Abstract
Intrapulmonary arteriovenous anastomoses (IPAVs) are large-diameter pathways that directly connect the arterial and venous networks, bypassing the pulmonary capillaries. Ubiquitously present in healthy humans, these pathways are recruited in experimental conditions by exercise, hypoxia, and catecholamines and have been previously shown to be closed by hyperoxia. Whether they play a role in pulmonary pathophysiology is unknown. Here, we describe IPAV recruitment associated with hypoxemia and right-to-left shunt in a patient with status asthmaticus, treated with agonists of the B2-adrenergic pathway. Our observation of IPAVs in a pediatric patient, mechanically ventilated with 100% O2, suggests that these pathways are recruited in clinically important circumstances and challenges the notion that IPAVs are always closed by alveolar hyperoxia.
Keywords: intrapulmonary shunting, asthma, gas exchange, β2-adrenergic receptor, pediatrics
Inducible intrapulmonary arteriovenous anastomoses (IPAVs) directly connect arteries and veins, bypassing the pulmonary capillary network. Distinct from the pathologic arteriovenous malformations (AVMs) observed in rare diseases of lung remodeling (eg, hereditary hemorrhagic telangiectasia), IPAVs are present in ∼90% of people. These conduits1 have been observed repeatedly in healthy humans, intact animals, and isolated lungs.1–7 Unlike pathologic AVMs, IPAVs are only transiently recruitable. Agitated saline contrast, 99mTc-labeled albumin macroaggregates, and solid microspheres have been used to demonstrate that these pathways open with moderate exercise or alveolar hypoxia. We and others have speculated that IPAVs might exist to modulate the distribution of perfusion in the face of high pulmonary pressures and flows.1,6–11
Experimentally, IPAV recruitment can be prevented or reversed by 100% O2 breathing.4 The fact that only brief periods of hyperoxia are needed suggests that O2 acts locally on the vasculature. Recently, Laurie et al12 echocardiographically observed IPAVs in a group of healthy adults infused with epinephrine, evidenced by the observation of agitated bubble contrast in the left ventricle >5 cardiac cycles after right heart opacification. Right-to-left bubble passage was eliminated by 100% O2 breathing. These authors speculate that IPAVs were initially recruited as a result of increased cardiac output and that O2 acts directly on IPAVs to close them.
It is unknown whether these pathways contribute to lung pathophysiology13 or whether their recruitment is of clinical consequence.14 Here, we describe IPAV recruitment associated with hypoxemia and right-to-left shunt in a patient with status asthmaticus, treated with multiple agonists of the B2-adrenergic pathway. Our observation of IPAVs in a pediatric patient, ventilated with 100% O2, suggests that these pathways are recruited in clinically relevant circumstances by commonly used drugs and challenges the notion that IPAVs are always closed by hyperoxia.
Clinical Presentation
A 2-year-old girl with a history of asthma presented to the emergency department in respiratory distress with cough, chest discomfort, wheezing, and fatigue. Her mother noted severe cough, audible wheezing, and 1 incident of posttussive emesis during the previous night. Despite the use of inhaled albuterol and 3 nebulized budesonide treatments at home, she continued to have labored breathing. Emergency department clinicians noted a low-grade fever, tachypnea, tachycardia, a visibly increased work of breathing, and waning mental status. Before this incident, her asthma had been managed at home with albuterol once daily. Although she had a prescription for budesonide (0.25 mg) twice daily, her mother reported use only during periods of acute illness.
Treatment and Observations
Upon admission to the PICU of the American Family Children’s Hospital (Madison, WI), she was noted to be in fulminant respiratory failure with profound respiratory acidosis (pH = 7.06 and Paco2 = 75 mm Hg). She was subsequently intubated to relieve her work of breathing and manage her acid-base status. She remained sedated and intubated in the PICU for 5 days. She was ventilated with a prolonged expiratory phase, mild permissive hypercapnia, and an elevated fraction of inspired oxygen (Fio2) aimed at achieving near-normal PaO2. She was treated empirically with ceftriaxone and azithromycin for pneumonia.
