Abstract
Dogs account for the majority of human exposures and deaths due to rabies virus (RABV) worldwide. In this report, we show that a replication-deficient RABV-based vaccine in which the matrix gene is deleted (RABV- M) is safe and induces rapid and potent VNA titers after a single inoculation in dogs. Average VNA titers peaked at 3.02 or 5.11 International Units (IU/ml) by 14 days post-immunization with a single dose of 106 or 107 focus forming units (ffu), respectively, of RABV- M. By day 70 post immunization, all dogs immunized with either dose of vaccine showed VNA titers >0.5 IU/ml, the level indicative of a satisfactory immunization. Importantly, no systemic or local reactions were noted in any dog immunized with RABV- M. The elimination of dog rabies through mass vaccination is hindered by limited resources, requirement for repeat vaccinations often for the life of a dog, and in some parts of the world, inferior vaccine quality. Our preliminary safety and immunogenicity data in dogs suggest that RABV- M might complement currently used inactivated RABV-based vaccines in vaccination campaigns by helping to obtain 100% response in vaccinated dogs, thereby increasing overall vaccination coverage.
Keywords: canine, rabies, replication-deficient RABV, vaccine
1. Introduction
Controlling rabies in domesticated or feral dogs is considered one of the most effective approaches to protect humans from rabies infection. In Central and South America, evidence suggests that continued mass vaccination of dogs helped to eliminate or contain rabies in that region of the world (1, 2). Nonetheless, over 90% of human rabies exposures, and over 99% of human rabies deaths, are still due to rabid dogs (2–5).
While the elimination of domesticated dog rabies through mass vaccination is considered feasible (2), limited resources, inferior vaccine quality in parts of the world, particularly in China, and low immunization coverage are considered important factors that hinder effective rabies control (5). When mass vaccination campaigns are implemented, they are often shown to be effective in achieving the WHO recommended rate of >70% canine vaccination rate (6). However, the turnover of the dog population, and the requirement for continual boost inoculations for the life of the dog, translates into millions of vaccinations or revaccinations every year (4). In some parts of the world, mass vaccination campaigns are not effective and programs would need to be implemented every 1 to 6 years to achieve the WHO recommended canine vaccination rate (7). Other regions of the world, such as in some rural areas of China where only 2–8% of the dogs are vaccinated (5), require mass vaccination programs to be initiated. To achieve the goal for improved and continued mass vaccination programs on a global scale, public health assistance and the availability of lower cost vaccine strategies are required (2, 7). One potential way to reduce the burden of public health dollars spent on the control of rabies virus in dogs is to reduce the number of inoculations required to protect each dog.
We previously showed that a replication-deficient RABV-based vaccine in which the matrix gene is deleted (RABV-ΔM) is immunogenic in mice and nonhuman primates (8–10). Furthermore, RABV- M is apathogenic and does not spread to the CNS of T and B cell-deficient mice (8). Due to the safety and immunogenicity profile of RABV- M, we tested the safety and antibody responses in dogs immunized with a single dose of RABV- M. We show that RABV- M is safe in dogs and results in potent anti-RABV immunity, indicating that RABV- M may help reduce the burden of canine rabies by helping to enhance vaccination coverage of pets and feral dogs and potentially reducing the need for repeated inoculations for protection.
2. Materials and Methods
2.1 Test Vaccine
The replication-deficient, matrix gene-deleted rabies virus-based vaccine (rRABV- M) used in these studies was cloned, recovered and characterized as described previously (8)Vaccine stocks were grown on BSR cells (a baby hamster kidney cell line) stably expressing RABV M (BSR-RABV M) (8, 11) in DMEM supplemented with 1% fetal bovine serum (FBS) for four days. Supernatants were collected and clarified by centrifugation at 900g, 10 minutes. Aliquots of the virus stock were frozen at −80° C until used. Viru1s was titered in triplicate using BSR-RABV M cells. Virus dilution were made in PBS on the day of immunization and kept on ice until administered. The virus stock was tested for sterility using general-purpose media. No growth was detected in fluid thioglycollate medium or trypticase soy broth (plus agar) inoculated with 100 μl virus stock and incubated at room temperature or at 35°C. Growth was detected in the positive control samples of fluid thioglycollate or trypticase soy broth at both temperatures; no growth was observed in the negative control using the same media at the same incubation temperatures.
