Abstract
OBJECTIVES:
The goals of this study were (1) to provide estimates of diagnostic stability for a sample of young children diagnosed with attention-deficit/hyperactivity disorder (ADHD) after undergoing comprehensive multidisciplinary assessments and (2) to identify baseline child and family characteristics that predict diagnostic stability over time.
METHODS:
Children aged 3 to 6 years, 11 months consecutively diagnosed with ADHD after multidisciplinary consultations at a tertiary care clinic between 2003 and 2008 were recontacted in 2012 and 2013 (N = 120). At follow-up, the primary outcome was the proportion of children who continued to meet diagnostic criteria for ADHD. To identify predictors of diagnostic stability, logistic regression models were used. In addition, a latent class model was used to independently classify subjects into distinct clusters.
RESULTS:
In this cohort, 70.4% of the children contacted at follow-up continued to meet diagnostic criteria for ADHD. Predictors of diagnostic stability included externalizing and internalizing symptoms at baseline, parental history of psychopathology, and family socioeconomic status. The latent class model independently identified 3 distinct profiles: (1) children who no longer met ADHD criteria; (2) children with persistent ADHD and high parental psychopathology; and (3) children with persistent ADHD and low family socioeconomic status.
CONCLUSIONS:
Young children who underwent comprehensive developmental and psychological assessments before receiving an ADHD diagnosis, had higher rates of diagnostic stability than in previous studies of community samples. Child and family factors that predict diagnostic stability have the potential to guide treatment planning for children diagnosed with ADHD before 7 years of age.
Keywords: attention-deficit/hyperactivity disorder, cohort, diagnostic stability, income-to-needs ratio, predictors, preschool, socioeconomic status
What’s Known on This Subject:
Approximately 50% of children diagnosed with attention-deficit/hyperactivity disorder (ADHD) at <7 years of age in the community do not meet criteria for ADHD over time. There is a need to examine predictors of diagnostic stability in young children with ADHD.
What This Study Adds:
Predictors of diagnostic stability from early to middle childhood include child’s baseline externalizing and internalizing symptoms, parental history of psychopathology, and socioeconomic status. These predictors may guide treatment planning at the time of ADHD diagnosis.
Attention-deficit/hyperactivity disorder (ADHD) is the most common neurobehavioral disorder of childhood, occurring in 3% to 9.5% of children ages 4 to 17 years.1–4 Diagnosis of ADHD in children aged <7 years presents significant challenges to clinicians because many behavioral manifestations of ADHD may be normative at such a young age.5–8 Consequently, longitudinal studies exploring ADHD symptoms in preschool-aged children have demonstrated a wide range of estimates for diagnostic stability over time.9–16 Previous research has defined “diagnostic stability” as the degree to which the original ADHD diagnosis is confirmed at the time of follow-up.
In studies that used community samples, diagnostic stability is ∼50% for children aged <7 years after a ≥2-year period.9–16 Most of these studies have used parent and/or teacher checklists to ascertain a diagnosis of ADHD; they lack comprehensive assessment to evaluate for alternative conditions, such as cognitive or language delays or sleep disorders. Studies using clinic-referred samples often use more rigorous ADHD diagnostic criteria and include some form of standardized assessment to rule out alternative diagnoses.17–21 These studies demonstrate a higher percentage (65%–89%) of preschool-aged children continuing to meet ADHD criteria 3 to 8 years later. Thus, differences in patient populations and diagnostic strategies used across studies may influence the accuracy of the initial ADHD diagnosis and estimates of diagnostic stability. Another potential reason for variable rates of diagnostic stability is that behavioral profiles that are relatively stable later in childhood may be more malleable early. During early childhood, adversity, parental socioeconomic status (SES), and enriched environments have been shown to shift risk for psychiatric and developmental disorders, including ADHD.22–30
Predictors of ADHD stability among children diagnosed during preschool or kindergarten are poorly understood. In the studies available, children with persistent ADHD have higher psychiatric comorbidities, family history of psychopathology, and exposure to childhood adversity than children who no longer meet criteria for ADHD at follow-up.14,15 However, in these studies, initial ADHD diagnoses have been assessed by using parent report of symptoms without in-depth evaluation of medical or developmental diagnoses that may mimic ADHD symptoms. To the best of our knowledge, the present study is the first study to identify predictors of diagnostic stability in children aged <7 years who received an ADHD diagnosis after medical, developmental, and psychological assessments.
Methods
Subjects
Participants were 120 children ages 3 to 6 years, 11 months consecutively diagnosed with ADHD at the Developmental Medicine Center of Boston Children’s Hospital between January 1, 2003, and December 31, 2008. These children were prospectively recontacted at a mean interval of 7 years (range: 5.6–9.5 years) after their initial diagnosis. Children who were adopted or had autism spectrum disorder (ASD), genetic syndromes, or brain injuries at the time of initial ADHD diagnosis were excluded. The Boston Children’s Hospital institutional review board approved all study procedures.
Identification of ADHD in Young Children at Time 1
At time 1, each child completed a multidisciplinary assessment in which developmental–behavioral pediatricians obtained a detailed medical and social history, and completed a physical examination on each child; pediatric psychologists assessed each child's developmental skills by using cognitive, language, and early academic standardized tests, reviewed day care and school intake questionnaires, and obtained ratings of child behavior by using the Behavior Assessment System for Children.31 The team of clinicians met subsequent to the assessment to review the child's diagnostic criteria for ADHD and to discuss alternative diagnoses and potential comorbid conditions; the clinicians then systematically recorded all findings in a formal report.
