Abstract
The perivascular adipose tissue (PVAT) has recently been recognized as a novel factor in vascular biology, with implications in the pathophysiology of cardiovascular disease. Composed mainly of adipocytes, PVAT releases a wide range of biologically active molecules that modulate the vascular smooth muscle cell contraction, proliferation and migration. PVAT exerts an anticontractile effect in various vascular beds which seems to be mediated by yet elusive PVAT-derived relaxing factor or factors (PVRF). Considerable progress has been made on deciphering the nature and mechanisms of action of PVRF, and the PVRFs proposed until now are reviewed here. However, complex pathways seem to regulate the PVAT function and more than one mechanism is probably responsible for PVAT actions in vascular biology.
This review will describe our current knowledge on the structure and function of PVAT, with a focus on its role in modulating vascular tone. Potential involvements of PVAT dysfunction in obesity, hypertension and atherosclerosis will be highlighted.
Keywords: perivascular adipose tissue, vascular contraction, adipokines, hypertension, obesity, atherosclerosis
Introduction
Prior to the 1980s, the importance of the endothelium in vascular biology was largely ignored. The discovery of endothelium-derived relaxing factor (EDRF) and subsequent identification of nitric oxide (NO) revolutionized the field, and today we recognize the crucial role of the endothelium in modulating the function of underlying vascular smooth muscle cells (VSMCs) via complex pathways mediated by a multitude of factors, such as endothelin-1, NO, prostacyclin, epoxyeicosatrienoic acids (EETs) as well as yet unidentified endothelium-derived hyperpolarizing factor (EDHF) and endothelium-derived contracting factor (EDCF). Additionally, endothelial dysfunction is now widely accepted as an important component of various cardiovascular diseases and pharmacological attempts to restore endothelium function are used in the treatment of these diseases.
A similar trajectory can be described for the perivascular adipose tissue (PVAT). After simple observations of PVAT effects on vascular function, the term adventitium-derived relaxing factor (ADRF), now PVAT-derived relaxing factor (PVRF), was introduced. Several candidate PVRFs have then been proposed and reports of dysfunctional PVAT in obesity and cardiovascular diseases have appeared. As we are beginning to uncover a wide range of PVAT mediators that influence vascular function in physiology and disease, we may envision a future PVAT-centered revolution in vascular biology to parallel the one kindled by the endothelium thirty years ago.
General considerations on adipose tissue
Adipose tissue is usually classified as either white or brown. Brown adipose tissue (BAT) is traditionally associated with non-shivering thermogenesis and is composed of metabolically active adipocytes equipped with numerous uncoupling protein-1 (UCP-1) expressing mitochondria, which render the characteristic brown color. White adipose tissue (WAT) is traditionally seen as a lipid storage site. Generally, WAT is less vascularized, less innervated and less metabolically active in comparison with BAT (1). Both BAT and WAT receive innervation from the sympathetic nervous system (SNS), while parasympathetic innervation is still under dispute (2). Typical WAT adipocytes are composed of a single large lipid vesicle, while BAT adipocytes contain smaller multilocular lipid vesicles. BAT and WAT adipocytes derive from different precursors. Thus, BAT adipocytes share origins with skeletal myocytes, while WAT adipocytes have a different, less clear origin (3,4). Interestingly, transdifferentiation appears possible, for example in response to β3 adrenergic or PPARγ agonist treatment. Thus, brown adipocytes sometimes found inside WAT (mixed adipose tissue) share progenitors with white adipocytes and not with “classical” BAT adipocytes (3,4). Several older concepts on adipose tissue are being overturned in recent years, such as the restriction of brown fat to particular ages or anatomical locations (5). Most importantly, the idea that adipose tissue is an inert structure with no functional significance other than connective support and lipid storage has now been definitively disproven. The current prevailing opinion is that the various fat depots (to which some have referred collectively as the “adipose organ”), are structures with important endocrine and paracrine functions regulated by complex mechanisms, and that possess plastic properties in response to nutritional or temperature stimuli (1,6,7).
Special characteristics of PVAT
First, PVAT is different from adipose tissue in general due to its specific location (surrounding blood vessels). PVAT surrounds most systemic blood vessels, with the notable exception of the cerebral circulation (8). PVAT lies on the outside of adventitia, without laminar structures or any organized barrier to separate the two. Depending on the vascular bed, PVAT can be mixed (such as aortic PVAT) or white (such as mesenteric PVAT) adipose tissue (8). Vascularization and innervation of PVAT also vary greatly with location, thus helping to explain local variations in the functional characteristics of PVAT. Besides adipocytes and cells associated with penetrating vasa vasorum, PVAT may contain infiltrating macrophages and T lymphocytes, the activity of which becomes relevant in pathophysiological situations.
Second, PVAT may be a functionally specialized type of adipose tissue, with different developmental and secretory properties. Thus, PVAT adipocytes appear to be inherently different from adipocytes in other fat depots, as evidenced in a study by Chaterjee et al (9) in which perivascular adipocytes from human coronary arteries were compared to adipocytes from perirenal and subcutaneous depots. The study found that PVAT adipocytes are less differentiated and have a secretory profile favoring pro-inflammatory cytokines, such as IL-6, IL-8 and MCP-1 with contrasting reduced adiponectin secretion, findings which prompted the authors to conclude that perivascular adipocytes contribute to adventitial inflammation and atherosclerosis development. The specialized nature of PVAT is further illustrated by differences in the secretory profiles depending on the vascular bed (10).
Due to these important differences, knowledge gained from studies focusing on adipose tissue is not necessarily applicable to PVAT. This is true of both the nature of specific molecules being released and of their expression levels.
Release of biologically active molecules from PVAT
It is now known that in addition to free fatty acids, adipose tissue releases hormone-like substances that disseminate in the blood stream and can act at a distance, e.g. adipose tissue-derived leptin acting on leptin receptors in the hypothalamus to regulate appetite. Some researchers have even observed the presence of fenestrated capillaries, a characteristic of endocrine organs, in normal mouse adipose tissue(11). The extent of endocrine effects of PVAT specifically is yet unclear, however PVAT has obvious paracrine effects on vascular structures.
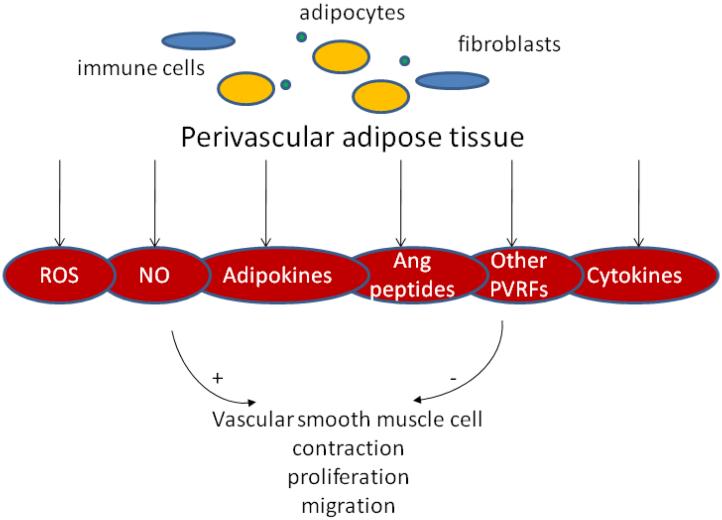
Whether acting in an endocrine or paracrine manner, PVAT releases a wide range of biologically active molecules (table 1). Adipokines are the specific adipocyte product and judging by their effect on cytokine levels they can be classified as pro-inflammatory (leptin, resistin, visfatin) and anti-inflammatory (adiponectin, adrenomedullin). As emerging modulators of vascular function, adipocytokines are now under intense scrutiny and several excellent reviews describe the advances in adipokine research (6,12-14). Newly identified adipokines (omentin, nesfatin, vaspin, chemerin) may also play a role on vascular function, as revealed in a recent review (15), although their expression in PVAT was not proven yet. In addition to adipokines, adipocytes in PVAT release classical chemokines/cytokines, such as IL-6, IL-8, MCP-1, and PAI-1 (14,16). The infiltrating macrophages and T lymphocytes present in PVAT or recruited in response to chemokine release by adipocytes not only release inflammatory mediators, but are likely the active partners of adipocytes in maintaining a balance of these factors as well as in regulating inflammatory responses to external stimuli. PVAT also releases angiotensin peptides, being equipped with an almost complete renin-angiotensin system (RAS), including angiotensinogen, converting enzymes and receptors (10,17). Release of reactive oxygen species (ROS), such as superoxide and hydrogen peroxide, as well as other gaseous molecules like H2S, was also evidenced in PVAT (18-20). Additionally, PVAT expresses a complex ROS/RNS machinery, containing among others NADPH oxidase, eNOS and all SOD isoforms. Finally, steroid hormone production (estradiol, cortisol) by PVAT is also possible, though not yet fully proven.