2.2. Animals
Male and female canines (Closed Colony Mongrels; Liberty Research, Inc.) between the ages of 4–13 months were used. No history of rabies vaccination were noted and confirmed negative by Rapid Fluorescent Focus Inhibition Test (RFFIT) prior to the onset of the study [< 0.2 International Units (IU)/ml]. Dogs were acclimated to housing for at least 7 days prior to the start of the study. Animals were determined to be healthy and no clinical signs of disease were noted prior to vaccination. Animals were handled in compliance with Merial Institutional Animal Care and Use Committee (IACUC) approvals.
2.3. Immunization and sample collection
Dogs were divided into two groups of 5 dogs/group: Group A and Group B dogs received a single dose of 106 or 107 focus forming units (ffu)/ml of vaccine, respectively, in a volume of 1 ml administered subcutaneously in the shoulder area. Dogs were monitored intermittently for one hour post-vaccination for any systemic adverse events, and injection site observations and rectal temperatures were taken on days 0, 1, 2, and 3 post-immunization. Blood was collected on days 7, 14, 28, 49, and 70 post-immunization and then processed for serum, divided into two aliquots and the serum stored frozen (−20°C) until testing for serology testing.
2.4. Immune assays and statistical analysis
Serum rabies antibody titers were quantified using RFFIT (10, 12–14). Personnel performing the laboratory tests were blind to the treatment groups. A titer value less than or equal to the assay detection limit (0.2 IU/ml) was replaced by 0.2 IU/ml for all analyses. Statistical analyses were conducted using SAS v9.1 Enterprise Guide (SAS Institute, Cary, NC). All tests were two sided and statistical significance was declared at a P value of 0.05 or less.
3. Results
3.1. Immunization of dogs with RABV- M does not result in systemic or local adverse events
Five 4–13 month-old mongrel dogs per group were injected subcutaneously with 106 or 107 ffu of RABV- M. No vaccination site reaction, pain upon palpation or injection site swelling was observed in either group. Rectal temperatures remained within normal limits for the duration of the study. In one dog (Group B), a rectal temperature of 40.4°C was recorded one day post-vaccination. However, this hyperthermia was probably not related to vaccination since this dog had a pre-vaccination baseline rectal temperature of 40.3°C. Dogs vaccinated with 106 or 107 ffu of RABV- M showed no clinical signs of disease for the duration of the study (i.e., 70 days post-injection). Together, the preliminary preclinical testing shows that RABV- M does not cause systemic or local adverse events in immunized dogs.
3.2. Immunization with RABV- M results in long lasting effective anti-RABV immunity in dogs
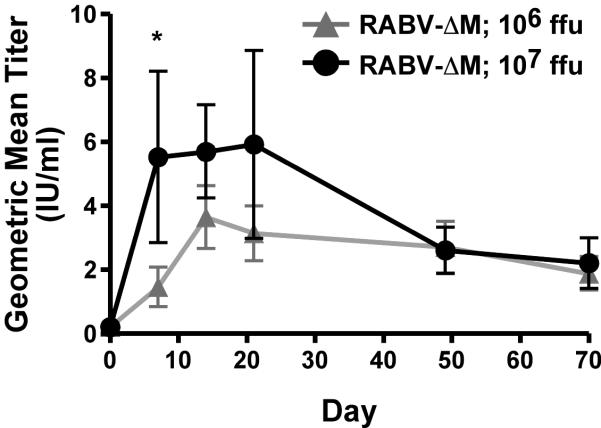
Serum rabies antibody titers were quantified using RFFIT from blood collected on days −29, 7, 14, 28, 49 and 70, relative to the day of vaccination. A VNA titer of 0.5 IU/ml is indicative of a satisfactory immunization of dogs by regulatory agencies from most areas of the world currently considered “rabies free” (15–17). As shown in the Table and Figure, animals immunized with either 106 or 107 ffu/dog showed VNA titers greater than 0.5 IU/ml by 14 days post-immunization, which corresponded to the peak average VNA titers in both groups of dogs. VNA titers then declined to a set point approximately two to eight times the indicative level of a satisfactory immunization at 70 days post-immunization. While peak VNA titers were higher in dogs immunized with 107 ffu compared to dogs immunized with 106 ffu, similar kinetics and longer-term VNA titers were detected in dogs immunized with 106 or 107 ffu of vaccine. No vaccine failures were noted in any animal. Together, this data indicates that RABV- M is highly immunogenic, safe, and induces potent anti-RABV immunity in dogs.