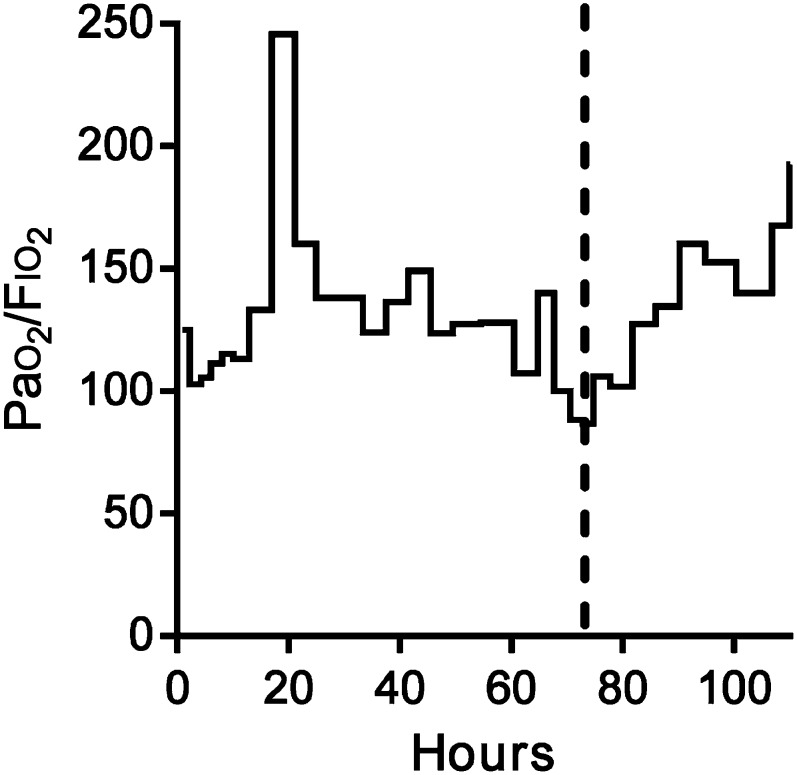
During the first 3 days, treatment strategies were added, aimed at improving her persistent airway dysfunction. Treatment ultimately included inhaled albuterol (20 mg/kg per hour) and ipratropium bromide (500 μg q4h), intravenous steroids, magnesium sulfate, aminophylline (1 mg/kg per hour), and terbutaline (5 μg/kg per minute), initiated sequentially over the first day. On the third day, marked worsening arterial hypoxemia (PaO2 = 52 mm Hg, arterial oxygen saturation [SaO2] = 88%) was noted despite optimal ventilator settings (Fig 1). A chest radiograph showed mild hyperinflation but no lung parenchymal disease. Furthermore, her SaO2 was not improved by increasing the Fio2 from 0.6 to 1.0, suggestive of a right-to-left shunt.
FIGURE 1.
Ratio between the alveolar O2 partial pressure of oxygen and the Fio2 (Pao2/Fio2) as an indicator of gas exchange efficiency. Treatment began at t = 0. Note the worsening of the Pao2-Fio2 ratio at 60 hours. The dashed line indicates the timing of the saline contrast echocardiogram shown in Fig 2 and the decision to discontinue this patient’s intravenous terbutaline. The cessation of terbutaline coincides with an improvement in Pao2/Fio2 over the subsequent 24 hours.
An echocardiogram was done to determine the source of right-to-left shunt. The echocardiogram was normal, without evidence of atrial or ventricular septal defects that would contribute to intracardiac right-to-left shunt. An agitated bubble contrast study showed a substantial, delayed bubble passage, indicative of an intrapulmonary shunt (Fig 2). She had no relevant history suggestive of an AVM with chronic hypoxemia. She was not polycythemic (hematocrit = 35% at admission) and had no liver disease, exercise intolerance, orthodeoxia, clubbing of the digits, or family history of congenital AVM.
