Abstract
BACKGROUND AND OBJECTIVES:
Preterm infants commonly have transient hypothyroxinemia of prematurity after birth, which has been associated with deficits in general intellectual functioning, memory, attention, and academic achievement. However, research has predominantly focused on thyroxine levels in the first 2 weeks of life and outcomes are limited to the preschool period. Our objective was to evaluate the relationships between free thyroxine (fT4) levels over the first 6 weeks after very preterm (VPT) birth with cognitive functioning and brain development at age 7 years.
METHODS:
A total of 83 infants born VPT (<30 weeks’ gestation) had fT4 concentrations measured postnatally and 2- and 6-week area under the curve (AUC) summary measures were calculated. Follow-up at age 7 years included a neuropsychological assessment and brain MRI. Univariable and multivariable regression modeling was used where AUC for fT4 was the main predictor of neurodevelopmental outcome at age 7 years.
RESULTS:
Multivariable modeling revealed that higher, not lower, postnatal fT4 levels (2-week AUC) were associated with poorer cognitive performances at age 7 years on tasks of verbal learning (P = .02), verbal memory (P = .03), and simple reaction time (P < .001). A similar pattern of results was found when the 6-week AUC was examined. No significant associations between postnatal fT4 levels and brain volumes at age 7 years were identified.
CONCLUSIONS:
Results are contradictory to previous observations and suggest that after adjustment for confounders, higher postnatal fT4 levels in VPT infants, rather than lower levels, may be a marker of adverse neuropsychological development in childhood.
Keywords: preterm infants, thyroxine, hormones, cognitive outcome, brain volumes
What’s Known on This Subject:
Preterm infants have transiently lowered thyroid hormone levels during the early postnatal period. Past research suggests that low thyroid hormone levels are related to cognitive and developmental deficits in children born preterm.
What This Study Adds:
Contrary to expectations, in this study of children born <30 weeks’ gestation, higher concentrations of free thyroxine over the first 6 weeks of life were associated with poorer cognitive function at 7 years of age.
Very preterm (VPT; <32 weeks’ gestation) children are at increased risk for a range of neuropsychological deficits, including impairments in general intellect,1 language,2 visuoperceptual reasoning,3 learning and memory,4,5 attention,6 and executive functioning.7 Furthermore, neuroimaging studies of preterm infants and children have reported volumetric reductions in gray and white matter tissue,8 as well as specific brain regions (cerebellum, basal ganglia, amygdala, hippocampi, and corpus callosum).9 Identifying mechanisms underpinning these negative outcomes is critical to improve the management and outcomes of these infants.
Transient hypothyroxinemia of prematurity (THOP) is a condition that primarily affects preterm infants born <30 weeks’ gestation and is characterized by low levels of circulating thyroid hormones (THs), despite normal levels of the pituitary hormone, thyroid stimulating hormone (TSH). During the first week of life, TH levels, for which thyroxine (T4) is the body’s main circulating TH, drop to a nadir below cord levels. The depth of the nadir and length of time before THOP resolves is related to gestational age (GA). This condition usually resolves within 2 to 3 weeks with progressive maturation of the hypothalamic-pituitary-thyroid axis.10,11 As no consensus exists for THOP reference ranges, prevalence rates vary by study definition, with 35%12 to 85%13 of VPT infants reported to be affected.
THs are critical for normal central nervous system (CNS) development,14 including cerebral neurogenesis (during early prenatal life)15; neural migration and differentiation, axonal and dendritic growth, and synaptogenesis16,17; gliogenesis (late fetal life until 6 months postnatally); dendritic arborization18; and myelogenesis (second trimester to 2 years postnatally).19 Consistent with this literature, the low levels of T4 associated with THOP have been found to be related to neurodevelopmental deficits in children born preterm, such as delayed development of motor, cognitive, language, and educational skills.20–24
Research to date has predominantly focused on TH levels in the first 2 weeks of life, and in most studies outcome assessments have been limited to infancy and the preschool period. The current study aimed to examine the relationship between fT4 over the first 6 weeks after VPT birth and cognitive functioning and brain development at age 7 years. Based on previous research it was hypothesized that low fT4 levels would be negatively associated with cognitive performance and brain volumes at age 7 years in a group of children born VPT.
METHOD
Study Design
Participants (n = 99) were born <30 weeks’ GA between June 2002 and December 2003 at the Royal Women’s Hospital (Melbourne, Victoria, Australia). Infants who had significant genetic or congenital abnormalities likely to affect brain growth or development were excluded. At age 7 years, 83 were assessed (the “assessed group”). Approval for the study was obtained from the Human Research Ethics Committees of the Royal Women’s Hospital and the Royal Children’s Hospital. Written consent was obtained from parents.
Procedure and Measures
Whole blood (0.5 mL) was collected from the cord at delivery, then on days 1, 4, 7, 14, 21, 28, and 42. Specimen collection was timed with routine morning blood sampling (7 to 9 am). Whole blood was centrifuged at 3000 rpm for 5 minutes. Plasma was collected and stored at −70°C; hormone assays were performed in batches. Free T4 was measured directly by competitive analog immunoassay using a chemiluminescent substrate on the Immulite 2000 (Siemens AG, Healthcare Sector, Erlangen, Federal Republic of Germany). The level of fT4 was interpolated from a stored standard curve calibrated for fT4 concentrations.25 The intra- and inter-assay coefficients of variation were acceptable (intra-assay = 5.1%; inter-assay range = 7.5% to 5.7%). Neonatal fT4 data were expressed as area under the curve (AUC) values as a summary measure of hormone exposure over 2 designated time periods: (1) birth to day 14, and (2) birth to day 42.