Table 1.
Adipose-tissue derived biologically active molecules with vascular effects
| Released by PVAT | |
|---|---|
| Adipokines | Leptin, adiponectin, resistin, visfatin, adrenomedullin, omentin (?), nesfatin (?), vaspin (?), chemerin (?) |
| Cytokines | IL-1, IL-6, IL-8, MCP-1, PAI-1, TNFα, RANTES |
| Gaseous molecules | Superoxide, hydrogen peroxide, NO, hydrogen sulfide |
| Expressed by PVAT | |
| RAS molecules | Angiotensinogen, angiotensin I, angiotensin II, angiotensin(1-7), ACE1, ACE2, (pro)renin receptor, AT receptors |
| ROS/oxidative stress | NADPH oxidase, SODs, eNOS, lipoxygenases |
| Adipocyte specific | Adipokines, adipokine receptors, free fatty acids, β3 adrenergic receptor, PPARy, UCP-1 |
| Inflammatory cell specific | Cytokines, cytokine receptors |
| Others | Metalloproteases, steroid hormones (?), complement 3 |
Although adipocytes constitute the majority of cells in PVAT, the other cell types present in PVAT (macrophages, T-cells, fibroblasts, capillary endothelial cells) also possess the capacity to produce biologically-active molecules. This is especially true of the infiltrating immune cells, which will secrete the aforementioned cytokines when activated. The crosstalk between adipocytes and the infiltrating immune cells in PVAT is a central feature of PVAT function, which becomes important especially in disease conditions (6,21-23). Few present studies have attempted to identify the cellular source of cytokines secreted from adipose tissue, which for example is the macrophage for most of the TNFα and IL-6 (23), with the macrophage being also the primary infiltrating cell type in the obese adipose tissue, ahead of lymphocytes and neutrophils (24). No such studies were performed for PVAT. The relative importance of macrophages in PVAT was illustrated by a study by Withers et al using a conditional macrophage ablation to demonstrate the dependence of PVAT function on macrophage infiltration in a mouse model of hypoxia (25). Additionally, important differences between brown and white adipocyte secretion may also exist. Therefore, until studies are performed to distinguish the exact source of PVRF, it is impossible to ascribe the effects of PVAT to adipocytes, whether white or brown, or any other cell type.
PVAT function
Typical experimental preparations of blood vessels for measurements of contraction begin by “cleaning” the vessel, which largely means removal of PVAT. This practice was justified by the belief that PVAT, as a connective tissue, provides only mechanical support to vessels, but does not play any role in vascular contraction. This view is still illustrated in almost any diagram of vascular structure/function with endothelium, smooth muscle and adventitia as the only blood vessel layers. Additionally, PVAT was removed also because it was believed that its presence would impair diffusion of the pharmacological agents used experimentally. An observation of PVAT-mediated decrease in contractile responses to norepinephrine in rat aorta, made by Soltis and Cassis in 1991(26), first pointed to the potential role of PVAT in vascular contraction. These studies were continued a decade later by Gollasch et al, who began analyzing the mechanism of the anticontractile effect of PVAT (27-31). Subsequently, more researchers, notably Gao et al (8,17,19,32-38), reproduced this effect and efforts began to identify the PVAT-derived relaxing factor (PVRF) and to study the changes of PVAT in various disease states.
Mechanisms of anticontractile effect of PVAT
Several mechanisms (table 2) have been proposed for the anticontractile effect of PVAT (figure 1). As with other common processes in vascular biology, probably no single mechanism would explain all experimental findings, the relative importance of different factors varying according to species, vascular bed and pathophysiological state (21,30,39).
Table 2.
Potential mechanisms of PVAT action on vascular contraction
| Mechanism | Effect | Condition | Vascular bed and species | References |
|---|---|---|---|---|
| K+ channels | ||||
| KATP | anticontractile | physiological | Rat aorta | (28),(27) |
| Kca | anticontractile | physiological | Rat aorta Human internal thoracic |
(19) (33) |
| Kv | anticontractile | physiological | Rat mesenteric | (29,99) |
| loss of anticontractile effect |
genetic hypertension |
Rat mesenteric | (10,81) | |
| Adiponectin | anticontractile | physiological | Rat aorta | (31) |
| Leptin | direct relaxing | physiological | Canine mesenteric Rabbit aorta Human forearm |
(42) (43) (44) |
| Superoxide | contractile | physiological | Rat aorta | (32) |
| Hydrogen peroxide | anticontractile | physiological | Rat aorta | (19) |
| NO | anticontractile | early diet-induced obesity |
Mouse mesenteric | (75) |
| Oxidative stress | blocks anticontractile effect |
obesity | Human subcutaneous fat arterioles |
(59) |
| diet-induced obesity genetic obesity |
Mouse mesenteric Mouse mesenteric |
(20) (70) |
||
| Inflammation | blocks anticontractile effect |
obesity | Human subcutaneous fat arterioles |
(59) |
| Hydrogen sulfide | anticontractile | physiological hypertension |
rat aorta | (18,99) |
| Angiotensin II | contractile | physiological | Rat mesenteric | (34) |
| Ang(1-7) | anticontractile | physiological | Rat aorta Rat vena cava |
(35) (38) |
| loss of anticontractile effect |
genetic hypertension |
Rat aorta | (37) | |
|
Mineralocorticoid
receptor |
blocks anticontractile effect |
Mouse mesenteric | (25) | |
| AMPK | endothelial dysfunction | obesity | Rat mesenteric | (71) |
|
Infiltrating
macrophages |
blocks anticontractile effect |
Mouse mesenteric | (25) | |
|
Changes in fatty acid
composition |
blocks anticontractile effect |
Metabolic syndrome |
Rat aorta | (76) |
| Hypoxia | blocks anticontractile effect anticontractile via KATP |
Rat aorta Mouse aorta |
(25) (57) |
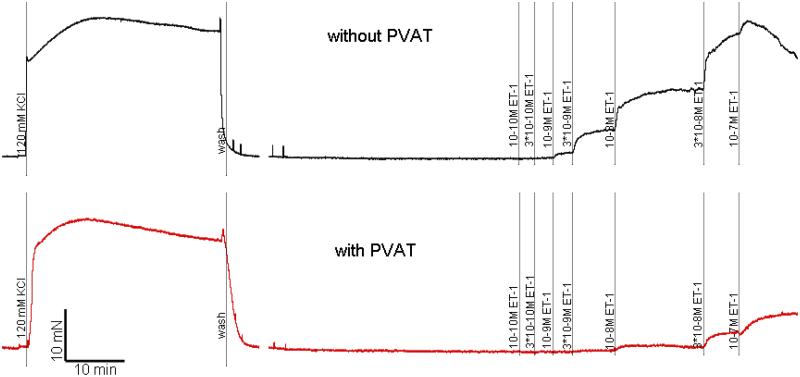
Figure 1.
