Introduction
The principle functions of arterial smooth muscle cells include contraction, relaxation and growth. Calcium signaling mechanisms govern the main functions of arterial smooth muscle and trigger specific responses.1-3 Cytosolic calcium concentrations and calcium signals are finely tuned by intracellular sources such as the sarcoplasmic reticulum, calcium-binding proteins and plasma membrane calcium permeable channels. In hypertension, these mechanisms are substantially modified, promoting a hypercontractile state and arterial wall remodeling. In this review, we will discuss various elements that are central to intracellular calcium handling and signaling in arterial smooth muscle cells. Emphasis will be given to most recent discoveries of components that link intracellular calcium stores to plasma membrane calcium entry channels. Additionally, we will propose a novel paradigm, suggesting that in hypertension, “alarm signals” generated by chronic innate immune system activation and transduced by pattern recognition receptors modulate calcium signaling mechanisms in arterial smooth muscle, promoting vascular dysfunction. Finally, new research directions in the context of calcium signaling in hypertension will be addressed.
Arterial Smooth Muscle Contractile Mechanism and Calcium Handling
Arterial smooth muscle contraction is regulated by receptor or mechanical activation of the contractile proteins actin and myosin.4 Changes in the membrane potential can also initiate contraction. The phosphorylation state of the light chain of myosin determines the contractile activity of arterial smooth muscle. Specifically, for contraction to occur, myosin light chain (MLC) kinase must phosphorylate Ser 19 of the 20 kDa regulatory MLC, enabling the interaction between myosin and actin.5, 6 The cycling of the myosin cross-bridges with actin is promoted by energy released from adenosine triphosphate (ATP) by myosin ATPase activity.7, 8 In some arteries, MLC is in a phosphorylated state in the absence of any external stimuli (i.e., vascular smooth muscle tone).
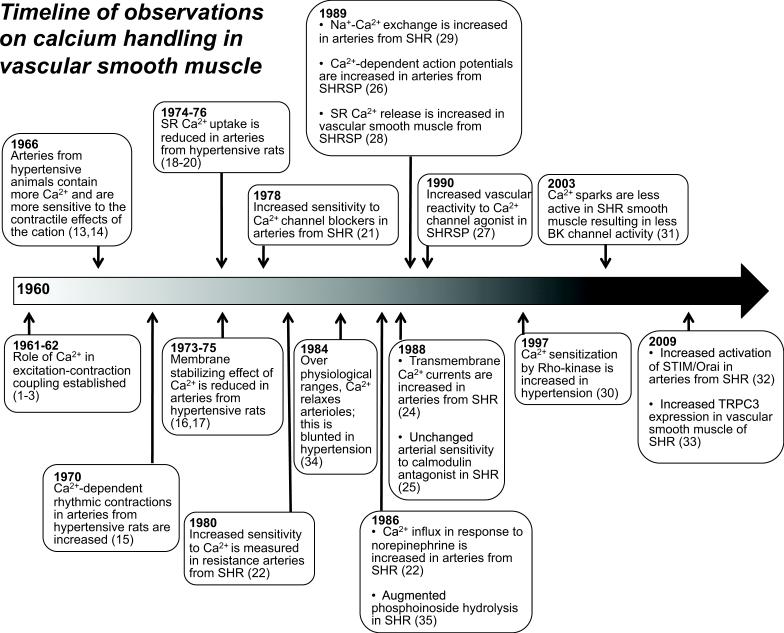
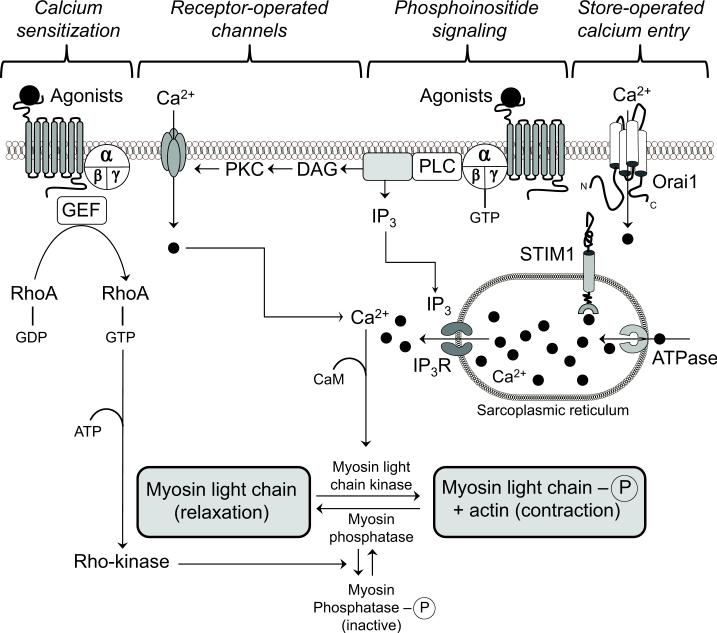
An increase in cytosolic calcium concentration is the trigger for vascular contraction.9 Hypertensive patients and animal models of hypertension exhibit augmented vascular contractile responses.10-12 A defect in the regulation of calcium and calcium signaling plays a role in hypertension-associated vascular dysfunction. Figure 1 illustrates a timeline of seminal scientific observations on calcium handling in arterial smooth muscle as it relates to hypertension1-3, 13-35 Abnormal calcium handling in arterial smooth muscle cells may involve increased calcium entry, increased calcium storage and/or decreased calcium extrusion. Figure 2 illustrates components of Ca2+ signaling in vascular arterial smooth muscle that participate in the contractile process.
Figure 1.
Timeline of observations on calcium handling in arterial smooth muscle related to hypertension (1960 to 2013). A clear role for calcium (Ca2+) as the activator for contraction in arterial smooth muscle was established in early 1960s. In the mid-60s, observations by several investigators demonstrated that arteries from hypertensive animals are more sensitive to the contractile effects of the cation. Over the following five decades, specifics about signaling cascades regulating intracellular Ca2+ in arterial smooth muscle of hypertensive animals and human subjects have been defined in greater detail. References for each observation are parenthetical. Abbreviations: Large conductance Ca2+-activated potassium channel (BK); sarcoplasmic reticulum (SR); spontaneously hypertensive rats (SHR); stroke-prone SHR (SHRSP); stromal interaction molecule (STIM) transient receptor potential cation channel (TPRC).