Additional perinatal variables of interest included GA (weeks), sex, and duration of intermittent positive pressure ventilation (IPPV), which was used as a general marker of illness. At term equivalent age, T1 and T2 images were acquired with a 1.5 Tesla MRI scanner at the Royal Children’s Hospital (Signa LX Echospeed System; General Electric, Milwaukee, WI). A validated global cerebral abnormality scoring system was rated by a neonatal neurologist who was blinded to TH status, with higher values reflecting more severe pathology.26
The 7-year follow-up included a detailed neuropsychological assessment and neuroimaging. Neuropsychological measures for the current study were selected to represent a broad range of cognitive domains (Table 1). Children who attempted a task but were too impaired to perform or had difficulty comprehending task instructions were assigned the lowest possible raw score for a subtest, or in the case of reaction time measures, the highest score recorded to signify a longer reaction time. The Social Risk Index parent
TABLE 1.
Neuropsychological Measures
| Test Battery | Description |
|---|---|
| WASI | The 4-subtest version of the WASI was administered to estimate general intellectual functioning (FSIQ). Normative mean = 100, SD = 15. |
| CELF-IV | The Core Language Index from the CELF-IV was administered and used as a measure of general language ability. Normative mean = 100, SD = 15. |
| WMTBC | Three subtests from the WMTBC were administered to assess elements of immediate and working memory: (1) Digit Recall involves the child repeating a sequence of numbers, assessing immediate verbal memory; (2) Backward Digit Recall involves the child repeating a presented sequence of numbers in reverse order, assessing verbal working memory; and (3) Block Recall requires the child to recall a sequence of blocks that the examiner taps on a standardized board, assessing spatial immediate memory. Raw scores for each task were used in the analyses. |
| CVLT-C | The CVLT-C is a verbal learning and memory task in which the child is required to recall a list of 15 words, which is presented 5 times. Raw total learning score (total words recalled from trial 1 to 5) and long delay recall score were used as measures of verbal learning and verbal memory, respectively. |
| CMS | Dot Locations subtest from the CMS was administered to assess spatial learning and memory. The child is required to learn and remember the spatial location of an array of dots, which is presented 3 times. Raw total learning score (total score from trials 1 to 3) and long delay recall score were used as measures of spatial learning and spatial memory, respectively. |
| TEA-Ch | Raw scores for 4 selected subtests were used to assess different attention domains: (1) Sky Search (number of correct targets) assesses selective attention; (2) Score! (number of correct trials) assesses sustained attention; (3) Creature Counting (number of correct trials) assesses shifting attention; and (4) Sky Search Dual Task (proportion of correct counting games and identified targets) assesses divided attention. |
| TOL | The TOL is a measure of planning, organization, and problem solving. The total raw score was used, which is a summary score for the 12 items where higher points are awarded for quicker completion times and points subtracted for planning errors. |
| CogState Research58 | Two subtests from the CogState Research battery were selected to assess processing speed: (1) Detection assesses simple reaction time; and (2) Identification assesses choice reaction time. The variable of interest for these 2 tasks was the mean of the log10 transformed reaction times for correct responses where lower scores indicated a better performance. |
CELF-IV, Clinical Evaluation of Language Fundamentals, 4th ed, Australian Standardized Edition51; CMS, Children’s Memory Scale54; CVLT-C, California Verbal Learning Test – Children’s Version53; TEA-Ch, Test of Everyday Attention for Children55; TOL, Tower of London56,57; WASI, Wechsler Abbreviated Scale of Intelligence50; WMTBC, Working Memory Test Battery for Children52
questionnaire was also administered with higher scores representing greater social disadvantage.27
Whole brain images were acquired at age 7 years on a 3 Tesla Siemens Magnetom Trio (Tim system) scanner, at the Royal Children’s Hospital, Melbourne, Australia (n = 65). Reasons for drop-out included: failing the mock scan (n = 7), being too impaired to participate (n = 4), parents not consenting to MRI (n = 3), failing to attend (n = 1), refusal by the child (n = 1), cochlear implant (n = 1), and extreme movement artifact (n = 1). Structural T1-weighted images were used for the purpose of the current study (flip angle = 9 , repetition time = 2.27 ms, echo time = 2.27 ms, field of view = 210 × 210 mm, matrix = 256 × 256, 0.8 mm3 isotropic voxels). Images were visually inspected and those that were considered of unacceptably poor quality due to artifact were excluded (n = 13). Data were processed by a single operator on Linux workstations using the automated FreeSurfer imaging processing suite (stable release version 4.4.0, http://surfer.nmr.mgh.harvard.edu). FreeSurfer output was inspected and manually edited as required. Cortical and cerebellar gray and white matter, thalamus, caudate, putamen, pallidum, hippocampus, and amygdala volumes were estimated for each hemisphere, and volumes from both hemispheres combined. The total volumes for the caudate, putamen, and pallidum were combined to represent basal ganglia volume. Cerebellar volume was the sum of cerebellar white and gray matter. Total brain tissue was the combined volumes of all brain structures, excluding cerebrospinal fluid.
Data Analysis
Data were analyzed by using Stata 12 (StataCorp, College Station, TX). Lost to follow-up analyses used t tests or Mann-Whitney U (continuous data) and χ2 (categorical data). Regression models were employed by using AUC for fT4 as the main predictor of outcome with models fitted in 2 ways: (1) cord to day 14 levels (14-day AUC), and (2) cord to day 42 (42-day AUC). Cognitive models were fitted controlling for GA, sex, social risk, duration of IPPV, and global neonatal brain abnormality score. Imaging model covariates included GA, sex, duration of IPPV, and global neonatal brain abnormality.
To account for the non-independent effect of twins/triplets, robust standard errors using the Huber/White/sandwich method were used for all analyses. Standardized β coefficients (β) (cut-points: 0.10 = weak, 0.30 = moderate, 0.50 = strong) were used as measures of the magnitude of effect. Also, the proportion of variance explained by an independent predictor (R2 for unadjusted analyses; sr2 for adjusted analyses) was investigated and expressed as a percentage, where larger values were more desirable.
