Abstract
Most ovarian cancer patients are diagnosed late in progression and often experience tumor recurrence and relapses due to drug resistance. Surface expression of matrix metalloprotease MMP-14 on ovarian cancer cells stimulates a tumor-stromal signaling pathway that promotes angiogenesis and tumor growth. In a cohort of 92 patients, we found that MMP-14 was increased in the serum of women with malignant ovarian tumors. Therefore, we investigated the preclinical efficacy of a MMP-14 monoclonal antibody that could inhibit the migratory and invasive properties of aggressive ovarian cancer cells in vitro. MMP-14 antibody disrupted ovarian tumor-stromal communication and was equivalent to Avastin in suppressing blood vessel growth in mice harboring matrigel plugs. These effects on angiogenesis correlated with down regulation of several important angiogenic factors. Further, mice with ovarian cancer tumors treated with anti-MMP-14 monotherapy showed a marked and sustained regression in tumor growth with decreased angiogenesis compared to IgG treated controls. In a model of advanced peritoneal ovarian cancer, MMP-14-dependent invasion and metastasis was effectively inhibited by intraperitoneal administration of monoclonal MMP-14 antibody. Together, these studies provide a preclinical proof-of-concept for MMP-14 targeting as an adjuvant treatment strategy for advanced ovarian cancer.
Keywords: ovarian cancer, angiogenesis, MMP-14, monoclonal antibody, antiangiogenic therapy
Introduction
Ovarian cancer remains the fifth leading cause of death in women and the most fatal gynecological cancer accounting for more than 15,000 deaths in the US annually 1. The vast majority of ovarian cancer patients are diagnosed in late stages and experience recurrences and relapses due to drug resistance despite initial response to surgical debulking and standard frontline chemotherapy 2. Additionally, there are no established and effective treatments for platinum refractory ovarian cancer. Therefore, identification of novel targeted therapies that block critical signaling pathways are important for improving survival in ovarian cancer 3.
Angiogenic and inflammatory markers such as IL (interleukin)-8, IL-6, GRO (growth related oncogene)-α, MCP (monocyte chemoattractant protein)-1, VEGF (vascular endothelial growth factor) and MMP (matrix metalloprotease)-1 are highly up regulated in epithelial ovarian cancers 4-7. These markers are postulated to play a pivotal role in tumor growth, inflammation, angiogenesis and metastasis and are associated with poor survival 8, 9. Angiogenesis inhibitors, as exemplified by the VEGF-antibody Avastin, have recently emerged as potential treatments for recurrent ovarian and primary peritoneal cancer10. But, anti-VEGF therapy has not improved overall survival 11 and one of the reasons is that decreased tumor size has been associated with increased invasiveness and propensity to metastasize 12.
Matrix metalloproteases are zinc-dependant endopeptidases that play a key role in cancer cell invasion, metastases and tumor angiogenesis 13, 14. Abundant evidence has indicted MMPs including MMP 1, 2, 9 and 14 as critical mediators of invasion, blood vessel penetration and metastasis of many solid tumors including ovarian cancer 4, 13, 15-18. Matrix metalloprotease-14, (MMP-14 or MT1-MMP), is a transmembrane collagenase, that is not detected in normal ovarian surface epithelium but is widely expressed on ovarian carcinomas of all histological types18.
High expression of epithelial MMP-14 on immunohistochemistry is associated with poorer prognosis and shorter disease survival in ovarian cancer patients 19. MMP-14 is critical to the acquisition of the invasive 20 and metastatic phenotype of breast, prostate, melanoma, renal and ovarian carcinomas 21-23. MMP-14 promotes a collagen-invasive phenotype24 in ovarian carcinoma which triggers peritoneal dissemination of metastatic ovarian tumors 23. MMP-14 is known to activate gelatinase MMP-2 25 that has been implicated in the mesothelial attachment and metastatic spread of ovarian cancer cells 20, 26. We have recently shown that ovarian cancer cell matrix metalloprotease (MMP)-14 leads to activation of proMMP-1 4 via MMP-9 which culminates in chemokine production, angiogenesis 4, 16, 27 and ovarian tumor growth 4, 27. Endothelial cell MMP-14 has been implicated in angiogenesis 28 however, the mechanism of cancer cell produced MMP-14 in stimulating angiogenesis in the ovarian tumor microenvironment is unknown.
A causal relationship between MMP expression and cancer progression, led to the evaluation of MMP inhibitors in several large clinical trials but met with limited efficacy and treatment failure 29, 30. These small molecule MMP inhibitors elicited debilitating musculoskeletal toxicities that precluded adequate dosing and poor patient compliance 31, 32. Toxicity was assumed to be due to the broad spectrum nature of these compounds which led to off target inhibition of protective MMPs such as MMP-8 and MMP-12 leading to worse clinical outcomes 29. Moreover, most of the patients recruited to the trials were in late stages (Stage III-IV) of cancer which could potentially miss the positive therapeutic benefits of blocking MMP pathway at earlier stages in cancer 33.
In this study we used a monoclonal antibody to inhibit MMP-14 on the ovarian carcinoma cells. MMP-14 inhibition led to suppression of both migratory and invasive propensity of ovarian carcinoma cells. MMP-14 antibody suppressed ovarian cancer cell-stimulated angiogenesis in vitro and in mice. Further, MMP-14 antibody treatment led to a marked regression in ovarian tumor growth and tumor blood vessel formation in a mouse xenograft model indicating that MMP-14 may be an attractive target for anti-angiogenic therapy in ovarian cancer and perhaps other solid tumors. Further, in a peritoneal model of aggressive ovarian cancer, combination intraperitoneal therapy of MMP-14 antibody and docetaxol inhibited invasion through the mouse diaphragm and thoracic metastasis. We believe, blockade of MMP-14 as monotherapy or as combinatorial therapy along with conventional chemotherapy has direct clinical relevance for adjuvant and metronomic treatment regimens in ovarian cancer.
Materials and Methods
Reagents
MMP-14 monoclonal antibodies were obtained from Calbiochem (EMD Biosciences, California), R&D (MAB 9181, 918) and Millipore (MAB 3319). Isotypic IgG1 control was purchased from R&D System and FITC conjugated rabbit anti-mouse from Invitrogen. MMP-7 (Ab-2), MMP-8 (Ab-1) and TIMP-1 (Ab-1) mouse monoclonal antibodies were obtained from Oncogene. Human MMP-14 ELISA was obtained from USCN (E92056Hu, Uscn Life Science, Inc) and human Quantikine MMP-9 and VEGF ELISA from R&D Systems and used as per manufacturer’s protocol. RNA interference reagents (RNAi) against Luciferase, MMP14 and MMP-9 have been described 4 and were obtained from Dharmacon.
Cell culture
Ovarian cancer cell lines OVCAR-4, SKOV-3 and OVCAR-3 were obtained from the National Cancer Institute and were grown in RPMI with 10% fetal bovine serum. Cells were serum starved overnight in 0.25% Bovine Serum Albumin (BSA) for migration and invasion assays. Human Umbilical Vein Endothelial cells (HUVECs) were bought from Cambrex (Lonza) and cultured in EBM2 medium with Bullet kit and 10% fetal bovine serum. HUVEC cells were serum starved for 1hour before tube formation. Gelatinase activity was assayed from conditioned media by measuring the cleavage of fluorescein conjugated DQ gelatin (Molecular Probes). Gelatinase assays contained 10 μg DQ gelatin in 50 mM Tris-HCL, pH 7.6, 150 mM NaCl, 5 mM CaCl2 and 0.2 mM NaN3 and cleavage was monitored at 538 nm using a fluorescence microplate reader with excitation at 485 nm at 37°C. Gelatinase activity was expressed as percentage of the control group. Experiments were repeated thrice in triplicates.