Figure 2.
Components of Ca2+ signaling in arterial smooth muscle that participate in the contractile process. The extracellular concentration of free Ca2+ is approximately 1.6 mmol/L whereas the intracellular concentration of the cation is 10,000-fold less (50-100 nmol/L). Abbreviations: adenosine triphosphate (ATP); diacylglycerol (DAG); guanine nucleotide exchange factor (GEF); guanosine diphosphate (GDP); guanosine triphosphate (GTP); inositol trisphosphate (IP3); IP3 receptor (IP3R); phosphoinosite phospholipase C (PLC); phosphatidylinositol 4,5-bisphosphate (PIP2); protein kinase C (PKC); stromal interaction molecule (STIM).
a. Calcium-dependent Contraction of Arterial Smooth Muscle
In vivo, the intracellular concentration of calcium is several orders of magnitude lower than that in the extracellular fluid and exhibits dynamic changes throughout the cell due to calcium flux. Strategic spatial positioning of intracellular calcium transporters and targets as well as spatial relationship of ion pumps and channels in the plasma membrane determine calcium flux and the fluctuations in intracellular calcium concentrations.36 The calcium/calmodulin complex activates MLC to phosphorylate the light chain of myosin. The increase in intracellular calcium concentration in response to specific stimuli occurs in a biphasic mode. In response to receptor-dependent or mechanical stimuli, intracellular calcium concentrations increase. The initial rapid increase in cytosolic calcium is due to calcium release from the sarcoplasmic reticulum, whereas the latter phase of calcium increase in the intracellular space is due to calcium entry from the extracellular space through plasma membrane calcium channels. Ligand-receptor interaction on the plasma membrane stimulates phospholipase C, which specifically catalyzes the formation of 2 second messengers, inositol triphosphate (IP3) and diacylglycerol (DAG). IP3 binds to receptors on the sarcoplasmic reticulum (IP3R) and initiates the release of calcium into the cytosol. This calcium binds to calmodulin, causing conformational changes, which allow the interaction of the calcium/calmodulin complex with the MLC. These events subsequently lead to activation of MLC kinase and phosphorylation of the regulatory MLC. DAG, along with calcium, activates protein kinase C (PKC), which phosphorylates specific target proteins (e.g. contractile proteins, regulatory proteins, channels, pumps, etc.) and has contraction-promoting effects.
b. Calcium-sensitization Mechanisms in Arterial Smooth Muscle
The force of contraction induced by ligand-receptor interaction in arterial smooth muscle is greater than that predicted by the actual calcium concentration in the cells, indicating the existence of calcium-independent mechanisms.37 In addition to MLC kinase, MLC phosphatase has a regulatory role in MLC phosphorylation.38-40 Activation of MLC phosphatase (or myosin phosphatase) promotes arterial smooth muscle relaxation via dephosphorylation of light chain of myosin. Myosin phosphatase has three subunits: a 37 kDa catalytic subunit (PP1c), a 20 kDa subunit, and a 110-130 kDa myosin-binding subunit (MYPT1). The binding of PP1c with MYPT1 inhibits the enzymatic activity of myosin phosphatase, allowing the light chain of myosin to remain phosphorylated, and thereby, promoting contraction.41
The calcium-sensitizing effect has been ascribed to the activation of the small G protein, RhoA, and its downstream effector Rho kinase.30 RhoA cycles between an inactive GDP-bound and an active GTP-bound state in response to various stimuli.42 Three classes of regulatory proteins facilitate activation/inactivation of RhoA: a) GTPase-activating proteins (GAPs) increase the intrinsic GTPase activity of RhoA to facilitate the return of the protein to its inactive state, b) guanine nucleotide dissociation inhibitors (GDIs) sequester the GDP-bound form of RhoA and prevent its binding to the membrane, c) Rho-specific guanine nucleotide exchange factors (RhoGEFs) enable the exchange of nucleotide to activate RhoA-GDP to Rho-GTP (active state).42 Upon activation, RhoA engages downstream effectors such as the enzyme Rho kinase, which phosphorylates the myosin-binding subunit of myosin phosphatase, inhibiting enzyme activity and promoting phosphorylation of MLC and contraction. Rho kinase is found in 2 isoforms: ROCK1 and ROCK2.43 Pharmacological inhibition of Rho kinase reduces blood pressure in experimental models of hypertension and induces relaxation in isolated arterial segments.30
c. Calcium Entry Mechanisms
Most calcium mobilization within the arterial smooth muscle cells is modulated by calcium entry channels, such as voltage-operated (VOC) and receptor-operated (ROC) channels, and store-operated calcium entry (SOCE) mechanisms. Other pathways, including purinergic receptors, transient receptor membrane potential (TRP) channels and Na+/Ca2+ exchanger (NCX), are also involved in calcium influx mechanisms but they will not be the focus of the current review.
Voltage-operated calcium channels
The function of VOC is regulated by membrane potential. Membrane hyperpolarization leads to VOC closure, whereas depolarization results in VOC opening and promotes contraction.44 L-Type VOC channels regulate the majority of agonist-induced calcium entry and depolarize in response to stretch, contributing to agonist-induced vasoconstriction and development of myogenic response and vascular tone, respectively. Increases in intracellular calcium concentration following VOC activation results in stimulation of calcium release from intracellular sources (e.g. sarcoplasmic reticulum) via activation of ryanodine receptors (RyR). This event leads to membrane depolarization, which further activates VOC and promotes calcium influx and constriction. Activation of cGMP-dependent protein kinase and further increases in intracellular calcium concentration act as negative feedback mechanisms that lead to the inhibition of VOC and cease of constriction.