4. Discussion
The ability for current inactivated RABV-based vaccines to induce protective immunity in dogs relies on many host- and vaccine-specific factors. The age, size and breed of the dog and the type of vaccine, number of vaccinations and the interval(s) between vaccination influence the quantity and persistence of RABV-specific immunity (18–20). Based on these variables, the frequency of dogs that seroconvert after a single inoculation with inactivated RABV-based vaccines above 0.5 IU/ml varies widely from study to study and can range from 50% to >90% (18, 20–22). In dogs that do respond to vaccination with inactivated RABV-based vaccines, antibody titers peak around 2–4 weeks post-immunization and then rapidly decline (23). By six weeks post-immunization, the risk of vaccine failure (i.e., <0.5 IU/ml) statistically increases compared to the risk of vaccine failure just two weeks prior (19). Within six months of vaccination, less than 25% of dogs showed antibody titers greater than 0.5 IU/ml (21). Conversely, dogs receiving multiple doses of vaccine have titers greater than 0.5 IU/ml more frequently and for longer periods of time than in dogs that received only a single inoculation (21). Taken together, data from research and conventional vaccine protocols in dogs that require repeated boosters indicate that the effectiveness of inactivated RABV-based vaccines relies on multiple inoculations over the life of the dog for antibody titers to persist above 0.5 IU/ml.
To help circumvent the problems associated with lowly persisting antibody titers in response to inactivated RABV-based vaccines, alternative approaches have been tested in dogs, including DNA vaccines (24, 25) or live viral vaccines (26–30) expressing the RABV G [reviewed in (31)]. Of particular note, canine adenovirus 2 (CAV2)-based vaccines that express the RABV G showed promise in dogs (30); however, up to 62% of domesticated dogs tested seropositive for canine adenovirus in South Africa, suggesting that CAV2 naturally circulates in dog populations. Pre-existing immunity to CAV2 severely impairs the protection afforded by CAV2-RABV G, making CAV2 not suitable for mass vaccination programs (32). Pseudorabies virus (PRV), a herpesvirus of swine, is safe in dogs and has limited pre-existing immunity. A single inoculation with PRV expressing the ectodomain of RABV G was immunogenic in dogs, however, immunity was less than conventional vaccines and expressing the full-length RABV G gene instead of only the RABV G ectodomain may help to improve the efficacy of a PRV-based vaccine in dogs (28). Recently, a novel vaccine vector based on recombinant Parapoxvirus Orf Virus (ORFV) showed promise in a wide range of animal species, including dogs (29). Dogs are non-permissive hosts to ORFV, which limit its host and tissue tropism, rendering this virus safe. VNA titers indicative of protective immunity were detected in dogs immunized with a single dose of ORFV expressing RABV G through day 28. A boost inoculation enhanced VNA titers presumably due to a lack of vector-specific humoral immunity (29). The question remains whether this newly developed virus will induce sustained antibody titers after only a single inoculation, beyond the 28 days tested.
In this report, we show that RABV- M, which is very safe even in immune-deficient mice (8), induces high and persistent VNA titers in dogs after a single inoculation. Peak antibody titers were detected in dogs immunized with RABV- M around day 14 post-immunization with either 106 or 107 ffu. Except for antibody titers measured on day 7 post-immunization, no statistical significance was observed in dogs immunized with either dose of vaccine up to and including on the last day of sample collection (i.e., 70 days post-immunization), suggesting RABV- M is effective at a range of vaccine doses. Importantly, no vaccine failures were detected in any dog immunized with either dose. The persistence of VNA titers induced by RABV- M has been demonstrated in other species as well, including mice and non-human primates (8), and it would be expected that RABV- M would induce long-lasting immunity in dogs as well. Based on the immunogenicity data presented in this report, the use of RABV- M in dogs may help to complement currently used inactivated RABV-based vaccines in mass vaccination programs. In all, inclusion of RABV- M into mass vaccination programs might help to reduce the frequency by which dogs need to be immunized, lowering costs and potentially saving human lives.