Detection of MMP-14 in patient samples
All patient blood samples were collected in full compliance with Tufts and Lahey Institutional Review Board (IRB) guidelines. Blood was collected from women presenting at Tufts Medical Center or Lahey clinic with benign or malignant pelvic masses, prior to surgery. Blood was processed to collect serum that was stored at −80 C till further use. Several serum samples were also obtained from Gynecology Oncology Group Tissue bank at Columbus, Ohio. Discarded patient fluids were collected from patients undergoing a peritoneal and pulmonary tap at Tufts Medical Center. Serum samples were diluted 10 fold and MMP-14 levels were quantified using ELISA (USCN E92056Hu). Groups were compared with one way Anova followed by T test. * p<0.05. Patient fluids and serum samples were normalized using Bradford assay. 2 μl of normalized samples (2.2mg/ml) were mixed with citrate buffer, EDTA and 5X sample buffer in a 10 μl volume, heated for 40 mins at 37°C and immunoblotted with monoclonal MMP-14 Ab (MAB 918).
Flow Cytometry
Flow cytometry was performed as described before 4 using a mouse monoclonal antibody specific to MMP-14 (R&D systems MAB9181).
Migration and Invasion
Chemotactic migration and invasion was conducted using an 8μm transwell apparatus (Corning) as described 16. 50,000 cells serum starved ovarian cancer cells were treated with 10ug/ml of antibody and placed in the upper chamber of the transwell in 200ul of 0.25%BSA in RPMI. The cells were migrated towards stromal matrix (fibroblast CM) or malignant ovarian ascites as previously described 16 for a period of 5h. For chemoinvasion, wells were coated with of 20μg of reconstituted matrix (matrigel, BD Biosciences) 4 and cells were migrated similarly for 20h. For collagen invasion assays, transwells were coated with 30μg of rat tail High Concentration Type I collagen (BD Biosciences) and cells were migrated for 40 hours. Migrated and invaded cells on the lower surface of the transwell were fixed, stained and counted and data was compared to the respective IgG controls. One way Anova followed by Students t test was used to compare the effect of treatment to the control. * P<0.05, * * P<0.005.
Tube formation assay
MatTek plates were chilled to 4°C and coated with 100 μl of matrigel per well. Freshly passaged HUVEC cells (35,000; P2-5) in EBM2 media with 0.25% BSA were plated on matrigel-coated MatTek plates and stimulated with CM from OVCAR-4 or SKOV-3 cells treated with either MMP-14 antibody IgG or RNAi. Endothelial tube formation was observed after 18 hours and photographed under phase contrast microscopy using an inverted Olympus microscope. Digital images were used to count total tubes per five 40x fields and quantified for tube length and branching complexity using NIH image J software. Measures were expressed in number of branch points and actual tubal length. * P<0.05, * * P<0.005.
RNA Interference
Cells were transfected with Luci, MMP-14 or MMP-9 RNAi using oligofectamine as previously described 4. 30 hrs after recovery, the cells were either used for RNA preparation or serum starved for conditioned media.
Matrigel plugs and ovarian cancer xenograft models
All animal experiments were conducted in full compliance with Tufts-NEMC Institutional Animal Care and Use Committee. Female NCR Nu/Nu mice (5-7 weeks) were purchased from Taconic farms. Mice were injected with 300μl matrigel plugs of equal parts CM and matrigel in their flanks subcutaneously. Animals were subsequently treated 24 hours later with either MMP-14 Ab, IgG or Avastin (2.5mg/kg twice weekly) in a 100 μL volume. At the end of 7 days, mice were euthanized and plugs were excised and fixed in 10% formalin/PBS and stained with CD31 (Chemicon, MAB 1398Z) for confocal microscopy as previously described 27. For ovarian xenograft experiments, mice were injected subcutaneously with 3.5 million OVCAR-4 cells in their flanks. Mice were randomized into 2 treatment groups and 24 h later injected with 2.5 mg/kg of IgG or MMP-14 antibody, i/p, twice weekly till Day 26. Tumor sizes were measured twice weekly initially and every other day after day 26 for the duration of the experiment. Tumor volume was calculated using the formula V=length x diameter2/2. Mice were euthanized on day 35 and tumors and tissues were collected. Tumors were stained for endothelial marker CD31 (760-4378, Ventana Medical Systems, automated as per manufacturer’s protocol) and evaluated by a pathologist in a blinded manner.
For the Peritoneal Ovarian Tumor Model, mice were injected into the peritoneal cavity with 1.5 million OVCAR-4 cells. Animals were treated 24 hours later with i.p. injections of 2.5 mg/kg of either IgG or MMP-14 antibody twice a week with concomitant docetaxel (5 mg/kg at day 21). At the end of 35 days, tumors and organs were excised from euthanized mice, fixed in 10% formalin/PBS and imbedded in paraffin. Tissue sections (5 μm) from omentum, diaphragm, and thoracic organs were prepared and stained with hematoxylin-eosin and examined in a blinded manner by two pathologists.
Results
Serum MMP-14 is increased in the patients of invasive ovarian cancer
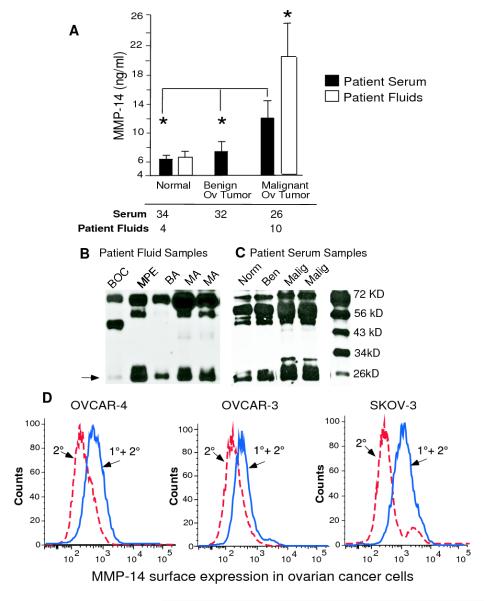
We have previously shown that MMP-14 initiates the activation of a matrix metalloprotease cascade in the ovarian tumor microenvironment that leads to angiogenesis and ovarian tumor growth 4. High expression of epithelial MMP-14 on immunohistochemistry was associated with poor outcome in ovarian cancer patients 19. Therefore we investigated if MMP-14 could be could be detected as a tumor biomarker in the serum and malignant ascites of ovarian carcinoma patients. These patients are known to produce copious amounts of peritoneal ascites and we explored if we could detect MMP-14 or a cleaved part of MMP-14 in malignant ovarian ascites. We collected fluids from 14 patients with malignant and benign disease (malignant ovarian ascites n=5, malignant ovarian cyst n=1, malignant pleural effusions from ovarian cancer patients n=4, benign ascites n=3, and benign ovarian cyst n=1) and found that the levels of MMP-14 were more than 3 fold increased in the malignant fluids of ovarian cancer patients compared to benign fluids (Fig. 1 A). Since MMP-14 was increased in the malignant ovarian ascites, we further explored if it could be detected in the serum of malignant ovarian cancer patients. In a cohort of 92 patients comprising of healthy females (n=34), those with benign ovarian tumors (n=32) and malignant ovarian cancer (serous epithelial cancer, Stages II-IV; n=26), we found that serum MMP-14 was significantly increased in malignant ovarian carcinoma patients (Fig. 1 A). To our knowledge, this is the first report of increased MMP-14 in the serum and malignant ascites of ovarian carcinoma patients and has important implications not only as a biomarker of the disease but also as a tumor marker for monitoring response to therapy. We also confirmed the presence of MMP-14 in these samples of malignant ovarian ascites, malignant pleural effusion and the serum of patients with ovarian carcinoma using immunoblot analysis (Fig. 1B and C). Immunoblotting for MMP-14 showed several fragments of MMP-14 exodomain as previously described 34. Our data correlates with the increased expression of MMP-14 found by immunohistochemistry in metastatic ovarian lesions as compared with primary ovarian tumors 23 and suggests an important role for MMP-14 in ovarian cancer progression. We also tested aggressive OVCAR-4, SKOV-3 or OVCAR-3 ovarian cancer cells and found a robust expression of MMP-14 by flow cytometry (Fig. 1D). This finding reiterates the fact that MMP-14 can be detected as a tumor biomarker in malignant ovarian cancer and emphasizes the importance of this MMP in ovarian carcinogenesis.
