Abstract
Transplantation of culture-expanded adult stem/progenitor cells often results in poor cellular engraftment, survival, and migration into sites of tissue injury. Mesenchymal cells including fibroblasts and stromal cells secrete factors that protect injured tissues, promote tissue repair, and support many types of stem/progenitor cells in culture. We hypothesized that secreted factors in conditioned medium (CdM) from adult bone marrow-derived multipotent stromal cells (MSCs) could be used to prime adult cardiac stem/progenitor cells (CSCs/CPCs) and improve graft success after myocardial infarction (MI). Incubation of adult rat CPCs in CdM from human MSCs isolated by plastic adherence or by magnetic sorting against CD271 (a.k.a., p75 low-affinity nerve growth factor receptor; p75MSCs) induced phosphorylation of STAT3 and Akt in CPCs, supporting their proliferation under normoxic conditions and survival under hypoxic conditions (1% oxygen). Priming CSCs with 30x p75MSC CdM for 30 min prior to transplantation into sub-epicardial tissue 1 day after MI markedly increased engraftment compared with vehicle priming. Screening CdM with neutralizing/blocking antibodies identified Connective Tissue Growth Factor (CTGF) and Insulin as key factors in p75MSC CdM that protected CPCs. Human CTGF peptide (CTGF-D4) and Insulin synergistically promoted CPC survival during hypoxia in culture. Similar to CdM priming, priming of CSCs with CTGF-D4 and Insulin for 30 min prior to transplantation promoted robust engraftment, survival and migration of CSC derivatives at 1 week and 1 month after MI. Our results indicate that short-term priming of human CSCs with CTGF-D4 and Insulin may improve graft success and cardiac regeneration in patients with MI.
Keywords: Stem cells, progenitor cells, MSCs, CSCs/CPCs, stromal cells, paracrine, CTGF, Insulin
Introduction
Poor graft success is a common problem after transplantation of cultured cells into injured tissues and occurs with transplants of adult stem/progenitor cells, embryonic stem (ES) cells, and ES cell derivatives [1–3]. Despite rapid progress in methods to identify, isolate and culture candidate cells for tissue repair, the inability to effectively graft culture-expanded cells to diseased or injured adult tissues remains a challenge for many anticipated forms of cell therapy. Cell grafts for solid, non-hematopoietic tissues and organs such as the heart are particularly inefficient, especially after ischemic injury. Upon transplantation, culture-expanded cells can exhibit low adhesion to host tissue, low survival, and/or low levels of migration [2,3]. Improving these stages of cell engraftment is critical because they typically precede differentiation and functional integration of transplanted cells into host tissue. Recent efforts to improve graft success have utilized genetic manipulation to over-express pro-survival factors such as Akt in transplanted cells or co-administer cells with accessory materials/scaffolds to support the graft [4,5].
Paracrine activity from mesenchymal cells such as fibroblasts and other stromal cells promotes tissue repair after injury [6,7] and also regulates, in part, stem cell niches [8]. In the bone marrow, endothelial cells and stromal derivatives from non-hematopoietic progenitor cells (multipotent stromal cells, MSCs) support hematopoietic stem cells (HSCs) by providing critical structural and regulatory components of the hematopoietic niche. The niche components include cellular substrate, e.g. extracellular matrix, as well as multiple growth factors, cytokines, and hormones that influence HSC self-renewal, proliferation, survival, and function [8–12]. Due to their supportive roles, feeder layers of stromal cells (e.g. MSCs or fibroblasts) are commonly used to support the culture of HSCs, other types of adult stem/progenitor cells, and ES cells [12–15].
MSCs are typically isolated from total bone marrow mononuclear cells simply based on their adhesion to tissue culture plastic. To standardize isolation methods, several investigators have sorted human MSCs from bone marrow aspirates based on cell surface epitopes such as CD271 (p75 low-affinity nerve growth factor receptor, p75MSCs) or CD133 (Prominin 1, CD133MSCs) [16–18]. In some cases, sorting MSCs based on markers appears to enrich subpopulations of MSCs that differ in terms of paracrine activity. Of clinical interest, the different repertoires of secreted proteins/peptides may enhance particular therapeutic applications. For example, secreted factors from the CD133MSC subpopulation was shown to provide greater protection of cerebral tissue after stroke compared with those from the p75MSC subpopulation [18]. In transplantation studies, co-infusion of human HSCs and p75MSCs into immunodeficient mice provided a 10 to 23 fold improvement in multi-lineage engraftment of bone marrow compared with co-infusion of HSCs and typical (non-selected) human MSCs [19].
CD271+ cells characteristic of bone marrow p75MSCs are rapidly mobilized into the blood of patients with acute MI [20]. We hypothesized that marrow-derived CD271+cells participate in cardiac repair/remodeling after MI, in part, through paracrine activity. We investigated the effects of stromal cell-derived ligands on cardiac stem/progenitor cells (CSCs/CPCs) and found that conditioned medium (CdM) from human p75MSCs, but not from CD133MSCs, supported the proliferation and survival of adult rat CSCs/CPCs. Furthermore, priming of CSCs in p75MSC CdM for 30 min prior to transplantation markedly improved CSC grafts after MI. By screening p75MSC CdM for molecules that protected CPCs under hypoxic conditions, we identified two ligands with synergistic effects on CSC survival and developed a novel priming tool to enhance graft success.
Results
CdM from human stromal cells induces proliferation of rat CPCs
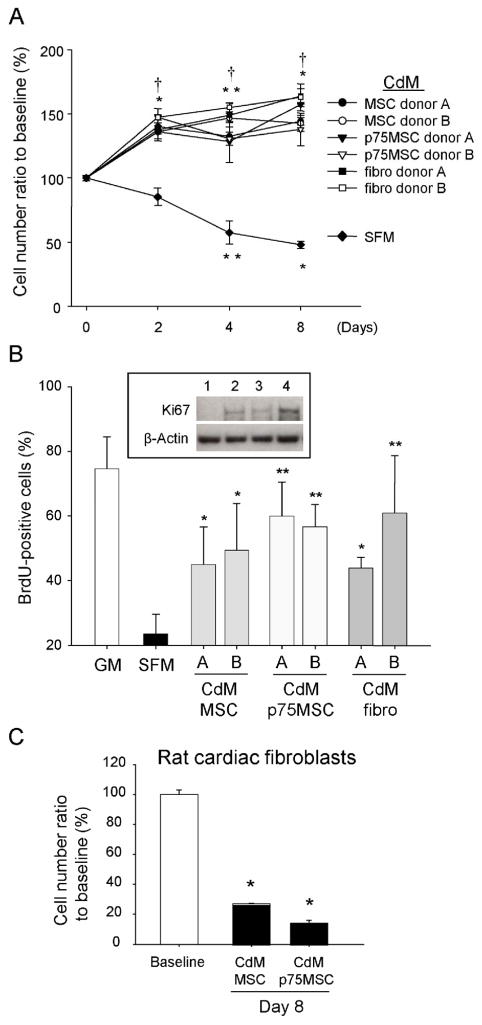
CdM was collected from human MSCs, p75MSCs, and dermal fibroblasts. CdM from each of the cell types supported CPC proliferation (Figure 1A and Supplemental Figure 1). We observed a concentration-dependent increase in CPC number when CPCs were incubated in 10x-concentrated CdM (10x CdM) from MSCs or p75MSCs (Supplemental Figure 2A). In contrast, when CPCs were incubated in CdM vehicle (serum-free α-MEM, SFM), their numbers gradually decreased (Figure 1A and B). To confirm DNA synthesis and active cell cycle status, we quantified incorporation of BrdU into CPCs 24 hrs after exposure to CdM. The percentage of BrdU-positive CPCs in CdM from MSCs, p75MSCs or fibroblasts was significantly greater than that for SFM-treated CPCs (Figure 1B). Immunoblotting demonstrated that Ki67 was expressed in CPCs treated with CdM but not in CPCs treated with SFM (Figure 1B). In contrast to its effects on CPCs, CdM from MSCs or p75MSCs did not support the proliferation of cardiac fibroblasts (Figure 1C).