Receptor-operated calcium channels
ROC channels are defined as channels where molecules are separate from the ligand-binding protein, are capable of activating a range of G protein coupled receptors via circulating ligands, and are neither VOC nor store-operated calcium channels (SOC).45 Following ligand binding, G protein coupled receptors, which are coupled to phospholipase C (PLC), activate ROC channels. This event leads to IP3 and DAG generation, subsequently promoting calcium release from the sarcoplasmic reticulum and PKC-associated activation of MLC kinase.46, 47 Members of the TRP channel family, including TRPC3, TRPC6 and TRPC7, have been shown to be components of ROC channels.48, 49
Store-operated calcium entry
The term store-operated calcium entry (SOCE) describes a cellular mechanism by which depletion of calcium content in the endo-/sarcoplasmic reticulum stimulates calcium influx via activation of plasma membrane calcium channels, replenishing intracellular calcium stores. In this mechanism, the endo-/sarcoplasmic reticulum acts as a capacitor.50 In non-excitable cells, SOCE is mediated by a highly calcium-sensitive, non-voltage-gated, inwardly rectifying current termed calcium release-activated calcium current (CRAC or ICRAC). Earlier studies demonstrated the existence of a diffusible messenger, calcium-influx factor (CIF). CIF production is restricted in the endoplasmic reticulum and its release is triggered by calcium depletion and/or a drop in intraluminal free calcium concentration. Upon its release from the endoplasmic reticulum, CIF induces displacement of inhibitory calmodulin from a plasma membrane variant of calcium-independent phospholipase A2 (iPLA2b), which transduces the signal to store-operated channels leading to their opening and activation of SOCE. Another pathway that has been shown to contribute to SOCE activation is NCX. When this exchanger is operating in the reverse mode it contributes to intracellular calcium depletion and subsequent activation of SOCE.51
Stromal interaction molecule 1 (STIM1) was first identified as a calcium sensor.52, 53 Currently, two members of the STIM family have been identified and characterized: STIM1 and STIM2. Upon calcium depletion STIM1 and STIM2 translocate towards junctional areas of the endoplasmic reticulum, known as puncta formations, which are in close proximity (10–25 nm) to the plasma membrane.54 STIM translocation is followed by activation of calcium release-activated calcium channels and SOCE in the plasma membrane. STIM2 plays a role in maintaining basal levels of calcium in the endoplasmic reticulum in the absence of agonist stimulation.55 In the presence of increasing agonist concentration, SOCE is mediated initially by STIM2 and incrementally by STIM1.56
Orai1 is a plasma membrane protein, an essential pore subunit of the CRAC channel57, 58 and the main means of communication between STIM1 and plasma membrane. The association of STIM1 with Orai1 triggers calcium influx, increases ICRAC, and is enhanced by thapsigargin,59 an inducer of calcium depletion in endo-/sarcoplasmic reticulum. However, the role of direct conformational coupling between STIM1 and Orai1 in SOCE activation and puncta formation has been challenged by evidence showing that Orai1 is not necessary for the accumulation of STIM1 in puncta as STIM1 accumulates in the absence and presence of Orai1.60 These recent data suggest the existence of additional intermediate elements. Accordingly, STIM1 and Orai1 interact with TRPC channels and TRPC channels may act as SOC in smooth muscle cells.61, 62
In contrast to non-excitable cells, where SOCE is mediated by ICRAC channels, in primary arterial smooth muscle cells, SOCE and agonist-induced contractile responses are mediated by nonselective cation channel. The presence of auxiliary mediators, such as iPLA2b and its lysophospholipid products, is required for signal transduction from STIM1 to plasma membrane SOC channels.63-65 Activation of nonselective cation SOC channels promotes calcium entry not only by mediating SOCE but also by playing the role of a depolarizing trigger for a secondary activation of VOC. This allows for further increases in calcium influx augmenting vasoconstriction.66, 67
The interaction of STIM1 and Orai1 plays a critical role in vascular contraction in various vascular beds and vessel types (i.e., aorta, coronary, and cerebral arteries).32, 64, 66, 68 RNA silencing of STIM1 and Orai1 reduced phenylephrine- and urotensin II-induced contractions in transfected coronary artery rings, whereas depolarization-induced contractions were not affected by downregulation of either Orai1 or STIM1.64 Further, thapsigargin-induced aortic contractions were attenuated following ex vivo treatment with Orai1 and STIM1 antibodies.32 Genetic manipulation of STIM1 further supports these data. Smooth muscle targeted STIM1 knock out mice had a 26% reduction in α1 adrenergic-induced aortic contraction in the absence of any effect on depolarization-induced contractile responses.32, 69
Aortae from spontaneous hypertensive stroke-prone rats (SHRSP) exhibited increased isometric force responses during the calcium-loading period on the depletion of intracellular calcium stores.70 Others have shown that the sarcoplasmic reticulum calcium store is larger in aortae from these animals due to enhanced influx of calcium across the sarcolemma.71 Activation of CRAC channels was enhanced in aortae from SHRSP compared to normotensive controls and CRAC/Orai1, through STIM1, contributed to augmented aortic contractility of hypertensive rats.32 We have proposed that sex differences in hypertension might be attributed to differences in STIM1/Orai1-mediated SOCE and intracellular calcium handling mechanisms.72 Force generation in aortae from genetically hypertensive rats was greater in males compared to females but this difference was abolished in the presence of antibodies against STIM1 and Orai1.72 Further, expression of these proteins was greater in male compared to female hypertensive rats. These studies were performed mostly in conduit arteries and the relevance of our findings to resistance vessels in the context of hypertension need to be examined.
Toll-like Receptors, Arterial Smooth Muscle Dysfunction, and Regulation of Calcium Handling in Hypertension: A New Paradigm
Long-term experimental efforts by several investigators, including our laboratory, have shed invaluable insight into the physiological mechanisms that are responsible for the pathogenesis of hypertension. Accordingly, previous studies have demonstrated evidence in support of “renocentric, neurocentric, and vasculocentric” views of the etiology of hypertension. These views need not be mutually exclusive. Most recently, low-grade inflammation and activation of the adaptive arm of the immune system have been implicated in hypertension, offering a potential link among the previously tested hypotheses and a new explanation for the multi-system effects of hypertension.73, 74
Recent studies show that host-derived molecules released to the extracellular space due to cell injury and/or death [damage-associated molecular patterns (DAMPs)] can trigger an inflammatory response via activation of the innate immune system. DAMPs include extracellular matrix components, plasma membrane, nuclear, and cytosolic proteins, and elements of damaged/fragmented organelles. Similar to pathogen-associated molecular patterns DAMPs stimulate pattern recognition receptors of the immune system, such as the Toll-like receptors (TLRs), eliciting an inflammatory response.
