Abstract
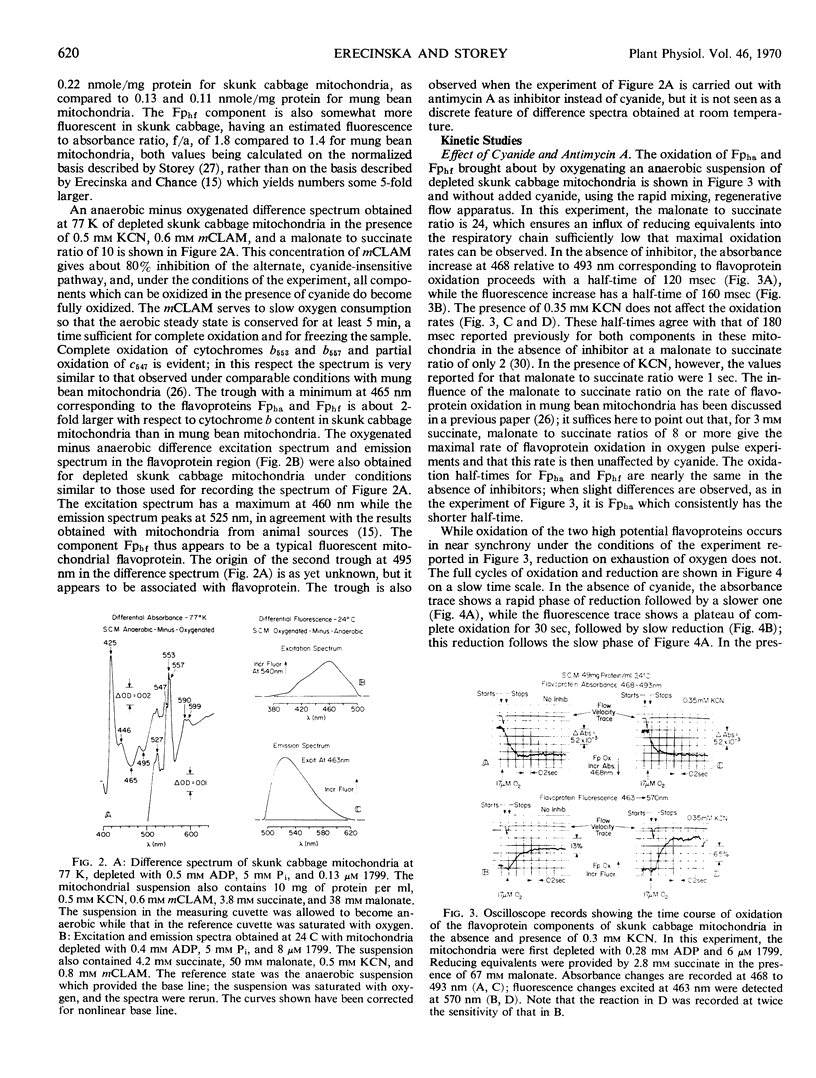
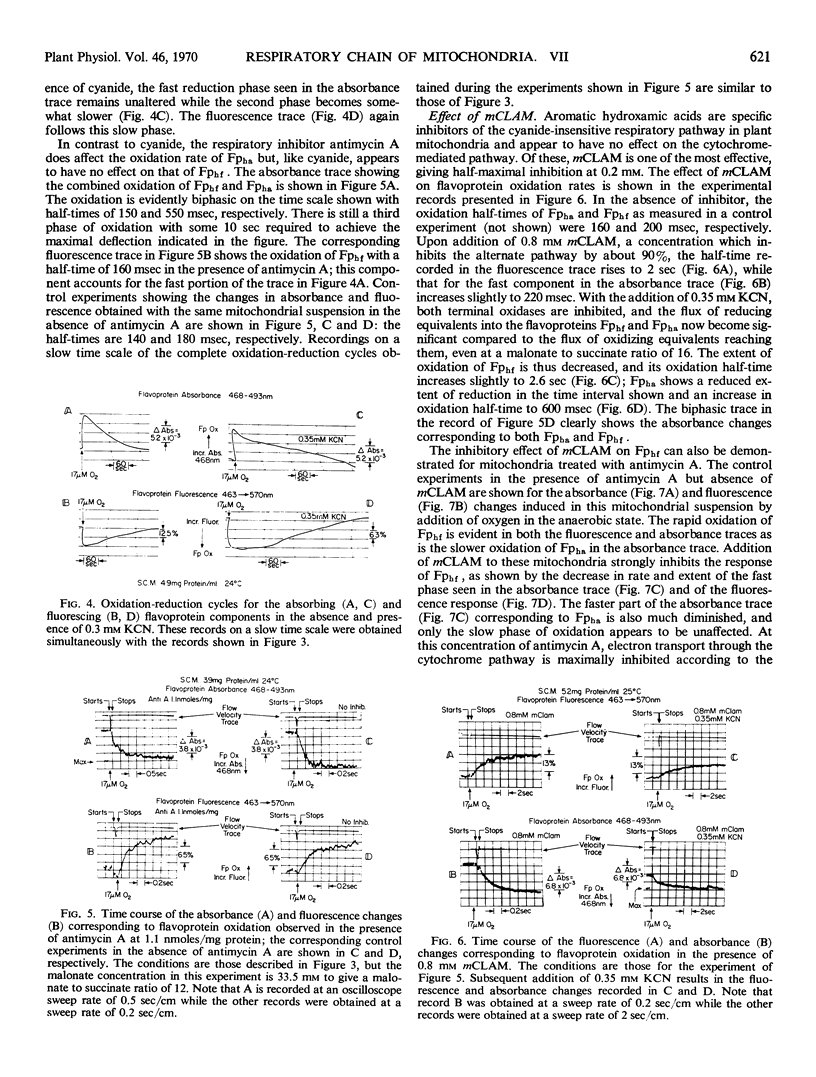
The oxidation kinetics of the two high potential flavo-proteins, one (Fphf) fluorescent and the other (Fpha) nonfluorescent, in mitochondria from skunk cabbage (Symplocarpus foetidus) spadices have been measured by combined spectrophotometry and fluorimetry. In the absence of respiratory inhibitors, both flavoproteins are oxidized at nearly the same rate with half-times between 120 and 160 milliseconds at 24 C. When slight differences in rate are observed, it is Fpha which consistently has the shorter half-time. The presence of 0.3 millimolar KCN has no perceptible effect on the oxidation rate of either component. Antimycin A (2 nanomoles per milligram of protein) increases the oxidation half-time of Fpha about 3-fold, but it has no effect on the oxidation half-time of Fphf. In contrast to these two inhibitors, m-chlorobenzhydroxamic acid—an inhibitor specific to the cyanide insensitive, alternate oxidase pathway in these mitochondria—increases the oxidation half-time of Fphf 10-fold to about 2 seconds, while increasing that of Fpha only some 20%. This result implies that the flavoprotein Fphf mediates electron transport to the alternate oxidase from the region of the mitochondrial respiratory chain encompassing Fpha, ubiquinone, and the cytochromes b. The oxidation rate of cytochrome b557 is unaffected by either m-chlorobenzhydroxamic acid or cyanide but is strongly inhibited by antimycin A. This result implies that cytochrome b557 plays no direct role in the respiratory pathway to the alternate oxidase and is different from cytochrome b7 found in mitochondria from the spadices of Arum maculatum.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENDALL D. S. Cytochromes and some respiratory enzymes in mitochondria from the spadix of Arum maculatum. Biochem J. 1958 Nov;70(3):381–390. doi: 10.1042/bj0700381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B., Bonner W. D. Kinetics of Cytochrome Oxidation in Skunk Cabbage Mitochondria. Plant Physiol. 1965 Nov;40(6):1198–1204. doi: 10.1104/pp.40.6.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B., Hackett D. P. The Electron Transfer System of Skunk Cabbage Mitochondria. Plant Physiol. 1959 Jan;34(1):33–49. doi: 10.1104/pp.34.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESTABROOK R. W. The low temperature spectra of hemoproteins. I. Apparatus and its application to a study of cytochrome c. J Biol Chem. 1956 Dec;223(2):781–794. [PubMed] [Google Scholar]
- HIGGINS J. Analysis of sequential reactions. Ann N Y Acad Sci. 1963 May 10;108:305–321. doi: 10.1111/j.1749-6632.1963.tb13382.x. [DOI] [PubMed] [Google Scholar]