Figure 1.
Human stromal cell CdM induces proliferation of adult rat CPCs. (A) Time course changes in the numbers of CPCs treated with CdM or SFM. CdM was assayed from 2 different donors for each cell type. Control cell number (48,896 cells) was regarded as 100%. Data are mean ± SEM, n = 3 to 7. *, P< 0.0001 vs baseline; **, P< 0.01 vs baseline; †, P< 0.0001 vs SFM. (B) Quantification of BrdU-positive CPCs after immunocytochemistry. Data for 2 human donors are shown for each cell type [donor A and donor B]. Inset, Immunoblot for Ki67 in CPCs (molecular weight, 359 kDa: lane 1, SFM; lane 2, 1x MSC CdM; lane 3, p75MSC CdM; lane 4, 1x fibroblast CdM). *, P< 0.05 vs SFM; **, P< 0.01 vs SFM. (C) MSC CdM does not support cardiac fibroblast proliferation. Control cell number (34,606 cells) was regarded as 100%. For B and C, data are mean ± SEM, n = 3. *, P< 0.0001 vs baseline. CdM, conditioned medium. SFM, serum-free α-MEM. GM, CPC growth medium (CSC medium with 2% FBS).
CdM from human MSCs activates STAT3 and Akt in CPCs
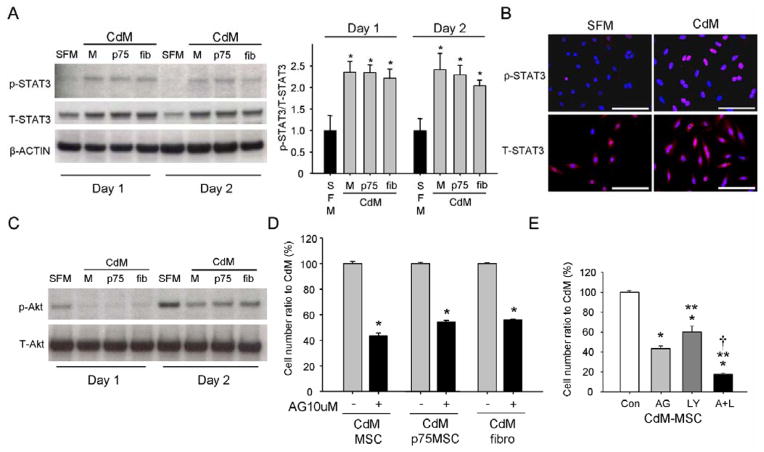
STAT3 activation is critical for self-renewal of ES cells, adult HSCs, and adult neural stem cells [22–24]. Accordingly, we assayed levels phosphorylated-STAT3 (p-STAT3; Tyr705) in CPCs exposed to CdMs from MSCs, p75MSCs, or fibroblasts. Levels of p-STAT3 were significantly higher in CPCs at 1 and 2 days after CdM treatment compared with SFM (Figure 2A). Immunocytochemistry demonstrated that p-STAT3 (Tyr 705) localized to the nuclei of CPCs treated with CdM (Figure 2B). In addition to p-STAT3, we observed phosphorylation of Akt (p-Akt; Ser473) after incubation of CPCs in CdM from each of the stromal cell types (Figure 2C). However, we also observed p-Akt in CPCs incubated in SFM alone (Figure 2C). Notably, auto-phosphorylation of signaling molecules affecting cell survival such as Akt, ERK1/2, and mTOR occurs in diverse cell types during serum or nutrient deprivation [25].
Figure 2.
STAT3 and Akt activation in CPCs treated with 1x CdM. (A) Left, Immunoblotting for phosphorylated STAT3 (p-STAT3) and total STAT3 (T-STAT3) in CPCs (molecular weight, 86 kDa). Beta-actin levels indicate loading. Right, Quantification of p-STAT3 levels (n = 3). The corrected values in SFM on day 1 and 2 were designated as “1”. *, P< 0.05 vs SFM. (B) Immunocytochemistry for p-STAT3 and T-STAT3. Note: p-STAT3 localizes to CPC nuclei (blue, DAPI). Scale bars = 100 μM. (C) Immunoblotting for phosphorylated Akt (p-Akt) and total Akt (T-Akt) in CPCs (molecular weight, 62 kDa). (D) Inhibitory effect of AG490 (10 μM) on CPC growth and survival in stromal cell CdM for 48 hrs. Control cell numbers (121,863 cells in MSC CdM, 115,342 cells in p75MSC CdM, and 118,682 cells in fibro CdM) were regarded as 100%. n= 3 replicates for 1 human donor per cell type. *, P< 0.0001 vs control. (E) Inhibitory effects of AG490 (10 μM) and LY294002 (10 μM) on CPCs incubated with 1x CdM for 48 hrs. Control cell numbers (121,863 cells) were regarded as 100%. Data are mean ± SEM, n = 3 to 6 replicates. *, P< 0.0001 vs control; **, P< 0.01 vs AG; †, P< 0.05 vs LY. Con: control, DMSO. AG: AG490, Jak2/STAT3 pathway inhibitor. LY: LY294002, inhibitor of PI3K/Akt pathway. A+L: AG490 + LY294002.
To determine whether p-STAT3 and/or p-Akt mediated the effects of CdM on CPCs, we treated CPCs with pharmacological inhibitors. AG490, the Jak2/STAT3 pathway inhibitor, reduced the number of CPCs treated with MSC CdM in a dose-dependent manner: control (CdM+DMSO), 100 ± 1.5%; 1 μM, 96.3 ± 0.9 %; 5 μM, 89.5 ± 0.9 %; 10 μM, 43.4 ± 2.4 % (cell number ratio to control cell number [121,863 cells], mean ± SEM, n = 3 to 6). The inhibitory effect of AG490 was also observed for CPCs treated with CdM from p75MSCs or fibroblasts (Figure 2D). LY294002, the phosphatidylinositol 3-kinase (PI3K)/Akt pathway inhibitor, decreased CPC number in CdM but to a lesser extent than did AG490 (Figure 2E). Combined treatment with AG490 and LY294002 was most effective in reducing CPC number in CdM (Figure 2E). Incubation of CPCs in “Stattic”, an inhibitor specific to STAT3, confirmed the role of STAT3 activation in CdM-mediated effects on CPC proliferation (Supplemental Figure 2B). AG490 treatment also significantly reduced the number of CPCs incubated in serum-free medium, however, the effect of the AG490 was nearly equal to that of LY294002 treatment (Supplemental Figure 2C). Of interest, LY294002 but not AG490 significantly diminished CPC numbers in growth medium, indicating that the factors promoting CPC growth in growth medium differed from the active factors in CdM that were STAT3-activating (Supplemental Figure 2C).
