Abstract
Though much attention has been given to the neural structures that underlie the long-term consolidation of contextual memories, little is known about the mechanisms responsible for the maintenance of memory precision. Here, we demonstrate a rapid time-dependent decline in memory precision in GABAB(1a) receptor knockout mice. First, we show that GABAB(1a) receptors are required for the maintenance, but not encoding, of a precise fear memory. We then demonstrate that GABAB(1a) receptors are required for the maintenance, but not encoding, of spatial memories. Our findings suggest that GABA-mediated presynaptic inhibition regulates the maintenance of memory precision as a function of memory age.
Individuals suffering from post-traumatic stress disorder (PTSD) often come to re-experience the traumatic event in the presence of stimuli that were not present at the time of the trauma but bear some similarity to that event. Furthermore, this generalization of fear to neutral cues increases over time, so that a multiplicity of stimuli serves as a reminder for the original trauma (Riccio et al. 1984; Zhou and Riccio 1996; Wiltgen and Silva 2007; Lissek et al. 2008; Jasnow et al. 2012). In short, the fear memory becomes imprecise and is reactivated by cues not present at the time of learning. Using contextual fear conditioning and novel object training in rodents, the current study directly investigated the precision of both a fear and a nonfear memory in order to gain an understanding of how memory precision develops and is maintained over time.
Contextual fear conditioning involves pairing a novel context (conditioned stimulus) with footshock (unconditioned stimulus) that serves to condition fear (assessed as freezing behavior) to that context. Many studies have demonstrated that shifting contextual cues shortly after contextual fear conditioning results in reduced freezing (i.e., the context shift effect). At early time points, rodents exhibit a contextually precise memory and can discriminate between the training and a neutral context. However, as the retention interval between training and testing increases, fear memories become less precise with animals exhibiting fear responses to neutral contexts (context specificity is lost 14- to 36-d post-training) (Zhou and Riccio 1996; Wiltgen and Silva 2007; Lissek et al. 2008; Jasnow et al. 2012). Though a significant amount of neurobehavioral research has investigated the mechanisms underlying the long-term consolidation of a contextual fear memory, very little is known about what underlies this loss in memory precision, or how memory precision is maintained over time.
Recent evidence suggests that GABAB receptor-mediated presynaptic inhibition may play a role in stimulus discrimination. GABAB receptors are G protein-coupled receptors that exist as heterodimers containing two subunits, GABAB1 and GABAB2 (Gassmann and Bettler 2012). The GABAB1 receptor exists in two isoforms, GABAB(1a) and GABAB(1b), with the isoforms being localized to presynaptic and postsynaptic terminals, respectively (Kulik et al. 2003; Vigot et al. 2006; Gassmann and Bettler 2012). Recently, GABAB(1a) receptors have been shown to play a role in cued-fear discrimination and novel object discrimination (Shaban et al. 2006; Vigot et al. 2006; Jacobson et al. 2007). Shaban et al. (2006) discovered that mice lacking presynaptic GABAB(1a) receptors exhibited increased nonassociative cortico-amygdala long-term potentiation (LTP) and impaired cued fear discrimination (i.e., animals could not distinguish between a fearful stimulus and a safe stimulus). These effects were specific to the loss of presynaptic inhibition. Deletion of postsynaptic GABAB(1b) receptors did not alter cortico-amygdala LTP or cued discrimination. However, in this study the mechanisms investigated were limited to afferent inputs to the lateral amygdala. In addition, testing at early time points following training were not examined. Thus, we sought to determine whether the loss of GABA-mediated presynaptic inhibition plays an integral role in hippocampal-dependent memory precision as a function of time.