FIGURE 2.
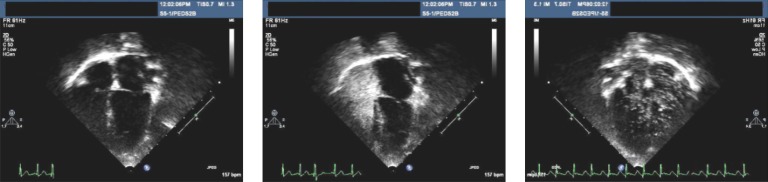
Saline contrast echocardiogram demonstrating right-to-left intrapulmonary shunting. The 4-chamber apical view is shown in the left panel. In the center panel, opacification of the right ventricle by agitated saline contrast is noted. Bubbles in left ventricle, 9 cardiac cycles after right ventricular opacification, are shown in the right panel.
Terbutaline was immediately discontinued, resulting in an improvement of SaO2 and PaO2 (PaO2 = 80 mm Hg, SaO2 = 96%) over the next 24 hours. Over the next few days, her clinical status improved. She was extubated on the fifth day but continued to demonstrate some mild hypoxemia. A repeated echocardiogram on the sixth day revealed only trace transpulmonary bubble passage. Over the next days the patient was transitioned from continuous therapy to an asthma control regimen (albuterol 90 μg, 2 puffs as needed, and fluticasone 220 μg twice daily). With the cessation of nebulized albuterol, her hypoxemia and right-to-left shunt completely resolved.
Discussion
Here, we present evidence of IPAV recruitment in a patient with life-threatening asthma, treated with agonists of the β2-adrenergic pathway. We present this case specifically because it suggests future hypotheses in the study of these novel pathways and demonstrates that their clinical importance may be underappreciated. Arteriovenous anastomoses exist ubiquitously in many systemic vascular beds,15–23 are important in modulating local blood flow, and are often regulated by catecholamines.24–26 In contrast, the purpose and regulation of IPAVs are less well understood. It is important to distinguish here between pathologic AVMs, which are rare and are found idiopathically or seen in the context of diseases such as hereditary hemorrhagic telangiectasia27 and hepatopulmonary syndrome,28 and IPAVs. We and others have documented the presence of transiently recruitable IPAVs in ∼90% of healthy humans.2–4,8,9,29–30 Although the size of IPAVs has never been directly determined in humans under physiologic conditions, data from isolated lung and intact animal studies suggest that these pathways are large (>200 μm) and are located early in the pulmonary vascular tree.1,31–33
Why Is This Case Relevant to the Clinician?
Allowing mixed venous blood to bypass the pulmonary capillaries subverts both the gas exchange and biological filter functions of the lung. During exercise, IPAV recruitment coincides with the onset of exercise-induced arterial hypoxemia.3,6,10 In a recent case report of an adult patient with beriberi heart, patent IPAVs were observed in association with arterial hypoxemia.14 Thiamine repletion resulted in IPAV closure and resolution of arterial hypoxemia.
Classically, lack of improvement of arterial hypoxemia with 100% O2 administration indicates right-to-left shunt.34 This shunt may be anatomic, caused by intracardiac defects or intrapulmonary arteriovenous anastomoses, or it may be physiologic, resulting from extreme ventilation-perfusion inequality. In the past, we have found several cases of unexpected anatomic shunt contributing to unexplained arterial hypoxemia and now routinely perform a saline contrast echocardiogram to distinguish between anatomic and physiologic shunt in patients with severe hypoxemia of unclear etiology.