Determination of Psychiatric Disorders at Time 2
After families were recruited for the follow-up time 2 study, they were scheduled for a visit in which parents completed the Diagnostic Interview Schedule for Children–Version IV (DISC-IV), Vanderbilt ADHD Diagnostic Parent Rating Scales, the parent global assessment (PGA), and the Children with Special Health Care Needs CSHCN Screener.32–40 The PGA is a 7-point scale ranging from 1 (no impairments) to 7 (needs 24-hour supervision due to severe impairments). For children who were being treated with stimulant medications, parents were asked to complete rating scales describing their child’s behavior on and off medication (eg, before the medication took effect in the morning). Parents also completed the Conflict Tactic Scales, Revised, which provides information about psychological and physical aggression in the family,41 and the Social Communication Questionnaire–Lifetime Form,42 which screens for difficulties in social and communication functioning. Parents were also asked about duration and intensity of behavioral therapy, school services, and medication use since time 1. Lastly, the Family Interview for Genetic Studies43 and demographic information not uniformly available from records (including time 1 parental education and income) were obtained. Teachers were also asked to complete the Vanderbilt ADHD Diagnostic Teacher Rating Scale.44
ADHD Case Definition at Time 2
Children who did not meet DSM-IV-TR criteria on both the DISC-IV and the Vanderbilt ADHD Diagnostic Parent Rating Scale, had a PGA score <3 (ie, minimal impairments), and <6 symptoms on the Vanderbilt ADHD Diagnostic Teacher Rating Scale were classified as no longer meeting criteria for ADHD at time 2. Nine children received an intermediate diagnosis on the DISC-IV, which indicated that symptoms and impairments were present but did not meet full diagnostic criteria for ADHD. Review of these 9 children showed that all of them had at least 4 symptoms in either the inattentive and/or hyperactive/impulsive criteria on the DISC-IV and the Vanderbilt ADHD Diagnostic Parent Rating Scale, impairments in multiple settings on the DISC-IV, a PGA score >3 (ie, moderate to severe impairments), and >6 symptoms on the Vanderbilt ADHD Diagnostic Teacher Rating Scale. For the purpose of this research study, we classified these intermediate cases as ADHD at time 2.
Covariates of Interest
All covariates were child and family characteristics at time 1 when each child initially received his or her ADHD diagnosis.
Child-level covariates included: (1) age at initial diagnosis; (2) gender; (3) presence of comorbid developmental, behavioral, or mental health diagnoses; (4) race/ethnicity; (5) composite nonverbal cognitive score on one of the following measures: the Wechsler Intelligence Scale for Children, the Wechsler Preschool and Primary Scale of Intelligence, the Differential Ability Scale, and the Stanford-Binet Intelligence Scales; (6) severity of externalizing symptoms (ie, attention problems, hyperactivity, aggression); and (7) severity of internalizing symptoms (ie, anxiety, depression, somatization). The severity of externalizing and internalizing symptoms was taken from the Behavior Assessment System for Children and stratified into a T score ≥70 and <70.
Family-level covariates included: (1) parental history of psychopathology; (2) parental education level; (3) income-to-needs ratio (a proxy for family economic resources); and (4) percentage of residents living below the poverty level based on the child’s address. Parental education level was a categorical variable divided into high school or less, some college, and at least a college degree. Income-to-needs ratio was calculated by dividing the subject’s household income by the US Census Bureau poverty threshold income for a family of that size.
Data Analysis
Statistical analyses were conducted by using SPSS version 21.0 (IBM SPSS Statistics, IBM Corporation, Armonk, NY [2012]) and Latent GOLD 4.5 (Statistical Innovations Inc, Belmont, MA [2008]). To compare means of continuous variables, t tests were used; χ2 statistics were used to compare proportions of categorical variables.
Logistic Regression
A multivariate logistic regression model was implemented to predict the stability of ADHD diagnosis from a combination of child-level and family-level factors. These factors were chosen on the basis of information from previous literature and were initially included in separate logistic regression models. A best synthesis model followed and included predictors with coefficients significant at P < .10.
Latent Class Model
Data were analyzed by means of latent class mixture modeling in an effort to identify the presence of subgroups that shared similar levels across a combination of independent variables. The latent class models were developed initially for dichotomous variables45 and were extended to include nominal46 and, subsequently, continuous indicators.47,48
Because the likelihood ratio χ2 statistic had been shown to be sensitive to sparse data, bootstrapping P values by using 500 replicated data sets was used to test the improvement in fit between 2 models in this study.49 The magnitude of R2 values, correct classifications based on group membership (stable versus unstable diagnosis), significance of independent variables in defining cluster group membership, and a significant reduction in the likelihood ratio test (L2) were all considered when comparing nested models.
Results
Study Participants
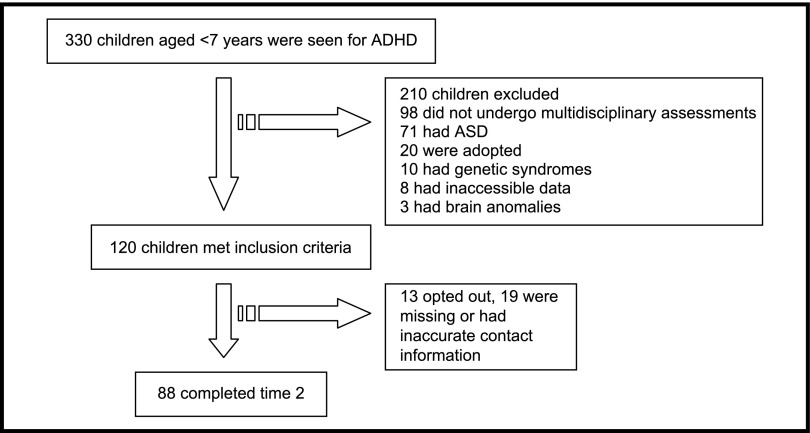
At time 1, a total of 330 children aged <7 years received an ADHD diagnosis in the Developmental Medicine Center of Boston Children’s Hospital. Of these, 120 met inclusion criteria and were seen by the multidisciplinary teams described earlier (Fig 1). Longitudinal follow-up was possible for 88 children (73.3% of the cohort). Table 1 presents baseline characteristics of the cohort. Characteristics of children who were and were not followed up at time 2 were compared; there was no significant difference between the 2 groups in age of initial ADHD diagnosis, current age, gender, nonverbal cognitive score, and percent living below the poverty level.