We recently reported that TLR4 was upregulated in resistance arteries of spontaneous hypertensive rats (SHR) and that TLR4 augmented activation contributed to increased contractile responses to norepinephrine and to elevated blood pressure levels in this rat model of hypertension.75 The cellular mechanism for these events was related to a COX-dependent mechanism since inhibition of COX-1 and COX-2 reduced contractile responses to norepinephrine in arteries from SHR but not in arteries from SHR treated with anti-TLR4.
Pathogen-associated molecular pattern recognition by TLRs and pathogen clearance after immune complex formation by engagement of Fc receptors are central mechanisms that trigger immune and inflammatory responses.76-79 In an analogous way necrotic cell death is increased in hypertension giving rise to DAMPs. Various DAMPs are associated with hypertension (e.g. mtDNA, Angiotensin II, oxidized lipoproteins, see ref. #80)80. There is cross talk between Fc receptors and TLRs to activate downstream signaling via PLC. Augmented PLC characterizes the accentuated vasoconstrictor response in hypertension, and this may be one mechanism where calcium signaling is amplified in hypertension. We propose that in hypertension, elevated levels of DAMPs activate TLRs, which then signal directly or through cells of the adaptive immune response to elicit an inflammatory process in organs and systems that regulate blood pressure.80-82
Future Research Directions: Promising Therapeutic Targets in Hypertension
Fibrocytes
From a traditional viewpoint, resident or adventitial fibroblasts have been thought to be activated by proinflammatory stimuli to proliferate and migrate to sites of vascular injury where they secrete collagen.83 However, recent work describes an important role for fibrocytes, cells that are implicated in chronic inflammation, fibrosis and wound healing. These cells are derived from bone marrow and are present in atherosclerotic lesions. They have inflammatory features of macrophages and vascular remodeling properties of fibroblasts. Additionally, fibrocytes develop α-actin expression and a contractile phenotype in tissue culture. Keeley et al84 observed increased circulating levels of fibrocytes in patients with hypertension and there was a strong correlation between left ventricular mass index and fibrocyte number (total and activated). Circulating fibrocytes are also increased in patients with pulmonary hypertension and are associated with remodeling of pulmonary vessels. In the cochlea, elevation of calcium in fibrocytes surrounding regulatory vessels of the spiral ligament results in propagation of a calcium signal in neighboring vascular cells.85 Fibrocytes may play an important role in vascular remodeling in systemic hypertension and they may contribute to disturbances in cellular calcium handling in the vascular wall.
Neuropilin
Neuropilins (NRPs) are transmembrane glycoprotein receptors for class III semaphorins and VEGF family members, that together with co-receptor plexins are involved in regulation of angiogenesis and axonal guidance.86 It was recently revealed that the adult expression of NRP2 is enriched in smooth muscle, where it mediates cytoskeletal rearrangement and a negative regulation of the Rho/ROCK pathway. Thus, NRP2 activation via semaphorin stimulation decreased active RhoA and phosphorylation of MLC in arterial smooth muscle cells and deletion of NRP2 increased contractile responses of bladder smooth muscle.87 However, the presence and functional importance of NRP2 has not been investigated in the vasculature of hypertensive animals or subjects.
Guanylyl cyclase or nucleotidyl cyclase
Most studies on isolated arteries from hypertensive animals and humans indicate that sodium nitroprusside-induced relaxation does not differ from normotensive values. This has been interpreted to indicate that soluble guanylyl cyclase (sGC) activity is unchanged in hypertension. However, the role of guanylyl cyclase in vasoconstrictor events may need to be re-evaluated. Chan and colleagues observed that hypoxia-induced contraction of porcine coronary arteries was inhibited by inhibition of sGC.88 Hypoxia also caused contraction in arteries treated with exogenous cyclic inosine 3',5'-monophosphate (cIMP) but not cyclic guanosine monophosphate (cGMP).89 Using liquid chromatography–mass spectrometry (HPLC-MS), an increased level of cIMP level was measured in arteries exposed to hypoxia. Altitude-induced systemic hypertension is related to hypoxia and it may be that second messengers in vascular arterial smooth muscle generated by a dysregulated sGC contribute to an exaggerated vasoconstriction. A recent study by Beste and co-workers90 demonstrates that sGC has a broader activity and may more correctly be termed nucleotidyl cyclase. They observed that purified recombinant rat sGC was capable of synthesizing seven cyclic purine and pyrimidine nucleotides. Study of the specificity of nucleotidyl cyclase activity in arteries from hypertensive animals may identify unique contractile signaling molecules related to this enzyme.
Conclusions/Perspectives
Recent evidence from our laboratory supports that in hypertension, elevated levels of DAMPs activate TLRs, which then signal directly or through cells of the adaptive immune response to elicit an inflammatory process in organs and systems that regulate blood pressure. We propose a new paradigm, according to which, the effects of TLR activation on vascular function are transduced via PLC-dependent amplification of calcium signaling, leading to excess vasoconstriction and contributing to vascular resistance. Additional molecular mechanisms presented in this review, involving circulating fibrocytes, neuropilins, and guanylyl cyclase, offer new ways of thinking about the origins of hypertension and hypertensive disorders; however, their effects on fundamental functions of arterial smooth muscle cells and calcium signaling warrant further investigation.
Supplementary Material
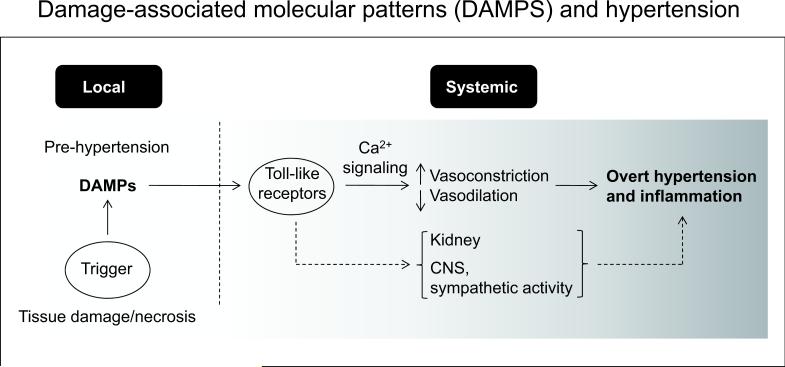
Figure 3.