Results
Sample and Hormone Characteristics
Table 2 outlines the sample characteristics of the assessed and imaged groups. The mean age of the assessed group was 7.5 years (range, 6.8–8.3 years). There were no characteristic differences between those who did and did not attend (data not shown). Sample characteristics for those children who had a useable MRI scan at age 7 years are also presented in Table 2; those in the imaged group had higher Full Scale IQ (FSIQ) scores than the non-imaged group (mean difference = 10.8; 95% confidence interval (CI) = 3.0 to 18.6; P = .007).
TABLE 2.
Perinatal, Demographic, and Hormone Characteristics
| 7-Year Cognitive Group (n = 83)a | 7-Year Imaging Group (n = 52)a | |
|---|---|---|
| Child characteristics | ||
| GA (wk), M (SD) | 27.3 (1.7) | 27.4 (1.6) |
| Birth weight (g), M (SD) | 975 (235) | 992 (216) |
| Males, n (%) | 35 (42.2) | 19 (36.5) |
| Singleton, n (%) | 49 (59.0) | 27 (51.9) |
| Perinatal medical factors | ||
| Small for GA, n (%) | 2 (2.4) | 2 (3.8) |
| Sepsis, n (%) | 34 (41.0) | 21 (40.4) |
| Patent ductus arteriosus, n (%) | 40 (48.2) | 23 (44.2) |
| Necrotizing enterocolitis, n (%) | 9 (10.8) | 4 (7.8) |
| Bronchopulmonary dysplasia, n (%) | 26 (31.7) | 15 (28.8) |
| Antenatal corticosteroids, n (%) | 76 (91.6) | 50 (96.2) |
| Postnatal corticosteroids, n (%) | 10 (12.0) | 4 (7.7) |
| IPPV (hours), median (25th and 75th percentile) | 98 (7, 431) | 88 (9, 376.5) |
| Cystic periventricular leukomalacia, n (%) | 1 (1.2) | 1 (1.9) |
| Grade III/IV intracentricular hemorrhage, n (%) | 3 (3.6) | 1 (1.9) |
| MRI brain injury, median (25th and 75th percentile) | 5 (3, 8) | 5 (3, 8) |
| Neonatal free thyroxine characteristics | ||
| 14-day AUC (pmol) | 154.1 (47.7) | 159.9 (46.8) |
| 42-day AUC (pmol) | 525.4 (114.4) | 535.0 (117.0) |
| 7-year measures | ||
| Social risk score at age 7 years, median (25th and 75th percentile) | 2 (1, 3) | 2 (0, 3) |
| FSIQ, M (SD) | 95.5 (17.8) | 99.5 (12.7)b |
Some sample sizes are less than the total sample owing to missing data.
Those in the image analysis group had higher FSIQs at age 7 years than those without imaging data (P = .007).
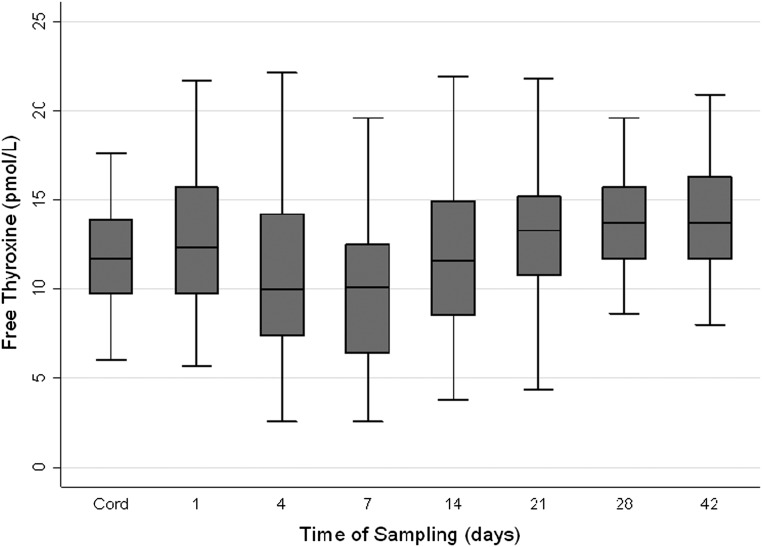
Importantly, there were no differences in fT4 levels between the assessed group and those lost to follow-up. The fT4 profile of the assessed cohort included a slight elevation in mean concentration over the first 24 hours of life, followed by a decrease to a nadir at day 4 to 7. Recovery from this transient state was noted over the next 2 to 3 weeks with concentrations rising slightly above cord concentrations by day 42 (see Fig 1). Those born less mature and sicker in infancy (with longer durations of assisted ventilation) were exposed to lower levels of fT4 over both the first 2 (GA: b-coefficient [95% CI] = 21.9 [17.9 to 25.4], P < .001; duration of IPPV: b-coefficient [95% CI] = −0.05 [−0.07 to −0.04], P < .001) and 6 (GA: b-coefficient [95% CI] = 47.0 [36.7 to 57.4], P < .001; duration of IPPV: b-coefficient [95% CI] = −0.11 [−0.15 to −0.07], P < .001) weeks of life.
FIGURE 1.
Boxplot of the fT4 profile from cord to day 42 for the assessed group. Values depicted as the median (solid line within box), 25th to 75th percentiles (margins of the box), and range of the data. Outliers were removed.
Cognitive Results
No relationships between fT4 and cognitive performance were observed at a univariable level. On multivariable analysis, higher levels of fT4 were associated with poorer performances on verbal learning, verbal memory, and simple reaction time tasks (Table 3). The 14-day AUC independently accounted for between 6.5% and 13% of the variance for these outcomes, with moderate to strong effect sizes observed.
TABLE 3.