FIGURE 1.
Study population flow diagram.
TABLE 1.
Characteristics of the Study Cohort
| Characteristic | Entire Cohort | Children With ADHD at Time 2 (n = 62) | Children Without ADHD at Time 2 (n = 26) | Pa | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Age at diagnosis, mo | 68.13 | 11.35 | 68.25 | 11.78 | 68.93 | 9.73 | .778 |
| Age at time 2, mo | 150.89 | 24.36 | 150.20 | 24.74 | 153.04 | 24.19 | .618 |
| Percent living below poverty level | 8.55 | 8.49 | 9.08 | 8.92 | 7.04 | 5.73 | .202 |
| Nonverbal cognitive composite | 93.16 | 17.36 | 90.00 | 15.87 | 95.11 | 14.08 | .154 |
| Income-to-needs ratio | 4.40 | 3.75 | 2.90 | 2.01 | 7.69 | 4.55 | <.001*** |
| N | % | N | % | N | % | ||
| Females | 21/120 | 17.50 | 11/62 | 17.74 | 4/26 | 15.38 | .788 |
| Presence of comorbid condition | 91/120 | 75.83 | 45/62 | 72.58 | 21/26 | 80.77 | .418 |
| At least 1 externalizing subscale with T score >70 | 59/120 | 49.20 | 39/62 | 62.90 | 6/26 | 23.08 | .001** |
| At least 1 internalizing subscale with T score >70 | 19/120 | 15.80 | 12/62 | 19.35 | 2/26 | 7.69 | .172 |
| Race (non-Hispanic white) | 64/88 | 72.73 | 42/62 | 67.74 | 22/26 | 84.62 | .105 |
| Maternal education (up to high school) | 18/88 | 20.45 | 16/62 | 25.81 | 3/26 | 11.54 | .138 |
| Paternal education (up to high school) | 27/88 | 30.68 | 26/62 | 41.94 | 4/26 | 15.38 | .017* |
| Parental report of psychopathology | 77/176 | 43.75 | 68/124 | 54.84 | 9/52 | 17.31 | <.001*** |
| Parental report of anxiety and/or depression | 60/176 | 34.09 | 54/124 | 43.55 | 6/52 | 11.54 | <.001*** |
| Parental report of bipolar disorder | 10/176 | 5.68 | 8/124 | 6.45 | 2/52 | 3.85 | .496 |
| Parental report of schizophrenia | 7/176 | 3.98 | 6/124 | 4.84 | 1/52 | 1.92 | .367 |
| Parental report of substance abuse | 24/176 | 13.64 | 16/124 | 12.90 | 8/52 | 15.38 | .662 |
| Parental report of ADHD | 93/176 | 52.84 | 82/124 | 66.13 | 11/52 | 21.15 | <.001*** |
P < .05, **P < .01, ***P < .001.
To compare children with and without ADHD at time 2, t tests and χ2 statistics were used.
Behavioral, Developmental, and Psychiatric Profiles of the Cohort
The cohort as a whole was found to have high rates of comorbid developmental disorders after multidisciplinary assessment as well as high rates of comorbid psychiatric disorders at follow-up (Table 2). After a mean interval of 7 years, 62 children (70.4% of the cohort) continued to meet diagnostic criteria for ADHD.
TABLE 2.
Comorbid Diagnoses of the Cohort of Young Children Diagnosed With ADHD
| Type of Comorbid Disordera | Time Point | ||
|---|---|---|---|
| Before Initial Multidisciplinary Assessment (N = 120) | Time 1 (N = 120) | Time 2 (n = 88) | |
| ADHD | 2 (1.67%) | 120 (100%) | 62 (70.40%) |
| Behavioral disordersb | 1 (0.82%) | 4 (3.28%) | 11 (12.50%) |
| Learning disorders | 0 | 21 (17.50%) | 31 (35.22%) |
| Tics disorder | 0 | 0 | 6 (6.81%) |
| Mood disordersc | 0 | 10 (8.33%) | 34 (38.60%) |
| Developmental disabilitiesd | 20 (16.67%) | 78 (65.00%) | 40 (45.45%) |
| Sleep disorders | 2 (1.67%) | 5 (4.17%) | 5 (5.68%) |
Disorders are not mutually exclusive.
Behavioral disorders include disruptive behavior disorder, oppositional defiant disorder, and conduct disorder.
Mood disorders include anxiety, obsessive-compulsive disorder, depression, bipolar disorder, and mood disorder, not otherwise specified.
Developmental disabilities include global developmental delay, intellectual disability, language/communication disorder, and motor/coordination disorder.
Prediction of Stable ADHD Diagnosis by Using Logistic Regression Analysis
Within the child-level model, significant predictors of ADHD stability included elevated externalizing symptoms (b = 2.21, P < .01; odds ratio [OR]: 9.0), elevated internalizing symptoms (B = 1.53, P < .05; OR: 4.6), and lower nonverbal composite score (B = –0.05, P < .05, OR: 0.94) (Table 3). Family-level predictors were positive parental history of psychopathology (B = 2.04, P < .05; OR: 7.6) and lower income-to-needs ratio (B = –0.57, P < .001; OR: 0.56). In the combined model, the significant predictors discussed earlier remained significant except for the child’s nonverbal cognitive score. All results were in the same direction and were more pronounced.