Insulin and Insulin-like growth factor 1 (IGF-1) bind to tyrosine kinase holoreceptors and promote cell survival and proliferation by signaling through the PI3K/Akt and Ras/MAP kinase pathways. Partial functional redundancy for Insulin and IGF-1 signaling is evidenced by signaling through IR/IGF-1R receptor heterodimers and bidirectional cross-talk by ligands and receptors [26]. Adult CPCs express the receptor for Insulin-like growth factor 1 (IGF-1) and injection of biotinylated IGF-1 nanofibers was shown to improve CPC grafts as well as endogenous myocardial regeneration after MI [27]. We observed that CSCs/CPCs did not proliferate in the absence of medium supplements containing Insulin (e.g. Insulin-Transferrin-Selenium [ITS] or fetal calf serum), even when in the presence of other mitogenic components from CSC/CPC growth medium such as LIF, EGF or bFGF (Supplemental Figure 3); this suggested that CdM from human stromal cells contained Insulin, IGF-1, or both.
CdM protects CPCs exposed to hypoxia
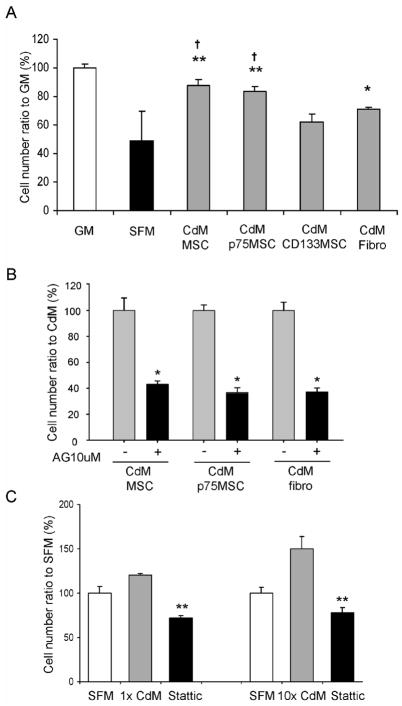
Because cells transplanted to the heart after MI may encounter hypoxic environments, we examined whether CdM could protect CPCs during exposure to 1% oxygen for 48 hrs ex vivo. Compared with cell survival in CPC growth medium (positive control; 100 ± 3.01%), survival of CPCs in SFM was 48.63 ± 21.13%, whereas that of CPCs incubated in MSC CdM was 87.515 ± 3.97% (P< 0.01 vs SFM), and in p75MSC CdM was 83.215 ± 4.005 % (P< 0.01 vs SFM). Survival of CPCs incubated in 1x CdM from MSC donors and p75MSC donors was not significantly different (n=4 donors per cell type, P= 0.206). Of note, CdM produced from a different subpopulation of bone marrow-derived MSCs, CD133MSCs, did not protect CPCs during culture under hypoxic conditions (P= 0.160 vs SFM). Skin fibroblast CdM significantly protected CPCs (70.79 ± 1.91%, P< 0.05 vs SFM), although both MSC CdM and p75MSC CdM were more protective than fibroblast CdM (each P< 0.01 vs fibro). Inhibition of STAT3 abolished CdM-mediated protection of CPCs under hypoxic conditions (AG490, Figure 3B; Stattic, Figure 3C).
Figure 3.
Protective effect of human stromal cell CdM on rat CPCs exposed to chronic hypoxia (1% oxygen for 48 hrs). (A) CPC numbers in GM, SFM and CdM after exposure to chronic hypoxia. Control cell number (58,616) was regarded as 100%. Data are mean ± SEM. CdM donors assayed for each cell type; n=3 (CD133MSC, Fibro), n=4 (p75MSC, MSC). *, P< 0.05 vs SFM; **, P< 0.01 vs SFM; †, P<0.01 vs CD133MSC CdM. (B) STAT3 inhibition with AG490 (10 μM) blocks CPC protection conferred by CdM during hypoxia. Control cell number (56,559 cells in MSC CdM, 92,120 cells in p75MSC CdM, and 74,511 cells in fibro CdM) was regarded as 100%. Data are mean ± SEM, n = 3 replicates for 1 human donor per cell type. *, P< 0.0001 vs CdM. AG: AG490, Jak2/STAT3 pathway inhibitor. (C) Survival of CPCs in p75MSC CdM and under hypoxic conditions for 48 hrs is dependent on signaling through STAT3 and abolished by incubation with the STAT3-specific inhibitor, “Stattic” (10 μM). n = 3 replicates for 1 human donor. **, P< 0.001 vs DMSO vehicle with 1x or 10x CdM. CdM, conditioned medium. SFM, serum-free α-MEM. GM, CPC growth medium (CSC medium with 2% FBS).
CPCs incubated in CdM retain their multipotent differentiation capacity
Differentiation assays were performed under normoxic conditions. Control CPCs cultured in CPC growth medium were negative for α-smooth muscle actin and von Willebrand Factor staining, whereas about 60% expressed α-sarcomeric actin (Supplemental Figure 4A, left and B). In contrast, clones of CPCs exposed to 1x CdM in culture for 4 days stained positively for α-sarcomeric actin, α-smooth muscle actin, and von Willebrand Factor (Supplemental Figure 4A and B). After 4 days in CdM, CPC-derived cells no longer expressed the CSC antigen, c-Kit, suggesting progress toward differentiation.
Effects of intra-arterial infusion of p75MSC CdM after MI
Based on our results with CdM and cultured CPCs, we examined whether infusion of CdM after MI would increase the number of endogenous CPCs. MI was produced in C57bl6 mice by permanent ligation of the left anterior descending coronary artery (LAD). The following day, mice were randomized to receive treatment with intra-arterial infusion (left ventricle lumen) of 30x p75MSC CdM or vehicle (SFM). Mice from each group were euthanized at 1 day or 1 week after treatment. Hearts obtained 1 day after treatment were sectioned and processed for TUNEL and immunohistochemistry to detect c-Kit. TUNEL assays showed that CdM infusion significantly reduced cardiac apoptosis/necrosis (SFM, 17.2 ± 8.1%; CdM, 3.7 ± 2.2%; P< 0.05, Supplemental Figure 5). Intra-muscular injection of porcine MSCs from bone marrow, but not their conditioned medium, was reported to increase the number of c-Kit+ cells in the hearts of pigs after MI [28]. After intra-arterial infusion of CdM from human p75MSCs, we detected rare c-Kit+ cells in heart sections from both CdM- and SFM-treated mice. However, because c-Kit+ cell number was variable and did not appear differ between mice that received CdM or SFM, we did not quantify c-Kit+ cells. Biochemical assays for residual myocardial Creatine Kinase (CK) activity in left ventricular (LV) homogenates indicated that CdM- and SFM-treated mice had similar size infarcts at 1 week after MI (Anterior LV, SFM, 4.66 ± 0.40; CdM, 5.00 ± 0.43, P=0.21; Posterior LV, SFM, 6.59 ±0.32; CdM, 6.34 ± 0.33, data expressed as IU CK/mg protein, mean ± SD, P=0.23; SFM, n=6; CdM, n=5).