Figure 1. MMP-14 is a biomarker of malignant ovarian cancer.
A. Serum samples were collected from females with malignant ovarian cancer (n= 26), females with benign ovarian masses (n= 32) and normal females (n= 34) and MMP-14 cellular exodomain levels were measured by ELISA. Various fluids were collected from 14 patients with benign or malignant disease. MMP-14 were measured by ELISA in malignant ovarian ascites (n=5), malignant ovarian cyst (n=1), pleural effusion of ovarian cancer patients (n=4), benign ascites (n=3) and benign ovarian cyst (n=1) B. Columns mean, bars SE; * P<0.05, * * P<0.01. B and C. Gels showing cleaved MMP-14 fragments in benign and malignant patient fluids. Patient serum and fluids (BOC=benign ovarian cyst, BA=benign ascites, MA=malignant ovarian ascites, MPE =malignant pleural effusion) were diluted, mixed with citrate buffer and immunoblotted with MAB 918 in 1:1000 dilution. The cleaved MMP-14 fragment seen at 27 K Da is most representative of the ELISA data shown above D. Surface expression of MMP-14 was measured in ovarian cancer cell lines OVCAR4, OVCAR-3 and SKOV-3 by flow cytometry using a MMP-14 monoclonal antibody. The red dotted line represents secondary alone and the blue solid line represents primary plus secondary antibody.
Monoclonal MMP-14 antibody inhibits migration and invasion of ovarian cancer cells
MMP-14, when over expressed, promotes cancer cell migration and invasion 24, 35 critical to the acquisition of the metastatic phenotype in ovarian cancer 23. MMP-14 silencing suppresses migration and invasion of tumor cells in vitro 36 and pharmacological inhibition of MMPs including MMP-14 using broad-spectrum MMP inhibitors such as prinimostat inhibits tumor progression 37. However, as broad spectrum MMP inhibitors have not been successful in clinical trials 38 we investigated if selective blockade of MMP-14 using a monoclonal antibody could inhibit the migratory ability of ovarian carcinoma cells. We treated OVCAR-4 and OVCAR-3 cells with monoclonal MMP-14 antibody (Ab) or corresponding IgG and allowed them to migrate towards fibroblast conditioned media (CM, Fig. 2A) or malignant ovarian ascites (Fig. 2B). Treatment with MMP-14 Ab significantly decreased migration by 46 to 69% of OVCAR-3 (P<0.05) and OVCAR-4 (P<0.005) cancer cells respectively, compared to IgG controls (Fig. 2A). We also used several other relevant monoclonal antibodies as controls. MMP-7 is a matrilysin important for inducing ovarian cancer invasion39, MMP-8 is tumor collagenase like MMP-14 which is known to have a protective role for cancers 40 but is not expressed in our ovarian cancer cells (Supplementary Fig S1A) and TIMP-1 (Tissue inhibitor of metalloproteinase-1) inhibits most MMPs except membrane tethered MMPs such as MMP-14, 16 and 25 41. Unlike MMP-14 Ab, antibodies against MMP-7, MMP-8 and TIMP1 did not suppress migration of either cell line (Fig. 2A) suggesting that the migratory ability of ovarian cancer cells was dependent on MMP-14. Anti-VEGF therapy is currently being used as frontline therapy in ovarian cancer 10 along with standard chemotherapeutic regimens but has not yet improved overall survival perhaps due to increased local invasion and metastasis 12. We therefore tested if antibodies against VEGF (Avastin) would inhibit migration of ovarian cancer cells. Anti VEGF antibody had no effect on the migration of OVCAR-4 cells while they had a non-significant decrease on the migration of OVCAR-3 cells (Fig. 2 A).
Figure 2. Blockade of MMP-14 inhibits migration and invasion of ovarian cancer cells.
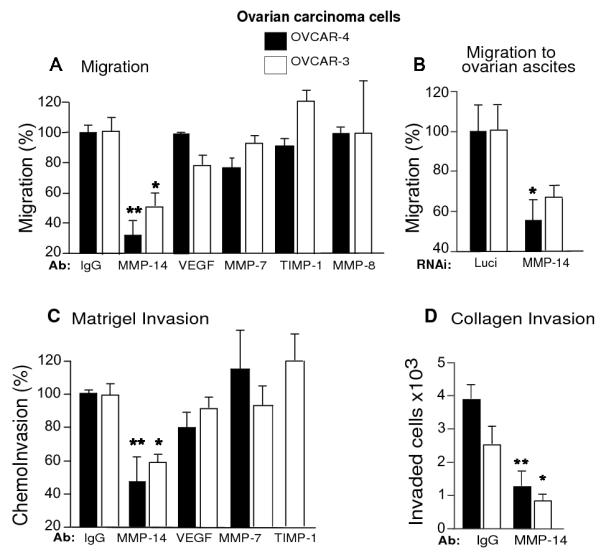
A. OVCAR-4 and OVCAR-3 cells were treated with 10 μg/ml of either IgG or MMP-14 Ab, Avastin (VEGF Ab), TIMP-1 Ab, MMP-7 Ab or MMP-8 Ab and migrated towards stromal matrix (fibroblast conditioned media) for 5 hours. B. OVCAR-4 and OVCAR-3 cells were treated with MMP-14 or luciferase RNAi, serum starved and migrated towards malignant ovarian ascites for 5 hours. C. Transwells were coated with 20 μg of matrigel. OVCAR-4 and OVCAR-3 cells were treated with either IgG, MMP-14, Avastin (VEGF Ab), MMP-7 or TIMP-1 antibody and migrated towards stromal matrix for 20 hours. D. Transwells were coated with Type 1 rat tail collagen, and cells were migrated towards stromal matrix for 40 hours. Columns mean, bars SE; * P<0.05, * * P<0.005.