Myograph tracing representative of isometric contractile force recordings in isolated rat mesenteric artery rings. Cumulative concentration response curve to endothelin-1 in the absence (above, black) and presence (below, red) of PVAT. The scale represents time in minutes (X axis) and force in mN (Y axis).
Soltis and Cassis hypothesized that PVAT increased the uptake of norepinephrine, explaining the observed reduced contractile response in the presence of PVAT (26). This idea would not explain subsequent observations of PVAT reducing contraction to a host of agonists, including phenylephrine, angiotensin II, thromboxane A2 agonists, serotonin or endothelin-1, some of which are not subject to uptake or extracellular inactivation.
Löhn et al created the term ADRF in their elegant article from 2002 (28) in which they proved that the anticontractile effect of PVAT in rat aorta is transferrable and adipocyte-derived. These authors also proposed the mechanism to be activation of KATP channels and have excluded contributions from NOS and from other K+ channels, such as large conductance Ca2+ channels, delayed rectifier and inwardly rectifying K+ channels. The same group went on to demonstrate that release of ADRF is calcium dependent and regulated by tyrosine kinase and PKA-dependent mechanisms, however independent of influences from vanilloid, cannabinoid and CGRP receptors, suggesting that nerve endings in PVAT are not involved in the anticontractile effect of PVAT(27). The PVAT anticontractile effect was then observed in the rat mesenteric bed, where the mechanism for ADRF release appeared to be KV channel-dependent (29).
The exact identity of PVRF or PVRFs has been chased ever since, several candidates appearing over time. Thus, Gao et al showed that the anticontractile effect in rat aorta has both endothelium-dependent and -independent components, involving NO release and KCa channel activation for the former, and the endothelium-independent one relying on hydrogen peroxide and sGC activation for the latter (19). Variations in the type of K+ channels involved in mediating the PVAT effects are probably also explained not only by species and vascular bed, but also by methodological differences between studies, Verlohren et al (29) being the only one to employ electrophysiological measurements. More such studies are needed to explore the role of K+ channels, and this is especially true of small vessels, which are particularly sensitive to the perivascular K+ environment (40).
In more recent papers, Gao et al. have proposed Ang(1-7) to be (one of) the PVRF released in rat aorta. Thus, Ang(1-7) expression was demonstrated in PVAT, and inhibition of the Mas receptor or ACE2 inhibitor treatment decreased the anticontractile effect of PVAT(35). These findings on Ang(1-7) were later reproduced in the rat vena cava, which displayed the same PVAT effect (38). Interestingly, the only report of eNOS expression in PVAT was also in a vein, the human saphenous vein (41).
Adipokines are also obvious candidates for PVRF, although quite a few of them have not yet been tested in vascular contractile experiments. Leptin, probably the longest studied adipokine, exerted a direct vasodilatory effect in the absence of PVAT in canine mesenteric vessels (42), rabbit aorta (43) and the human forearm (44) and leptin receptors are present in endothelium, where they may mediate vasorelaxation via both NO-dependent and independent mechanisms (43,45,46). Leptin has directly inhibited angiotensin II vasoconstriction in rat aorta (47) and this effect was mediated by stimulation of iNOS activity in VSMCs (48). Among other effects of leptin infusion in vivo, it produces acute hypotension, supporting a direct vasodilatory role. However chronic leptin infusion lead to endothelial dysfunction (49) and hypertension (50), highlighting the dual role of leptin as detrimental and beneficial factor in the cardiovascular system, depending on plasma levels and duration of exposure (21). Other studies were not able to reproduce these findings or had conflicting results and a final conclusion on leptin effects on the vasculature is lacking (46). Adiponectin was proposed as a candidate PVRF by Gollasch et al, who showed that recombinant adiponectin reduced 5-HT-mediated contraction of rat aorta. However, contractile responses to 5-HT in the mesenteric bed of adiponectin deficient mice were not different from wild type mice, dismissing adiponectin as PVRF(31). This does not, however, exclude multiple protective effects of adiponectin on vascular function, including insulin-sensitizing, anti-atherogenic and anti-inflammatory actions. Vascular function is likely influenced by adiponectin via multiple mechanisms, including AMPK-mediated activation of eNOS(51). Additionally, expression of adiponectin by endothelium following injury supports the idea of crosstalk mechanisms between PVAT and endothelium (52). With two circulating active forms (full length and cleavage-generated globular adiponectin), capable of multimerization, and recently discovered two receptors, adiponectin is probably part of a more complex pathway with roles in immunity, insulin resistance and inflammation (6,12,53). The less investigated resistin may also play a role in the PVAT effect on vascular contraction, however probably only in an indirect manner, such as ROS-mediated, by regulating protein expression of other mediators, or by stimulating macrophage infiltration and the release of other cytokines (21,54,55). The full picture of resistin action is still lacking, a putative receptor has not been identified yet and indeed even its main cellular source is not entirely clear (6). Visfatin was excluded from exerting direct effects on vascular contraction (56), however this adipokine may play important roles in VSMC proliferation and atherosclerosis pathogenesis (see below). Additionally, newly identified adipokines such as omentin, chemerin, nesfatin and vaspin may also have anticontractile effects, however their expression in PVAT remains to be demonstrated (15).
A recent report demonstrated the loss of the anticontractile effect of PVAT in the presence of aldosterone in rat mesenteric arteries (25), similarly to the initial Soltis and Cassis report, where the same was observed with deoxycorticosterone acetate (DOCA) in the rat aorta (26). The same loss of PVAT function was observed during hypoxia, and the authors of this paper used a conditional macrophage ablation mouse model to demonstrate that these effects are mediated by infiltrating macrophages in PVAT and that aldosterone receptor antagonism reverses both aldosterone and hypoxia effects, restoring PVAT anticontractile function (25). Another group published the opposite results with respect to hypoxia, with the relaxing effect of aortic PVAT being enhanced by exposure to hypoxic conditions via KATP dependent and endothelium-independent mechanisms (57). One poorly explored mechanism for the PVAT effects on vascular contraction is sympathetic nervous system (SNS) activity, which innervates BAT and may be part of a brain-adipocyte axis in which signals from adipose tissue such as leptin work to modulate food intake, while at the same time influencing SNS activity, which will in turn regulate vasoconstriction (21).
A few results of the studies performed in animals were reproduced in human vessels in healthy conditions. The anticontractile effect of PVAT was observed in the internal thoracic artery, acting via KCa channels (33,58). The protective effects of adiponectin in healthy human PVAT is supported by the results of a study in which adiponectin receptor fragment blocked the vasorelaxant effect of PVAT in small arteries (59).
Alterations in mechanisms of PVAT vasoactivity may occur in pathophysiological conditions such as obesity and hypertension, in which the most consistent finding has been a loss of the anticontractile effect of PVAT.
Pro-contractile effects of PVAT
Interestingly, some authors have found either opposed or negative effects of PVAT on vascular contraction, thus contesting the now accepted dogma (60). The first account of PVAT effects on vascular function by Soltis and Cassis (26) included data demonstrating contraction of intact rat aorta to electrical field stimulation that was absent in tissues without PVAT. Additionally, in their first paper on PVAT, Gao et al actually proposed a role of PVAT in promoting vasoconstriction elicited by perivascular nerve stimulation of rat superior mesenteric artery. This effect was mediated via superoxide production by NADPH oxidase in PVAT adipocytes (32) and reversed by treatment with an ACE inhibitor or AT1 receptor blocker (17). The role of ROS was further emphasized in subsequent publications by the same group, however the vasoconstrictor effect was not reproduced, instead the authors studied the already established anticontractile effect.
In contrast to work by Gao et al showing NO-mediated, endothelium-dependent mechanisms for the anticontractile effects of PVAT (19), work by Tune et al. has demonstrated that canine coronary PVAT decreases endothelium-dependent relaxation via PKCβ-mediated phosphorylation of eNOS at Thr-495 in physiological conditions (61), and this effect may be exacerbated during metabolic syndrome (62) (see below).