To test the involvement of GABAB(1a) receptors in context memory precision, we used mice lacking the GABAB(1a) isoform (GABAB(1a)−/−). The knockout mice were created on a BALB/c background using the procedure outlined in Vigot et al. (2006). GABAB(1a) knockout mice expressed a global deletion of the presynaptic GABAB1 receptor, while WT mice exhibited normal expression of the receptor and were littermates of the GABAB(1a)−/− animals on a BALB/c background. All animals underwent fear conditioning in the training context (Context A) during which five 1-sec 0.8-mA shocks were delivered with a 90-sec intertrial interval. Animals were tested for conditioned fear in either the training context (Context A) or a novel context (Context B) 2 h, 24 h, or 5 d following training. The sample size was six animals per context group per genotype at each time point (a total of 36 wild-type [WT] and 36 GABAB(1a)−/− mice).
All context conditioning procedures were performed in four identical conditioning chambers (7-in W × 7-in D × 12-in H) containing two Plexiglas walls (front and back) and two aluminum sidewalls and a stainless-steel shock-grid floor (Coulbourn Instruments). All fear conditioning chambers were placed inside sound-attenuating chambers and contained cameras mounted on top of each chamber that recorded training and testing sessions. Context A consisted of the context chamber (two Plexiglas and two aluminum walls), with polka dots on the back wall, white noise projected through a wall speaker, dim illumination (house light), and the grid floors were cleaned with 70% ethanol. Context B consisted of identical chambers, minus the polka dots, was not illuminated, and contained no white noise. A flat, brown Plexiglas floor replaced the grid floor and was washed with 70% Quatricide.
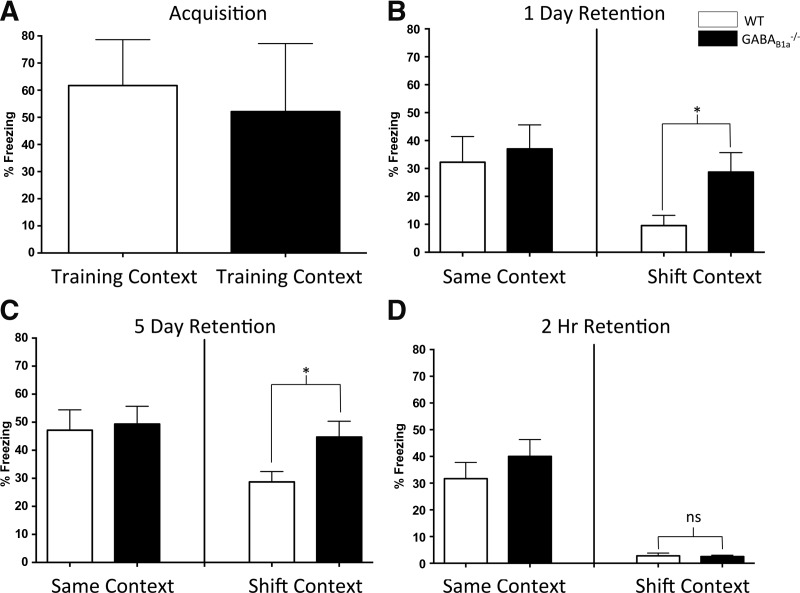
A factorial analysis of variance (ANOVA) was conducted on freezing scores, defined as the absence of all movement minus that associated with breathing, at 24 h following training and yielded nonsignificant main effects of context (F(1,22) = 2.93, ns) and genotype (F(1,22) = 0.75, ns) as well as a nonsignificant interaction between context and genotype (F(1,22) = 0.49, ns). Although the ANOVA yielded a nonsignificant interaction, we were interested in differences in context discrimination between the WT animals and the GABAB(1a)−/− animals. Therefore, we chose to conduct independent samples t-tests to conduct direct comparisons between WT and GABAB(1a)−/− animals within the contexts. At 24-h post-training, wild-type mice exhibited significantly more freezing in Context A than in Context B (t(10) = 3.04, P < 0.05), illustrating normal context discrimination (i.e., the context shift was present). However, GABAB(1a)−/− mice exhibited a loss of context discrimination, as they exhibited significantly higher levels of freezing in Context B than wild-type mice (t(12) = −2.19, P < 0.05) (Fig. 1C). This lack of context discrimination cannot be accounted for by a difference in acquisition strength between the two genotypes. Both wild-type and GABAB(1a)−/− mice exhibited equivalent levels of freezing during fear training (t(24) = 1.16, P > 0.20) (Fig. 1A).