In the peritoneal cavity, malignant ovarian ascites serves as a rich chemoattractant for the migratory ovarian carcinoma cells. To examine the role of MMP-14 in migration to ascites, we treated OVCAR-4 or OVCAR-3 cells with MMP-14 RNAi 36 and migrated them towards malignant ovarian ascites. MMP-14 silencing of OVCAR-4 and OVCAR-3 cells led to a 60-80% decrease in MMP-14 levels at the mRNA level (Supplementary Fig. S 1B) and 80% to 95% decrease by flow cytometry (Supplementary Fig. S 1C and D respectively) which was confirmed by immunoblotting (Supplementary Fig. S 1 E and F). MMP-14 RNAi treated OVCAR-4 and OVCAR-3 cells showed decreased migration towards ascites compared to the corresponding luciferase treated cells (Fig. 2B), consistent with the MMP-14 Ab data, suggesting that MMP-14 is important for the movement of ovarian cancer cells in the peritoneal cavity.
We further explored the effect of MMP-14 antibody on ovarian cancer cell invasion. MMP-14 antibody treated cells showed a more than 50% reduction in the invasion of OVCAR-4 and a 40% reduction in the invasion of OVCAR-3 cells through reconstituted basement membrane (matrigel) compared to corresponding IgG controls. Antibodies against VEGF (Avastin), MMP-7 and TIMP1 had no significant effect on matrigel invasion (Fig. 2C). We also tested invasion through reconstituted Type I collagen matrices which is highly dependent on MMP-14 activity42. We migrated MMP-14 Ab treated OVCAR-4 and OVCAR-3 cells through a Type I collagen matrix and found that MMP-14 antibody significantly inhibited more than 60% of the invasive propensity of OVCAR-4 (P<0.005) and OVCAR-3 cells compared to respective IgG treated controls (p< 0.05; Fig 2 D). These data suggest that selectively blocking MMP-14 may be physiologically relevant for inhibiting the migratory and invasive potential of ovarian carcinoma cells.
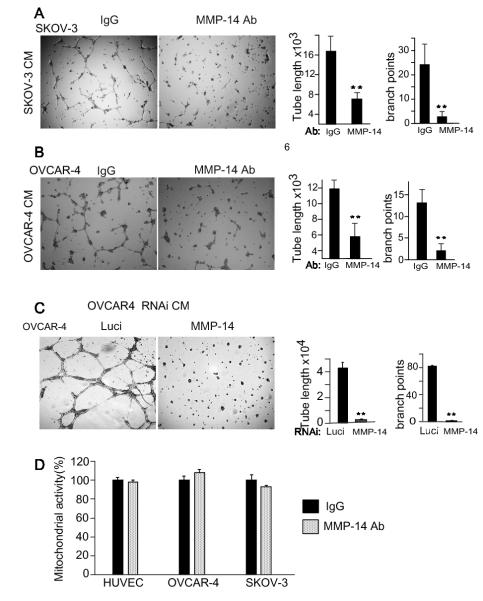
Blockade of cancer cell MMP-14 inhibits endothelial angiogenesis
Endothelial cell MMP-14 is known to be important for blood vessel formation 43 because MMP-14 knockout mice display skeletal defects due to impaired vascularization of the cartilage and endothelial cells from these mice show decreased fibroblast growth factor (FGF2) induced capillary tube formation 44. We postulated that the cancer cell MMP-14 was regulating the release of angiogenic factors in the ovarian tumor microenvironment and stimulating blood vessel formation. To test the role of cancer cell MMP-14 in cancer cell stimulated angiogenesis, we treated OVCAR-4 or SKOV-3 cells overnight with either MMP-14 blocking Ab or IgG (10 μg/ml), collected serum starved CM and stimulated HUVECs on matrigel coated plates. HUVECs stimulated with CM from MMP-14 Ab treated OVCAR-4 or SKOV-3 cells showed 60-83% reduction in branching complexity and ≥ 50% reduction in tubal length compared to those stimulated with IgG treated CM (Fig. 3A and B). Further, to ensure that these effects were a function of blockade of MMP-14 on the cancer cell, we also collected serum starved CM from MMP-14 and Luci RNAi treated OVCAR-4 cells (Supplementary Fig. S1C and E) and stimulated tube formation. MMP-14 gene silencing of the ovarian cancer cells completely blocked carcinoma cell stimulated angiogenesis (Fig. 3 C) confirming that endothelial tube formation was at least partly regulated through cancer cell MMP-14. We performed a mitochondrial activity assay (MTT) to rule out that MMP-14 Ab treatment affected the viability of treated cells. MMP-14 Ab and IgG treated endothelial and cancer cells did not show any differences in viability after 72 hours of treatment (Fig. 3 D). This suggests that the cancer cell MMP-14 was regulating the production or release of angiogenic factors in the ovarian tumor microenvironment that promoted endothelial tube formation and branching complexity.
Figure 3. Blockade of MMP-14 disrupts tumor-stromal communication and angiogenesis A and B.
Ovarian cancer cell stimulated angiogenesis is inhibited by MMP-14 antibody. OVCAR-4 and SKOV-3 cells were treated overnight with either MMP-14 Ab or IgG (10μg/ml) in serum starved CM. Ovarian cancer cell CM was collected 18 hours later and used to stimulate HUVECs on matrigel coated MatTEK plates. C. OVCAR-4 ovarian cancer cells were treated with RNAi against MMP-14 and luciferase, and serum starved CM was collected to stimulate HUVEC cells. Results from A, B and C were quantified using NIH Image J. Tubal length and branch points were compared between the treatment groups using a T Test. * P<0.05, **P<0.01 D. HUVECs, OVCAR-4 and SKOV-3 ovarian cancer cells were treated with either 10 μg/ml of IgG or MMP-14 antibody and allowed to grow for 72 hours. Mitochondrial activity for all the cells was assessed after 72 hours and expressed as a percent of IgG controls.
Further, we characterized the MMP-14 antibodies used in this study (Calbiochem and R&D Systems) and found that they blocked the conversion of proMMP-2 to active MMP-2 (Supplementary Fig S2 B). Further, MMP-14 Ab (R&D Systems) was specific in recognizing MMP-14 protein and did not cross react with other MMPs such as MMP-3, 7 and 8 (Supplementary Fig S2C).