Conventional teaching suggests that the worsening hypoxemia with β agonist therapy is caused by ventilation-perfusion mismatch, although our case suggests that transient intrapulmonary shunt may be an important contributor. With this patient’s first echocardiogram, we noted abundant right-to-left bubble passage, suggesting that a component of her hypoxemia was attributable to anatomic, intrapulmonary shunting. With the discontinuation of terbutaline, her hypoxemia improved over the next 24 hours but did not completely resolve. Another saline contrast echocardiogram was performed on the sixth day to better define the cause of her remaining mild hypoxemia. It would have been reasonable to suspect that this remaining mild hypoxemia was the result of an idiopathic pulmonary AVM. Here, we performed the second echocardiogram as an alternative to immediately considering angiography to identify a suspected AVM. It proved invaluable in identifying the contributors to her hypoxemia. Because the repeat echocardiogram demonstrated only trivial bubble passage, we were able to conclude that her remaining hypoxemia was the result of ventilation-perfusion mismatch. Saline contrast echocardiography is a low-risk tool35,36 to determine whether shunt has an anatomic component, and the risk of systemic embolization is negligible, even in patients with anatomic right-to-left shunt.
Given the complexity of the airway disease in our patient, it is difficult to precisely partition the fraction of the hypoxemia caused by intrapulmonary shunting. However, it is noteworthy that her worsening hypoxemia coincided with the use of agonists of an adrenergic pathway and that it improved rapidly after the discontinuation of terbutaline. In Fig 1, we note initial improvement in the Pao2-Fio2 ratio at hour 20 that coincides with the initiation of multiple bronchodilators, including continuous terbutaline. We attribute this improvement to bronchodilation and better ventilation-perfusion matching. After a subsequent period of worsening hypoxemia, intrapulmonary shunting was identified, and her terbutaline was discontinued. The steady, progressive improvement in her hypoxemia over 24 hours is consistent with the washout of terbutaline, which has a half-life of ∼2 hours in these circumstances.37
The other characteristic of this case that is important is that this patient needed a long period of mechanical ventilation with sedation. Recently, a multicenter study demonstrated that immobility in the PICU, with central venous catheterization, is associated with a risk of deep vein thrombosis (24.7 per 1000 catheter-days).38 IPAVs provide a route by which these thrombi could bypass the pulmonary circulation, resulting in arterial embolization. Whether IPAVs influence stroke risk in the PICU warrants additional investigation.
What Does This Case Teach Us About IPAV Regulation?
Previous studies in our laboratory have shown that alveolar hyperoxia consistently closes IPAVs. Importantly, this is the first time that IPAVs have been observed in coincidence with alveolar hyperoxia. Indeed, there are notable differences between our patient and previous physiologic studies that suggest future hypotheses to be explored. Laurie et al12 used epinephrine, which is a mixed adrenergic agonist. However, our patient was treated with specific B2-adrenergic pathway agonists (albuterol and terbutaline). It should be considered that the IPAVs may be recruited directly by B2-receptor–mediated dilation and that our observations are related to the high specificity of the agents used. Again, we noted a progressive improvement after the discontinuation of terbutaline.
We can also speculate on the importance of our patient’s extended treatment in overcoming the effects of hyperoxia. Laurie et al demonstrated that both the level of hypoxia and the duration of the exposure influence IPAVs such that a mild hypoxic stimulus can be effective if it is prolonged.30 Similarly, exposure to adrenergic stimuli may overcome the effects of hyperoxia if the dosing is prolonged. It is possible that if they had prolonged the infusion of epinephrine, they would also have observed IPAV recruitment despite the 100% O2 breathing.
Our patient also received treatment with aminophylline and magnesium sulfate, and we cannot rule out the possibility that they were synergistic culprits in this case. Terbutaline and albuterol stimulate the B2 receptor, thereby increasing the concentration of intracellular cyclic adenosine monophosphate. Aminophylline prolongs the lifetime of this second messenger by inhibiting phosphodiesterase. Thus, aminophylline may increase the magnitude of the response by slowing the conversion of cyclic adenosine monophosphate. Indeed, Butler and Hills39 observed an increase in transpulmonary bubble passage in air-embolized dogs that were pretreated with aminophylline. Magnesium sulfate is a B2 pathway–independent pulmonary vasodilator that might be considered in future investigations.40 The multiple agents used here also reflect this patient’s life-threatening illness. We cannot rule out that her asthma, or the infection she was treated empirically for, may have contributed to IPAVs recruitment.
