodes, Vietnam. Emerg Infect Dis. 2010;16:1905–9. 10.3201/eid1612.100422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffman GL, Schubert G. Some parasites of exotic fishes. In: Courtenay WR Jr, Stauffer JR Jr, editors. Distribution, biology, and management of exotic fishes. Baltimore: The Johns Hopkins University Press; 1984. p. 233–61. [Google Scholar]
- 17.Font WF. The global spread of parasites: what do Hawaiian streams tell us? Bioscience. 2003;53:1061–7. 10.1641/0006-3568(2003)053[1061:TGSOPW]2.0.CO;2 [DOI] [Google Scholar]
- 18.Peeler EJ, Oidtmann BC, Midtlyng PJ, Miossec L, Gozlan RE. Non-native aquatic animal introductions have driven disease emergence in Europe. Biol Invasions. 2011;13:1291–303. 10.1007/s10530-010-9890-9 [DOI] [Google Scholar]
- 19.Berra TM. Freshwater fish distribution. San Diego (CA): Academic Press; 2001. [Google Scholar]
- 20.Collins TM, Trexler JC, Nico LG, Rawlings TA. Genetic diversity in a morphologically conservative invasive taxon: multiple swamp eel introductions in the southeastern United States. Conserv Biol. 2002;16:1024–35. 10.1046/j.1523-1739.2002.01182.x [DOI] [Google Scholar]
- 21.Matsumoto S, Kon T, Yamaguchi M, Takeshima H, Yamazaki Y, Mukai T. Cryptic diversification of the swamp eel Monopterus albus in East and Southeast Asia with special reference to the Ryukyuan populations. Ichthyol Res. 2010;57:71–7. 10.1007/s10228-009-0125-y [DOI] [Google Scholar]
- 22.Jayaram KC. The freshwater fishes of the Indian region. Delhi (India): Narendra Publishing House; 1999. [Google Scholar]
- 23.Liem KF. Functional morphology of the integumentary, respiratory and digestive systems on the synbranchoid fish, Monopterus albus. Copeia.1967;1967:375–88.
- 24.Chan STH, Wright A, Phillips JG. The atretic structures in the gonads of the rice-field eel (Monopterus albus) during natural sex-reversal. J Zool. 1967;153:527–39. 10.1111/j.1469-7998.1967.tb04981.x [DOI] [Google Scholar]
- 25.Le XT, Pham TLH, Dekumyoy P, Nguyen HH, Le HK, Tran THV, et al. Gnathostoma infection in South Vietnam. Southeast Asian J Trop Med Public Health. 2004;35(Suppl 1):S97–9. [Google Scholar]
- 26.Herman JS, Chiodini PL. Gnathostomiasis, another emerging imported disease. Clin Microbiol Rev. 2009;22:484–92. 10.1128/CMR.00003-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bertoni-Ruiz F, Lamothe y Argumedo MR, García-Prieto L, Osorio-Sarabia D, León-Régagnon V. Systematics of the genus Gnathostoma (Nematoda: Gnathostomatidae) in the Americas. Revista Mexicana de Biodiversidad. 2011;82:453–64. [Google Scholar]
- 28.Mosqueda Cabrera MA, Miranda ES, Calderón LC, Nájera HEO. Finding advanced third-stage larvae of Gnathostoma turgidum Stossich, 1902 in Mexico form natural and experimental host and contributions to the life cycle description. Parasitol Res. 2009;104:1219–25. 10.1007/s00436-008-1318-4 [DOI] [PubMed] [Google Scholar]
- 29.Anantaphruti M, Stasuban P, Daengsvang S, Vajrasthira S. Electron microscopy of the advanced third-stage larvae of Gnathostoma spinigerum. Southeast Asian J Trop Med Public Health. 1982;13:531–40 . [PubMed] [Google Scholar]
- 30.Almeyda-Artigas RJ, Bargues MD, Mas-Coma S. ITS-2 rDNA sequencing of Gnathostoma species (Nematoda) and the elucidation of the species causing human gnathostomiasis in the Americas. J Parasitol. 2000;86:537–44 . [DOI] [PubMed] [Google Scholar]
- 31.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–9. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sneath PHA, Sokal RR. Numerical taxonomy. San Francisco: Freeman; 1973. [Google Scholar]
- 33.Yang MS, Xiong BX. Age and growth of Monopterus albus Zuiew, 1793 (Synbranchidae). J Appl Ichthyology. 2010;26:488–90. 10.1111/j.1439-0426.2009.01358.x [DOI] [Google Scholar]
- 34.United States Government Accountability Office (GAO). Live animal imports. [cited 2011 Sep 9]. GAO Highlights 2010; GAO-11–9 November. http://www.gao.gov/assets/320/312037.pdf
- 35.Rojekittikhun W, Chaiyasith T, Nuamtanong S, Komalamisra C. Gnathostoma infection in fish caught for local consumption in Nakhon Nayok Province, Thailand. I. Prevalence and fish species. Southeast Asian J Trop Med Public Health. 2004;35:523–30 . [PubMed] [Google Scholar]
- 36.Katchanov J, Sawanyawisuth K, Chotmongkol V, Nawa Y. Neurognathostomiasis, a neglected neglected parasitosis of the central nervous system. Emerg Infect Dis. 2011;17:1174–80. 10.3201/eid1707.101433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Catalano M, Kaswan D, Levi MH. Wider range for parasites that cause eosinophilic meningitis. Clin Infect Dis. 2009;49:1283. 10.1086/605687 [DOI] [PubMed] [Google Scholar]
- 38.Jarell AD, Dans MJ, Elston DM, Mathison BA, Ruben BS. Gnathostomiasis in a patient who frequently consumes sushi. Am J Dermatopathol. 2011;33:e91–3. 10.1097/DAD.0b013e31821cf4a6 [DOI] [PubMed] [Google Scholar]
- 39.Rahman MM. Gnathostomiasis: a rare nematode infection. Mymensingh Med J. 2006;15:105–7 . [DOI] [PubMed] [Google Scholar]
- 40.Samad MA. Public health threat caused by zoonotic diseases in Bangladesh. Bangl J Vet Med. 2011;9:95–120. [Google Scholar]