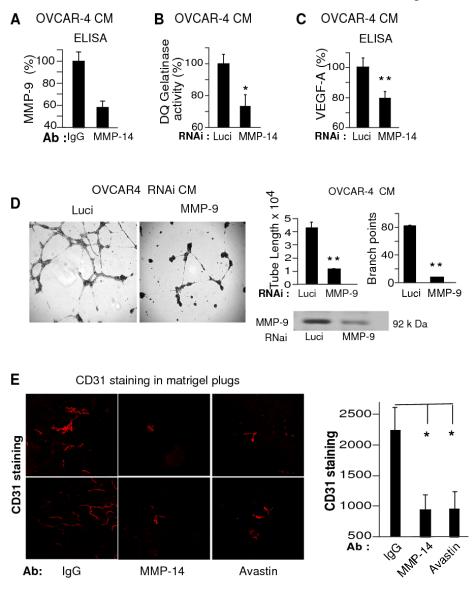
Cancer cell MMP-14 regulates angiogenesis in the ovarian tumor microenvironment
Cancer cell MMP-14 is known to convert proMMP-2 to MMP-2 25. We have previously shown that MMP-14 on the ovarian cancer cells regulates activation of proMMP-1 to MMP-1, which up regulates chemokine production and ovarian cancer angiogenesis 4. Therefore we speculated that cancer cell MMP-14 was regulating angiogenesis by modulating the activity of other MMPs or angiogenic factors 45 in the ovarian tumor microenvironment. Having seen the unequivocal effects of MMP-14 Ab and MMP-14 RNAi on in vitro cancer cell stimulated angiogenesis, we explored the angiogenic factors being regulated through MMP-14. It is well established that MMP-9 and VEGF are important for tumor angiogenesis 46 but it is not well known that MMP-14 regulates MMP-947. We hypothesized that MMP-14 was suppressing blood vessel formation by down regulating production or release of MMP-9 or VEGF, or both of these factors. We tested MMP-14 Ab treated OVCAR-4 cell CM and confirmed the decrease in MMP-9 levels by ELISA (Fig 4A). Since active MMP-9 is required for the release of VEGF from the cancer cells and for promoting angiogenesis 46 we further tested if MMP-14 inhibition decreased MMP-9 activity by measuring in situ gelatinase (largely MMP-2 and MMP-9) activity from RNAi treated OVCAR-4 cell CM. MMP-14 gene suppression significantly reduced gelatin activity (Fig. 4B), part of which can be attributed to MMP-9 activity as OVCAR-4 cells lack MMP-2 (Supplementary Fig S2 A and B).
Figure 4. MMP-14 regulates ovarian cancer angiogenesis through MMP-9 and VEGF pathways.
A. OVCAR-4 cells were treated with MMP-14 Ab or MMP-14 RNAi along with respective controls. Serum starved CM was collected to assess levels of MMP-9 by ELISA (A), gelatin cleavage activity using DQ gelatinase assay (B) and VEGF by ELISA (C) D. OVCAR-4 cells were treated with either MMP-9 or luciferase RNAi and CM was used to stimulate endothelial tube formation. Results were quantified as tube length and branch points. * P<0.05, * * P<0.005 E. Matrigel plugs of 300 μl were injected into the flanks of female NCR nu/nu mice subcutaneously (s/c). The plugs consisted of equal volumes of OVCAR-4 CM and matrigel along with HUVECs. On day 2 mice were treated with either MMP-14 (n=8) or corresponding IgG antibody (n=8) or Avastin (n=7) in a dose of 2.5 mg/kg, i.p. twice a week for 7 days. At the end of 7 days, mice were euthanized and the matrigel plugs were excised, fixed and stained for CD31. Figure shows CD31 stained microscopic sections of matrigel plugs and quantification of CD31 counts from the three treatment groups. Columns mean, bars SE; * P<0.05, * * P<0.005.
We further tested whether MMP-14 blockade, also had an effect on VEGF levels. MMP-14 knockdown also significantly decreased VEGF A (165) levels in serum starved OVCAR-4 CM (Fig. 4 C) establishing the importance of MMP-14 in ovarian tumor angiogenesis. To ensure that these effects were a function of decreased MMP-9, we also collected serum starved CM from OVCAR-4 cells that had been treated with MMP-9 and Luci RNai (Fig 4 D and Supplementary Fig. S3 A) and stimulated tube formation. MMP-9 gene silencing of the ovarian cancer cell demonstrated a more than 75% decrease in tubal length and branching complexity (Fig. 4D). This suggested that at least a part of the MMP-14 regulated ovarian cancer angiogenesis was mediated through suppression of MMP-9 (Fig 4 A and B, Supplementary Fig. S3B) especially as MMP-14 ab added directly on the HUVECs did not significantly suppress tube formation (Supplementary Fig. S3C).
Having seen the importance of cancer cell MMP-14 in ovarian cancer angiogenesis and the inhibitory effects of MMP-14 antibody on in vitro tube formation, we tested if this would be true for in vivo angiogenesis. We subcutaneously injected matrigel plugs into the flanks of female nude mice. The plugs consisted of equal volumes of OVCAR-4 CM and matrigel along with HUVECs. The mice were then treated on Day 2 with either MMP-14 Ab or corresponding IgG antibody or Avastin (VEGF) twice a week at 2.5 mg/kg, intraperitoneally (i.p.). At the end of 7 days, matrigel plugs were excised, fixed overnight and stained with CD31, a marker for endothelial cells27. Both MMP-14 and VEGF antibody treated mice showed a marked decrease in vascularization of matrigel plugs as compared to the IgG treated controls (Fig. 4 E). The matrigel plug experiment not only established the efficacy of the MMP-14 antibody in vivo but also the adequate dose required for inhibition of blood vessels suggesting that blocking MMP-14 may be a therapeutic strategy to inhibit ovarian cancer angiogenesis.
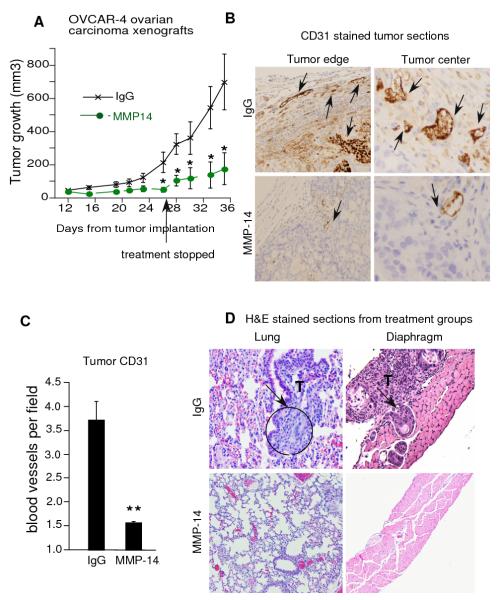
MMP-14 antibody inhibits ovarian tumor growth and angiogenesis
Small molecule MMP inhibitors have not been successful in clinical trials due to their broad spectrum effects. As shown above, monoclonal MMP-14 Ab had striking effects on in vivo ovarian cancer angiogenesis and did not bind non specifically to other MMPs such as MMP-8 (Supplementary Fig. S 2C); therefore we further tested if these effects could be translated into decreased tumor growth. We injected 3.5 million OVCAR-4 ovarian carcinoma cells subcutaneously into the flanks of nude mice. Mice were randomized 24 hours later into two treatment groups and treated with either MMP-14 antibody or corresponding IgG (2.5mg/kg, twice per week, i.p.). MMP-14 Ab treated mice demonstrated a significant reduction in tumor growth (p<0.06) from day 26 onwards (Fig 5A). As antibodies usually have long lasting effects, we stopped treatment in both groups at Day 27 and let the tumors grow for another week. After halting treatment, the IgG treated mice had exponential tumor growth whereas the MMP-14 Ab treated mice continued to show a significant reduction (p<0.01) in tumor growth compared to the IgG treated mice. Further, within the MMP-14 Ab treatment group there was no significant increase in tumor size between the day 28 and day 35 time point demonstrating the long lasting effect of the MMP-14 Ab treatment on tumor growth. Mice were euthanized and tumors collected for CD31 immunohistochemistry on day 35. MMP-14 treated mice showed a marked reduction in blood vessels (P<0.001) both at the tumor edge and the tumor center compared to the IgG treated controls (Fig. 5 B and C). We correlated decreased angiogenesis with reduced tumor growth in MMP-14 Ab treated tumors compared to their IgG treated counterparts. Further, we examined the livers of mice in both treatment groups and found no obvious signs of toxicity (data not shown). These findings suggest that MMP-14 is an important regulator of ovarian cancer angiogenesis and tumor growth and could potentially be a novel target for anti-angiogenic therapy in ovarian cancer.