This is the first time that IPAV opening has been noted in a child. During childhood, the sensitivity of the pulmonary vasculature to oxygen, vasodilators, and vasoconstrictors evolves. It is possible that hyperoxia may be effective in countering the effects of IPAV recruiting agents in adults but that it does not have the same efficacy in children. McMullan et al41 demonstrated that IPAVs are patent in late in gestation in lambs but close or regress postnatally. Studies of IPAV sensitivity have not been completed during this dynamic developmental period.
Conclusions
IPAVs may be recruited by therapies commonly used in the pediatric intensive care setting, resulting in worsening hypoxemia. The consequences of IPAVs opening and potential mechanistic differences between the adult and child are important topics of future investigation.
Acknowledgment
The authors are grateful to Erica White for her assistance in the preparation of this manuscript.
Glossary
- AVM
arteriovenous malformation
- Fio2
fraction of inspired oxygen
- IPAV
intrapulmonary arteriovenous anastomosis
- Sao2
arterial oxygen saturation
Footnotes
Dr Eldridge provided the primary care of this patient; Drs Bates and Eldridge discussed the interpretation of this patient’s findings and treatment during her admission to the PICU; and all authors reviewed the patient’s data after discharge, interpreted these data, and prepared and reviewed the manuscript.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by funding from the National Institutes of Health (5R01HL086897 and 5T32HL007654) and the American Heart Association (postdoctoral fellowship, Dr Bates). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Bates ML, Fulmer BR, Farrell ET, et al. Hypoxia recruits intrapulmonary arteriovenous pathways in intact rats but not isolated rat lungs. J Appl Physiol. 2012;112(11):1915–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lovering AT, Haverkamp HC, Romer LM, Hokanson JS, Eldridge MW. Transpulmonary passage of 99mTc macroaggregated albumin in healthy humans at rest and during maximal exercise. J Appl Physiol. 2009;106(6):1986–1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lovering AT, Romer LM, Haverkamp HC, Pegelow DF, Hokanson JS, Eldridge MW. Intrapulmonary shunting and pulmonary gas exchange during normoxic and hypoxic exercise in healthy humans. J Appl Physiol. 2008;104(5):1418–1425 [DOI] [PubMed] [Google Scholar]
- 4.Lovering AT, Stickland MK, Amann M, et al. Hyperoxia prevents exercise-induced intrapulmonary arteriovenous shunt in healthy humans. J Physiol. 2008;586(pt 18):4559–4565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lovering AT, Stickland MK, Kelso AJ, Eldridge MW. Direct demonstration of 25- and 50-microm arteriovenous pathways in healthy human and baboon lungs. Am J Physiol Heart Circ Physiol. 2007;292(4):H1777–H1781 [DOI] [PubMed] [Google Scholar]
- 6.Stickland MK, Lovering AT. Exercise-induced intrapulmonary arteriovenous shunting and pulmonary gas exchange. Exerc Sport Sci Rev. 2006;34(3):99–106 [DOI] [PubMed] [Google Scholar]