TABLE 3.
Logistic Regression Analysis for the Prediction of Stability of an ADHD diagnosis From Child-Level, Family-Level, and Combined Model Predictors
| Predictors | B | SE | P | OR | 95% Confidence Interval | |
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| Child-level factors | ||||||
| Gender (girls) | –0.672 | 0.918 | .464 | 0.511 | 0.084 | 3.086 |
| Current age | –0.012 | 0.014 | .414 | 0.988 | 0.961 | 1.017 |
| Nonverbal cognitive composite | –0.052 | 0.025 | .034* | 0.949 | 0.904 | 0.996 |
| Elevated externalizing behaviors | 2.21 | 0.711 | .002** | 9.094 | 2.257 | 36.639 |
| Elevated internalizing behaviors | 1.53 | 0.674 | .023* | 4.606 | 1.230 | 17.250 |
| Age at initial diagnosis | 0.027 | 0.031 | .392 | 1.027 | 0.966 | 1.092 |
| Race (white) | 0.652 | 0.681 | .338 | 1.920 | 0.505 | 7.292 |
| Presence of comorbid diagnoses | −1.35 | 0.812 | .097 | 0.260 | 0.053 | 1.276 |
| Family-level factors | ||||||
| Gendera | –0.622 | 0.888 | .484 | 0.537 | 0.094 | 3.060 |
| Current agea | 0.013 | 0.014 | .360 | 1.013 | 0.986 | 1.040 |
| Parent history of psychopathology | 2.04 | 0.952 | .032* | 7.683 | 0.413 | 0.773 |
| Income-to-needs ratio | –0.572 | 0.160 | <.001*** | 0.565 | 0.079 | 1.805 |
| Parental education (up to high school) | –0.974 | 0.798 | .222 | 0.377 | 1.190 | 49.611 |
| Percent living in poverty | –0.021 | 0.048 | .658 | 0.979 | 0.892 | 1.075 |
| Combined model, including child-level and family-level factors | ||||||
| Gendera | –1.17 | 1.325 | .378 | 0.311 | 0.023 | 4.179 |
| Current agea | 0.018 | 0.017 | .305 | 1.018 | 0.984 | 1.053 |
| Nonverbal cognitive composite | –0.053 | 0.034 | .121 | 0.948 | 0.886 | 1.014 |
| Elevated externalizing behaviors | 2.33 | 1.041 | .025* | 10.297 | 1.339 | 79.192 |
| Elevated internalizing behaviors | 2.33 | 1.157 | .044* | 10.319 | 1.069 | 99.621 |
| Presence of comorbid diagnoses | –1.18 | 1.077 | .275 | 0.309 | 0.037 | 2.551 |
| Parental history of psychopathology | 3.55 | 1.460 | .015* | 34.643 | 1.980 | 606.272 |
| Income-to-needs ratio | –0.749 | 0.216 | .001** | 0.473 | 0.309 | 0.723 |
P < .05, **P < .01, ***P < .001.
Gender and current age were forced into the model.
Exploring the Presence of Subgroups Based on ADHD Diagnostic Stability By Using Latent Class Modeling
A set of ancillary analyses was conducted to evaluate whether specific clusters of individuals and profiles would explain the stability of ADHD diagnosis (Table 4). A 1-class, baseline model was used as a reference point, and this model was compared with a 2-class model. The 2-class model provided a good fit to the data and suggested that the group of children with stable ADHD diagnosis had baseline characteristics different from the group of children with unstable ADHD diagnosis (R2 = 90.25%). Although the 2-class model provided a good fit to the data, examination of the bivariate residuals suggested that some were above the expected value of 1.0, and the probability of misfit was equal to 3.4%. Thus, on the basis of findings from previous research,50,51 the model was fit with the addition of a 3-class model, in an attempt to minimize those residual covariations and to improve model fit. The difference between a 2-class and a 3-class model was evaluated by using the log-likelihood –2LL statistic based on the bootstrap distribution and 500 replications (Table 5), suggesting that a 3-class model provided significantly improved fit (–2LLDiff = 165.76, P < .001).
TABLE 4.
Percentage of Sample Belonging to Each Class and Significance of Predictor
| Fixed Effect | Class 1 Stable Diagnosis | Class 2 Stable Diagnosis | Class 3 Unstable Diagnosis | Z Test | P | R2,a |
|---|---|---|---|---|---|---|
| Cluster size, n (%) | 33 (38.4) | 29 (33.7) | 2 (27.9) | |||
| Stability of ADHD diagnosis | 3.009 | .0047** | 65.8% | |||
| Stable | 99.5% | 82.06% | 8.71% | |||
| Parental history of psychopathology | 2.223 | .08 | 8.92% | |||
| Present | 91.09% | 93.86% | 69.86% | |||
| Externalizing symptoms | 2.360 | .0096** | 14.19% | |||
| T score >70 in ≥1 subscale | 53.63% | 42.82% | 27.34% | |||
| Internalizing symptoms | 2.108 | .11 | 9.30% | |||
| T score >70 in ≥1 subscale | 22.52% | 23.82% | 5.63% | |||
| Parents going to college | 1.894 | .011* | 60.98% | |||
| Yes | 4.65% | 88.94% | 78.38% | |||
| Income-to-needs ratio | 4.810 | <.001*** | 55.83% | |||
| Income greater than expected for a family of that size | 6.44% | 20.89% | 41.92% |
P < .05, **P < .01, ***P < .001.
These values indicate amount of variance of each variable explained by using the 3-class solution.
TABLE 5.