Figure 5. MMP-14 antibody inhibits tumor growth, angiogenesis, invasion and metastasis in mice.
A. OVCAR-4 ovarian cancer cells (3.5 million) were injected into the flanks of nude mice and the mice were treated with either IgG or MMP-14 antibody, i.p. 24 hours after implantation. Treatment was stopped from Day 27 onwards but tumors were monitored and measured by calipers every other day till day 35. B and C. Tumors were stained with CD31 antibody and blood vessels counted in sections from each tumor. Intense staining of individual endothelial cells was scored as a blood vessel in a blinded manner from paraffin embedded tissue sections. Vascular density was determined by counting 5-9 fields (160X magnification) from at least 5 tumors in each group. CD 31 stained blood vessels are demonstrated by black arrows in tumor edge and center. Columns mean, bars SE; * P<0.05, * * P<0.005. D. Female NCR Nu/Nu mice were injected i.p. with 1.5 million OVCAR-4 cells and randomized into treatment groups of IgG (n=5) or MMP-14 Ab (n=4, 2.5 mg/kg/day, twice a week, i.p.), starting on day 2. Both groups received docetaxel (5 mg/kg/week, i.p.) starting on day 21 and a repeat dose after 7 days. Mice were euthanized and diaphragm, peritoneal, and thoracic organs were harvested, stained with hematoxylin & eosin and analyzed by two independent pathologists blinded to the treatment groups. Representative sections from lung and diaphragm are shown and tumor areas are marked by arrows.
Intraperitoneal combination of MMP-14 antibody Plus Docetaxol Inhibits Invasion and Metastasis of Ovarian Cancer in Nude Mice
Patients whose cancer has spread throughout the peritoneal cavity have a poor survival rate, thus inhibition of peritoneal dissemination and metastatic progression is critical for the successful treatment of ovarian cancer. Therefore, we assessed whether inhibition of MMP-14 using a monoclonal antibody in combination with docetaxel was able to slow progression of peritoneal ovarian cancer in mice. We have previously developed a histological staging system that used the diaphragm as a marker of the progressive invasion and metastasis from the peritoneal cavity/omentum to the thoracic organs 4. We injected 1.5 million aggressive OVCAR-4 ovarian cancer cells into the peritoneal cavity of mice. Starting on day 2, mice received either IgG or MMP-14 Ab (2.5mg/kg, i.p. twice a week) and two doses of docetaxel (5 mg/kg) i.p. on day 21 and day 28 until the end of the experimental period. At the 35 d time point, all of the IgG-treated mice had complete penetration through the diaphragm and metastasis to the lungs and mediastinum, confirming that the OVCAR-4 cells were extremely aggressive and metastatic in vivo (Fig. 5 D and Supplementary Fig. S4A). By comparison, mice treated with MMP-14 antibody had a marked decrease in metastatic progression (p=0.01) and the cancer remained confined to the peritoneal surface of the diaphragm in 75 % of the mice (Fig. 5 D, Supplementary Fig S4A). And while we did not see a significant reduction in ascites volume between the two treatment groups, the ascites in the MMP-14 treated mice was straw colored as opposed to the bloody ascites in the IgG treated mice (Supplementary Fig. S4B). MMP-14 Ab conferred significant (P = 0.01) protection against invasion into the diaphragm and thoracic metastases suggesting that MMP-14 targeted therapy maybe clinically relevant for inhibiting progression of advanced ovarian cancer.
Discussion
MMP-14: a new anti angiogenic molecular therapeutic target in human ovarian cancer
Ovarian cancers critically depend on blood vessels for their expansive growth and belong to the group of highly angiogenic tumors that respond to even monotherapy with anti angiogenic drugs48. Bevacizumab, a monoclonal antibody against VEGF (vascular endothelial growth factor), has been increasingly used upfront for therapy in ovarian cancer 10. Despite early benefits seen with these anti VEGF pathway targeting drugs, the clinical benefits in terms of overall survival have been modest and associated with enhanced local invasion and metastatic progression 12. Therefore it is possible that therapies that target multiple, distinct angiogenic pathways are superior to those targeting singular angiogenic pathways. We believe MMP-14 could be such a target in ovarian cancer.
MMP-14 has been associated with poor overall survival in breast cancer 22 and in this study we found MMP-14 to be increased in the serum of malignant ovarian cancer patients compared to normal women and those with benign ovarian masses confirming its importance for ovarian carcinogeneis. MMP-14 is a 66 kDa membrane tethered MMP and unlike other MMPs it is not known to be secreted into the tumor milieu. However, we postulated that other MMPs and serine proteases present in the ovarian tumor microenvironment might cleave the extracellular domain of MMP-14 49 and allow for its detection in ovarian cancer patient fluids and serum. We confirmed the increased presence of cleaved MMP-14 in malignant ovarian ascites, pleural effusions from ovarian cancer patients and serum of these patients using ELISA and immunoblot analysis using a MMP-14 monoclonal antibody. To our knowledge this is the first study to report the detection of MMP-14 in the serum and malignant fluids of ovarian cancer patients. Detection of MMP-14 in the serum is important for monitoring relapse and recurrence of the disease, remission and potentially, response to anti-MMP-14 targeted therapy.
Recently, there have been several reports of proinvasive consequences of antiangiogenic tumor therapy 50 that led the authors to suggest that a combination of different treatment regimens which include anti invasive therapy may be more effective in blocking tumor progression. MMP-14, which is expressed on the invadopodia of cancer cells 20 could be such a therapeutic target. However targeting MMPs has been challenging and like other systemic therapies broad-spectrum MMP inhibitors failed to produce enduring efficacy in terms of either tumor shrinkage or long-term survival benefit 30, 32. In this study, we used commercially available monoclonal antibody against MMP-14 to significantly impair the migratory and invasive ability of MMP-14 expressing ovarian cancer cells. This is especially relevant for the spread of ovarian cancer cells within the rich milieu of the peritoneal cavity23. These results demonstrate that anti migratory and anti invasive property of the MMP-14 antibody could be a significant advantage over the VEGF antibody for long term anti angiogenic therapy.
Using a monoclonal antibody or RNAi against ovarian cancer cell MMP-14 we disrupted tumor-stromal communication that normally stimulated in vitro and in vivo angiogenesis. In vivo, MMP-14 antibody was equivalent to Avastin in blocking blood vessel growth within matrigel plugs in mice. We confirmed that these effects were not due to inhibition of cellular proliferation but due to production of angiogenic factors. Cancer cell MMP-14 not only regulated MMP-9 and VEGF production and but also MMP-9 activity which is important for VEGF release 46. While OVCAR-4 cells do not express MMP-1 we have previously shown that MMP-14 on the ovarian cancer cell, along with MMP-9 activates stromal proMMP1 to MMP-1 4 which is known to be important for tumor angiogenesis 9, 16 directly and through the up regulation of several chemokines 27.