- 7.Stickland MK, Lovering AT, Eldridge MW. Exercise-induced arteriovenous intrapulmonary shunting in dogs. Am J Respir Crit Care Med. 2007;176(3):300–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eldridge MW, Dempsey JA, Haverkamp HC, Lovering AT, Hokanson JS. Exercise-induced intrapulmonary arteriovenous shunting in healthy humans. J Appl Physiol. 2004;97(3):797–805 [DOI] [PubMed] [Google Scholar]
- 9.Lovering AT, Stickland MK, Eldridge MW. Intrapulmonary shunt during normoxic and hypoxic exercise in healthy humans. Adv Exp Med Biol. 2006;588:31–45 [DOI] [PubMed] [Google Scholar]
- 10.Stickland MK, Welsh RC, Haykowsky MJ, et al. Intra-pulmonary shunt and pulmonary gas exchange during exercise in humans. J Physiol. 2004;561(pt 1):321–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.La Gerche A, MacIsaac AI, Burns AT, et al. Pulmonary transit of agitated contrast is associated with enhanced pulmonary vascular reserve and right ventricular function during exercise. J Appl Physiol. 2010;109(5):1307–1317 [DOI] [PubMed] [Google Scholar]
- 12.Laurie SS, Elliott JE, Goodman RD, Lovering AT. Catecholamine-induced opening of intrapulmonary arteriovenous anastomoses in healthy humans at rest. J Appl Physiol. 2012;113(8):1213–1222 [DOI] [PubMed] [Google Scholar]
- 13.Bates ML. A potential role for intrapulmonary shunt pathways in pathology. J Appl Physiol. 2009;107(3):1000–1001; discussion 1997–1008, 1998 [DOI] [PubMed]
- 14.Nakano S, Sujino Y, Tanno J, et al. Inducible intrapulmonary arteriovenous shunt in a patient with beriberi heart. Am J Respir Crit Care Med. 2013;187(3):332–333 [DOI] [PubMed] [Google Scholar]
- 15.Schlegel JU. Arteriovenous anastomoses in the endometrium in man. Acta Anat (Basel). 1945–1946;1(3):284–325 [DOI] [PubMed] [Google Scholar]
- 16.Prinzmetal M, Ornitz EM, Jr, Simkin B, Bergman HC. Arterio-venous anastomoses in liver, spleen, and lungs. Am J Physiol. 1948;152(1):48–52 [DOI] [PubMed] [Google Scholar]
- 17.Simkin B, Bergman HC, Silver H.Prinzmetal M,. Renal arteriovenous anastomoses in rabbits, dogs and human subjects. Arch Intern Med (Chic). 1948;81(2): 115–125 [DOI] [PubMed] [Google Scholar]
- 18.Barrie HJ, Klebanoff SJ, Cates GW. Direct medullary arterioles and arteriovenous anastomoses in the arcuate sponges of the kidney. Lancet. 1950;1(6593):23. [DOI] [PubMed] [Google Scholar]
- 19.Walder DN. Arteriovenous anastomoses in the stomach wall. Lancet. 1950;1(6596):162. [DOI] [PubMed] [Google Scholar]
- 20.Staubesand J. Various types of arteriovenous anastomoses [in German]. Anat Anz. 1951;97(suppl):68–75 [PubMed] [Google Scholar]
- 21.Doby T. The number of direct arteriovenous anastomoses in the kidney. Acta Med Acad Sci Hung. 1952;3(2):207–210 [PubMed] [Google Scholar]
- 22.Piiper J, Schneider PW, Schoedel W. Arteriovenous anastomoses [in German]. Klin Wochenschr. 1954;32(23–24):540–542 [DOI] [PubMed] [Google Scholar]
- 23.Vajda J, Herpai Z, Raposa T. Arterio-venous anastomoses in small intestine. Acta Morphol Hung. 1966;14(3–4):335 [Google Scholar]
- 24.Baker CH, Davis DL, Sutton ET. Blood flow distribution with adrenergic and histaminergic antagonists. Proc Soc Exp Biol Med. 1989;190(3):260–267 [DOI] [PubMed] [Google Scholar]
- 25.Amenta F, Mione MC, Napoleone P. The autonomic innervation of the vasa nervorum. J Neural Transm. 1983;58(3–4):291–297 [DOI] [PubMed] [Google Scholar]