PVAT function in VSMC proliferation/migration
In addition to its impact on VSMC contraction, PVAT is involved in the proliferative and migration function of VSMCs (63,64) (figure 2). Secretion of visfatin by PVAT adipocytes was higher in aortic PVAT compared to subcutaneous or visceral fat depots, and it was correlated with phosphoribosyltransferase enzymatic activity and nicotineaminde mononucleotide (NMN) production. Although lacking a direct vasoactive effect, visfatin has been shown to act as a VSMC growth factor, stimulating proliferation of VSMC via insulin-independent NMN-mediated activation of Erk1/2 and p38 pathways (56). Leptin and resistin, also acting via MAPK and/or PI3K pathways, have been shown to increase
Figure 2.
Effects of perivascular adipose tissue on VSMCs.
VSMC proliferation and migration in vitro (6,55). Free fatty acids (FFA) released by PVAT also stimulate VSMC proliferation in vitro (65). Additionally, although not yet directly proven, other PVAT products such as ROS, angiotensin peptides and steroid hormones, may stimulate VSMC growth and migration, thus potentially implicating PVAT as a pathogenetic factor of atherosclerosis. Adiponectin decreases proliferation in VSMC (65), probably by similar mechanisms as in endothelial cells (66), thus promoting a beneficial antiangiogenic effect that may be reduced in pathophysiological conditions in which circulating adiponectin is decreased.
PVAT in pathophysiological states
The vast majority of studies on PVAT were performed in animals. Conclusions from these studies may not necessarily be applicable to human physiology and pathophysiology, therefore in the following sections we will discuss the few human data available separately.
I. Obesity, insulin resistance, diabetes
In both human pathology and animal models, there is a continuum of metabolic alterations linking obesity with insulin resistance and diabetes, with clear distinction between them often lacking, which is why for the purpose of this review these particular states will be addressed together.
In humans, obesity is a chronic disease presently at a stage of worldwide pandemic. Due to the link between obesity and cardiovascular disease risk and mortality, global efforts are currently underway to combat this condition. A chronic, low-grade systemic inflammatory state is characteristic of obesity evolving chronically. These facts explain the recent interest in obesity-induced alterations in adipose tissue in general and PVAT in particular (67,68). Although worldwide still classified according to BMI, obesity is now being redefined as an increase in adiposity. However, besides pure mass increase, the changes in adipose tissue accompanying obesity are also in adipocyte type (brown vs white), fatty acid composition of lipid vesicles, cell size, potentially cell number, inflammatory cell infiltration, ECM remodeling and other structural modifications. These are naturally followed by functional alterations, imbalance in adipocytokine production and potential oxidative stress, hypoxia and inflammation (60). Fulfilling the predictions from other fat depots (69), a study by Greenstein et al. (20,59,70,71) showed that total PVAT mass is also increased in obese humans. In addition, PVAT anticontractile effect was diminished and signs of local inflammation and hypoxia observed in PVAT were mimicked by application of IL-6 or TNF-α and rescued with SOD and catalase, as well as with TNF-α antagonists. Highlighting the importance of PVAT, it has been shown that PVAT mass in humans was associated with visceral fat mass and negatively correlated with insulin sensitivity (72). Moreover, evidence from the Framingham Heart Study (73) shows that periaortic fat mass correlates with hypertension, diabetes and aortic/coronary calcification, even if corrected for BMI (but not if corrected for visceral adipose tissue).
In animal models of obesity, total PVAT mass and adipocyte size is also increased (20,59,70,71). Since PVAT releases PVRF with anticontractile effects in normal conditions and this effect is adipose mass-dependent (29), it would be expected that PVAT would confer a protective effect to vascular function in obesity via increased PVRF release. However, as previously mentioned, the opposite occurs and the PVAT anticontractile effect is lost in obesity. Various explanations have been proposed for this puzzling finding, mostly centered around the idea of impaired adipokine secretion and activation of detrimental pathways such as inflammation and oxidative stress. The influence of PVAT on the link between inflammation and insulin resistance in obesity was recently reviewed extensively elsewhere (74).
Release of leptin is uniformly increased in animal models of obesity, and when specific PVAT leptin production was measured in models of high fat diet (HFD)-induced obesity, it followed the same trend (9,20). In a swine model of metabolic syndrome, the coronary endothelial dysfunction was exacerbated in the presence of PVAT, via leptin and PKCβ-dependent mechanisms (62). The inhibition of VSMC Ca2+ signaling and vasoconstriction by leptin observed in normal animals was lost in the Zucker rat (47). On the other hand, adiponectin release from PVAT was reduced in both HFD and genetic models of obesity (9,70). In the New Zealand obese mouse (NZO), in addition to changes in PVAT mass, there was evidence of macrophage infiltration, increased ROS formation, decreased expression of superoxide dismutases (SOD) and signs of eNOS uncoupling (70). On the contrary, early diet-induced obesity was correlated with increased NO production by mesenteric PVAT in a mouse model (75). The role of ROS in obesity-induced PVAT dysfunction was demonstrated in a study by Ketonen et al in which impaired endothelium-dependent relaxation in diet-induced obese mice was restored by PVAT removal or treatment with ROS scavengers. The same study found increased leptin, MCP-1 and NADPH oxidase expression in PVAT from obese mice, and the increased ROS production was corrected by treatment with apocynin, suggesting a role for PVAT NADPH-produced superoxide in mediating the obesity-associated endothelial dysfunction (20). In a study of chronic (6 months) HFD treatment in rats, the authors similarly improved endothelial dysfunction by removing PVAT. HFD was accompanied by decreases in AMPK and eNOS and upregulation of mTOR. By creating a VSMC-adipocyte co-culture, these authors demonstrated a decrease in AMPK phosphorylation and an increase in mTOR phosphorylation in VSMCs co-cultured with adipocytes from HFD treated rats (71). Finally, the role of fatty acid (FA) composition was highlighted in a recent article of fructose-fed rat model of metabolic syndrome, in which the PVAT anticontractile effect was diminished in aorta and the ratio of saturated to mono or polyunsaturated FA was increased, antioxidant enzyme expression was decreased and markers of oxidative stress were increased in PVAT (76). In another study, the increased norepinephrine-induced contraction in fructose-fed rats was corrected with losartan in both the presence and absence of PVAT(77). Contrary to previously mentioned reports, PVAT anticontractile effect was increased in a rat model of type I diabetes (36).
Overall, it appears that alterations in PVAT function in obesity and the metabolic syndrome are indeed accompanied by alterations in the release of adipokines, inflammation and oxidative stress. The initiating factor as well as the sequence of pathological events is yet unclear, however increased free fatty acid release from hypertrophied adipocytes is a clear candidate hypothesis, leading to the activation of a cascade of inflammatory pathways and oxidative stress that feed forward each other (78).