Further, mice bearing OVCAR-4 ovarian cancer tumors when treated with MMP-14 antibody as monotherapy showed a marked regression in tumor growth. Even a week after the treatment had been stopped, there was no significant growth in the MMP-14 antibody treated tumors whereas the IgG treated cohort continued to grow exponentially. The MMP-14 antibody treated tumors showed a marked reduction in CD31 stained blood vessels compared to the IgG controls providing proof of concept for targeting MMP-14 for anti-angiogenic therapy in ovarian cancer. Additionally, in an aggressive, orthotopic mouse model of ovarian cancer, MMP-14 Ab along with docetaxol, inhibited invasion through the diaphragm and thoracic metastasis. We believe that MMP-14 blockade as monotherapy or as combinatorial therapy with standard-of-care drugs may have direct clinical relevance for advanced ovarian cancer patients and implications for adjuvant therapeutic regimens not only for ovarian cancer but also for other solid tumors.
Supplementary Material
Acknowledgements
We would like to acknowledge all the authors whose important contributions could not be cited due to reference limits. We thank Ms. Susan Turner, Dr. Katie Wakeley and Dr. John Yang for their help in collecting the patient samples, Dr. Goli Javid for help with the histopathological analysis, Ms Namrata Nammi for technical assistance, Dr. Katie O’Callaghan for reading the manuscript and Prof Phil Hinds for helpful suggestions.
Grant Support. This work was funded by Liz Tilberis award from Ovarian Cancer Research Foundation (AA), NIH T32 CA009429 training grant (RA), Beauchamp Memorial Fund (CZ) and NIH HL064701 (AK).
Footnotes
Conflict of interest: Authors declare no conflict of interest
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Ozols RF, Bookman MA, Connolly DC, et al. Focus on epithelial ovarian cancer. Cancer Cell. 2004;5(1):19–24. doi: 10.1016/s1535-6108(04)00002-9. [DOI] [PubMed] [Google Scholar]
- 3.Bast RC, Jr., Hennessy B, Mills GB. The biology of ovarian cancer: new opportunities for translation. Nat Rev Cancer. 2009;9(6):415–28. doi: 10.1038/nrc2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agarwal A, Covic L, Sevigny LM, et al. Targeting a metalloprotease-PAR1 signaling system with cell-penetrating pepducins inhibits angiogenesis, ascites, and progression of ovarian cancer. Mol Cancer Ther. 2008;7(9):2746–57. doi: 10.1158/1535-7163.MCT-08-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Negus RP, Stamp GW, Relf MG, et al. The detection and localization of monocyte chemoattractant protein-1 (MCP-1) in human ovarian cancer. J Clin Invest. 1995;95(5):2391–6. doi: 10.1172/JCI117933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merritt WM, Lin YG, Spannuth WA, et al. Effect of interleukin-8 gene silencing with liposome-encapsulated small interfering RNA on ovarian cancer cell growth. J Natl Cancer Inst. 2008;100(5):359–72. doi: 10.1093/jnci/djn024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kulbe H, Chakravarty P, Leinster DA, et al. A dynamic inflammatory cytokine network in the human ovarian cancer microenvironment. Cancer Res. 2012;72(1):66–75. doi: 10.1158/0008-5472.CAN-11-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blackburn JS, Rhodes CH, Coon CI, Brinckerhoff CE. RNA interference inhibition of matrix metalloproteinase-1 prevents melanoma metastasis by reducing tumor collagenase activity and angiogenesis. Cancer Res. 2007;67(22):10849–58. doi: 10.1158/0008-5472.CAN-07-1791. [DOI] [PubMed] [Google Scholar]
- 10.Burger RA. Experience with bevacizumab in the management of epithelial ovarian cancer. J Clin Oncol. 2007;25(20):2902–8. doi: 10.1200/JCO.2007.12.1509. [DOI] [PubMed] [Google Scholar]
- 11.Sood AK, Coleman RL, Ellis LM. Moving beyond anti-vascular endothelial growth factor therapy in ovarian cancer. J Clin Oncol. 2011;30(4):345–7. doi: 10.1200/JCO.2011.38.8413. [DOI] [PubMed] [Google Scholar]
- 12.Paez-Ribes M, Allen E, Hudock J, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15(3):220–31. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freije JM, Balbin M, Pendas AM, Sanchez LM, Puente XS, Lopez-Otin C. Matrix metalloproteinases and tumor progression. Adv Exp Med Biol. 2003;532:91–107. doi: 10.1007/978-1-4615-0081-0_9. [DOI] [PubMed] [Google Scholar]
- 14.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141(1):52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coticchia CM, Curatolo AS, Zurakowski D, et al. Urinary MMP-2 and MMP-9 predict the presence of ovarian cancer in women with normal CA125 levels. Gynecol Oncol. 2011 doi: 10.1016/j.ygyno.2011.07.034. [DOI] [PubMed] [Google Scholar]
- 16.Boire A, Covic L, Agarwal A, Jacques S, Sherifi S, Kuliopulos A. PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell. 2005;120(3):303–13. doi: 10.1016/j.cell.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 17.Belotti D, Paganoni P, Manenti L, et al. Matrix metalloproteinases (MMP9 and MMP2) induce the release of vascular endothelial growth factor (VEGF) by ovarian carcinoma cells: implications for ascites formation. Cancer Res. 2003;63(17):5224–9. [PubMed] [Google Scholar]
- 18.Barbolina MV, Adley BP, Ariztia EV, Liu Y, Stack MS. Microenvironmental regulation of membrane type 1 matrix metalloproteinase activity in ovarian carcinoma cells via collagen-induced EGR1 expression. J Biol Chem. 2007;282(7):4924–31. doi: 10.1074/jbc.M608428200. [DOI] [PubMed] [Google Scholar]
- 19.Kamat AA, Fletcher M, Gruman LM, et al. The clinical relevance of stromal matrix metalloproteinase expression in ovarian cancer. Clin Cancer Res. 2006;12(6):1707–14. doi: 10.1158/1078-0432.CCR-05-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sato H, Takino T, Okada Y, et al. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994;370(6484):61–5. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- 21.Petrella BL, Lohi J, Brinckerhoff CE. Identification of membrane type-1 matrix metalloproteinase as a target of hypoxia-inducible factor-2 alpha in von Hippel-Lindau renal cell carcinoma. Oncogene. 2005;24(6):1043–52. doi: 10.1038/sj.onc.1208305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van de Vijver MJ, He YD, van’t Veer LJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347(25):1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 23.Moss NM, Barbolina MV, Liu Y, Sun L, Munshi HG, Stack MS. Ovarian cancer cell detachment and multicellular aggregate formation are regulated by membrane type 1 matrix metalloproteinase: a potential role in I.p. metastatic dissemination. Cancer Res. 2009;69(17):7121–9. doi: 10.1158/0008-5472.CAN-08-4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hotary KB, Allen ED, Brooks PC, Datta NS, Long MW, Weiss SJ. Membrane type I matrix metalloproteinase usurps tumor growth control imposed by the three-dimensional extracellular matrix. Cell. 2003;114(1):33–45. doi: 10.1016/s0092-8674(03)00513-0. [DOI] [PubMed] [Google Scholar]