Nested Latent Class Models Suggesting the Superiority of a 3-Class Solution Over Competing 1- and 2-Class Models for Explaining Stability in ADHD Diagnosis
| Model Tested | BICLLa | LL | Class Errorb, P |
|---|---|---|---|
| 1-class independence model | 1278.330 | −610.212 | <.0001*** |
| 2-class | 1199.742 | −553.100 | .039* |
| 3-class | 1183.840 | −527.332 | .732 |
P < .05, ***P < .001.
BICLL, Bayesian Information Criterion based on log-likelihood.
A nonsignificant model indicates no difference between the hypothetical and obtained model.
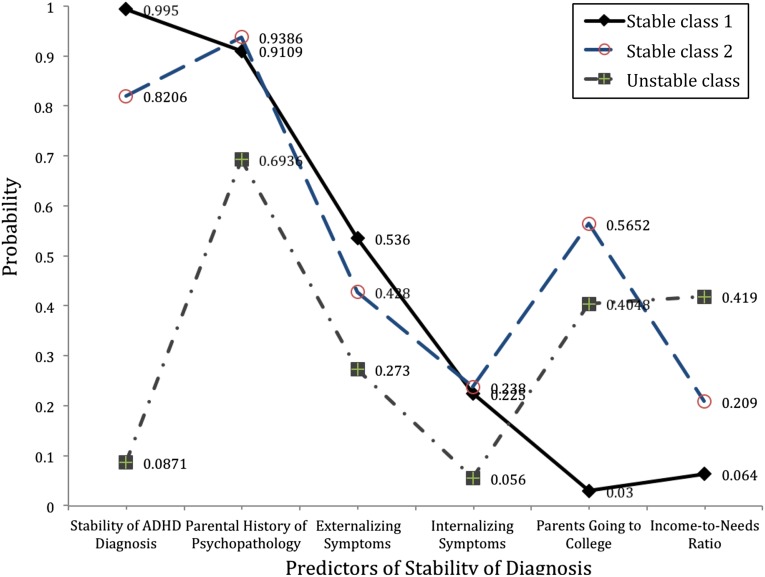
In the 3-class model, there were 2 classes of children that belonged to the stable ADHD group and 1 class of children that fit into the unstable ADHD group (Fig 2). Only 8.14% were considered misclassified cases. Class 1 represented children with a 99.5% probability of having a stable ADHD diagnosis. These were children with elevated internalizing and externalizing symptoms, had parents with a history of psychopathology and low educational level, and also came from families with low family economic resources. Class 2 described a cluster of children with an 82% probability of having a stable ADHD diagnosis. The major difference, however, was that children in class 2 tended to have parents with more psychopathology but a higher probability of attending college and higher income-to-needs ratio compared with class 1 children. Last, class 3 described children who no longer met criteria for ADHD. These children were characterized by relatively low levels of internalizing and externalizing symptoms during early childhood, came from families with higher economic resources, and had parents who were more likely to attend college.
FIGURE 2.
Latent class model displaying the presence of 2 stable diagnosis classes and 1 unstable diagnosis class.
Profiles of Children With Remitted ADHD
Of the 26 children who did not meet diagnostic criteria for ADHD at follow-up, new diagnoses emerged in 15 children. Specifically, 2 children met criteria for anxiety disorders, 10 had new diagnoses of ASD, and another 3 had new learning disorders. The remaining children did not meet diagnostic criteria for any developmental, learning, or behavioral disorder, including ADHD, and did not shift from one externalizing disorder to another, which suggests remittance of ADHD symptoms at time 2. For children who were reported to have new ASD by parents, psychological reports were available for the majority of these children. Their Social Communication Questionnaire total scores (median: 17.40 points) were significantly higher compared with those without a new ASD diagnosis (median: 5.93 points; P < .001).
Discussion
Several important findings emerged from the present cohort study. First, ADHD stability over a 7-year period was 70% when young children underwent comprehensive developmental and psychological evaluations. Although diagnostic stability in this cohort was higher overall than previous studies of community samples9–16, this study found that diagnosing ADHD in young children is not an easy task, even for professionals with expertise in the pediatric ADHD population. More than one-half of children who no longer met criteria for ADHD were later found to have alternative developmental diagnoses, including anxiety, ASD, and learning disorders. However, there was a group of children who no longer met criteria for ADHD and did not have any new onset of psychiatric disorders, suggesting ADHD remittance over time.
Clinicians caring for young children with ADHD symptoms, therefore, should be equipped to monitor learning, behavioral, and mood difficulties over time. Importantly, children with an early ADHD diagnosis should have continuity of care with clinicians who have expertise in ADHD or should receive a comprehensive reevaluation during school age.
Although it is not surprising that elevated externalizing symptoms are associated with stability in ADHD diagnosis, we additionally observed that children with a greater risk of ADHD persistence had higher internalizing symptoms at a young age. This finding is consistent with previous literature on older children with ADHD,52–54 and it points to the importance of screening for internalizing symptoms at initial diagnostic visits, even when parental concerns are about disruptions due to externalizing symptoms.