Relationship Between Free Thyroxine 14-Day AUC/42-Day AUC and 7-Year Cognitive Outcomes
| Cognitive outcomes | 14-Day AUC (unadjusted; n = 83a) | 14-day AUC (adjusted; n = 79a) | 42-day AUC (unadjusted; n = 83a) | 42-day AUC (adjusted; n = 79a) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | R2 (%) | p | β | sr2 (%) | p | β | R2 (%) | p | β | sr2 (%) | p | |
| General intellectual ability | ||||||||||||
| FSIQ | 0.17 | 2.9 | 0.19 | −0.08 | 0.3 | 0.69 | 0.09 | 0.8 | 0.45 | −0.09 | 0.4 | 0.61 |
| Language | ||||||||||||
| General language ability | 0.02 | 0.03 | 0.90 | −0.25 | 2.7 | 0.17 | −0.03 | 0.09 | 0.81 | −0.20 | 2.0 | 0.25 |
| Verbal working memory, learning, and memory | ||||||||||||
| Immediate verbal memory | −0.03 | 0.09 | 0.83 | −0.40 | 6.6 | 0.06 | −0.05 | 0.3 | 0.57 | −0.23 | 2.6 | 0.13 |
| Working memory | −0.003 | <0.001 | 0.98 | −0.15 | 0.9 | 0.48 | −0.01 | 0.01 | 0.93 | −0.04 | 0.1 | 0.81 |
| Verbal learning | −0.04 | 0.2 | 0.72 | −0.42 | 7.5 | 0.02 | −0.08 | 0.7 | 0.42 | −0.30 | 4.5 | 0.04 |
| Verbal memory | −0.06 | 0.4 | 0.61 | −0.40 | 6.5 | 0.03 | −0.13 | 1.6 | 0.28 | −0.35 | 6.2 | 0.06 |
| Spatial working memory, learning, and memory | ||||||||||||
| Immediate spatial memory | −0.07 | 0.5 | 0.61 | −0.31 | 4.1 | 0.11 | −0.08 | 0.7 | 0.44 | −0.12 | 0.7 | 0.43 |
| Spatial learning | −0.09 | 0.9 | 0.51 | −0.26 | 2.8 | 0.17 | −0.05 | 0.2 | 0.60 | 0.03 | 0.04 | 0.85 |
| Spatial memory | −0.11 | 1.3 | 0.44 | −0.21 | 1.9 | 0.21 | −0.07 | 0.5 | 0.49 | 0.06 | 0.2 | 0.62 |
| Attention and executive functioning | ||||||||||||
| Selective attention | −0.03 | 0.1 | 0.80 | −0.33 | 4.4 | 0.09 | −0.11 | 1.1 | 0.35 | −0.28 | 4.0 | 0.06 |
| Sustained attention | 0.005 | <0.001 | 0.97 | −0.15 | 0.9 | 0.35 | 0.08 | 0.7 | 0.46 | 0.10 | 0.4 | 0.56 |
| Shifting attention | 0.19 | 3.7 | 0.08 | 0.11 | 0.5 | 0.50 | 0.17 | 2.9 | 0.13 | 0.12 | 0.8 | 0.46 |
| Divided attention | 0.06 | 0.4 | 0.58 | −0.07 | 0.2 | 0.68 | 0.04 | 0.2 | 0.71 | −0.02 | 0.01 | 0.90 |
| Planning | 0.008 | 0.01 | 0.95 | −0.19 | 1.6 | 0.29 | −0.02 | 0.06 | 0.82 | −0.13 | 0.8 | 0.43 |
| Speed of information processing and reaction time | ||||||||||||
| Simple reaction timeb | 0.19 | 3.66 | 0.13 | 0.56 | 13.0 | <0.001 | 0.22 | 4.7 | 0.05 | 0.40 | 7.7 | 0.002 |
| Complex reaction timeb | −0.0003 | <0.001 | 0.99 | 0.24 | 2.4 | 0.10 | 0.05 | 0.2 | 0.67 | 0.21 | 2.1 | 0.16 |
Covariates for adjusted analyses included GA, gender, social risk, duration of IPPV, and neonatal brain injury.
Some sample sizes are less than the total sample owing to missing data.
Task is reverse-scored, where higher scores signify a slower reaction time.
As expected, the pattern of results obtained when the 42-day AUC was examined was less profound than the 14-day AUC analyses. However, a similar profile was observed with higher levels of fT4 over the first 6 weeks of life related to poorer cognitive performance (Table 3).
Brain Volume Results
Despite a consistent pattern of results from the cognitive models, no unadjusted or adjusted associations were found between early postnatal fT4 levels and measures of 7-year brain volume (Table 4). Consequently, fT4 levels only explained a limited percentage of variance within the models (14-day AUC, 0.01%–1.7%; 42-day AUC, 0.1%–4.0%).
TABLE 4.
Relationship Between Free Thyroxine 14-Day AUC/42-Day AUC and 7-Year Imaging Outcomes
| Imaging outcomes | 14-Day AUC (unadjusted; n = 52a) | 14-Day AUC (adjusted; n = 52a) | 42-Day AUC (unadjusted; n = 52a) | 42-Day AUC (adjusted; n = 52a) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | R2 (%) | p | β | sr2 (%) | p | β | R2 (%) | p | β | sr2 (%) | p | |
| Total brain tissue | 0.06 | 0.4 | 0.73 | −0.01 | <0.001 | 0.96 | −0.06 | 0.4 | 0.68 | −0.13 | 0.9 | 0.42 |
| Cortical gray matter | 0.04 | 0.2 | 0.82 | −0.07 | 0.2 | 0.69 | −0.10 | 1.1 | 0.54 | −0.22 | 2.6 | 0.22 |
| Cortical white matter | 0.01 | 0.01 | 0.95 | −0.08 | 0.3 | 0.64 | −0.10 | 1.0 | 0.47 | −0.17 | 1.6 | 0.24 |
| Cerebellum | 0.03 | 0.1 | 0.83 | −0.18 | 1.2 | 0.26 | −0.04 | 0.2 | 0.76 | −0.16 | 1.5 | 0.29 |
| Thalamus | −0.08 | 0.7 | 0.51 | −0.11 | 0.5 | 0.41 | −0.14 | 2.0 | 0.34 | −0.09 | 0.4 | 0.60 |
| Basal ganglia | −0.04 | 0.2 | 0.77 | −0.18 | 1.3 | 0.22 | −0.15 | 2.2 | 0.23 | −0.25 | 3.4 | 0.12 |
| Hippocampus | −0.008 | 0.01 | 0.96 | −0.01 | <0.001 | 0.96 | −0.09 | 0.8 | 0.54 | −0.10 | 0.6 | 0.55 |
| Amygdala | 0.04 | 0.2 | 0.77 | −0.13 | 0.6 | 0.55 | −0.03 | 0.1 | 0.84 | −0.14 | 1.1 | 0.40 |
Covariates for adjusted analyses included: GA, gender, duration of IPPV, and neonatal brain injury.