Figure 1.
Presynaptic GABAB(1a) receptors are required for the maintenance, but not initial encoding, of a contextually precise memory. (A) Average freezing levels during the last two shock presentations of contextual fear conditioning for the 24-h retention group. Wild-type (WT) and GABAB(1a)−/− mice exhibit equivalent levels of fear acquisition following training. Acquisition data for the 5-d and 2-h retention groups were identical and therefore not shown. (B) GABAB(1a)−/− mice freeze at equivalently high levels compared to WT mice when tested in the Same context (Context A) 24 h following fear conditioning. When tested in the Shift context (Context B), GABAB(1a)−/− mice exhibit significantly more freezing (P < 0.05) than WT mice. Thus, WT mice exhibit a normal contextually precise memory, whereas GABAB(1a)−/− mice exhibit an imprecise context fear memory. (C) At 5-d post-training, WT animals retain a contextually precise memory (significantly lower levels of freezing in the Shift context compared to the Same context). GABAB(1a)−/− mice exhibit a contextually imprecise memory as evidenced by significantly more freezing in the Shift context compared to WT animals. (D) Both WT and GABAB(1a)−/− mice exhibit a contextually precise memory when tested in the Shift context 2-h post-training. Asterisks denote a statistically significant difference. (*) P < 0.05.
A factorial ANOVA was conducted on freezing behavior at 5-d post-training and yielded nonsignificant main effects of context (F(1,26) = 3.71, P = 0.065) and genotype (F(1,26) = 2.31, ns) as well as a nonsignificant interaction between context and genotype (F(1,26) = 1.32, ns). An independent samples t-test indicates that the knockout-induced loss of discrimination was relatively stable, as GABAB(1a)−/− mice exhibited a complete loss of discrimination at 5-d post-training compared to wild-type animals that continued to exhibit lower freezing levels in the novel context (i.e., more context discrimination) (t(12) = −2.47, P < 0.05) (Fig. 1D).
These data suggest that presynaptic GABAB receptors are required for the discrimination of contextual cues and support earlier findings that these receptors are important in the precision of fear memory (Shaban et al. 2006). However, since GABAB(1a)−/− mice failed to discriminate at 1-d or 5-d post-fear conditioning, it is plausible that the knockout of these presynaptic receptors resulted in an inability to perceive differences in the contextual stimuli. Therefore, we conducted a 2-h post-training test to determine whether GABAB(1a)−/− mice could perceive differences in the two contexts at an early time point. A factorial ANOVA was conducted on freezing behavior at 2-h post-training and yielded a significant main effect of context (F(1,34) = 35.85, P < 0.001) and a nonsignificant main effect of genotype (F(1,34) = 0.33, ns) as well as a nonsignificant interaction between context and genotype (F(1,34) = 0.53, ns). At 2-h post-training, GABAB(1a)−/− mice exhibited normal context discrimination, as they showed similar low levels of freezing behavior compared to WT mice in Context B (t(19) = 0.23, ns) (Fig. 1B). These data suggest that mice lacking GABAB(1a) receptors can initially discriminate between contextual cues, indicating a precise memory at an early time point. Previous electrophysiological and behavioral data suggested that the loss of GABA-mediated presynaptic inhibition shifted the threshold for generalization. If this were the case, however, GABAB(1a)−/− mice would not be able to distinguish contexts at any time point. Rather, our data suggest that GABA-mediated presynaptic inhibition is more likely involved in the maintenance, but not the acquisition or consolidation, of contextually precise memories.