II. Hypertension
The typical vascular dysfunction encountered in hypertension, whether present as one of the causal factors or as a consequence of remodeling in the face of chronically increased pressure, was so far studied from the point of view of deregulations of the endothelial, the smooth muscle and even adventitial layers. Only a handful of studies exist on the function of PVAT during hypertension, and they have all been completed in animals. Two consistent findings are shared by most of these studies: decreased PVAT mass and decreased PVAT anticontractile effects. PVAT adipocyte size is decreased in several animal models of hypertension: the spontaneously hypertensive rat (SHR) (79), the angiotensin II hypertensive rat (34), and the DOCA-salt rat (80). Total PVAT mass (79,81) is also reduced in the SHR compared to the control Wistar-Kyoto (WKY) rat. The function of PVAT is impaired in SHR aorta (82) and isolated mesenteric arteries as well as perfused whole mesenteric bed (79,81), where the anticontractile effect of PVAT is diminished or lost. This reduced anticontractile effect is not due to endothelial dysfunction as it is still observable in endothelium-denuded arteries (therefore alteration is likely due to changes in the endothelium-independent PVRF)(34). Interestingly, the Gao et al group observed potentiation of responses to AngII in the presence of PVAT in control rats, and this PVAT contractile effect was reduced in aorta from AngII-treated rats (34). PVAT mass and PVAT adipocyte size were also decreased in AngII hypertension as compared to controls (34). The same group reported opposite findings in the SHR model, in which PVAT intact aorta contracted more to phenylephrine than the control WKY rats, thus exhibiting the previously published loss of anticontractile effect compared to control. Relaxation induced by transfer of bath solution from PVAT intact vessels was also diminished in SHR aorta, suggesting that the decreased PVAT mass is not responsible for the loss of function. This relaxation was inhibited by Ang(1-7) receptor antagonism (37). Interestingly, the PVAT anticontractile effect was restored by systemic atorvastatin treatment (82). Fetal and neonatal exposure to nicotine induced hypertension in rats and this hypertension was associated with loss of the anticontractile effect of PVAT, as well as an increase in periaortic brown adipocytes (83).
Not many studies explored the changes in adipocytokine secretion during hypertension. Different from obesity, leptin secretion is decreased in mesenteric PVAT from SHR (79). The leptin-induced decrease in VSMC Ca2+ signaling and angiotensin II-mediated vasoconstriction is also lost in the SHR (84). In the only published study of PVAT in the DOCA-salt model of hypertension, Ruan et al performed a different kind of experiment by using liquid chromatography-tandem mass spectrometry to identify the secretome of aortic PVAT. By this method, the most abundant secretory protein in PVAT was identified as complement 3 (C3). PVAT-conditioned media and recombinant C3 induced adventitial fibroblast migration and differentiation via JNK activation that was counteracted by treatment with C3 antagonist and a neutralizing antibody. The PVAT C3 expression and JNK phosphorylation was increased in DOCA compared to sham rats and this expression associated with adventitial remodeling observed in the DOCA arteries (80).
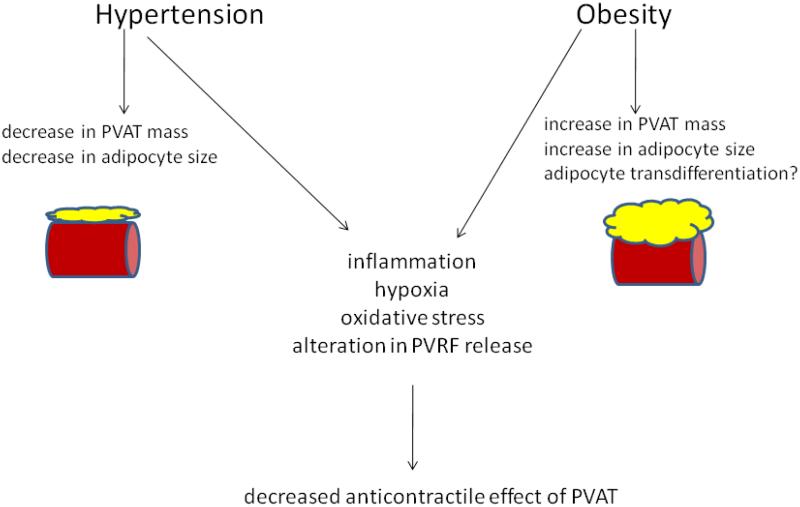
Therefore, the hypertensive PVAT, although reduced in size, seems to be affected by similar alterations in function as the obese PVAT. The extent of similarities between the PVAT dysfunction in obesity and hypertension has yet to be investigated (figure 3).
Figure 3.
Alterations of PVAT structure and function during obesity and hypertension lead to decreased anticontractile effects of PVAT. Although similar imbalances in adipokine release and inflammatory events occur in PVAT during atherosclerosis, there appear to be no changes in contractile function.
III. Atherosclerosis
Atherosclerosis is a chronic inflammatory condition and in view of the list of inflammatory mediators released by PVAT (table 1), it is understandable why PVAT is being investigated as a link in the pathogenetic events leading to atherosclerosis. The “outside to inside” view on atherogenesis is supported by a series of studies suggesting that alterations in PVAT function that follow an increase in PVAT mass lead to release of proinflammatory and chemotactic mediators from PVAT, macrophage and inflammatory cell infiltration with ultimate pro-atherogenic consequences (16,85,86).
Because of the clinical manifestations of atherosclerosis, most human studies are focused on the epicardial adipose tissue (18,90,91), which contains, although it is not identical to, the peri-coronary adipose tissue. An increase in epicardial adipose tissue was correlated in several clinical studies with parameters of coronary artery disease (CAD), such as the existence of plaque, the degree of stenosis, clinical CAD, or cardiovascular events (85). Adipokine secretion was shown to be impaired in epicardial adipose tissue from CAD patients, with increases in IL-6, PAI-1, TNFα, adrenomedullin, visfatin and leptin, and decreases in adiponectin (92-97). Macrophage and T-cell infiltration in PVAT in the vicinity of atherosclerotic plaques was demonstrated in human aorta (22). However, since increases in human epicardial adipose tissue are also associated with obesity and increases in visceral adipose tissue, the causal relationship is harder to establish between peri-coronary PVAT dysfunction and atherosclerosis. A very convincing argument would be the fact that intramyocardial segments of coronary arteries, lacking PVAT, are more likely to be devoid of atherosclerosis (98).
In animal studies, the involvement of PVAT on the vascular tone of left circumflex artery was excluded in a porcine model of atherosclerosis (87), However, direct vasomotor effects of PVAT may also play a role in this setting. Pro-inflammatory adipokines were increased and anti-inflammatory adipokines were decreased in PVAT following balloon injury in animal models of endovascular injury (88). Increased adipokine secretion and inflammatory cell infiltration have also been demonstrated in PVAT from atherosclerotic apolipoprotein E knockout (apoE−/−) mice (89).
Conclusion
The importance of PVAT as a paracrine modulator of vascular function is becoming increasingly apparent. Complex mechanisms of PVAT action on vascular contraction relying on both secretion of PVRF and plastic properties of PVAT are probably involved in the pathogenesis of vascular dysfunction in obesity/metabolic syndrome, hypertension and atherosclerosis. Irrespective of the changes in PVAT mass present in these conditions, in all cases PVAT dysfunction seems to contribute to the impairment the underlying vessel function. The most likely common denominator present in these conditions is inflammation. However, the differences between PVAT and other fat depots are still poorly defined and a clear beneficial/detrimental role of PVAT has not been established. Whether PVAT is a feasible target of therapies designed to restore its function in order to improve vascular function in these diseases is therefore a separate question, one that will require more complete mechanistic studies.
Acknowledgments
Grant support: NIH postdoctoral training grant T32HL066993 (TS), NIH RO1 grants DK83685, HL71138 (RCW).