Some sample sizes are less than the total sample owing to missing data.
Discussion
In the current study the concentrations of fT4 over the first 6 weeks of life in infants <30 weeks’ GA were similar to previous reports, but the surprising finding was that higher concentrations rather than lower concentrations of fT4 were associated with poorer cognitive function at age 7 years. The association between fT4 and cognitive outcome was not explained by effects on brain volumes at age 7 years.
Free Thyroxine Concentrations Over the First 6 Weeks of Life
The daily mean (SD) of fT4 ranged from 9.9 (4.1) to 14.0 (3.0) pmol/L over the 8 sampling days, with the lowest levels observed on day 7, consistent with other reports and with the phenomenon of THOP.28,29 For example, 1 study investigated a group of 128 infants born 23 to 29 weeks’ gestation whose fT4 profile included a gradual decrease from 15 pmol/L at <5 hours of age, reached a nadir of 9.7 pmol/L on day 7, and then increased to 11.0 pmol/L on day 14.29 Both GA and increased duration of IPPV (marker of illness) affected fT4 levels in the current study, consistent with past reports.11,29 Of note, 2 more recent studies observed this GA/illness trend only when total T4, not fT4 levels, were considered.28,30
Free Thyroxine and Cognition
There was little evidence that low postnatal fT4 levels over different periods of exposure were associated with cognitive deficits at age 7 years across a broad range of neuropsychological domains in children born VPT. Contrary to expectations, higher fT4 levels were related to adverse cognitive performances on tasks of verbal learning, verbal memory, and simple reaction time, after adjustment for confounding variables. Given the sparse nature of the literature, the best support for the aforementioned findings rests in studies of neonatal thyrotoxicosis and results from TH supplementation trials. Neonatal thyrotoxicosis is rare, usually arising in mothers who have Graves’ disease in which the exposure to elevated levels of THs appears to be detrimental.31 Although long-term outcome studies following this condition are limited and mixed, elevated rates of intellectual and psychological impairment32,33 have been reported. Furthermore, in a model using rats made hyperthyroid during the first 24 days of life, the animals performed less well on a learning task as adults compared with controls.34 It is important to note that the levels of fT4 observed in our study did not approach levels consistent with those seen in children who have neonatal thyrotoxicosis. In the same way as causation for deficit following THOP has been extrapolated from understanding mechanisms in congenital hypothyroidism, relative elevation of fT4 may be on the spectrum of maldevelopment linked to neonatal thyrotoxicosis.
With regard to TH supplementation trials, 1 study included a randomized, placebo-controlled, double-blind trial of T4 supplementation that involved 200 infants born <30 weeks’ gestation and 3 follow-up investigations.35 At age 2 years, T4-treated infants born <27 weeks’ gestation scored 18 points higher on a general development examination than those in the placebo group. However, for those in the T4-treated group born at 27 weeks’ gestation or later, a detrimental effect of the supplementation was found, with the T4-treated group scoring 10 points lower than their placebo counterparts.35 At the 5.7- and 10.5-year follow-ups a similar gestational difference was noted. In another study T4 supplementation was positively associated with outcome in children born <29 weeks’ gestation (especially in the 25/26-week GA group), but more developmental problems were observed in those T4-treated children born >29 weeks’ gestation.36 Although these findings support the notion that elevated T4 levels may be problematic for later outcome in VPT subgroups, it is important to note that conclusions were based largely on a set of posteriori analyses, which have innate limitations.
Further support for the finding that elevated fT4 levels may be of concern rests in an endocrine model of critical illness.37–39 This model outlines the hypothetical response of the endocrine system to both acute and chronic illness phases. In summary, the acute phase is considered transient, adaptive, and beneficial, and hormone variations during this time are thought to promote the redirection of energy to vital organs such as the brain. The chronic phase is a harmful, maladaptive response of the body’s natural defense mechanisms. Thyroid hormones have been monitored over these phases and have been noted to drop significantly in the first hours after illness onset with the magnitude of the drop reflecting the severity of illness. Moreover, TSH concentrations remain unchanged, suggesting that the feedback mechanisms are altered at the hypothalamic-pituitary level. If illness remains prolonged, THs continue to be reduced, but are then accompanied by diminished TSH secretion.
The application of this model to the preterm population is interesting. It suggests that the low levels of THs, without a reduction in TSH, may be a protective reaction to illness, with the body potentially treating preterm birth itself as an acute illness state. Findings from the current study further support this premise in that low fT4 levels in our group of VPT infants were not associated with adverse later childhood outcomes. In contrast, higher fT4 levels fall outside the expected endocrine response to acute illness.
It is possible that our cohort of VPT infants may have been healthier than groups examined in past studies. Many of the published studies began before the late 1990s when advances in perinatal, neonatal, and obstetric care, such as increased use of antenatal corticosteroids and surfactant therapy, were not regularly used.40,41 It is therefore possible that experiencing chronic illness endocrine patterns during early postnatal life may affect later outcome.