- 25.Wang P, Nie J, Pei D. The hemopexin domain of membrane-type matrix metalloproteinase-1 (MT1-MMP) Is not required for its activation of proMMP2 on cell surface but is essential for MT1-MMP-mediated invasion in three-dimensional type I collagen. J Biol Chem. 2004;279(49):51148–55. doi: 10.1074/jbc.M409074200. [DOI] [PubMed] [Google Scholar]
- 26.Kenny HA, Kaur S, Coussens LM, Lengyel E. The initial steps of ovarian cancer cell metastasis are mediated by MMP-2 cleavage of vitronectin and fibronectin. J Clin Invest. 2008;118(4):1367–79. doi: 10.1172/JCI33775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agarwal A, Tressel SL, Kaimal R, et al. Identification of a metalloprotease-chemokine signaling system in the ovarian cancer microenvironment: implications for antiangiogenic therapy. Cancer Res. 2010;70(14):5880–90. doi: 10.1158/0008-5472.CAN-09-4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Devy L, Huang L, Naa L, et al. Selective inhibition of matrix metalloproteinase-14 blocks tumor growth, invasion, and angiogenesis. Cancer Res. 2009;69(4):1517–26. doi: 10.1158/0008-5472.CAN-08-3255. [DOI] [PubMed] [Google Scholar]
- 29.Overall CM, Kleifeld O. Tumour microenvironment - opinion: validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat Rev Cancer. 2006;6(3):227–39. doi: 10.1038/nrc1821. [DOI] [PubMed] [Google Scholar]
- 30.Roy R, Yang J, Moses MA. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J Clin Oncol. 2009;27(31):5287–97. doi: 10.1200/JCO.2009.23.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drummond AH, Beckett P, Brown PD, et al. Preclinical and clinical studies of MMP inhibitors in cancer. Ann N Y Acad Sci. 1999;878:228–35. doi: 10.1111/j.1749-6632.1999.tb07688.x. [DOI] [PubMed] [Google Scholar]
- 32.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295(5564):2387–92. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 33.Bergers G, Javaherian K, Lo KM, Folkman J, Hanahan D. Effects of angiogenesis inhibitors on multistage carcinogenesis in mice. Science. 1999;284(5415):808–12. doi: 10.1126/science.284.5415.808. [DOI] [PubMed] [Google Scholar]
- 34.Hernandez-Barrantes S, Toth M, Bernardo MM, et al. Binding of active (57 kDa) membrane type 1-matrix metalloproteinase (MT1-MMP) to tissue inhibitor of metalloproteinase (TIMP)-2 regulates MT1-MMP processing and pro-MMP-2 activation. J Biol Chem. 2000;275(16):12080–9. doi: 10.1074/jbc.275.16.12080. [DOI] [PubMed] [Google Scholar]
- 35.Kajita M, Itoh Y, Chiba T, et al. Membrane-type 1 matrix metalloproteinase cleaves CD44 and promotes cell migration. J Cell Biol. 2001;153(5):893–904. doi: 10.1083/jcb.153.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ueda J, Kajita M, Suenaga N, Fujii K, Seiki M. Sequence-specific silencing of MT1-MMP expression suppresses tumor cell migration and invasion: importance of MT1-MMP as a therapeutic target for invasive tumors. Oncogene. 2003;22(54):8716–22. doi: 10.1038/sj.onc.1206962. [DOI] [PubMed] [Google Scholar]
- 37.Littlepage LE, Sternlicht MD, Rougier N, et al. Matrix metalloproteinases contribute distinct roles in neuroendocrine prostate carcinogenesis, metastasis, and angiogenesis progression. Cancer Res. 2010;70(6):2224–34. doi: 10.1158/0008-5472.CAN-09-3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bissett D, O’Byrne KJ, von Pawel J, et al. Phase III study of matrix metalloproteinase inhibitor prinomastat in non-small-cell lung cancer. J Clin Oncol. 2005;23(4):842–9. doi: 10.1200/JCO.2005.03.170. [DOI] [PubMed] [Google Scholar]
- 39.Chang MC, Chen CA, Chen PJ, et al. Mesothelin enhances invasion of ovarian cancer by inducing MMP-7 through MAPK/ERK and JNK pathways. Biochem J. 2012;442(2):293–302. doi: 10.1042/BJ20110282. [DOI] [PubMed] [Google Scholar]
- 40.Balbin M, Fueyo A, Tester AM, et al. Loss of collagenase-2 confers increased skin tumor susceptibility to male mice. Nat Genet. 2003;35(3):252–7. doi: 10.1038/ng1249. [DOI] [PubMed] [Google Scholar]
- 41.Maskos K, Bode W. Structural basis of matrix metalloproteinases and tissue inhibitors of metalloproteinases. Mol Biotechnol. 2003;25(3):241–66. doi: 10.1385/MB:25:3:241. [DOI] [PubMed] [Google Scholar]
- 42.Even-Ram S, Yamada KM. Cell migration in 3D matrix. Curr Opin Cell Biol. 2005;17(5):524–32. doi: 10.1016/j.ceb.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 43.Chun TH, Sabeh F, Ota I, et al. MT1-MMP-dependent neovessel formation within the confines of the three-dimensional extracellular matrix. J Cell Biol. 2004;167(4):757–67. doi: 10.1083/jcb.200405001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holmbeck K, Bianco P, Caterina J, et al. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99(1):81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- 45.Sounni NE, Roghi C, Chabottaux V, et al. Up-regulation of vascular endothelial growth factor-A by active membrane-type 1 matrix metalloproteinase through activation of Src-tyrosine kinases. J Biol Chem. 2004;279(14):13564–74. doi: 10.1074/jbc.M307688200. [DOI] [PubMed] [Google Scholar]
- 46.Bergers G, Brekken R, McMahon G, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2(10):737–44. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Toth M, Chvyrkova I, Bernardo MM, Hernandez-Barrantes S, Fridman R. Pro-MMP-9 activation by the MT1-MMP/MMP-2 axis and MMP-3: role of TIMP-2 and plasma membranes. Biochem Biophys Res Commun. 2003;308(2):386–95. doi: 10.1016/s0006-291x(03)01405-0. [DOI] [PubMed] [Google Scholar]
- 48.Spannuth WA, Sood AK, Coleman RL. Angiogenesis as a strategic target for ovarian cancer therapy. Nat Clin Pract Oncol. 2008;5(4):194–204. doi: 10.1038/ncponc1051. [DOI] [PubMed] [Google Scholar]
- 49.Pepper MS. Role of the matrix metalloproteinase and plasminogen activator-plasmin systems in angiogenesis. Arterioscler Thromb Vasc Biol. 2001;21(7):1104–17. doi: 10.1161/hq0701.093685. [DOI] [PubMed] [Google Scholar]
- 50.Kunkel P, Ulbricht U, Bohlen P, et al. Inhibition of glioma angiogenesis and growth in vivo by systemic treatment with a monoclonal antibody against vascular endothelial growth factor receptor-2. Cancer Res. 2001;61(18):6624–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.