Highlights
A replication-deficient rabies virus-based vaccine is proposed as a new vaccine against canine rabies virus.
Rapid and potent virus neutralizing antibodies were induced in dogs immunized with the replication-deficient rabies virus-based vaccine.
The replication-deficient rabies virus-based vaccine was safe in vaccinated dogs.
Figure 1.
Kinetics analyses of VNA responses in dogs immunized with RABV- M. Neutralization titers, defined as the inverse of the highest serum dilution that neutralizes 50% of the challenge virus (Challenge Virus Strain 11), were normalized to international units/ml (IU/ml) using the WHO anti-RV antibody reference standard. Virus neutralizing antibodies peaked on average around day 14 post-immunization and all dogs maintained VNA titers greater than 0.5 IU/ml for the duration of the study. Except for day 7, antibody titers were similar for each dilution at the indicated time points (*P value < 0.05). Error bars represent the standard error of the mean (SEM).
Table 1.
Virus neutralizing antibody titers in dogs immunized with RABV-ΔM
| Immunization Groupsa | VNA Titers per day post-immunization (International Units/ml)b | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | Vaccine | Dose | ID | Pre-screen | d7 | d14 | d21 | d49 | d70 |
| A | RABV-ΔM | 106 ffu | 1171704 | <= 0.2 | 3.2 | 7 | 6.2 | 5.4 | 3.7 |
| 1171706 | <= 0.2 | 0.7 | 2.9 | 2.7 | 1.4 | 0.8 | |||
| 1171904 | <= 0.2 | <= 0.2 | 0.9 | 2.7 | 2.7 | 2 | |||
| 1172303 | <= 0.2 | 0.5 | 3.7 | 3.2 | 3.2 | 2 | |||
| 1271803 | <= 0.2 | 2.7 | 3.7 | 0.9 | 0.8 | 0.9 | |||
| B | RABV-ΔM | 107 ffu | 1171702 | <= 0.2 | 16 | 6.2 | 4 | 2.9 | 0.9 |
| 1171703 | <= 0.2 | 3.2 | 11.2 | 17.6 | 4.3 | 4 | |||
| 1172307 | <= 0.2 | 0.7 | 3.7 | 3.7 | 4 | 4.3 | |||
| 1271401 | <= 0.2 | 4 | 4 | 2.3 | 0.8 | 0.9 | |||
| 1271801 | <= 0.2 | 3.7 | 3.4 | 2 | 1 | 0.9 | |||
Groups of 5 dogs per group were immunized with either 106 focus forming units (ffu) (Group A) or 107 ffu (Group B) of RABV-ΔM subcutaneously
Virus neutralizing antibody (VNA) titers were evaluated on the indicated days post-immunization by the Rapid Fluorescent Focus Inhibition Test (RFFIT) and displayed as International Units/ml.
Acknowledgements
We thank Marsha Royston for her technical assistance.
Funding and conflict of interest statement This study was supported in-part by 1) the National Institute of Allergy and Infectious Diseases (NIAID) Division of Intramural Research grant R01AI079211 to JPM. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. 2) Merial Inc. The funder was involved in study design, data analysis, and conduct of the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Belotto A, Leanes LF, Schneider MC, Tamayo H, Correa E. Overview of rabies in the Americas. Virus Res. 2005;111:5–12. doi: 10.1016/j.virusres.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Lembo T, Hampson K, Kaare MT, Ernest E, Knobel D, Kazwala RR, Haydon DT, Cleaveland S. The feasibility of canine rabies elimination in Africa: dispelling doubts with data. PLoS Negl Trop. Dis. 2010;4:e626. doi: 10.1371/journal.pntd.0000626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coetzee P, Nel LH. Emerging epidemic dog rabies in coastal South Africa: a molecular epidemiological analysis. Virus Res. 2007;126:186–195. doi: 10.1016/j.virusres.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 4.CDC 2012 http://www.cdc.gov/rabies/location/usa/cost.html.