Free Thyroxine and Brain Volumes
In the current study, there was little evidence of an association between fT4 exposure over the first weeks of life and brain volumes at age 7 years in children born VPT. This was unexpected, especially for white matter volume. THs are important for (1) the maturation of oligodendrocytes42; (2) the accumulation of oligodendrocytes within white matter tracts43; (3) the regulation and production of components of myelin such as basic myelin protein43; and (4) the compaction of myelin within the axonal sheath.44 The role of THs in myelination also appears to be time-dependent, with different brain regions affected at different developmental stages.45
There are limited MRI studies investigating the impact of TH status on brain morphology. In one study, MRI and a subjective rating system were used to investigate myelination and CNS morphology in infants who had congenital hypothyroidism before TH replacement therapy.46 No differences in myelination patterns were found between those who had congenital hypothyroidism and control subjects; both groups also had normal MRI examinations. In another study of very low birth weight infants, cerebral white matter damage diagnosed by cranial ultrasound was twice as common in infants exposed to low total T4 levels compared with peers exposed to higher total T4 levels.47
A limitation of our study was use of a direct immunoassay to measure fT4 levels; immunoassays can produce variable results, with a tendency to overestimate fT4 at high protein concentrations and underestimate fT4 at low protein concentrations, which tend to be found in sick infants.48 Therefore, direct dialysis methods are now considered to be the “gold standard” for measuring TH levels.49
The current study is the first of its kind to comprehensively examine the association of postnatal fT4 and brain volumetrics in childhood. Myelination in the human brain is largely complete by 2 years of postnatal age. It is possible that an early delay in myelination did occur as a result of disturbed thyroid levels, but that imaging at 7 years of age missed the window in which to detect this delay. If there was any scope for catch-up in myelination, then this may well have occurred by 7 years of age, explaining the absence of association between fT4 levels and global white matter volumes. Therefore, more research is needed to truly understand this relationship, especially given the findings from the cognitive models detailed above. Finally it must be noted that our imaged group had higher FSIQ scores than those who attended 7-year follow-up and did not have a usable MRI scan. This bias may have skewed our imaging data by not allowing examination of those children who had more severe cognitive difficulties who were unable to participate in scanning procedures.
Conclusions
The fT4 profile observed in our group of VPT infants was consistent with the transiently altered levels commonly reported within the literature. However, novel elements of our study included the use of brain volumetrics and the unexpected relationship between higher postnatal fT4 levels and reduced cognitive performances at age 7 years. Conclusions supported an endocrine model of critical illness and suggest that future investigations should potentially be directed toward those infants exhibiting unexpected endocrine patterns after VPT birth.
Glossary
- AUC
area under the curve
- CI
confidence interval
- CNS
central nervous system
- FSIQ
Full Scale IQ
- fT4
free thyroxine
- GA
gestational age
- IPPV
intermittent positive pressure ventilation
- T4
thyroxine
- THOP
transient hypothyroxinemia of prematurity
- THs
thyroid hormones
- TSH
thyroid stimulating hormone
- VPT
very preterm
Footnotes
Dr Scratch conceptualized and designed the study, analyzed the data, and drafted the initial manuscript; Dr Hunt collected the blood specimens for hormone analysis, was involved in initial cerebral MRI interpretation, assisted with the conceptualization and design of the study, supervised data collection at all time points, and reviewed and revised the manuscript; Dr Thompson supervised the collection and analysis of the MRI scans and reviewed and revised the manuscript; Ms Ahmadzai performed the post-acquisition analysis of the MRI scans and reviewed and revised the manuscript; Dr Doyle assisted with the conceptualization and design of the study, supervised data collection at all time points, and reviewed and revised the manuscript; Dr Inder was involved in initial cerebral MRI interpretation, assisted with the conceptualization and design of the study, and reviewed and revised the manuscript; Dr Anderson assisted with the conceptualization and design of the study, supervised data collection at all time points, and reviewed and revised the manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by Australia’s National Health and Medical Research Council (project grants 237117, 491209, and Senior Research Fellowship to Dr Anderson, 628371), National Institutes of Health (RO1, HD058056), and the Victorian Government’s Operational Infrastructure Support Program. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJS. Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA. 2002;288(6):728–737 [DOI] [PubMed] [Google Scholar]
- 2.Barre N, Morgan A, Doyle LW, Anderson PJ. Language abilities in children who were very preterm and/or very low birth weight: a meta-analysis. J Pediatr. 2011;158(5):766–774, e1 [DOI] [PubMed] [Google Scholar]
- 3.Atkinson J, Braddick O. Visual and visuocognitive development in children born very prematurely. Prog Brain Res. 2007;164:123–149 [DOI] [PubMed] [Google Scholar]