List of abbreviations
- 5-HT
5-hydroxytryptamine
- ADRF
adventitium-derived relaxing factor
- BAT
brown adipose tissue
- DOCA
deoxycorticosterone acetate
- ECM
extracellular matrix
- EDCF
endothelium-derived contracting factor
- EDHF
endothelium-derived hyperpolarizing factor
- EDRF
endothelium-derived relaxing factor
- EET
epoxyeicosatrienoic acids
- IL
interleukin
- MAPK
mitogen-activated protein kinase
- MCP
monocyte chemotactic protein
- NADPH
nicotinamide adenine dinucleotide phosphate
- NOS
nitric oxide (NO) synthase
- PI3K
phosphoinositide 3 kinase
- PVAT
perivascular adipose tissue
- PVRF
PVAT-derived relaxing factor
- RAS
renin-angiotensin system
- ROS
reactive oxygen species
- SHR
spontaneously hypertensive rat
- SNS
sympathetic nervous system
- SOD
superoxide dismutase
- UCP-1
uncoupling protein-1
- VSMC
vascular smooth muscle cell
- WAT
white adipose tissue
- WKY
Wistar-Kyoto rat
References
- 1.Cinti S. Prostaglandins Leukot Essent Fatty Acids. 2005;73(1):9–15. doi: 10.1016/j.plefa.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Berthoud HR, Fox EA, Neuhuber WL. Am J Physiol Regul Integr Comp Physiol. 2006;291(5):R1240–1242. doi: 10.1152/ajpregu.00428.2006. [DOI] [PubMed] [Google Scholar]
- 3.Lazar MA. Science. 2008;321(5892):1048–1049. doi: 10.1126/science.1164094. [DOI] [PubMed] [Google Scholar]
- 4.Richard D, Carpentier AC, Dore G, Ouellet V, Picard F. Int J Obes (Lond) 2010;34(Suppl 2):S59–66. doi: 10.1038/ijo.2010.241. [DOI] [PubMed] [Google Scholar]
- 5.Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K, Kawai Y, Tsujisaki M. Diabetes. 2009;58(7):1526–1531. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tilg H, Moschen AR. Nat Rev Immunol. 2006;6(10):772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 7.Kershaw EE, Flier JS. J Clin Endocrinol Metab. 2004;89(6):2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 8.Gao YJ. Curr Pharm Des. 2007;13(21):2185–2192. doi: 10.2174/138161207781039634. [DOI] [PubMed] [Google Scholar]
- 9.Chatterjee TK, Stoll LL, Denning GM, Harrelson A, Blomkalns AL, Idelman G, Rothenberg FG, Neltner B, Romig-Martin SA, Dickson EW, Rudich S, Weintraub NL. Circ Res. 2009;104(4):541–549. doi: 10.1161/CIRCRESAHA.108.182998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galvez-Prieto B, Bolbrinker J, Stucchi P, de Las Heras AI, Merino B, Arribas S, Ruiz-Gayo M, Huber M, Wehland M, Kreutz R, Fernandez-Alfonso MS. J Endocrinol. 2008;197(1):55–64. doi: 10.1677/JOE-07-0284. [DOI] [PubMed] [Google Scholar]
- 11.Cao R, Brakenhielm E, Wahlestedt C, Thyberg J, Cao Y. Proc Natl Acad Sci U S A. 2001;98(11):6390–6395. doi: 10.1073/pnas.101564798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohashi K, Ouchi N, Matsuzawa Y. Am J Hypertens. 2011 doi: 10.1038/ajh.2010.216. [DOI] [PubMed] [Google Scholar]
- 13.Bhalla V, Kalogeropoulos A, Georgiopoulou V, Butler J. Biomark Med. 2010;4(3):445–452. doi: 10.2217/bmm.10.17. [DOI] [PubMed] [Google Scholar]
- 14.Rajsheker S, Manka D, Blomkalns AL, Chatterjee TK, Stoll LL, Weintraub NL. Curr Opin Pharmacol. 2010;10(2):191–196. doi: 10.1016/j.coph.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamawaki H. Biol Pharm Bull. 2011;34(3):307–310. doi: 10.1248/bpb.34.307. [DOI] [PubMed] [Google Scholar]
- 16.Thalmann S, Meier CA. Cardiovasc Res. 2007;75(4):690–701. doi: 10.1016/j.cardiores.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Lu C, Su LY, Lee RM, Gao YJ. Eur J Pharmacol. 2010;634(1-3):107–112. doi: 10.1016/j.ejphar.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Fang L, Zhao J, Chen Y, Ma T, Xu G, Tang C, Liu X, Geng B. J Hypertens. 2009;27(11):2174–2185. doi: 10.1097/HJH.0b013e328330a900. [DOI] [PubMed] [Google Scholar]
- 19.Gao YJ, Lu C, Su LY, Sharma AM, Lee RM. Br J Pharmacol. 2007;151(3):323–331. doi: 10.1038/sj.bjp.0707228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ketonen J, Shi J, Martonen E, Mervaala E. Circ J. 2010;74(7):1479–1487. doi: 10.1253/circj.cj-09-0661. [DOI] [PubMed] [Google Scholar]
- 21.Guzik TJ, Marvar PJ, Czesnikiewicz-Guzik M, Korbut R. J Physiol Pharmacol. 2007;58(4):591–610. [PubMed] [Google Scholar]
- 22.Henrichot E, Juge-Aubry CE, Pernin A, Pache JC, Velebit V, Dayer JM, Meda P, Chizzolini C, Meier CA. Arterioscler Thromb Vasc Biol. 2005;25(12):2594–2599. doi: 10.1161/01.ATV.0000188508.40052.35. [DOI] [PubMed] [Google Scholar]
- 23.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr. J Clin Invest. 2003;112(12):1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. J Clin Invest. 2003;112(12):1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Withers SB, Agabiti-Rosei C, Livingstone DM, Little MC, Aslam R, Malik RA, Heagerty AM. Arterioscler Thromb Vasc Biol. 2011 doi: 10.1161/ATVBAHA.110.221705. [DOI] [PubMed] [Google Scholar]
- 26.Soltis EE, Cassis LA. Clin Exp Hypertens A. 1991;13(2):277–296. doi: 10.3109/10641969109042063. [DOI] [PubMed] [Google Scholar]
- 27.Dubrovska G, Verlohren S, Luft FC, Gollasch M. Am J Physiol Heart Circ Physiol. 2004;286(3):H1107–1113. doi: 10.1152/ajpheart.00656.2003. [DOI] [PubMed] [Google Scholar]
- 28.Lohn M, Dubrovska G, Lauterbach B, Luft FC, Gollasch M, Sharma AM. FASEB J. 2002;16(9):1057–1063. doi: 10.1096/fj.02-0024com. [DOI] [PubMed] [Google Scholar]
- 29.Verlohren S, Dubrovska G, Tsang SY, Essin K, Luft FC, Huang Y, Gollasch M. Hypertension. 2004;44(3):271–276. doi: 10.1161/01.HYP.0000140058.28994.ec. [DOI] [PubMed] [Google Scholar]
- 30.Gollasch M, Dubrovska G. Trends Pharmacol Sci. 2004;25(12):647–653. doi: 10.1016/j.tips.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 31.Fesus G, Dubrovska G, Gorzelniak K, Kluge R, Huang Y, Luft FC, Gollasch M. Cardiovasc Res. 2007;75(4):719–727. doi: 10.1016/j.cardiores.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 32.Gao YJ, Takemori K, Su LY, An WS, Lu C, Sharma AM, Lee RM. Cardiovasc Res. 2006;71(2):363–373. doi: 10.1016/j.cardiores.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 33.Gao YJ, Zeng ZH, Teoh K, Sharma AM, Abouzahr L, Cybulsky I, Lamy A, Semelhago L, Lee RM. J Thorac Cardiovasc Surg. 2005;130(4):1130–1136. doi: 10.1016/j.jtcvs.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 34.Lee RM, Ding L, Lu C, Su LY, Gao YJ. Can J Physiol Pharmacol. 2009;87(11):944–953. doi: 10.1139/y09-088. [DOI] [PubMed] [Google Scholar]