- 5.Hu RL, Fooks AR, Zhang SF, Liu Y, Zhang F. Inferior rabies vaccine quality and low immunization coverage in dogs (Canis familiaris) in China. Epidemiol. Infect. 2008;136:1556–1563. doi: 10.1017/S0950268807000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davlin SL, Vonville HM. Canine rabies vaccination and domestic dog population characteristics in the developing world: a systematic review. Vaccine. 2012;30:3492–3502. doi: 10.1016/j.vaccine.2012.03.069. [DOI] [PubMed] [Google Scholar]
- 7.Durr S, Meltzer MI, Mindekem R, Zinsstag J. Owner valuation of rabies vaccination of dogs, Chad. Emerg. Infect. Dis. 2008;14:1650–1652. doi: 10.3201/eid1410.071490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cenna J, Hunter M, Tan GS, Papaneri AB, Ribka EP, Schnell MJ, Marx PA, McGettigan JP. Replication-deficient rabies virus-based vaccines are safe and immunogenic in mice and nonhuman primates. J Infect Dis. 2009;200:1251–60. doi: 10.1086/605949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dorfmeier CL, Lytle AG, Dunkel AL, Gatt A, McGettigan JP. Protective Vaccine-Induced CD4+ T Cell-Independent B Cell Responses against Rabies Infection. J. Virol. 2012 doi: 10.1128/JVI.00615-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dorfmeier CL, Shen S, Tzvetkov EP, McGettigan JP. Reinvestigating the role of IgM in rabies virus post-exposure vaccination. J. Virol. 2013 doi: 10.1128/JVI.00995-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mebatsion T, Weiland F, Conzelmann KK. Matrix protein of rabies virus is responsible for the assembly and budding of bullet-shaped particles and interacts with the transmembrane spike glycoprotein G. J Virol. 1999;73:242–50. doi: 10.1128/jvi.73.1.242-250.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dorfmeier CL, Lytle AG, Dunkel AL, Gatt A, McGettigan JP. Protective vaccine-induced CD4(+) T cell-independent B cell responses against rabies infection. J. Virol. 2012;86:11533–11540. doi: 10.1128/JVI.00615-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorfmeier CL, Tzvetkov EP, Gatt A, McGettigan JP. Investigating the Role for IL-21 in Rabies Virus Vaccine-induced Immunity. PLoS Negl Trop. Dis. 2013;7:e2129. doi: 10.1371/journal.pntd.0002129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cenna J, Tan GS, Papaneri AB, Dietzschold B, Schnell MJ, McGettigan JP. Immune modulating effect by a phosphoprotein-deleted rabies virus vaccine vector expressing two copies of the rabies virus glycoprotein gene. Vaccine. 2008;26:6405–14. doi: 10.1016/j.vaccine.2008.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Briggs DJ, Schweitzer K. Importation of dogs and cats to rabies-free areas of the world. Vet. Clin. North Am. Small Anim. Pract. 2001;31:573–83. viii. doi: 10.1016/s0195-5616(01)50610-5. [DOI] [PubMed] [Google Scholar]
- 16.Moore SM, Hanlon CA. Rabies-specific antibodies: measuring surrogates of protection against a fatal disease. PLoS Negl Trop. Dis. 2010;4:e595. doi: 10.1371/journal.pntd.0000595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fooks AR, McElhinney LM, Brookes SM, Johnson N, Keene V, Parsons G, Soldan A. Rabies antibody testing and the UK Pet Travel Scheme. Vet. Rec. 2002;150:428–430. [PubMed] [Google Scholar]
- 18.Berndtsson LT, Nyman AK, Rivera E, Klingeborn B. Factors associated with the success of rabies vaccination of dogs in Sweden. Acta Vet. Scand. 2011;53 doi: 10.1186/1751-0147-53-22. 22-0147-53-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mansfield KL, Burr PD, Snodgrass DR, Sayers R, Fooks AR. Factors affecting the serological response of dogs and cats to rabies vaccination. Vet. Rec. 2004;154:423–426. doi: 10.1136/vr.154.14.423. [DOI] [PubMed] [Google Scholar]