Finally, we observed that family factors, including parental history of psychopathology and family economic resources, are predictive of ADHD diagnostic stability. The idea that family contexts contribute to the maintenance of preschool-aged problem behaviors and long-term impairments is not new. Previous research studies have shown that variables of maternal psychopathology, family climate, and SES are predictive of stability of preschool psychiatric disorder.14,15 Given the convergence of these findings in these previous samples and our own research, it seems that factors related to the family should be explored more explicitly in clinical settings. Furthermore, these findings indicate that parent-training programs may have a potentially important role in ADHD treatment in early childhood and may enhance child-specific treatments in this young age range. Evidence-based parent management training has been shown to help parents actively acquire parenting skills that target relationship building and improve parent–child interactions, as well as lower children’s externalizing behaviors.55,56 The American Academy of Pediatrics therefore recommends that primary care physicians prescribe such evidence-based therapy as a first line of treatment for preschool-aged children.57
Interestingly, parental education emerged in the latent class model as the most discriminating variable in the prediction of ADHD stability (R2 = 60.98%). One possible explanation is that parents with different levels of education provide a different day-to-day environment for children and that these environmental factors mediate changes in the prefrontal cortex, decreasing ADHD symptoms over time. This speculation is bolstered by recent data pointing to the impact of early environments, particularly those linking the effects of SES on prefrontal cortex development and neurocognitive measures associated with ADHD symptoms.23,24,58 Potential mediators of the relationships between low parental education and high ADHD persistence include differences in the quality of the home environment (eg, use of complex language, enrichment activities), quality of parent–child interactions, and degree of family conflicts. The influences of these variables on diagnostic stability in ADHD are beyond the scope of the present study but are of critical importance to explore in future studies.
Several limitations should be noted when interpreting our findings. One limitation is that this cohort consisted of children referred to a single tertiary care clinic. However, these children were not a convenience sample or recruited from advertisement. Our cohort consisted of every child aged <7 years diagnosed with ADHD in our specialty clinic in 2003 to 2008; almost all of them were referred to this clinic because pediatricians would not otherwise give a diagnosis of ADHD or start treatment at such a young age. Another limitation is that we were not able to recontact every child diagnosed at time 1. However, a comparison of demographic data indicated that subjects lost to follow-up did not differ from subjects with full participation. Lastly, families in this cohort had a higher mean income compared with the mean income of the US population, even when family size was considered. Use of a cohort with higher SES may have biased the study toward less severe cases and, consequently, may have produced more conservative results.
Conclusions
Although ADHD symptoms may improve in a subset of children, for many, ADHD symptoms are chronic and associated with many psychiatric comorbid conditions. Those with higher risk for long-term ADHD have more externalizing symptoms and internalizing symptoms at baseline, more parental psychopathology, and fewer family economic resources. This study adds to the growing literature demonstrating that: (1) child-specific therapies may not suffice as the only armamentarium for practitioners treating children with ADHD; and (2) the preschool years may serve as an important, sensitive period for intervention.
Glossary
- ADHD
attention-deficit/hyperactivity disorder
- ASD
autism spectrum disorder
- DISC-IV
Diagnostic Interview Schedule for Children–Version IV
- OR
odds ratio
- PGA
parent global assessment
- SES
socioeconomic status
Footnotes
Dr Law conceptualized and designed the study, coordinated and supervised data collection, conducted the initial analyses, drafted the initial manuscript, and reviewed and revised the manuscript; Dr Sideridis critically reviewed the initial analyses, conducted all remaining analyses, and reviewed and revised the manuscript; Dr Albers Prock supervised data collection and reviewed and revised the manuscript; Dr Sheridan conceptualized and designed the study with Dr Law, supervised data collection, reviewed all analyses completed by Drs Law and Sideridis, and critically reviewed and revised the manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Support for this research was provided by the Society for Developmental and Behavioral Pediatrics (research award 85260) and by the National Institute of Mental Health (5K01MH092555). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Centers for Disease Control and Prevention (CDC) . Mental health in the United States. Prevalence of diagnosis and medication treatment for attention-deficit/hyperactivity disorder—United States, 2003. MMWR Morb Mortal Wkly Rep. 2005;54(34):842–847 [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) . Increasing prevalence of parent-reported attention-deficit/hyperactivity disorder among children—United States, 2003 and 2007. MMWR Morb Mortal Wkly Rep. 2010;59(44):1439–1443 [PubMed] [Google Scholar]