Priming of CSCs with CdM from p75MSCs improves graft success after MI
Based on our observations with p75MSC CdM and its ability to increase proliferation and survival of cultured CSCs/CPCs, we next determined whether prior exposure of CSCs to p75MSC CdM could foster the grafting of CSCs to the injured heart. MI was produced in Fischer rats by permanent ligation of the LAD. One day after MI, syngeneic GFP-positive rat CSCs were primed for 30 min on ice in p75MSC CdM (30x CdM, n=6 rats) or vehicle (SFM, n=5 rats). The chest wall was re-opened and rats were randomized to treatment with co-injections of CSCs/CdM or CSCs/SFM (125,000 cells/5 μl injection, 2 sub-epicardial injections, 1 per border zone; please refer to Supplemental Figure 6 to view injection site). At 1 week and 1 month after MI, rats were euthanized and their hearts were processed as frozen serial-sections from apex to base. For each heart, we quantified GFP+ cells in the tissue section with the most GFP+ cells. At 1 week after MI, for 2 rats in the CSCs/SFM co-injection group, we did not detect GFP+ cells. Furthermore, hearts from CSC/SFM rats with the highest level of engraftment contained less than 40 GFP+ cells/section (Figure 4A). In contrast, many sections from rats co-injected with CSCs/CdM contained several thousand GFP+ cells (Figure 4B–E). After priming of CSCs in p75MSC CdM, GFP+ cells grafted into sub-epicardial locations, proliferated (see Ki67 stain, Figure 4C), and migrated into zones with infarction (Figure 4C–E and Supplemental Figure 6). Furthermore, after 1 week, derivatives from CSCs primed in CdM engrafted into blood vessel walls as CD31-positive endothelial cells (Figure 4F). They also generated smooth muscle cells and myofibroblasts (smooth muscle alpha actin-positive, Figure 4G). Although they grafted into sub-epicardial locations after MI, GFP+ cells derived from vehicle-primed CSCs did not stain for Ki67 and had not entered myocardial tissue from sub-epicardial tissue at 1 week after MI and treatment (Figure 4A). At 1 month after MI, GFP+ cells were detected in infarct and border zones in multiple tissue sections and differentiated to CD31-positive endothelial cells. Whereas engraftment was extensive in 4 out of 4 animals that were injected with CSCs primed in p75MSC CdM, only 1 out of the 5 control animals had detectable cell engraftment at 1 month after MI. However, similar to control cell grafts at 1 week after MI, this animal had only a few GFP+ cells in the sub-epicardial tissue and none in the myocardial tissue. (Supplemental Figure 7A–C″).
Figure 4.
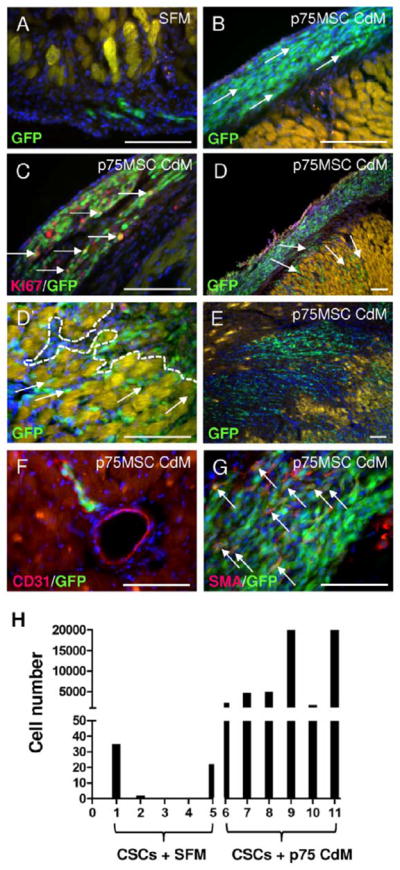
Priming of adult rat CSCs with human p75MSC CdM markedly improves CSC graft success 1 week after MI. At 1 day after MI, CSCs were primed in 30x CdM or vehicle (SFM) for 30 min on ice prior to co-injection (2 sub-epicardial injections per rat, 1 per border zone). (A) Image of the largest cell graft for control rats (n = 5), at 1 week after MI and injections of CSCs/SFM. Yellow autofluorescence indicates host-derived myocytes. (B–D) Images of sub-epicardial grafts from three different rats, 1 week after MI and injections of CSCs/CdM (n = 6). (C) Ki67 staining (red) of proliferating GFP+ CSC derivatives in sub-epicardial tissue after CSC/CdM injections. (D, D′) For hearts treated with CSCs/CdM, GFP+ cells were observed to migrate between apparently healthy cardiac myocytes in order to reach distant zones of necrotic myocardium with infarction. White arrows in (D) indicate change in CSC orientation during migration from sub-epicardium into myocardium after MI; compare to orientation in (B). The dashed white line in D′ indicates edge of infarction. (E) After CdM-priming, GFP+ CSC derivatives migrated into areas of necrosis with few remaining viable myocytes. (F) CdM-primed CSC derivatives differentiate into CD31-positive (red) vascular endothelial cells to repair blood vessels. (G) CdM-primed CSC derivatives differentiate into smooth muscle alpha actin-positive (SMA, red) smooth muscle cells and myofibroblasts (arrows indicate co-localizations with GFP). (H) Quantification of GFP+ cells from individual tissue sections with the most engraftment in hearts that received CSCs/SFM (rats 1 – 5) or CSCs/CdM (rats 6 – 11). Note: We stopped counting after 20,000 GFP+ cells for two CSC/CdM-treated rats, but observed thousands of additional GFP+ cells. Images A, B′, C, D′, F, G: Scale bars = 100 μM; D, E: Scale bars = 50 μM.