- Hackett D. P., Haas D. W. Oxidative Phosphorylation and Functional Cytochromes in Skunk Cabbage Mitochondria. Plant Physiol. 1958 Jan;33(1):27–32. doi: 10.1104/pp.33.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuma H., Bonner W. D. Properties of Higher Plant Mitochondria. III. Effects of Respiratory Inhibitors. Plant Physiol. 1967 Nov;42(11):1535–1544. doi: 10.1104/pp.42.11.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lance C., Bonner W. D. The respiratory chain components of higher plant mitochondria. Plant Physiol. 1968 May;43(5):756–766. doi: 10.1104/pp.43.5.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragan C. I., Garland P. B. The intra-mitochondrial localization of flavoproteins previously assigned to the respiratory chain. Eur J Biochem. 1969 Oct;10(3):399–410. doi: 10.1111/j.1432-1033.1969.tb00704.x. [DOI] [PubMed] [Google Scholar]
- Storey B. T., Bahr J. T. The respiratory chain of plant mitochondria. I. Electron transport between succinate and oxygen in skunk cabbage mitochondria. Plant Physiol. 1969 Jan;44(1):115–125. doi: 10.1104/pp.44.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T., Bahr J. T. The respiratory chain of plant mitochondria. II. Oxidative phosphorylation in skunk cabbage mitochondria. Plant Physiol. 1969 Jan;44(1):126–134. doi: 10.1104/pp.44.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T. The Respiratory Chain of Plant Mitochondria. III. Oxidation Rates of the Cytochromes c and b in Mung Bean Mitochondria Reduced With Succinate. Plant Physiol. 1969 Mar;44(3):413–421. doi: 10.1104/pp.44.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T. The Respiratory Chain of Plant Mitochondria: VI. Flavoprotein Components of the Respiratory Chain of Mung Bean Mitochondria. Plant Physiol. 1970 Jul;46(1):13–20. doi: 10.1104/pp.46.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T. The Respiratory Chain of Plant Mitochondria: VIII. Reduction Kinetics of the Respiratory Chain Carriers of Mung Bean Mitochondria with Reduced Nicotinamide Adenine Dinucleotide. Plant Physiol. 1970 Oct;46(4):625–630. doi: 10.1104/pp.46.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T. The respiratory chain of plant mitochondria. IV. Oxidation rates of the respiratory carriers of mung bean mitochondria in the presence of cyanide. Plant Physiol. 1970 Apr;45(4):447–454. doi: 10.1104/pp.45.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban P. F., Klingenberg M. On the redox potentials of ubiquinone and cytochrome b in the respiratory chain. Eur J Biochem. 1969 Jul;9(4):519–525. doi: 10.1111/j.1432-1033.1969.tb00640.x. [DOI] [PubMed] [Google Scholar]