Damage-associated molecular patterns (DAMPs) and hypertension. We hypothesize that in the pre-hypertensive state, necrotic cell injury during hypoxic and ischemic events leads to the local release of DAMPs. DAMPs activate Toll-like receptors (TLRs) in the arterial smooth muscle cell leading to disturbed Ca2+ signaling. This altered Ca2+ pattern leads to increased vasoconstriction and reduced vasodilation contributing to overt hypertension. Additionally, activation of TLRs leads to a generalized inflammatory state, characteristic of hypertension. Considerable evidence indicates that activation of TLRs in the kidney and central (CNS) and sympathetic nervous system contribute to hypertension. Thus, activation of the innate immune response provides a common pathway to explain “renocentric, neurocentric, and vasculocentric” views of the etiology of hypertension.
Acknowledgements
We would like to express our sincere appreciation to Dr. Nathan Tykocki for input on the timeline of observations on calcium handling in arterial smooth muscle.
Sources of Funding
This study was supported in part by the National Institutes of Health (R01 HL071138, R01 DK083685), the Society for Women's Health Research, and the American Heart Association (13SDG17050056).
Footnotes
Conflict(s) of Interest/Disclosure(s) Statement
None.
References
- 1.Bohr DF, Goulet PL. Role of electrolytes in the contractile machinery of vascular smooth muscle. Am J Cardiol. 1961;8:549–556. doi: 10.1016/0002-9149(61)90133-3. [DOI] [PubMed] [Google Scholar]
- 2.Briggs AH, Melvin S. Ion movements in isolated rabbit aortic strips. American Journal of Physiology. 1961;201:365–368. [Google Scholar]
- 3.Waugh WH. Role of calcium in contractile excitation of vascular smooth muscle by epinephrine and potassium. Circ Res. 1962;11:927–940. doi: 10.1161/01.res.11.6.927. [DOI] [PubMed] [Google Scholar]
- 4.Somlyo AV, Somlyo AP. Intracellular signaling in vascular smooth muscle. Adv Exp Med Biol. 1993;346:31–38. doi: 10.1007/978-1-4615-2946-0_4. [DOI] [PubMed] [Google Scholar]
- 5.Adelstein RS, Conti MA. Phosphorylation of platelet myosin increases actin-activated myosin ATPase activity. Nature. 1975;256:597–598. doi: 10.1038/256597a0. [DOI] [PubMed] [Google Scholar]
- 6.Gallagher PJ, Herring BP, Stull JT. Myosin light chain kinases. J Muscle Res Cell Motil. 1997;18:1–16. doi: 10.1023/a:1018616814417. [DOI] [PubMed] [Google Scholar]
- 7.Hai CM, Murphy RA. Ca2+, crossbridge phosphorylation, and contraction. Annu Rev Physiol. 1989;51:285–298. doi: 10.1146/annurev.ph.51.030189.001441. [DOI] [PubMed] [Google Scholar]
- 8.Kamm KE, Stull JT. The function of myosin and myosin light chain kinase phosphorylation in smooth muscle. Annu Rev Pharmacol Toxicol. 1985;25:593–620. doi: 10.1146/annurev.pa.25.040185.003113. [DOI] [PubMed] [Google Scholar]
- 9.Himpens B, Missiaen L, Casteels R. Ca2+ homeostasis in vascular smooth muscle. J Vasc Res. 1995;32:207–219. doi: 10.1159/000159095. [DOI] [PubMed] [Google Scholar]
- 10.Angus JA, Jennings GL, Sudhir K. Enhanced contraction to noradrenaline, serotonin and nerve stimulation but normal endothelium-derived relaxing factor response in skin small arteries in human primary hypertension. Clin Exp Pharmacol Physiol Suppl. 1992;19:39–47. doi: 10.1111/j.1440-1681.1992.tb02809.x. [DOI] [PubMed] [Google Scholar]
- 11.Giachini FR, Sullivan JC, Lima VV, Carneiro FS, Fortes ZB, Pollock DM, Carvalho MH, Webb RC, Tostes RC. Extracellular signal-regulated kinase 1/2 activation, via downregulation of mitogen-activated protein kinase phosphatase 1, mediates sex differences in desoxycorticosterone acetate-salt hypertension vascular reactivity. Hypertension. 2010;55:172–179. doi: 10.1161/HYPERTENSIONAHA.109.140459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Supiano MA, Hogikyan RV, Sidani MA, Galecki AT, Krueger JL. Sympathetic nervous system activity and alpha-adrenergic responsiveness in older hypertensive humans. Am J Physiol. 1999;276:E519–528. doi: 10.1152/ajpendo.1999.276.3.E519. [DOI] [PubMed] [Google Scholar]
- 13.Tobian L, Chesley G. Calcium content of arteriolar walls in normotensive and hypertensive rats. Proc Soc Exp Biol Med. 1966;121:340–343. doi: 10.3181/00379727-121-30773. [DOI] [PubMed] [Google Scholar]
- 14.Hinke JA. Effect of Ca++ upon contractility of small arteries from DOCA-hypertensive rats. Circulation Research. 1966;18-19:23–34. [Google Scholar]
- 15.Bohr DF, Sitrin M. Regulation of vascular smooth muscle contraction. Changes in experimental hypertension. Circ Res. 1970;27(Suppl 2):83–90. [PubMed] [Google Scholar]
- 16.Holloway ET, Bohr DF. Reactivity of vascular smooth muscle in hypertensive rats. Circ Res. 1973;33:678–685. doi: 10.1161/01.res.33.6.678. [DOI] [PubMed] [Google Scholar]
- 17.Hansen TR, Bohr DF. Hypertension, transmural pressure, and vascular smooth muscle response in rats. Circ Res. 1975;36:590–598. doi: 10.1161/01.res.36.5.590. [DOI] [PubMed] [Google Scholar]
- 18.Aoki K, Ikeda N, Yamashita K, Hotta K. ATPase activity and Ca 2 ion interaction of myofibrils and sarcoplasmic reticulum isolated from the hearts of spontaneously hypertensive rats. Jpn Heart J. 1974;15:475–484. doi: 10.1536/ihj.15.475. [DOI] [PubMed] [Google Scholar]
- 19.Moore L, Hurwitz L, Davenport GR, Landon EJ. Energy-dependent calcium uptake activity of microsomes from the aorta of normal and hypertensive rats. Biochim Biophys Acta. 1975;413:432–443. doi: 10.1016/0005-2736(75)90126-1. [DOI] [PubMed] [Google Scholar]