CdM contains human CTGF and bovine Insulin that activate CSCs/CPCs
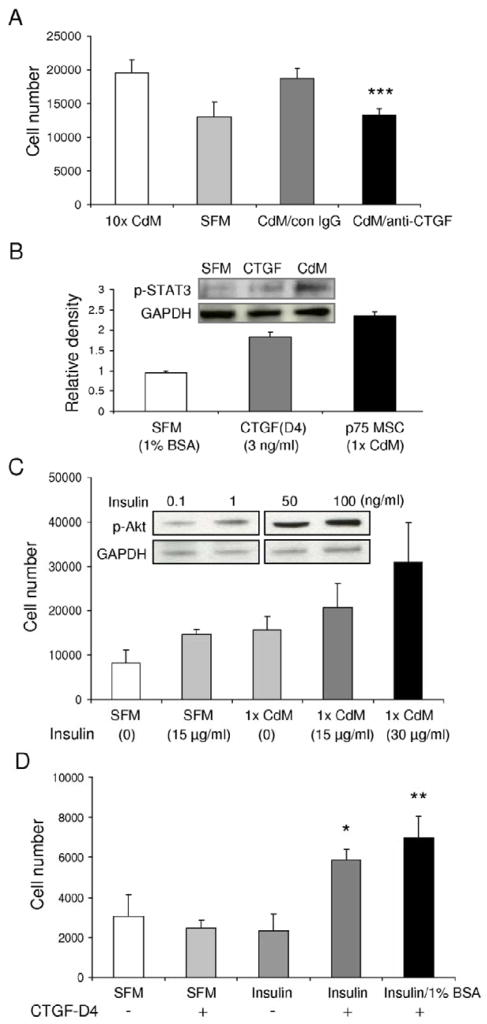
To identify factors in p75MSC CdM that promoted the proliferation and survival of CPCs, we examined Affymetrix gene expression profiles from human p75MSCs that were freshly sorted from marrow aspirates and from p75MSCs cultured adherently for 2 passages [18]. Antibody blocking/neutralization studies for selected secreted factors were carried out with 10x p75MSC CdM and CPCs exposed to hypoxic conditions (1% oxygen, 48 hrs). Under hypoxia, neutralizing antisera specific to human CTGF prevented CdM from protecting CPCs (P< 0.001, Figure 5A). Of interest, addition of human CTGF alone to SFM containing 1% BSA significantly induced p-STAT3 in CPCs (See inset, Figure 5B). By ELISA, 10x p75MSC CdM contained significantly more CTGF than did CD133MSC CdM (p75MSC, 1.29 ± 0.03 ng/ml; CD133MSC, 0.59 ± 0.13 ng/ml; P< 0.01), but did not differ in CTGF levels in comparison to MSC CdM (0.81 ± 0.14 ng/ml) or fibroblast CdM (1.1 ± 0.36 ng/ml). To our surprise, we did not detect IGF-1 in CdM, but we did detect Insulin. The amount of Insulin was not significantly different for CdMs from MSC, p75MSC, CD133MSC, or fibroblasts (about 2–3 ng/ml). Since the growth medium for MSCs and fibroblasts contained FCS (with bovine Insulin) and we did not detect human mRNA for Insulin by gene chip or by RT-PCR with cDNA from MSCs, we expect that the Insulin present in CdM was bovine in origin. Neutralizing antibodies specific to Insulin significantly reduced CdM-mediated protection of CPCs during hypoxia exposure, albeit not as much as did blocking CTGF (MTS assay[Abs490]: non-specific IgG, 0.378 ± 0.021; anti-Insulin, 0.270 ± 0.034; mean ± SD, n=4, P< 0.05). Addition of recombinant human Insulin to CPCs induced p-Akt in a dose-responsive manner and increased also CPC survival and proliferation under hypoxic conditions (Figure 5C). Notably, CPC protection assays with human Insulin or IGF-1 alone (30 ng/ml, each) demonstrated that they were equivalent in their ability to rescue CPCs exposed to hypoxia (control [SFM with 1% BSA]: 9004 ± 12 cells; Insulin: 13,507 ± 1,473 cells; IGF-1, 14,894 ± 559 cells; mean ± SD, n=3, P= 0.24).
Figure 5.
CTGF and Insulin are key factors present in p75MSC CdM that promote the survival and proliferation of CPCs. (A) Incubation of 10x p75MSC CdM with antisera specific to human CTGF ablates its ability to protect CPCs during 48 hrs of hypoxia. Non-specific IgG (con IgG) or anti-CTGF was added to separate aliquots of CdM (10 μg/ml, each). ***, P< 0.001 vs. con IgG. (B) CTGF (3 ng/ml, 30 min incubation) induces p-STAT3 in CPCs compared with incubation in vehicle (1% BSA). Levels of p-STAT3 after 30 min incubation in 1x CdM are shown for comparison. Inset, Representative blot. (C) Effects of Insulin on survival and growth of CPCs under normoxic conditions. Inset, Increasing Insulin concentration has dose-responsive effect on p-Akt levels in CPCs (30 min incubation). (D) Dual incubation of CPCs with C-terminal domain 4 peptide (CTGF D4) and Insulin (1 ng/ml, each) has synergistic effects on CPC survival during 48 hrs of hypoxia. *, P< 0.05 vs SFM; **, P< 0.01 vs SFM. For A–D, n = 3–5. CdM, conditioned medium. SFM, serum-free α-MEM.
CTGF (CCN2, IGFBP8) consists of 4 domains and the C-terminal (4th) domain alone was reported to increase cell adhesion and proliferation [29,30]. In experiments with recombinant peptides, we found that combined treatment with human C-terminal CTGF (CTGF-D4) and Insulin had synergistic effects on CPC survival and proliferation under hypoxic conditions. For example, CTGF-D4 or Insulin alone (1 ng/ml in SFM, each) did not protect CPCs against 48 hr of hypoxia (Figure 5D). In contrast, addition of both CTGF- D4 and Insulin to SFM (1 ng/ml, each) provided significant protection of CPCs against hypoxia (P< 0.05, Figure 5D). CTGF-D4/Insulin-mediated protection of CPCs was enhanced by including 1% BSA as a carrier (P< 0.01, Figure 5D). Clones of CPCs exposed to CTGF-D4 (3ng/ml) and Insulin (30ng/ml) in culture for 4 days stained positively for α-sarcomeric actin, α-smooth muscle actin, and von Willebrand Factor at ratios similar to those observed after 4 day CPC differentiation in 1x CdM (Supplemental Figure 4B). These results indicated that the multipotency of CPCs was retained after exposure to CTGF-D4 and Insulin.
A defined combination of CTGF-D4/Insulin promotes CSC grafts after MI
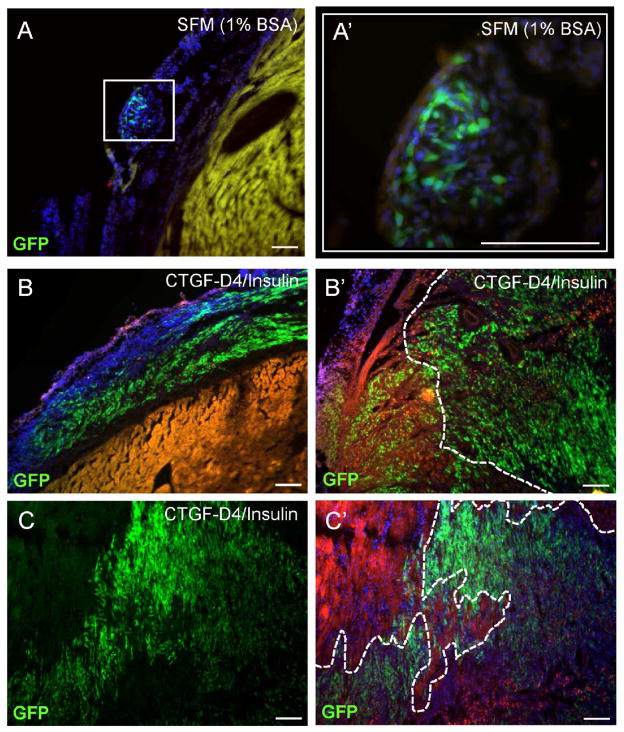
Having observed synergistic protective effects after CTGF-D4/Insulin treatment of cultured CPCs exposed to simulated ischemia, we hypothesized that a priming mixture based on the CTGF-D4/Insulin ratio found in 30x p75MSC CdM would promote CSC engraftment when using to prime CSCs. One day after MI, GFP CSCs were incubated on ice with SFM containing 1% BSA, CTGF-D4 (3 ng/ml), and Insulin (30 ng/ml), or with vehicle (SFM with 1% BSA) for 30 min prior to co-injection into border zone areas of rats randomized to treatment (125,000 cells/5 μl injection, 2 sub–epicardial injections, 1 per border zone). As before, all rats were euthanized 1 week after MI and their hearts were processed as serial sections. Whereas few rats that received co-injections of CSCs/vehicle had detectable GFP+ cells after 1 week (1/7 rats, Figure 6A and A′), all rats that received CSCs/CTGF-D4/Insulin exhibited a level of engraftment consistent with results obtained by priming with 30x p75MSC CdM (5/5 rats, Figure 6B–C′). Similar to CSCs primed in CdM, CSCs primed in CTGF-D4/Insulin grafted into sub-epicardial locations, proliferated, and provided GFP+ CSC derivatives that migrated into host myocardium, reaching areas of infarction with few remaining viable myocytes (Figure 6B′–C′). At one month after MI, 4 out of 5 rats that received CSCs primed in CTGF-D4/Insulin exhibited widespread engraftment cells of GFP+ derivatives. Similar to priming of CSCs in CdM, CTGF-D4/Insulin priming facilitated survival, migration and differentiation of CSC derivatives to CD31-positive and smooth muscle actin positive cells in infarct area at 1 month (Supplemental Figure 7D–E′).