- 35.Lee RM, Lu C, Su LY, Gao YJ. J Hypertens. 2009;27(4):782–790. doi: 10.1097/HJH.0b013e328324ed86. [DOI] [PubMed] [Google Scholar]
- 36.Lee RM, Lu C, Su LY, Werstuck G, Gao YJ. J Hypertens. 2009;27(1):118–131. doi: 10.1097/HJH.0b013e3283163cc9. [DOI] [PubMed] [Google Scholar]
- 37.Lu C, Su LY, Lee RM, Gao YJ. Eur J Pharmacol. 2011 doi: 10.1016/j.ejphar.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 38.Lu C, Zhao AX, Gao YJ, Lee RM. Eur J Pharmacol. 2011 Jan 13; doi: 10.1016/j.ejphar.2010.12.028. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 39.Brandes RP. Br J Pharmacol. 2007;151(3):303–304. doi: 10.1038/sj.bjp.0707229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Girouard H, Bonev AD, Hannah RM, Meredith A, Aldrich RW, Nelson MT. Proc Natl Acad Sci U S A. 107(8):3811–3816. doi: 10.1073/pnas.0914722107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dashwood MR, Dooley A, Shi-Wen X, Abraham DJ, Dreifaldt M, Souza DS. Interact Cardiovasc Thorac Surg. 2007;12(2):170–173. doi: 10.1510/icvts.2010.247874. [DOI] [PubMed] [Google Scholar]
- 42.Mohammed MM, Myers DS, Sofola OA, Hainsworth R, Drinkhill MJ. Clin Exp Pharmacol Physiol. 2007;34(8):771–774. doi: 10.1111/j.1440-1681.2007.04648.x. [DOI] [PubMed] [Google Scholar]
- 43.Sahin AS, Bariskaner H. Fundam Clin Pharmacol. 2007;21(6):595–600. doi: 10.1111/j.1472-8206.2007.00541.x. [DOI] [PubMed] [Google Scholar]
- 44.Nakagawa K, Higashi Y, Sasaki S, Oshima T, Matsuura H, Chayama K. Hypertens Res. 2002;25(2):161–165. doi: 10.1291/hypres.25.161. [DOI] [PubMed] [Google Scholar]
- 45.Kimura K, Tsuda K, Baba A, Kawabe T, Boh-oka S, Ibata M, Moriwaki C, Hano T, Nishio I. Biochem Biophys Res Commun. 2000;273(2):745–749. doi: 10.1006/bbrc.2000.3005. [DOI] [PubMed] [Google Scholar]
- 46.Rahmouni K, Haynes WG. Curr Diab Rep. 2005;5(4):260–266. doi: 10.1007/s11892-005-0020-5. [DOI] [PubMed] [Google Scholar]
- 47.Fortuno A, Rodriguez A, Gomez-Ambrosi J, Muniz P, Salvador J, Diez J, Fruhbeck G. Endocrinology. 2002;143(9):3555–3560. doi: 10.1210/en.2002-220075. [DOI] [PubMed] [Google Scholar]
- 48.Rodriguez A, Fortuno A, Gomez-Ambrosi J, Zalba G, Diez J, Fruhbeck G. Endocrinology. 2007;148(1):324–331. doi: 10.1210/en.2006-0940. [DOI] [PubMed] [Google Scholar]
- 49.Knudson JD, Dincer UD, Zhang C, Swafford AN, Jr., Koshida R, Picchi A, Focardi M, Dick GM, Tune JD. Am J Physiol Heart Circ Physiol. 2005;289(1):H48–56. doi: 10.1152/ajpheart.01159.2004. [DOI] [PubMed] [Google Scholar]
- 50.Shek EW, Brands MW, Hall JE. Hypertension. 1998;31(1 Pt 2):409–414. doi: 10.1161/01.hyp.31.1.409. [DOI] [PubMed] [Google Scholar]
- 51.Zhu W, Cheng KK, Vanhoutte PM, Lam KS, Xu A. Clin Sci (Lond) 2008;114(5):361–374. doi: 10.1042/CS20070347. [DOI] [PubMed] [Google Scholar]
- 52.Okamoto Y, Arita Y, Nishida M, Muraguchi M, Ouchi N, Takahashi M, Igura T, Inui Y, Kihara S, Nakamura T, Yamashita S, Miyagawa J, Funahashi T, Matsuzawa Y. Horm Metab Res. 2000;32(2):47–50. doi: 10.1055/s-2007-978586. [DOI] [PubMed] [Google Scholar]
- 53.Lam KS, Xu A. Curr Diab Rep. 2005;5(4):254–259. doi: 10.1007/s11892-005-0019-y. [DOI] [PubMed] [Google Scholar]
- 54.Cho Y, Lee SE, Lee HC, Hur J, Lee S, Youn SW, Lee J, Lee HJ, Lee TK, Park J, Hwang SJ, Kwon YW, Cho HJ, Oh BH, Park YB, Kim HS. J Am Coll Cardiol. 2010;57(1):99–109. doi: 10.1016/j.jacc.2010.07.035. [DOI] [PubMed] [Google Scholar]
- 55.Shyu KG, Lien LM, Wang BW, Kuan P, Chang H. Clin Sci (Lond) 2011;120(3):121–129. doi: 10.1042/CS20100226. [DOI] [PubMed] [Google Scholar]
- 56.Wang P, Xu TY, Guan YF, Su DF, Fan GR, Miao CY. Cardiovasc Res. 2009;81(2):370–380. doi: 10.1093/cvr/cvn288. [DOI] [PubMed] [Google Scholar]
- 57.Maenhaut N, Boydens C, Van de Voorde J. Eur J Pharmacol. 2010;641(2-3):207–212. doi: 10.1016/j.ejphar.2010.05.058. [DOI] [PubMed] [Google Scholar]
- 58.Malinowski M, Deja MA, Golba KS, Roleder T, Biernat J, Wos S. Eur J Cardiothorac Surg. 2008;33(2):225–231. doi: 10.1016/j.ejcts.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 59.Greenstein AS, Khavandi K, Withers SB, Sonoyama K, Clancy O, Jeziorska M, Laing I, Yates AP, Pemberton PW, Malik RA, Heagerty AM. Circulation. 2009;119(12):1661–1670. doi: 10.1161/CIRCULATIONAHA.108.821181. [DOI] [PubMed] [Google Scholar]
- 60.Achike FI, To NH, Wang H, Kwan CY. Clin Exp Pharmacol Physiol. 2011;38(1):1–10. doi: 10.1111/j.1440-1681.2010.05460.x. [DOI] [PubMed] [Google Scholar]
- 61.Payne GA, Bohlen HG, Dincer UD, Borbouse L, Tune JD. Am J Physiol Heart Circ Physiol. 2009;297(1):H460–465. doi: 10.1152/ajpheart.00116.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Payne GA, Borbouse L, Kumar S, Neeb Z, Alloosh M, Sturek M, Tune JD. Arterioscler Thromb Vasc Biol. 2010;30(9):1711–1717. doi: 10.1161/ATVBAHA.110.210070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barandier C, Montani JP, Yang Z. Am J Physiol Heart Circ Physiol. 2005;289(5):H1807–1813. doi: 10.1152/ajpheart.01259.2004. [DOI] [PubMed] [Google Scholar]
- 64.Engeli S. Am J Physiol Heart Circ Physiol. 2005;289(5):H1794–1795. doi: 10.1152/ajpheart.00762.2005. [DOI] [PubMed] [Google Scholar]
- 65.Lamers D, Schlich R, Greulich S, Sasson S, Sell H, Eckel J. J Cell Mol Med. 2010 doi: 10.1111/j.1582-4934.2010.01099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Y, Lam KS, Xu JY, Lu G, Xu LY, Cooper GJ, Xu A. J Biol Chem. 2005;280(18):18341–18347. doi: 10.1074/jbc.M501149200. [DOI] [PubMed] [Google Scholar]
- 67.Zhang H, Zhang C. Am J Biomed Sci. 2009;1(2):133–142. doi: 10.5099/aj090200133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yudkin JS, Eringa E, Stehouwer CD. Lancet. 2005;365(9473):1817–1820. doi: 10.1016/S0140-6736(05)66585-3. [DOI] [PubMed] [Google Scholar]