- 20.Webb RC, Bhalla RC. Altered calcium sequestration by subcellular fractions of vascular smooth muscle from spontaneously hypertensive rats. J Mol Cell Cardiol. 1976;8:651–661. doi: 10.1016/0022-2828(76)90050-x. [DOI] [PubMed] [Google Scholar]
- 21.Lederballe Pedersen O, Mikkelsen E, Andersson KE. Effects of extracellular calcium on potassium and noradrenaline induced contractions in the aorta of spontaneously hypertensive rats--increased sensitivity to nifedipine. Acta Pharmacol Toxicol (Copenh) 1978;43:137–144. doi: 10.1111/j.1600-0773.1978.tb02247.x. [DOI] [PubMed] [Google Scholar]
- 22.Mulvany MJ, Nyborg N. An increased calcium sensitivity of mesenteric resistance vessels in young and adult spontaneously hypertensive rats. Br J Pharmacol. 1980;71:585–596. doi: 10.1111/j.1476-5381.1980.tb10977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Breemen C, Cauvin C, Johns A, Leijten P, Yamamoto H. Ca2+ regulation of vascular smooth muscle. Fed Proc. 1986;45:2746–2751. [PubMed] [Google Scholar]
- 24.Rusch NJ, Hermsmeyer K. Calcium currents are altered in the vascular muscle cell membrane of spontaneously hypertensive rats. Circ Res. 1988;63:997–1002. doi: 10.1161/01.res.63.6.997. [DOI] [PubMed] [Google Scholar]
- 25.Asano M, Masuzawa K, Kojima M, Aoki K, Matsuda T. Comparison of inhibitory effects of calcium channel blockers and that of a calmodulin antagonist in strips of mesenteric arteries from spontaneously hypertensive and normotensive rats. Jpn J Pharmacol. 1988;48:77–90. doi: 10.1254/jjp.48.77. [DOI] [PubMed] [Google Scholar]
- 26.Lamb FS, Webb RC. Regenerative electrical activity and arterial contraction in hypertensive rats. Hypertension. 1989;13:70–76. doi: 10.1161/01.hyp.13.1.70. [DOI] [PubMed] [Google Scholar]
- 27.Bruner CA, Webb RC. Increased vascular reactivity to Bay K 8644 in genetic hypertension. Pharmacology. 1990;41:24–35. doi: 10.1159/000138696. [DOI] [PubMed] [Google Scholar]
- 28.Moriyama K, Osugi S, Shimamura K, Sunano S. Caffeine-induced contraction in arteries from stroke-prone spontaneously hypertensive rats. Blood Vessels. 1989;26:280–289. doi: 10.1159/000158777. [DOI] [PubMed] [Google Scholar]
- 29.Ashida T, Kuramochi M, Omae T. Increased sodium-calcium exchange in arterial smooth muscle of spontaneously hypertensive rats. Hypertension. 1989;13:890–895. doi: 10.1161/01.hyp.13.6.890. [DOI] [PubMed] [Google Scholar]
- 30.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 31.Amberg GC, Santana LF. Downregulation of the BK channel beta1 subunit in genetic hypertension. Circ Res. 2003;93:965–971. doi: 10.1161/01.RES.0000100068.43006.36. [DOI] [PubMed] [Google Scholar]
- 32.Giachini FR, Chiao CW, Carneiro FS, Lima VV, Carneiro ZN, Dorrance AM, Tostes RC, Webb RC. Increased activation of stromal interaction molecule-1/Orai-1 in aorta from hypertensive rats: a novel insight into vascular dysfunction. Hypertension. 2009;53:409–416. doi: 10.1161/HYPERTENSIONAHA.108.124404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu D, Yang D, He H, Chen X, Cao T, Feng X, Ma L, Luo Z, Wang L, Yan Z, Zhu Z, Tepel M. Increased transient receptor potential canonical type 3 channels in vasculature from hypertensive rats. Hypertension. 2009;53:70–76. doi: 10.1161/HYPERTENSIONAHA.108.116947. [DOI] [PubMed] [Google Scholar]
- 34.Overbeck HW. Attenuated arteriolar dilator responses to calcium in genetically hypertensive rats. Hypertension. 1984;6:647–653. doi: 10.1161/01.hyp.6.5.647. [DOI] [PubMed] [Google Scholar]
- 35.Heagerty AM, Ollerenshaw JD, Swales JD. Abnormal vascular phosphoinositide hydrolysis in the spontaneously hypertensive rat. Br J Pharmacol. 1986;89:803–807. doi: 10.1111/j.1476-5381.1986.tb11185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clark JH, Kinnear NP, Kalujnaia S, Cramb G, Fleischer S, Jeyakumar LH, Wuytack F, Evans AM. Identification of functionally segregated sarcoplasmic reticulum calcium stores in pulmonary arterial smooth muscle. J Biol Chem. 2010;285:13542–13549. doi: 10.1074/jbc.M110.101485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 38.Solaro RJ. Myosin light chain phosphatase: a Cinderella of cellular signaling. Circ Res. 2000;87:173–175. doi: 10.1161/01.res.87.3.173. [DOI] [PubMed] [Google Scholar]
- 39.Somlyo AP, Somlyo AV. Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol. 2000;522(Pt 2):177–185. doi: 10.1111/j.1469-7793.2000.t01-2-00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Somlyo AP, Wu X, Walker LA, Somlyo AV. Pharmacomechanical coupling: the role of calcium, G-proteins, kinases and phosphatases. Rev Physiol Biochem Pharmacol. 1999;134:201–234. doi: 10.1007/3-540-64753-8_5. [DOI] [PubMed] [Google Scholar]
- 41.Ishihara N, Matsuo H, Murakoshi H, Laoag-Fernandez JB, Samoto T, Maruo T. Increased apoptosis in the syncytiotrophoblast in human term placentas complicated by either preeclampsia or intrauterine growth retardation. Am J Obstet Gynecol. 2002;186:158–166. doi: 10.1067/mob.2002.119176. [DOI] [PubMed] [Google Scholar]
- 42.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 43.Amano M, Fukata Y, Kaibuchi K. Regulation and functions of Rho-associated kinase. Exp Cell Res. 2000;261:44–51. doi: 10.1006/excr.2000.5046. [DOI] [PubMed] [Google Scholar]