- 3.Froehlich TE, Lanphear BP, Epstein JN, Barbaresi WJ, Katusic SK, Kahn RS. Prevalence, recognition, and treatment of attention-deficit/hyperactivity disorder in a national sample of US children. Arch Pediatr Adolesc Med. 2007;161(9):857–864 [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC). Attention-deficit/hyperactivity disorder (ADHD). Data and statistics. Available at: www.cdc.gov/ncbddd/adhd/data.html. Accessed October 10, 2013
- 5.Kadesjö C, Kadesjö B, Hägglöf B, Gillberg C. ADHD in Swedish 3- to 7-year-old children. J Am Acad Child Adolesc Psychiatry. 2001;40(9):1021–1028 [DOI] [PubMed] [Google Scholar]
- 6.Pineda D, Ardila A, Rosselli M, et al. Prevalence of attention-deficit/hyperactivity disorder symptoms in 4- to 17-year-old children in the general population. J Abnorm Child Psychol. 1999;27(6):455–462 [DOI] [PubMed] [Google Scholar]
- 7.Smidts DP, Oosterlaan J. How common are symptoms of ADHD in typically developing preschoolers? A study on prevalence rates and prenatal/demographic risk factors. Cortex. 2007;43(6):710–717 [DOI] [PubMed]
- 8.Campbell SB. Behavior problems in preschool children: a review of recent research. J Child Psychol Psychiatry. 1995;36(1):113–149 [DOI] [PubMed] [Google Scholar]
- 9.Campbell SB, Ewing LJ, Breaux AM, Szumowski EK. Parent-referred problem three-year-olds: follow-up at school entry. J Child Psychol Psychiatry. 1986;27(4):473–488 [DOI] [PubMed] [Google Scholar]
- 10.Campbell SB, Ewing LJ. Follow-up of hard-to-manage preschoolers: adjustment at age 9 and predictors of continuing symptoms. J Child Psychol Psychiatry. 1990;31(6):871–889 [DOI] [PubMed] [Google Scholar]
- 11.Pierce EW, Ewing LJ, Campbell SB. Diagnostic status and symptomatic behavior of hard-to-manage preschool children in middle childhood and early adolescence. J Clin Child Psychol. 1999;28(1):44–57 [DOI] [PubMed] [Google Scholar]
- 12.McGee R, Partridge F, Williams S, Silva PA. A twelve-year follow-up of preschool hyperactive children. J Am Acad Child Adolesc Psychiatry. 1991;30(2):224–232 [DOI] [PubMed] [Google Scholar]
- 13.Palfrey JS, Levine MD, Walker DK, Sullivan M. The emergence of attention deficits in early childhood: a prospective study. J Dev Behav Pediatr. 1985;6(6):339–348 [PubMed] [Google Scholar]
- 14.Lavigne JV, Arend R, Rosenbaum D, Binns HJ, Christoffel KK, Gibbons RD. Psychiatric disorders with onset in the preschool years: II. Correlates and predictors of stable case status. J Am Acad Child Adolesc Psychiatry. 1998;37(12):1255–1261 [DOI] [PubMed] [Google Scholar]
- 15.Tandon M, Si X, Luby J. Preschool onset attention-deficit/hyperactivity disorder: course and predictors of stability over 24 months. J Child Adolesc Psychopharmacol. 2011;21(4):321–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harvey EA, Youngwirth SD, Thakar DA, Errazuriz PA. Predicting attention-deficit/hyperactivity disorder and oppositional defiant disorder from preschool diagnostic assessments. J Consult Clin Psychol. 2009;77(2):349–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lahey BB, Pelham WE, Stein MA, et al. Validity of DSM-IV attention-deficit/hyperactivity disorder for younger children. J Am Acad Child Adolesc Psychiatry. 1998;37(7):695–702 [DOI] [PubMed] [Google Scholar]
- 18.Lahey BB, Pelham WE, Loney J, et al. Three-year predictive validity of DSM-IV attention deficit hyperactivity disorder in children diagnosed at 4-6 years of age. Am J Psychiatry. 2004;161(11):2014–2020 [DOI] [PubMed] [Google Scholar]
- 19.Beitchman JH, Wekerle C, Hood J. Diagnostic continuity from preschool to middle childhood. J Am Acad Child Adolesc Psychiatry. 1987;26(5):694–699 [DOI] [PubMed] [Google Scholar]
- 20.Barkley RA, Fischer M, Edelbrock CS, Smallish L. The adolescent outcome of hyperactive children diagnosed by research criteria: I. An 8-year prospective follow-up study. J Am Acad Child Adolesc Psychiatry. 1990;29(4):546–557 [DOI] [PubMed] [Google Scholar]
- 21.Riddle MA, Yershova K, Lazzaretto D, et al. The Preschool Attention-Deficit/Hyperactivity Disorder Treatment Study (PATS) 6-year follow-up. J Am Acad Child Adolesc Psychiatry. 2013;52(3):264–278.e2 [DOI] [PMC free article] [PubMed]
- 22.Nelson CA, Sheridan MA. Lessons from neuroscience research for understanding causal links between family and neighborhood characteristics and educational outcomes. In: Duncan G, Murnane RJ, ed. Whither Opportunity?: Rising Inequality, Schools, and Children's Life Chances. New York, NY: Russell Sage Foundation; 2011 [Google Scholar]
- 23.Sarsour K, Sheridan M, Jutte D, Nuru-Jeter A, Hinshaw S, Boyce WT. Family socioeconomic status and child executive functions: the roles of language, home environment, and single parenthood. J Int Neuropsychol Soc. 2011;17(1):120–132 [DOI] [PubMed] [Google Scholar]
- 24.Sheridan MA, Sarsour K, Jutte D, D’Esposito M, Boyce WT. The impact of social disparity on prefrontal function in childhood. PLoS ONE. 2012;7(4):e35744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noble KG, Norman MF, Farah MJ. Neurocognitive correlates of socioeconomic status in kindergarten children. Dev Sci. 2005;8(1):74–87 [DOI] [PubMed] [Google Scholar]
- 26.Hackman DA, Farah MJ. Socioeconomic status and the developing brain. Trends Cogn Sci. 2009;13(2):65–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benjet C, Borges G, Medina-Mora ME. Chronic childhood adversity and onset of psychopathology during three life stages: childhood, adolescence and adulthood. J Psychiatr Res. 2010;44(11):732–740 [DOI] [PubMed] [Google Scholar]
- 28.Raizada RD, Kishiyama MM. Effects of socioeconomic status on brain development, and how cognitive neuroscience may contribute to levelling the playing field. Front Hum Neurosci. 2010;4:3 [DOI] [PMC free article] [PubMed]
- 29.Galera C, Bouvard MP, Lagarde E, et al. Childhood attention problems and socioeconomic status in adulthood: 18-year follow-up. Br J Psychiatry. 2012;201(1):20–25 [DOI] [PMC free article] [PubMed]