Figure 6.
Priming of cultured CSCs in CTGF-D4 and Insulin promotes graft success after MI. (A, A′) Few control rats (1/7) injected with CSCs primed for 30 min in vehicle (SFM with 1% BSA) had detectable GFP+ cells at 1 week after MI and sub-epicardial injections. Note: GFP+ cells of control rats do not exit sub-epicardial graft site. (B–C′) All rats (5/5) that received CSCs primed with CTGF-D4 (3 ng/ml)/Insulin (30 ng/ml) demonstrated robust engraftment of GFP+ cells at 1 week after MI. (B, B′) Sub-epicardial engraftment (B) and extensive migration/integration (B′) of GFP+ cells 1 week after MI in a representative animal that received CSCs primed with CTGF-D4/Insulin. (C, C′) Engraftment of GFP+ cells 1 week after MI in a second representative animal that received CSCs primed with CTGF-D4/Insulin. Image demonstrates integration into area with infarction. FITC channel (C′) shows extent of migration into infarct. Note: White dashes in B′ and C′ indicate infarct border. Area beyond dashes has few viable myocytes that remain from host. Images A, B, B′, C, C′: Scale bars = 50 μM; image A′: Scale bar = 100 μM.
Discussion
Pre-conditioning strategies that expose cells to hypoxia, heat-shock, protein/peptide growth factors, chemicals and drugs have been shown to increase the survival of adult stem/progenitor cells, ES cells, and ES cell-derivatives after transplantation to the heart [31–36]. Although significant in terms of effect, many reported strategies result in modest cell grafts that are unlikely to repair the large tracts of necrotic tissue generated after MI. Our results indicate that short-term priming in p75MSC CdM or CTGF-D4/Insulin may boost graft success for clinical application of CSCs and perhaps also cultured stem/progenitor cells derived from other tissues or sources.
Priming in IGF-1 was reported to improve the survival of cardiac cell grafts with adult and embryonic stem cells [36]. We found that adult rat CPCs were protected equally well by Insulin or IGF-1 during hypoxia in culture. Although human p75MSC CdM did not have detectable IGF-1, we found that it contained sufficient residual bovine Insulin to significantly improve CPC survival under hypoxic conditions. CdM contains Insulin because MSCs internalize fetal calf serum components such as albumin, IgG, and Insulin from their growth medium. Even after multiple washes, they can release some components back into the base medium used for CdM production [37].
CTGF is a secreted “matricellular” protein with multiple functions in mammalian development and tissue remodeling/repair after injury, including angiogenesis and fibrosis [38–41]. During pancreatic development, CTGF promotes the proliferation of beta cell progentitors in islets [42]. Cardiac expression of CTGF increases significantly after MI and it is expressed by interstitial fibroblasts and cardiac myocytes [43]. By interacting with the extracellular matrix, integrins and several cell surface receptors (e.g. LRP-1, LRP-6, TrkA), CTGF mediates numerous cellular functions including: adhesion, proliferation, migration, differentiation, and survival [44–46]. To regulate coincident processes after injury such as angiogenesis and fibrosis, CTGF physically associates with numerous other secreted proteins including VEGFA, Slit3, von Willebrand Factor, PDGF-B, BMP-4, IGF-1, IGF-2, TGF alpha and TGF beta[30, 38–41]. Although we do not identify them here, it will be of great interest to determine which CTGF binding partners control graft success.
CTGF controls fibrosis in multiple tissues after injury, in part, by interacting with TGF beta, IGF-1 or IGF-2 and promoting the differentiation of fibroblasts into myofibroblasts. The N-terminal (1st) and 2nd domains of CTGF interact with IGFs and TGF beta or BMP4, respectively. Notably, due to its numerous binding partners, the effects of CTGF are context-dependent. In the presence of cellular mitogens such as EGF, CTGF does not induce fibrosis, even when pro-fibrotic mediators like TGF beta or IGF-2 are present [40,41]. Importantly, the CTGF-D4/Insulin priming method reported here is unlikely to promote myofibroblast differentiation or fibrosis from transplanted CSCs as CTGF-D4 is known to promote cell adhesion and proliferation, but lacks N-terminal functions in fibrosis [29, 30, 40, 41].
To date, clinical trials or animal models designed to graft cells to the heart after MI primarily administer cells intravenously, intra-arterially, or intra-muscularly. Here we developed a tangential injection method to deliver CSCs to the sub-epicardial space lying outside and adjacent to the injured myocardium. In addition to priming CSCs in p75MSC CdM or CTGF-D4/Insulin, we found that the border zone sub-epicardial space was important for cell injections. Notably, CSCs injected into the sub-epicardial space of healthy hearts, or, into the sub-epicardial space lying above normal, uninjured tissue in hearts with MI did not proliferate or migrate in the manner observed for border zone injections after MI (data not shown). These observations indicate that the border zone sub-epicardial environment may itself contain factors that promote cardiac graft success. Whereas our injections were performed in an open-chest fashion, for a less-invasive procedure in patients it may be possible to use catheter-based cell injection to reach the sub-epicardial space through the myocardium.
Multiple tissue-specific cell types express integrins and cell surface receptors are known to interact with CTGF and Insulin, including stem/progenitor cells of adult tissues. In addition to improving cell grafts for the heart, we are hopeful that priming with CdM from stromal progenitors or with CTGF-D4/Insulin will promote graft success for other solid tissues after injury.
Materials and Methods
Preparation of human stromal progenitor cells and fibroblasts
Human MSCs, p75MSCs and CD133MSCs were prepared with protocols approved by an Institutional Review Board. For the experiments outlined here, we used banked vials of stromal progenitor cells that were characterized previously for the same human donors [18]. Human dermal fibroblasts were obtained from the cell bank of the Tulane Center for Gene Therapy; they were isolated from skin punch biopsy samples under a protocol that was approved by an Institutional Review Board [18,47].
Preparation of serum-free conditioned medium (CdM)
Passage 4 to 8 human MSCs, p75MSCs, CD133MSCs, or dermal fibroblasts were cultured in 150 cm2 dishes with complete culture medium [18]. For the present study, the various human cell types were plated at 1000 cells/cm2 for seeding, underwent about 3 population doublings per passage, and were frozen down at each passage. To generate CdM, cells at 80 to 90% confluence were washed twice with PBS and incubated with 20 mls of fresh serum-free α-MEM in standard conditions without any supplements or growth factors for 48 hrs. CdM was then collected, filtered, and stored at − 80 C°. For some experiments, CdM was concentrated up to 10- or 30-fold with the use of a Labscale™ TFF diafiltration system (Pellicon XL 5 kDa cut-off filters, Millipore, Bedford, MA).