- 44.Jackson WF. Ion channels and vascular tone. Hypertension. 2000;35:173–178. doi: 10.1161/01.hyp.35.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miwa S, Kawanabe Y, Okamoto Y, Masaki T. Ca2+ entry channels involved in endothelin-1-induced contractions of vascular smooth muscle cells. J Smooth Muscle Res. 2005;41:61–75. doi: 10.1540/jsmr.41.61. [DOI] [PubMed] [Google Scholar]
- 46.Kuno M, Gardner P. Ion channels activated by inositol 1,4,5-trisphosphate in plasma membrane of human T-lymphocytes. Nature. 1987;326:301–304. doi: 10.1038/326301a0. [DOI] [PubMed] [Google Scholar]
- 47.Morris AP, Gallacher DV, Irvine RF, Petersen OH. Synergism of inositol trisphosphate and tetrakisphosphate in activating Ca2+-dependent K+ channels. Nature. 1987;330:653–655. doi: 10.1038/330653a0. [DOI] [PubMed] [Google Scholar]
- 48.Saleh SN, Albert AP, Peppiatt CM, Large WA. Angiotensin II activates two cation conductances with distinct TRPC1 and TRPC6 channel properties in rabbit mesenteric artery myocytes. J Physiol. 2006;577:479–495. doi: 10.1113/jphysiol.2006.119305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tai K, Hamaide MC, Debaix H, Gailly P, Wibo M, Morel N. Agonist-evoked calcium entry in vascular smooth muscle cells requires IP3 receptor-mediated activation of TRPC1. Eur J Pharmacol. 2008;583:135–147. doi: 10.1016/j.ejphar.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 50.Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 51.Naghdi S, Waldeck-Weiermair M, Fertschai I, Poteser M, Graier WF, Malli R. Mitochondrial Ca2+ uptake and not mitochondrial motility is required for STIM1-Orai1-dependent store-operated Ca2+ entry. J Cell Sci. 2010;123:2553–2564. doi: 10.1242/jcs.070151. [DOI] [PubMed] [Google Scholar]
- 52.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr., Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Soboloff J, Spassova MA, Tang XD, Hewavitharana T, Xu W, Gill DL. Orai1 and STIM reconstitute store-operated calcium channel function. J Biol Chem. 2006;281:20661–20665. doi: 10.1074/jbc.C600126200. [DOI] [PubMed] [Google Scholar]
- 55.Brandman O, Liou J, Park WS, Meyer T. STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell. 2007;131:1327–1339. doi: 10.1016/j.cell.2007.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thiel M, Lis A, Penner R. STIM2 drives Ca2+ oscillations through store-operated Ca2+ entry caused by mild store depletion. J Physiol. 2013;591:1433–1445. doi: 10.1113/jphysiol.2012.245399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 58.Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gwozdz T, Dutko-Gwozdz J, Zarayskiy V, Peter K, Bolotina VM. How strict is the correlation between STIM1 and Orai1 expression, puncta formation, and ICRAC activation? Am J Physiol Cell Physiol. 2008;295:C1133–1140. doi: 10.1152/ajpcell.00306.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ambudkar IS, Ong HL, Liu X, Bandyopadhyay BC, Cheng KT. TRPC1: the link between functionally distinct store-operated calcium channels. Cell Calcium. 2007;42:213–223. doi: 10.1016/j.ceca.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 62.Liao Y, Erxleben C, Yildirim E, Abramowitz J, Armstrong DL, Birnbaumer L. Orai proteins interact with TRPC channels and confer responsiveness to store depletion. Proc Natl Acad Sci U S A. 2007;104:4682–4687. doi: 10.1073/pnas.0611692104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bolotina VM. Orai, STIM1 and iPLA2beta: a view from a different perspective. J Physiol. 2008;586:3035–3042. doi: 10.1113/jphysiol.2008.154997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dominguez-Rodriguez A, Diaz I, Rodriguez-Moyano M, Calderon-Sanchez E, Rosado JA, Ordonez A, Smani T. Urotensin-II signaling mechanism in rat coronary artery: role of STIM1 and Orai1-dependent store operated calcium influx in vasoconstriction. Arterioscler Thromb Vasc Biol. 2012;32:1325–1332. doi: 10.1161/ATVBAHA.111.243014. [DOI] [PubMed] [Google Scholar]
- 65.Smani T, Zakharov SI, Csutora P, Leno E, Trepakova ES, Bolotina VM. A novel mechanism for the store-operated calcium influx pathway. Nat Cell Biol. 2004;6:113–120. doi: 10.1038/ncb1089. [DOI] [PubMed] [Google Scholar]
- 66.Park KM, Trucillo M, Serban N, Cohen RA, Bolotina VM. Role of iPLA2 and store-operated channels in agonist-induced Ca2+ influx and constriction in cerebral, mesenteric, and carotid arteries. Am J Physiol Heart Circ Physiol. 2008;294:H1183–1187. doi: 10.1152/ajpheart.01148.2007. [DOI] [PubMed] [Google Scholar]
- 67.Poteser M, Wakabayashi I, Rosker C, Teubl M, Schindl R, Soldatov NM, Romanin C, Groschner K. Crosstalk between voltage-independent Ca2+ channels and L-type Ca2+ channels in A7r5 vascular smooth muscle cells at elevated intracellular pH: evidence for functional coupling between L-type Ca2+ channels and a 2-APB-sensitive cation channel. Circ Res. 2003;92:888–896. doi: 10.1161/01.RES.0000069216.80612.66. [DOI] [PubMed] [Google Scholar]
- 68.Smani T, Dominguez-Rodriguez A, Hmadcha A, Calderon-Sanchez E, Horrillo-Ledesma A, Ordonez A. Role of Ca2+-independent phospholipase A2 and store-operated pathway in urocortin-induced vasodilatation of rat coronary artery. Circ Res. 2007;101:1194–1203. doi: 10.1161/CIRCRESAHA.107.159053. [DOI] [PubMed] [Google Scholar]