- 26.Waris T, Kyösola K, Partanen S. The adrenergic innervation of arteriovenous anastomoses in the subcutaneous fascia of rat skin. Scand J Plast Reconstr Surg. 1980;14(3):215–220 [DOI] [PubMed] [Google Scholar]
- 27.Velthuis S, Buscarini E, van Gent MW, et al. Grade of pulmonary right-to-left shunt on contrast echocardiography and cerebral complications: a striking association. Chest. 2013;144(2):542–548 [DOI] [PubMed] [Google Scholar]
- 28.Khabbaza JE, Krasuski RA, Tonelli AR. Intrapulmonary shunt confirmed by intracardiac echocardiography in the diagnosis of hepatopulmonary syndrome. Hepatology. 2013;58(4):1514–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elliott JE, Choi Y, Laurie SS, Yang X, Gladstone IM, Lovering AT. Effect of initial gas bubble composition on detection of inducible intrapulmonary arteriovenous shunt during exercise in normoxia, hypoxia, or hyperoxia. J Appl Physiol. 2011;110(1):35–45 [DOI] [PubMed] [Google Scholar]
- 30.Laurie SS, Yang X, Elliott JE, Beasley KM, Lovering AT. Hypoxia-induced intrapulmonary arteriovenous shunting at rest in healthy humans. J Appl Physiol. 2011;110(5):1502. [DOI] [PubMed] [Google Scholar]
- 31.Tobin CE. Arteriovenous shunts in the peripheral pulmonary circulation in the human lung. Thorax. 1966;21(3):197–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tobin CE, Zariquiey MO. Arteriovenous shunts in the human lung. Proc Soc Exp Biol Med. 1950;75(3):827–829 [DOI] [PubMed] [Google Scholar]
- 33.Ragaglia G, Deluca F, Pastena L. Arteriovenous anastomoses of the lung [in Italian]. Rass Int Clin Ter. 1965;45:412–417 [PubMed] [Google Scholar]
- 34.West JB. Respiratory Physiology: The Essentials. 8th ed. Philadelphia, PA: Wolters Kluwer; 2008 [Google Scholar]
- 35.Parker JM, Weller MW, Feinstein LM, et al. Safety of ultrasound contrast agents in patients with known or suspected cardiac shunts. Am J Cardiol. 2013;112(7):1039–1045 [DOI] [PubMed] [Google Scholar]
- 36.Marriott K, Manins V, Forshaw A, Wright J, Pascoe R. Detection of right-to-left atrial communication using agitated saline contrast imaging: experience with 1162 patients and recommendations for echocardiography. J Am Soc Echocardiogr. 2013;26(1):96–102 [DOI] [PubMed] [Google Scholar]
- 37.Lebovitz DJ, Smith PG, O’Riordan M, Reed MD. Pharmacokinetic properties and tolerability of single-dose terbutaline in patients with severe asthma treated in the pediatric intensive care unit. Curr Ther Res. 2004;65(1):98–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Faustino EV, Spinella PC, Li S, et al. Incidence and acute complications of asymptomatic central venous catheter-related deep venous thrombosis in critically ill children. J Pediatr. 2013;162(2):387–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Butler BD, Hills BA. The lung as a filter for microbubbles. J Appl Physiol. 1979;47(3):537–543 [DOI] [PubMed] [Google Scholar]
- 40.Weinberger B, Weiss K, Heck DE, Laskin DL, Laskin JD. Pharmacologic therapy of persistent pulmonary hypertension of the newborn. Pharmacol Ther. 2001;89(1):67–79 [DOI] [PubMed] [Google Scholar]
- 41.McMullan DM, Hanley FL, Cohen GA, Portman MA, Riemer RK. Pulmonary arteriovenous shunting in the normal fetal lung. J Am Coll Cardiol. 2004;44(7):1497–1500 [DOI] [PubMed] [Google Scholar]