- 20.Gazi A, Seyyal A. Detection of Neutralising Antibody Titration in Vaccinated Owned and Stray Dogs against Rabies Virus. J. Fac. Vet. Med. Istanbul Univ. 2011;37:97. [Google Scholar]
- 21.Cliquet F, Verdier Y, Sagne L, Aubert M, Schereffer JL, Selve M, Wasniewski M, Servat A. Neutralising antibody titration in 25,000 sera of dogs and cats vaccinated against rabies in France, in the framework of the new regulations that offer an alternative to quarantine. Rev. Sci. Tech. 2003;22:857–866. doi: 10.20506/rst.22.3.1437. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe I, Yamada K, Aso A, Suda O, Matsumoto T, Yahiro T, Ahmed K, Nishizono A. Relationship between virus-neutralizing antibody levels and the number of rabies vaccinations: a prospective study of dogs in Japan. Jpn. J. Infect. Dis. 2013;66:17–21. doi: 10.7883/yoken.66.17. [DOI] [PubMed] [Google Scholar]
- 23.Minke JM, Bouvet J, Cliquet F, Wasniewski M, Guiot AL, Lemaitre L, Cariou C, Cozette V, Vergne L, Guigal PM. Comparison of antibody responses after vaccination with two inactivated rabies vaccines. Vet. Microbiol. 2009;133:283–286. doi: 10.1016/j.vetmic.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 24.Osorio JE, Tomlinson CC, Frank RS, Haanes EJ, Rushlow K, Haynes JR, Stinchcomb DT. Immunization of dogs and cats with a DNA vaccine against rabies virus. Vaccine. 1999;17:1109–1116. doi: 10.1016/s0264-410x(98)00328-4. [DOI] [PubMed] [Google Scholar]
- 25.Lodmell DL, Ewalt LC, Parnell MJ, Rupprecht CE, Hanlon CA. One-time intradermal DNA vaccination in ear pinnae one year prior to infection protects dogs against rabies virus. Vaccine. 2006;24:412–416. doi: 10.1016/j.vaccine.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Tims T, Briggs DJ, Davis RD, Moore SM, Xiang Z, Ertl HC, Fu ZF. Adult dogs receiving a rabies booster dose with a recombinant adenovirus expressing rabies virus glycoprotein develop high titers of neutralizing antibodies. Vaccine. 2000;18:2804–2807. doi: 10.1016/s0264-410x(00)00088-8. [DOI] [PubMed] [Google Scholar]
- 27.Zhang S, Liu Y, Fooks AR, Zhang F, Hu R. Oral vaccination of dogs (Canis familiaris) with baits containing the recombinant rabies-canine adenovirus type-2 vaccine confers long-lasting immunity against rabies. Vaccine. 2008;26:345–350. doi: 10.1016/j.vaccine.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 28.Yuan Z, Zhang S, Liu Y, Zhang F, Fooks AR, Li Q, Hu R. A recombinant pseudorabies virus expressing rabies virus glycoprotein: safety and immunogenicity in dogs. Vaccine. 2008;26:1314–1321. doi: 10.1016/j.vaccine.2007.12.050. [DOI] [PubMed] [Google Scholar]
- 29.Amann R, Rohde J, Wulle U, Conlee D, Raue R, Martinon O, Rziha HJ. A new rabies vaccine based on a recombinant ORF virus (parapoxvirus) expressing the rabies virus glycoprotein. J. Virol. 2013;87:1618–1630. doi: 10.1128/JVI.02470-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu R, Zhang S, Fooks AR, Yuan H, Liu Y, Li H, Tu C, Xia X, Xiao Y. Prevention of rabies virus infection in dogs by a recombinant canine adenovirus type-2 encoding the rabies virus glycoprotein. Microbes Infect. 2006;8:1090–1097. doi: 10.1016/j.micinf.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 31.Ertl HC. Novel vaccines to human rabies. PLoS Negl Trop. Dis. 2009;3:e515. doi: 10.1371/journal.pntd.0000515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wright N, Jackson FR, Niezgoda M, Ellison JA, Rupprecht CE, Nel LH. High prevalence of antibodies against canine adenovirus (CAV) type 2 in domestic dog populations in South Africa precludes the use of CAV-based recombinant rabies vaccines. Vaccine. 2013 doi: 10.1016/j.vaccine.2013.06.089. [DOI] [PubMed] [Google Scholar]