- 69.Mancarella S, Potireddy S, Wang Y, Gao H, Gandhirajan RK, Autieri M, Scalia R, Cheng Z, Wang H, Madesh M, Houser SR, Gill DL. Targeted STIM deletion impairs calcium homeostasis, NFAT activation, and growth of smooth muscle. FASEB J. 2013;27:893–906. doi: 10.1096/fj.12-215293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kanagy NL, Ansari MN, Ghosh S, Webb RC. Recycling and buffering of intracellular calcium in vascular smooth muscle from genetically hypertensive rats. J Hypertens. 1994;12:1365–1372. [PubMed] [Google Scholar]
- 71.Peel SE, Liu B, Hall IP. ORAI and store-operated calcium influx in human airway smooth muscle cells. Am J Respir Cell Mol Biol. 2008;38:744–749. doi: 10.1165/rcmb.2007-0395OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Giachini FR, Lima VV, Filgueira FP, Dorrance AM, Carvalho MH, Fortes ZB, Webb RC, Tostes RC. STIM1/Orai1 contributes to sex differences in vascular responses to calcium in spontaneously hypertensive rats. Clin Sci (Lond) 2012;122:215–226. doi: 10.1042/CS20110312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, Vinh A, Weyand CM. Inflammation, immunity, and hypertension. Hypertension. 2011;57:132–140. doi: 10.1161/HYPERTENSIONAHA.110.163576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bomfim GF, Dos Santos RA, Oliveira MA, Giachini FR, Akamine EH, Tostes RC, Fortes ZB, Webb RC, Carvalho MH. Toll-like receptor 4 contributes to blood pressure regulation and vascular contraction in spontaneously hypertensive rats. Clin Sci (Lond) 2012;122:535–543. doi: 10.1042/CS20110523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barton GM, Kagan JC. A cell biological view of Toll-like receptor function: regulation through compartmentalization. Nat Rev Immunol. 2009;9:535–542. doi: 10.1038/nri2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ivashkiv LB. A signal-switch hypothesis for cross-regulation of cytokine and TLR signalling pathways. Nat Rev Immunol. 2008;8:816–822. doi: 10.1038/nri2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rittirsch D, Flierl MA, Day DE, Nadeau BA, Zetoune FS, Sarma JV, Werner CM, Wanner GA, Simmen HP, Huber-Lang MS, Ward PA. Cross-talk between TLR4 and FcgammaReceptorIII (CD16) pathways. PLoS Pathog. 2009;5:e1000464. doi: 10.1371/journal.ppat.1000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xia YC, Schuliga M, Shepherd M, Powell M, Harris T, Langenbach SY, Tan PS, Gerthoffer WT, Hogarth PM, Stewart AG, Mackay GA. Functional expression of IgG-Fc receptors in human airway smooth muscle cells. Am J Respir Cell Mol Biol. 2011;44:665–672. doi: 10.1165/rcmb.2009-0371OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McCarthy CG, Goulopoulou S, Wenceslau CF, Spitler KM, Matsumoto T, Webb RC. Toll-Like Receptors and Damage-Associated Molecular Patterns: Novel links between inflammation and hypertension. Am J Physiol Heart Circ Physiol. 2013 doi: 10.1152/ajpheart.00328.2013. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bomfim GF, Szasz T, Carvalho MH, Webb RC. The Toll way to hypertension: role of the innate immune response. Endocrinol Metabol Synd. 2011;2:117. doi: 10.4172/2161-1017.1000117. [Google Scholar]
- 82.Goulopoulou S, Matsumoto T, Bomfim GF, Webb RC. Toll-like receptor 9 activation: a novel mechanism linking placenta-derived mitochondrial DNA and vascular dysfunction in pre-eclampsia. Clin Sci (Lond) 2012;123:429–435. doi: 10.1042/CS20120130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Reilkoff RA, Bucala R, Herzog EL. Fibrocytes: emerging effector cells in chronic inflammation. Nat Rev Immunol. 2011;11:427–435. doi: 10.1038/nri2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Keeley EC, Mehrad B, Janardhanan R, Salerno M, Hunter JR, Burdick MM, Field JJ, Strieter RM, Kramer CM. Elevated circulating fibrocyte levels in patients with hypertensive heart disease. J Hypertens. 2012;30:1856–1861. doi: 10.1097/HJH.0b013e32835639bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dai M, Shi X. Fibro-vascular coupling in the control of cochlear blood flow. PLoS One. 2011;6:e20652. doi: 10.1371/journal.pone.0020652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bagri A, Tessier-Lavigne M. Neuropilins as Semaphorin receptors: in vivo functions in neuronal cell migration and axon guidance. Adv Exp Med Biol. 2002;515:13–31. [PubMed] [Google Scholar]
- 87.Bielenberg DR, Seth A, Shimizu A, Pelton K, Cristofaro V, Ramachandran A, Zwaans BM, Chen C, Krishnan R, Seth M, Huang L, Takashima S, Klagsbrun M, Sullivan MP, Adam RM. Increased smooth muscle contractility in mice deficient for neuropilin 2. Am J Pathol. 2012;181:548–559. doi: 10.1016/j.ajpath.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chan CK, Mak J, Gao Y, Man RY, Vanhoutte PM. Endothelium-derived NO, but not cyclic GMP, is required for hypoxic augmentation in isolated porcine coronary arteries. Am J Physiol Heart Circ Physiol. 2011;301:H2313–2321. doi: 10.1152/ajpheart.00258.2011. [DOI] [PubMed] [Google Scholar]
- 89.Chen C, Zhang X, Ying L, Dou D, Vanhoutte PM, Gao Y. Soluble guanylyl cyclase-derived inosine 3':5'-cyclic monophosphate as a mediator of hypoxic contraction or porcine coronary artery. 11th International Symposium of Mechanisms of Vasodilatation. 2013 In Press. [Google Scholar]
- 90.Beste KY, Burhenne H, Kaever V, Stasch JP, Seifert R. Nucleotidyl cyclase activity of soluble guanylyl cyclase alpha1beta1. Biochemistry. 2012;51:194–204. doi: 10.1021/bi201259y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.