Isolation and culture of adult rat cardiac stem/progenitor cells
Adult CSCs were isolated from the ventricles of Fischer 344 rats and labeled with retroviral vector for GFP [21]. CSCs were cultured as floating spheres in DMEM/F12 supplemented with bFGF (10 ng/ml), EGF (20 ng/ml), LIF (10 ng/ml) and ITS. To grow adherent CPCs, CSCs were plated at 500 cells/cm2 and cultured in CSC medium supplemented with 2% FBS (CPC growth medium).
Short-term priming of CSCs
CSCs were cultured as spheres in serum-free CSC growth medium. CSC spheres were trypsinized and centrifuged at 1000 x g for 8 min. After re-suspension in 1x PBS, cells were passed through a 40 micron filter to isolate single CSCs (cell strainer, Fisher Scientific). Cells were counted on a hemocytometer, centrifuged again, and re-suspended in 30x p75MSC CdM or Alpha-MEM (CdM vehicle control), or CTGF-D4 (3ng/ml)/Insulin (30ng/ml)/1% BSA in α-MEM, or 1% BSA in α-MEM (vehicle control for recombinant peptides). CSCs were incubated in the above conditions for 30 minutes on ice prior to sub-epicardial injection.
Myocardial infarction surgery and CSC transplantation in rats
Fischer 488 rats (males, 7 weeks of age) were weighed, shaved, anesthetized under 4% isoflurane, and endotracheally-intubated. Rats were ventilated at a respiration rate of 65 beats per min under a peak inspiration pressure of 15 cm H2O (Kent Scientific). Body temperature was maintained at 37°C with a heating pad (Gaymar). Through a dermal incision, a blunt dissection of the fascia was performed and the intercostal muscles were separated. The heart was exposed by retraction of the pericardium to expose the LAD. The LAD was occluded with a 6-0 nylon suture and occlusion was confirmed by blanching of the anterior free wall of the LV. The animals were allowed to recover off the ventilator.
After 24 hours, rats were re-intubated, ventilated, and the chest wall was re-opened. Hearts were exposed to reveal the border zones of the infarct. For each rat, we performed 2 sub-epicardial injections of CSCs (5 μl each, one per border zone) with a 30 gauge Hamilton syringe. The needle was introduced tangentially to the wall of the LV and with the bevel facing upward. The syringe was advanced only as far as the bevel edge to access the sub-epicardial surface of the heart and so as not target the underlying myocardium of the LV. After the injections, the chest wall was closed and rats recovered for 7 days or 1 month prior to euthanization.
An expanded Methods section with detailed descriptions for isolation of human bone marrow-derived MSCs and p75MSCs, isolation and culture of adult rat cardiac fibroblasts, cell culture in CdM and evaluation of cell number, immunocytochemistry, DNA replication assays, immunoblotting, ELISAs for IGF-1, Insulin, and CTGF, MI surgery in mice, infusion of p75MSC CdM after MI in mice, TUNEL Assay, Creatine Kinase (CK) activity assay, immunohistochemistry, and statistical analysis is available in the online-only Data Supplement.
Supplementary Material
Acknowledgments
This work was supported in part by NIH grants HL077570 and HL085210 (to J.L.S.). Special thanks to Alexander Aronshtam, Ph.D., Keara McElroy-Yaggy, Patricia Baumann, Dagnija Neimane, and Calvin Yang for technical assistance.
Footnotes
Author Information. The authors have no conflicting financial interests
Y.I, K.S.R, C.N.P.: and J.L.S.: designed research; Y.I, K.S.R.: C.N.P.: A.K.M.T.Z, IC.: and J.L.S.: performed research, Y.I, K.S.R.: C.N.P, B.E.S.: and J.L.S.: analyzed data; J.K.: and P.A.: contributed vital reagents; Y.I, K.S.R, C.N.P, B.E.S.: and J.L.S.: wrote the paper.
References
- 1.Mohsin S, Siddiqi S, Collins B, et al. Empowering adult stem cells for myocardial regeneration. Circ Res. 2011;109(12):1415–1428. doi: 10.1161/CIRCRESAHA.111.243071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shimada IS, Spees JL. Stem and progenitor cells for neurological repair: minor issues, major hurdles, and exciting opportunities for paracrine-based therapeutics. J Cell Biochem. 2011;112(2):374–380. doi: 10.1002/jcb.22963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robey TE, Saiget MK, Reinecke H, et al. Systems approaches to preventing transplanted cell death in cardiac repair. J Mol Cell Cardiol. 2008;45(4):567–581. doi: 10.1016/j.yjmcc.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Segers VF, Lee RT. Biomaterials to enhance stem cell function in the heart. Circ Res. 2011;109(8):910–922. doi: 10.1161/CIRCRESAHA.111.249052. [DOI] [PubMed] [Google Scholar]
- 5.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9(9):1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 6.Kinnaird T, Stabile E, Burnett MS, et al. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 7.Gnecchi M, Zhang Z, Ni A, et al. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103(11):1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding L, Saunders TL, Enikolopov G, et al. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481(7382):457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Méndez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–34. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sacchetti B, Funari A, Michienzi S, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131(2):324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 11.Stier S, Ko Y, Forkert R, et al. Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. J Exp Med. 2005;201(11):1781–1791. doi: 10.1084/jem.20041992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dexter TM, Allen TD, Lajtha LG. Conditions controlling the proliferation of haemopoietic stem cells in vitro. J Cell Physiol. 1977;91(3):335–344. doi: 10.1002/jcp.1040910303. [DOI] [PubMed] [Google Scholar]
- 13.Messina E, De Angelis L, Frati G, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–921. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 14.Kim CF, Jackson EL, Woolfenden AE, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 15.Richards M, Fong CY, Chan WK, et al. Human feeders support prolonged undifferentiated growth of human inner cell masses and embryonic stem cells. Nat Biotechnol. 2002;20:933–936. doi: 10.1038/nbt726. [DOI] [PubMed] [Google Scholar]
- 16.Quirici N, Soligo D, Bossolasco P, et al. Isolation of bone marrow mesenchymal stem cells by anti-nerve growth factor receptor antibodies. Exp Hematol. 2002;30:783–791. doi: 10.1016/s0301-472x(02)00812-3. [DOI] [PubMed] [Google Scholar]
- 17.Cattoretti G, Schiro R, Orazi A, et al. Bone marrow stroma in humans: anti-nerve growth factor receptor antibodies selectively stain reticular cells in vivo and in vitro. Blood. 1993;81:1726–1738. [PubMed] [Google Scholar]