- 69.Montani JP, Carroll JF, Dwyer TM, Antic V, Yang Z, Dulloo AG. Int J Obes Relat Metab Disord. 2004;28(Suppl 4):S58–65. doi: 10.1038/sj.ijo.0802858. [DOI] [PubMed] [Google Scholar]
- 70.Marchesi C, Ebrahimian T, Angulo O, Paradis P, Schiffrin EL. Hypertension. 2009;54(6):1384–1392. doi: 10.1161/HYPERTENSIONAHA.109.138305. [DOI] [PubMed] [Google Scholar]
- 71.Ma L, Ma S, He H, Yang D, Chen X, Luo Z, Liu D, Zhu Z. Hypertens Res. 2010;33(5):446–453. doi: 10.1038/hr.2010.11. [DOI] [PubMed] [Google Scholar]
- 72.Rittig K, Staib K, Machann J, Bottcher M, Peter A, Schick F, Claussen C, Stefan N, Fritsche A, Haring HU, Balletshofer B. Diabetologia. 2008;51(11):2093–2099. doi: 10.1007/s00125-008-1128-3. [DOI] [PubMed] [Google Scholar]
- 73.Lehman SJ, Massaro JM, Schlett CL, O'Donnell CJ, Hoffmann U, Fox CS. Atherosclerosis. 2010;210(2):656–661. doi: 10.1016/j.atherosclerosis.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meijer RI, Serne EH, Smulders YM, van Hinsbergh VW, Yudkin JS, Eringa EC. Curr Diab Rep. 2011;11(3):211–217. doi: 10.1007/s11892-011-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gil-Ortega M, Stucchi P, Guzman-Ruiz R, Cano V, Arribas S, Gonzalez MC, Ruiz-Gayo M, Fernandez-Alfonso MS, Somoza B. Endocrinology. 2010;151(7):3299–3306. doi: 10.1210/en.2009-1464. [DOI] [PubMed] [Google Scholar]
- 76.Rebolledo A, Rebolledo OR, Marra CA, Garcia ME, Roldan Palomo AR, Rimorini L, Gagliardino JJ. Cardiovasc Diabetol. 2010;9(1):65. doi: 10.1186/1475-2840-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huang F, Lezama MA, Ontiveros JA, Bravo G, Villafana S, del-Rio-Navarro BE, Hong E. Clin Exp Hypertens. 2010;32(2):98–104. doi: 10.3109/10641960902993129. [DOI] [PubMed] [Google Scholar]
- 78.Zhang H, Zhang C. Obesity (Silver Spring) 2010;18(11):2071–2076. doi: 10.1038/oby.2010.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Galvez B, de Castro J, Herold D, Dubrovska G, Arribas S, Gonzalez MC, Aranguez I, Luft FC, Ramos MP, Gollasch M, Fernandez Alfonso MS. Arterioscler Thromb Vasc Biol. 2006;26(6):1297–1302. doi: 10.1161/01.ATV.0000220381.40739.dd. [DOI] [PubMed] [Google Scholar]
- 80.Ruan CC, Zhu DL, Chen QZ, Chen J, Guo SJ, Li XD, Gao PJ. Arterioscler Thromb Vasc Biol. 2010;30(12):2568–2574. doi: 10.1161/ATVBAHA.110.215525. [DOI] [PubMed] [Google Scholar]
- 81.Galvez-Prieto B, Dubrovska G, Cano MV, Delgado M, Aranguez I, Gonzalez MC, Ruiz-Gayo M, Gollasch M, Fernandez-Alfonso MS. Hypertens Res. 2008;31(7):1415–1423. doi: 10.1291/hypres.31.1415. [DOI] [PubMed] [Google Scholar]
- 82.Zeng ZH, Zhang ZH, Luo BH, He WK, Liang LY, He CC, Su CJ. Clin Exp Hypertens. 2009;31(4):355–363. doi: 10.1080/10641960902977916. [DOI] [PubMed] [Google Scholar]
- 83.Gao YJ, Holloway AC, Su LY, Takemori K, Lu C, Lee RM. Eur J Pharmacol. 2008;590(1-3):264–268. doi: 10.1016/j.ejphar.2008.05.044. [DOI] [PubMed] [Google Scholar]
- 84.Rodriguez A, Fruhbeck G, Gomez-Ambrosi J, Catalan V, Sainz N, Diez J, Zalba G, Fortuno A. J Hypertens. 2006;24(8):1589–1597. doi: 10.1097/01.hjh.0000239295.17636.6e. [DOI] [PubMed] [Google Scholar]
- 85.Verhagen SN, Visseren FL. Atherosclerosis. 2011;214(1):3–10. doi: 10.1016/j.atherosclerosis.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 86.Vela D, Buja LM, Madjid M, Burke A, Naghavi M, Willerson JT, Casscells SW, Litovsky S. Arch Pathol Lab Med. 2007;131(3):481–487. doi: 10.5858/2007-131-481-TROPFI. [DOI] [PubMed] [Google Scholar]
- 87.Bunker AK, Laughlin MH. J Appl Physiol. 2010;108(3):490–497. doi: 10.1152/japplphysiol.00999.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Takaoka M, Suzuki H, Shioda S, Sekikawa K, Saito Y, Nagai R, Sata M. Arterioscler Thromb Vasc Biol. 2010;30(8):1576–1582. doi: 10.1161/ATVBAHA.110.207175. [DOI] [PubMed] [Google Scholar]
- 89.Lohmann C, Schafer N, von Lukowicz T, Sokrates Stein MA, Boren J, Rutti S, Wahli W, Donath MY, Luscher TF, Matter CM. Atherosclerosis. 2009;207(2):360–367. doi: 10.1016/j.atherosclerosis.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 90.Sacks HS, Fain JN. Am Heart J. 2007;153(6):907–917. doi: 10.1016/j.ahj.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 91.Spiroglou SG, Kostopoulos CG, Varakis JN, Papadaki HH. J Atheroscler Thromb. 2010;17(2):115–130. doi: 10.5551/jat.1735. [DOI] [PubMed] [Google Scholar]
- 92.Hug C, Lodish HF. Curr Opin Pharmacol. 2005;5(2):129–134. doi: 10.1016/j.coph.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 93.Silaghi A, Achard V, Paulmyer-Lacroix O, Scridon T, Tassistro V, Duncea I, Clement K, Dutour A, Grino M. Am J Physiol Endocrinol Metab. 2007;293(5):E1443–1450. doi: 10.1152/ajpendo.00273.2007. [DOI] [PubMed] [Google Scholar]
- 94.Baker AR, Silva NF, Quinn DW, Harte AL, Pagano D, Bonser RS, Kumar S, McTernan PG. Cardiovasc Diabetol. 2006;5:1. doi: 10.1186/1475-2840-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cheng KH, Chu CS, Lee KT, Lin TH, Hsieh CC, Chiu CC, Voon WC, Sheu SH, Lai WT. Int J Obes (Lond) 2008;32(2):268–274. doi: 10.1038/sj.ijo.0803726. [DOI] [PubMed] [Google Scholar]
- 96.Eiras S, Teijeira-Fernandez E, Shamagian LG, Fernandez AL, Vazquez-Boquete A, Gonzalez-Juanatey JR. Cytokine. 2008;43(2):174–180. doi: 10.1016/j.cyto.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 97.Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, Sarov-Blat L, O'Brien S, Keiper EA, Johnson AG, Martin J, Goldstein BJ, Shi Y. Circulation. 2003;108(20):2460–2466. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 98.Ishikawa Y, Akasaka Y, Ito K, Akishima Y, Kimura M, Kiguchi H, Fujimoto A, Ishii T. Atherosclerosis. 2006;186(2):380–389. doi: 10.1016/j.atherosclerosis.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 99.Schleifenbaum J, Kohn C, Voblova N, Dubrovska G, Zavarirskaya O, Gloe T, Crean CS, Luft FC, Huang Y, Schubert R, Gollasch M. J Hypertens. 2010;28(9):1875–1882. doi: 10.1097/HJH.0b013e32833c20d5. [DOI] [PubMed] [Google Scholar]