- 4.Taylor GH, Klein NM, Minich NM, Hack M. Verbal memory deficits in children with less than 750 g birth weight. Child Neuropsychol. 2000;6(1):49–63 [DOI] [PubMed] [Google Scholar]
- 5.Omizzolo C, Scratch SE, Stargatt R, et al. Neonatal brain abnormalities and memory and learning outcomes at 7 years in children born very preterm [published online ahead of print June 27, 2013]. Memory. 10.1080/09658211.2013.809765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson PJ, De Luca CR, Hutchinson E, Spencer-Smith MM, Roberts G, Doyle LW, Victorian Infant Collaborative Study Group . Attention problems in a representative sample of extremely preterm/extremely low birth weight children. Dev Neuropsychol. 2011;36(1):57–73 [DOI] [PubMed] [Google Scholar]
- 7.Aarnoudse-Moens CSH, Smidts DP, Oosterlaan J, Duivenvoorden HJ, Weisglas-Kuperus N. Executive function in very preterm children at early school age. J Abnorm Child Psychol. 2009;37(7):981–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inder TE, Wells SJ, Mogridge NB, Spencer C, Volpe JJ. Defining the nature of the cerebral abnormalities in the premature infant: a qualitative magnetic resonance imaging study. J Pediatr. 2003;143(2):171–179 [DOI] [PubMed] [Google Scholar]
- 9.Peterson BS, Vohr B, Staib LH, et al. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA. 2000;284(15):1939–1947 [DOI] [PubMed] [Google Scholar]
- 10.Mercado M, Yu VYH, Francis I, Szymonowicz W, Gold H. Thyroid function in very preterm infants. Early Hum Dev. 1988;16(2-3):131–141 [DOI] [PubMed] [Google Scholar]
- 11.van Wassenaer AG, Kok JH, Dekker FW, de Vijlder JJM. Thyroid function in very preterm infants: influences of gestational age and disease. Pediatr Res. 1997;42(5):604–609 [DOI] [PubMed] [Google Scholar]
- 12.Uhrmann S, Marks KH, Maisels MJ, Kulin HE, Kaplan M, Utiger R. Frequency of transient hypothyroxinaemia in low birthweight infants. Potential pitfall for neonatal screening programmes. Arch Dis Child. 1981;56(3):214–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paul DA, Leef KH, Stefano JL, Bartoshesky L. Low serum thyroxine on initial newborn screening is associated with intraventricular hemorrhage and death in very low birth weight infants. Pediatrics. 1998;101(5):903–907 [DOI] [PubMed] [Google Scholar]
- 14.Zoeller RT, Rovet J. Timing of thyroid hormone action in the developing brain: clinical observations and experimental findings. J Neuroendocrinol. 2004;16(10):809–818 [DOI] [PubMed] [Google Scholar]
- 15.Ares S, Quero J, Escobar-Morreale H, et al. Thyroid hormone metabolism in premature infants and their neurodevelopment. In: Escobar GMd, Vijlder JJMd, Butz S, Hostalek U, eds. The Thyroid and Brain. Stuttgart, Germany: Schattauer GmbH; 2003:85–96 [Google Scholar]
- 16.Balázs R, Kovács S, Cocks WA, Johnson AL, Eayrs JT. Effect of thyroid hormone on the biochemical maturation of rat brain: postnatal cell formation. Brain Res. 1971;25(3):555–570 [DOI] [PubMed] [Google Scholar]
- 17.Khamsi F, Eayrs JT. A study of the effects of thyroid hormones on growth and development. Growth. 1966;30(2):143–156 [PubMed] [Google Scholar]
- 18.Nicholson JL, Altman J. Synaptogenesis in the rat cerebellum: effects of early hypo- and hyperthyroidism. Science. 1972;176(4034):530–532 [DOI] [PubMed] [Google Scholar]
- 19.Nunez J. Effects of thyroid hormones during brain differentiation. Mol Cell Endocrinol. 1984;37(2):125–132 [DOI] [PubMed] [Google Scholar]
- 20.Simic N, Asztalos EV, Rovet J. Impact of neonatal thyroid hormone insufficiency and medical morbidity on infant neurodevelopment and attention following preterm birth. Thyroid. 2009;19(4):395–401 [DOI] [PubMed] [Google Scholar]
- 21.Meijer WJ, Verloove-Vanhorick SP, Brand R, van den Brande JL. Transient hypothyroxinaemia associated with developmental delay in very preterm infants. Arch Dis Child. 1992;67(7):944–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reuss ML, Paneth N, Pinto-Martin JA, Lorenz JM, Susser M. The relation of transient hypothyroxinemia in preterm infants to neurologic development at two years of age. N Engl J Med. 1996;334(13):821–827 [DOI] [PubMed] [Google Scholar]
- 23.Den Ouden AL, Kok JH, Verkerk PH, Brand R, Verloove-Vanhorick SP. The relation between neonatal thyroxine levels and neurodevelopmental outcome at age 5 and 9 years in a national cohort of very preterm and/or very low birth weight infants. Pediatr Res. 1996;39(1):142–145 [DOI] [PubMed] [Google Scholar]
- 24.Delahunty C, Falconer S, Hume R, et al. Scottish Preterm Thyroid Group . Levels of neonatal thyroid hormone in preterm infants and neurodevelopmental outcome at 5 1/2 years: millennium cohort study. J Clin Endocrinol Metab. 2010;95(11):4898–4908 [DOI] [PubMed] [Google Scholar]
- 25.Wosilait WD. A theoretical analysis of the distribution of thyroxine among sites on thyroid binding globulin, thyroid binding prealbumin, and serum albumin. Res Commun Chem Pathol Pharmacol. 1977;16(3):541–548 [PubMed] [Google Scholar]
- 26.Kidokoro H, Neil JJ, Inder TE. New MR imaging assessment tool to define brain abnormalities in very preterm infants at term. AJNR Am J Neuroradiol. 2013;34(11):2208–2214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts G, Howard K, Spittle AJ, Brown NC, Anderson PJ, Doyle LW. Rates of early intervention services in very preterm children with developmental disabilities at age 2 years. J Paediatr Child Health. 2008;44(5):276–280 [DOI] [PubMed] [Google Scholar]
- 28.Dilli D, Oğuz SS, Andiran N, Dilmen U, Büyükkağnici U. Serum thyroid hormone levels in preterm infants born before 33 weeks of gestation and association of transient hypothyroxinemia with postnatal characteristics. J Pediatr Endocrinol Metab. 2010;23(9):899–912 [PubMed] [Google Scholar]