- 18.Bakondi B, Shimada IS, Perry A, et al. CD133 identifies a human bone marrow stem/progenitor cell sub-population with a repertoire of secreted factors that protect against stroke. Mol Ther. 2009;17(11):1938–1947. doi: 10.1038/mt.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuçi S, Kuçi Z, Kreyenberg H, et al. CD271 antigen defines a subset of multipotent stromal cells with immunosuppressive and lymphohematopoietic engraftment-promoting properties. Haematologica. 2010;95(4):651–659. doi: 10.3324/haematol.2009.015065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iso Y, Yamaya S, Sato T, et al. Distinct Mobilization of Circulating CD271+ Mesenchymal Progenitors from Hematopoietic Progenitors During Aging and After Myocardial Infarction. Stem Cells Trans Med 2012. 2012;1:462–468. doi: 10.5966/sctm.2011-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114(6):763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 22.Niwa H, Burdon T, Chambers I, et al. Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev. 1998;12(13):2048–2060. doi: 10.1101/gad.12.13.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chung YJ, Park BB, Kang YJ, et al. Unique effects of Stat3 on the early phase of hematopoietic stem cell regeneration. Blood. 2006;108(4):1208–1215. doi: 10.1182/blood-2006-01-010199. [DOI] [PubMed] [Google Scholar]
- 24.Yoshimatsu T, Kawaguchi D, Oishi K, et al. Non-cell-autonomous action of STAT3 in maintenance of neural precursor cells in the mouse neocortex. Development. 2006;133:2553–2563. doi: 10.1242/dev.02419. [DOI] [PubMed] [Google Scholar]
- 25.Pirkmajer S, Chibalin AV. Serum starvation: caveat emptor. Am J Physiol Cell Physiol. 2011;301(2):C272–279. doi: 10.1152/ajpcell.00091.2011. [DOI] [PubMed] [Google Scholar]
- 26.DeBosch BJ, Muslin AJ. Insulin signaling pathways and cardiac growth. J Mol Cell Cardiol. 2008;44(5):855–864. doi: 10.1016/j.yjmcc.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Padin-Iruegas ME, Misao Y, Davis ME, et al. Cardiac progenitor cells and biotinylated insulin-like growth factor-1 nanofibers improve endogenous and exogenous myocardial regeneration after infarction. Circulation. 2009;120(10):876–887. doi: 10.1161/CIRCULATIONAHA.109.852285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hatzistergos KE, Quevedo H, Oskouei BN, et al. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107(7):913–922. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steffen CL, Ball-Mirth DK, Harding PA, et al. Characterization of cell-associated and soluble forms of connective tissue growth factor (CTGF) produced by fibroblast cells in vitro. Growth Factors. 1998;15(3):199–213. doi: 10.3109/08977199809002117. [DOI] [PubMed] [Google Scholar]
- 30.Gao R, Brigstock DR. Connective tissue growth factor (CCN2) induces adhesion of rat activated hepatic stellate cells by binding of its C-terminal domain to integrin alpha(v)beta(3) and heparan sulfate proteoglycan. J Biol Chem. 2004;279(10):8848–8855. doi: 10.1074/jbc.M313204200. [DOI] [PubMed] [Google Scholar]
- 31.Kim HW, Haider HK, Jiang S, Ashraf M. Ischemic preconditioning augments survival of stem cells via miR-210 expression by targeting caspase-8-associated protein 2. J Biol Chem. 2009;284(48):33161–33168. doi: 10.1074/jbc.M109.020925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pasha Z, Wang Y, Sheikh R, et al. Preconditioning enhances cell survival and differentiation of stem cells during transplantation in infarcted myocardium. Cardiovasc Res. 2008;77(1):134–142. doi: 10.1093/cvr/cvm025. [DOI] [PubMed] [Google Scholar]
- 33.Lu G, Haider HK, Jiang S, Ashraf M. Sca-1+ stem cell survival and engraftment in the infarcted heart: dual role for preconditioning-induced connexin-43. Circulation. 2009;119(19):2587–2596. doi: 10.1161/CIRCULATIONAHA.108.827691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kofidis T, de Bruin JL, Yamane T, et al. Stimulation of paracrine pathways with growth factors enhances embryonic stem cell engraftment and host-specific differentiation in the heart after ischemic myocardial injury. Circulation. 2005;111(19):2486–2493. doi: 10.1161/01.CIR.0000165063.09283.A8. [DOI] [PubMed] [Google Scholar]
- 35.Kofidis T, de Bruin JL, Yamane T, et al. Insulin-like growth factor promotes engraftment, differentiation, and functional improvement after transfer of embryonic stem cells for myocardial restoration. Stem Cells. 2004;22(7):1239–1245. doi: 10.1634/stemcells.2004-0127. [DOI] [PubMed] [Google Scholar]
- 36.Martinez EC, Wang J, Gan SU, Kofidis T, et al. Ascorbic acid improves embryonic cardiomyoblast cell survival and promotes vascularization in potential myocardial grafts in vivo. Tissue Eng Part A. 2010;16(4):1349–1361. doi: 10.1089/ten.TEA.2009.0399. [DOI] [PubMed] [Google Scholar]
- 37.Spees JL, Gregory CA, Singh H, et al. Internalized antigens must be removed to prepare hypoimmunogenic mesenchymal stem cells for cell and gene therapy. Mol Ther. 2004;9(5):747–756. doi: 10.1016/j.ymthe.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 38.Shimo T, Nakanishi T, Nishida T, et al. Connective tissue growth factor induces the proliferation, migration, and tube formation of vascular endothelial cells in vitro, and angiogenesis in vivo. J Biochem. 1999;126(1):137–145. doi: 10.1093/oxfordjournals.jbchem.a022414. [DOI] [PubMed] [Google Scholar]
- 39.Pi L, Shenoy AK, Liu J, et al. CCN2/CTGF regulates neovessel formation via targeting structurally conserved cystine knot motifs in multiple angiogenic regulators. FASEB J. 2012;26(8):3365–3379. doi: 10.1096/fj.11-200154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grotendorst GR, Rahmanie H, Duncan MR. Combinatorial signaling pathways determine fibroblast proliferation and myofibroblast differentiation. FASEB J. 2004;18:469–479. doi: 10.1096/fj.03-0699com. [DOI] [PubMed] [Google Scholar]
- 41.Grotendorst GR, Duncan MR. Individual domains of connective tissue growth factor regulate fibroblast proliferation and myofibroblast differentiation. FASEB J. 2005;19(7):729–38. doi: 10.1096/fj.04-3217com. [DOI] [PubMed] [Google Scholar]
- 42.Guney MA, Petersen CP, Boustani A, Menon R, Warfield C, Grotendorst GR, Means AL, Economides AN, Gannon M, et al. Connective tissue growth factor acts within both endothelial cells and beta cells to promote proliferation of developing beta cells. Proc Natl Acad Sci U S A. 2011;108(37):15242–15247. doi: 10.1073/pnas.1100072108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohnishi H, Oka T, Kusachi S, et al. Increased expression of connective tissue growth factor in the infarct zone of experimentally induced myocardial infarction in rats. J Mol Cell Cardiol. 1998;30:2411–2422. doi: 10.1006/jmcc.1998.0799. [DOI] [PubMed] [Google Scholar]
- 44.Segarini PR, Nesbitt JE, Li D, et al. Carmichael DF. The low density lipoprotein receptor-related protein/alpha2-macroglobulin receptor is a receptor for connective tissue growth factor. J Biol Chem. 2001;276(44):40659–40667. doi: 10.1074/jbc.M105180200. [DOI] [PubMed] [Google Scholar]
- 45.Mercurio S, Latinkic B, Itasaki N, et al. Connective-tissue growth factor modulates WNT signalling and interacts with the WNT receptor complex. Development. 2004;131(9):2137–2147. doi: 10.1242/dev.01045. [DOI] [PubMed] [Google Scholar]
- 46.Wahab NA, Weston BS, Mason RM. Connective tissue growth factor CCN2 interacts with and activates the tyrosine kinase receptor TrkA. J Am Soc Nephrol. 2005;16(2):340–351. doi: 10.1681/ASN.2003100905. [DOI] [PubMed] [Google Scholar]
- 47.Spees JL, Olson SD, Whitney MJ, Prockop DJ. Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci USA. 2006;103(5):1283–1288. doi: 10.1073/pnas.0510511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.