- 30.Poulton R, Caspi A, Milne BJ, et al. Association between children’s experience of socioeconomic disadvantage and adult health: a life-course study. Lancet. 2002;360(9346):1640–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reynolds CR, Kamphaus RW. The Clinician's Guide to the Behavior Assessment System for Children. New York, NY: The Guildford Press; 2002 [Google Scholar]
- 32.Schwab-Stone M, Fisher P, Piacentini J, Shaffer D, Davies M, Briggs M. The Diagnostic Interview Schedule for Children-Revised Version (DISC-R): II. Test-retest reliability. J Am Acad Child Adolesc Psychiatry. 1993;32(3):651–657 [DOI] [PubMed] [Google Scholar]
- 33.Schwab-Stone M, Fallon T, Briggs M, Crowther B. Reliability of diagnostic reporting for children aged 6-11 years: a test-retest study of the Diagnostic Interview Schedule for Children-Revised. Am J Psychiatry. 1994;151(7):1048–1054 [DOI] [PubMed] [Google Scholar]
- 34.Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39(1):28–38 [DOI] [PubMed] [Google Scholar]
- 35.Wolraich ML, Lambert W, Doffing MA, Bickman L, Simmons T, Worley K. Psychometric properties of the Vanderbilt ADHD diagnostic parent rating scale in a referred population. J Pediatr Psychol. 2003;28(8):559–567 [DOI] [PubMed] [Google Scholar]
- 36.Biederman J, Melmed RD, Patel A, et al. SPD503 Study Group . A randomized, double-blind, placebo-controlled study of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics. 2008;121(1). Available at: www.pediatrics.org/cgi/content/full/121/1/e73 [DOI] [PubMed] [Google Scholar]
- 37.Kollins SH, Jain R, Brams M, et al. Clonidine extended-release tablets as add-on therapy to psychostimulants in children and adolescents with ADHD. Pediatrics. 2011;127(6): Available at: www.pediatrics.org/cgi/content/full/127/6/e1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belhassan M, Zeitoun JD, Lefevre JH, et al. Infliximab infusion time in patients with inflammatory bowel diseases: is longer really safer? Clin Res Hepatol Gastroenterol. 2013;37(2):189–192 [DOI] [PubMed]
- 39.Bethell CD, Read D, Stein RE, Blumberg SJ, Wells N, Newacheck PW. Identifying children with special health care needs: development and evaluation of a short screening instrument. Ambul Pediatr. 2002;2(1):38–48 [DOI] [PubMed]
- 40.van Dyck P, Kogan MD, Heppel D, Blumberg SJ, Cynamon ML, Newacheck PW. The National Survey of Children’s Health: a new data resource. Matern Child Health J. 2004;8(3):183–188 [DOI] [PubMed] [Google Scholar]
- 41.Straus MA. Conflict tactics scales. In: Jackson NA, ed. Encyclopedia of Domestic Violence. New York: NY: Routledge, Taylor & Francis Group; 2007:190–197 [Google Scholar]
- 42.Chandler S, Charman T, Baird G, et al. Validation of the social communication questionnaire in a population cohort of children with autism spectrum disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(10):1324–1332 [DOI] [PubMed] [Google Scholar]
- 43.Maxwell E. Manual for the FIGS. Bethesda, MA: National Institute of Mental Health, Clinical Neurogenetics Branch; 1992
- 44.Wolraich ML, Feurer ID, Hannah JN, Baumgaertel A, Pinnock TY. Obtaining systematic teacher reports of disruptive behavior disorders utilizing DSM-IV. J Abnorm Child Psychol. 1998;26(2):141–152 [DOI] [PubMed] [Google Scholar]
- 45.Lazarsfeld PF, Henry NW. Latent Structure Analysis. New York, NY: Houghton; 1968 [Google Scholar]
- 46.Goodman LA. Exploratory latent structure analysis using both identifiable and unidentifiable models. Biometrika. 1974;61(2):215–231 [Google Scholar]
- 47.Moustaki I. A latent trait and a latent class model for mixed observed variables. Br J Math Stat Psychol. 1996;49(2):313–334 [Google Scholar]
- 48.Wolfe JH. Pattern clustering by multivariate cluster analysis. Multivariate Behav Res. 1970;5:329–350 [DOI] [PubMed] [Google Scholar]
- 49.von Davier M. Bootstrapping goodness-of-fit statistics for sparse categorical data results of a Monte Carlo study. Methods of Psychological Research Online. 1997;2(2):29–48 [Google Scholar]
- 50.Magidson J, Vermunt JK. Latent class models for clustering: a comparison with K-means. Can J Marketing Res. 2002;20:37–44 [Google Scholar]
- 51.Magidson JV. J. K. Latent class factor and cluster models, bi-plots and related graphical displays. Sociol Methodol. 2001;31:223–264 [Google Scholar]
- 52.Barkley RA, Anastopoulos AD, Guevremont DC, Fletcher KE. Adolescents with ADHD: patterns of behavioral adjustment, academic functioning, and treatment utilization. J Am Acad Child Adolesc Psychiatry. 1991;30(5):752–761 [DOI] [PubMed] [Google Scholar]
- 53.Fischer M, Barkley RA, Fletcher KE, Smallish L. The adolescent outcome of hyperactive children: predictors of psychiatric, academic, social, and emotional adjustment. J Am Acad Child Adolesc Psychiatry. 1993;32(2):324–332 [DOI] [PubMed] [Google Scholar]
- 54.Yoshimasu K, Barbaresi WJ, Colligan RC, et al. Childhood ADHD is strongly associated with a broad range of psychiatric disorders during adolescence: a population-based birth cohort study. J Child Psychol Psychiatry. 2012;53(10):1036–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bajwa J, Charach M, Duclos D. Adverse effects of rifampicin in dogs and serum alanine aminotransferase monitoring recommendations based on a retrospective study of 344 dogs. Vet Dermatol. 2013;Oct 28. [DOI] [PubMed] [Google Scholar]
- 56.Charach A, Carson P, Fox S, Ali MU, Beckett J, Lim CG. Interventions for preschool children at high risk for ADHD: a comparative effectiveness review. Pediatrics 2013;131(5). Available at: www.pediatrics.org/cgi/content/full/131/5/e1584 [DOI] [PubMed]
- 57.Wolraich M, Brown L, Brown RT, et al. Subcommittee on Attention-Deficit/Hyperactivity Disorder. Steering Committee on Quality Improvement and Management . ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1007–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA. 2009;301(21):2252–2259 [DOI] [PubMed] [Google Scholar]