- 29.Biswas S, Buffery J, Enoch H, Bland JM, Walters D, Markiewicz M. A longitudinal assessment of thyroid hormone concentrations in preterm infants younger than 30 weeks’ gestation during the first 2 weeks of life and their relationship to outcome. Pediatrics. 2002;109(2):222–227 [DOI] [PubMed] [Google Scholar]
- 30.Simpson J, Williams FLR, Delahunty C, et al. Scottish Preterm Thyroid Group . Serum thyroid hormones in preterm infants and relationships to indices of severity of intercurrent illness. J Clin Endocrinol Metab. 2005;90(3):1271–1279 [DOI] [PubMed] [Google Scholar]
- 31.Kopp P, van Sande J, Parma J, et al. Brief report: congenital hyperthyroidism caused by a mutation in the thyrotropin-receptor gene. N Engl J Med. 1995;332(3):150–154 [DOI] [PubMed] [Google Scholar]
- 32.Hollingsworth DR, Mabry CC. Congenital graves disease. Four familial cases with long-term follow-up and perspective. Am J Dis Child. 1976;130(2):148–155 [DOI] [PubMed] [Google Scholar]
- 33.Daneman D, Howard NJ. Neonatal thyrotoxicosis: intellectual impairment and craniosynostosis in later years. J Pediatr. 1980;97(2):257–259 [DOI] [PubMed] [Google Scholar]
- 34.Eayrs JT. Effect of neonatal hyperthyroidism on maturation and learning in the rat. Anim Behav. 1964;12:195–199 [Google Scholar]
- 35.van Wassenaer AG, Kok JH, de Vijlder JJM, et al. Effects of thyroxine supplementation on neurologic development in infants born at less than 30 weeks’ gestation. N Engl J Med. 1997;336(1):21–26 [DOI] [PubMed] [Google Scholar]
- 36.Briët JM, van Wassenaer AG, Dekker FW, de Vijlder JJM, van Baar A, Kok JH. Neonatal thyroxine supplementation in very preterm children: developmental outcome evaluated at early school age. Pediatrics. 2001;107(4):712–718 [DOI] [PubMed] [Google Scholar]
- 37.Van den Berghe G, de Zegher F, Bouillon R. Clinical review 95: Acute and prolonged critical illness as different neuroendocrine paradigms. J Clin Endocrinol Metab. 1998;83(6):1827–1834 [DOI] [PubMed] [Google Scholar]
- 38.Van den Berghe G. Novel insights into the neuroendocrinology of critical illness. Eur J Endocrinol. 2000;143(1):1–13 [DOI] [PubMed] [Google Scholar]
- 39.Vanhorebeek I, Van den Berghe G. The neuroendocrine response to critical illness is a dynamic process. Crit Care Clin. 2006;22(1):1–15, v [DOI] [PubMed] [Google Scholar]
- 40.Johansson S, Cnattigius S. Epidemiology of preterm birth. In: Nosarti C, Murray RM, Hack M, eds. Neurodevelopmental Outcomes of Preterm Birth: From Childhood to Adult Life. New York: Cambridge University Press; 2010:1–16 [Google Scholar]
- 41.Darlow BA, Cust AE, Donoghue DA. Improved outcomes for very low birthweight infants: evidence from New Zealand national population based data. Arch Dis Child Fetal Neonatal Ed. 2003;88(1):F23–F28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernandez M, Pirondi S, Manservigi M, Giardino L, Calzà L. Thyroid hormone participates in the regulation of neural stem cells and oligodendrocyte precursor cells in the central nervous system of adult rat. Eur J Neurosci. 2004;20(8):2059–2070 [DOI] [PubMed] [Google Scholar]
- 43.Schoonover CM, Seibel MM, Jolson DM, et al. Thyroid hormone regulates oligodendrocyte accumulation in developing rat brain white matter tracts. Endocrinology. 2004;145(11):5013–5020 [DOI] [PubMed] [Google Scholar]
- 44.Ferreira AA, Nazário JC, Pereira MJS, Azevedo NL, Barradas PC. Effects of experimental hypothyroidism on myelin sheath structural organization. J Neurocytol. 2004;33(2):225–231 [DOI] [PubMed] [Google Scholar]
- 45.Ibarrola N, Rodríguez-Peña A. Hypothyroidism coordinately and transiently affects myelin protein gene expression in most rat brain regions during postnatal development. Brain Res. 1997;752(1-2):285–293 [DOI] [PubMed] [Google Scholar]
- 46.Siragusa V, Boffelli S, Weber G, et al. Brain magnetic resonance imaging in congenital hypothyroid infants at diagnosis. Thyroid. 1997;7(5):761–764 [DOI] [PubMed] [Google Scholar]
- 47.Leviton A, Paneth N, Reuss ML, et al. Hypothyroxinemia of prematurity and the risk of cerebral white matter damage. J Pediatr. 1999;134(6):706–711 [DOI] [PubMed] [Google Scholar]
- 48.Baloch Z, Carayon P, Conte-Devolx B, et al. Guidelines Committee, National Academy of Clinical Biochemistry . Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003;13(1):3–126 [DOI] [PubMed] [Google Scholar]
- 49.Nelson JC, Weiss RM, Wilcox RB. Underestimates of serum free thyroxine (T4) concentrations by free T4 immunoassays. J Clin Endocrinol Metab. 1994;79(1):76–79 [DOI] [PubMed] [Google Scholar]
- 50.Wechsler D. Wechsler Abbreviated Scale of Intelligence. London, UK: Psychological Corporation; 1999 [Google Scholar]
- 51.Semel E, Wiig EH, Secord W. Clinical Evaluation of Language Fundamentals. 4th ed. Australian Standardised Edition. Marrackville, Australia: Harcourt Assessment; 2006 [Google Scholar]
- 52.Pickering SJ, Gathercole SE. Working Memory Test Battery for Children. London, UK: The Psychological Corporation; 2001 [Google Scholar]
- 53.Delis DC, Kramer JH, Kaplan E, Ober BA. The California Verbal Learning Test - Children's Version. San Antonio, TX: The Psychological Corporation; 1994 [Google Scholar]
- 54.Cohen MJ. Children's Memory Scale. Bloomington, MN: NCS Pearson, Inc; 1997 [Google Scholar]
- 55.Manly T, Robertson I, Anderson V, Nimmo-Smith I. Test of Everyday Attention for Children (TEA-Ch). Cambridge, UK: Thames Valley Test Company; 1999 [DOI] [PubMed] [Google Scholar]
- 56.Shallice T. Specific impairments of planning. Philos Trans R Soc Lond B Biol Sci. 1982;298(1089):199–209 [DOI] [PubMed] [Google Scholar]
- 57.Anderson PJ, Anderson V, Lajoie G. The Tower of London test: validation and standardization for pediatric populations. Clin Neuropsychol. 1996;10(1):54–65 [Google Scholar]
- 58.CogState Ltd. Melbourne, Australia