The surprising finding that GABAB(1a)−/− mice can initially discriminate between contexts led us to investigate the time course of the involvement of these receptors in additional discrimination tasks. It has been previously reported that animals lacking presynaptic GABAB1 receptors were unable to discriminate objects in a novel object recognition task (Vigot et al. 2006; Jacobson et al. 2007). In an attempt to replicate and extend this finding, we placed both GABAB(1a)−/− (n = 15) and WT (n = 15) mice in an open field arena on three consecutive days with two identical objects (200-mL glass beakers) and allowed animals to explore for 10 min. Two hours following the third and final day of object exposure, all animals were placed back into the arena for 3 min in the presence of one familiar object and one novel object (plastic funnel) (novel object recognition test). The location of the novel object was counterbalanced so that half of the animals were presented with the novel object on the right side of the apparatus and half of the animals saw the novel object on the left side of the apparatus. Following the 3-min novel object recognition test, the animals were removed from the arena, the arena was cleaned, and the animals were placed back into the arena for 3 min in the presence of two familiar objects (novel object location test). One of the familiar objects was moved to a new location within the arena. The object that was moved was counterbalanced so that half of the animals saw the left object moved and half of the animals saw the right object moved. The order of testing was counterbalanced such that half of the animals received the novel object recognition test first and half of the animals received the novel object location test first.
Twenty-four hours following the third and final day of object exposure, all animals were placed back into the arena for 5 min for an additional novel object recognition test as described above. All animals also received an additional novel object location test for 5 min as described above. Again, the order of testing was counterbalanced such that half of the animals received the novel object recognition test first and half received the novel object location test first. The amount of time spent exploring the novel object/location and familiar object/location was expressed as a discrimination index (DI) using Stefanko et al.’s (2009) equation, DI = (tnovel − tfamiliar)/(tnovel + tfamiliar) × 100%. Discrimination indexes were calculated for each animal and averaged for each group. Higher values on the DI indicate more time spent exploring the novel object/location. The novel object recognition and location procedures were conducted in four identical open field arenas (46 cm × 46 cm × 39 cm) containing four Plexiglas sidewalls (Coulbourn Instruments). The chambers were placed in a dimly lit room (red lighting) with cameras (Coulbourn Instruments) mounted on the wall above each chamber to record activity. Chamber floors were cleaned with 70% ethanol to remove potential olfactory cues left by other animals.
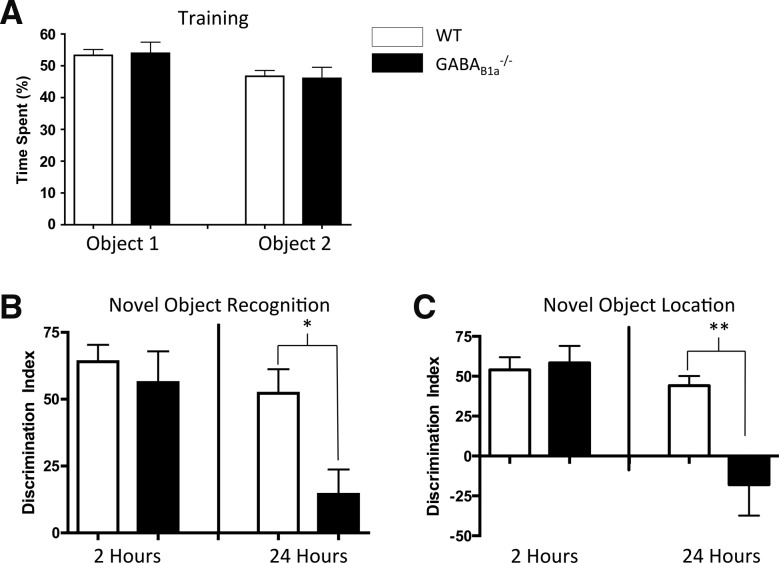
We replicated the finding from Vigot et al. (2006) showing that GABAB(1a)−/− mice failed to discriminate between a familiar and novel object when tested for novel object discrimination 24 h following training (a 2 (genotype) × 2 (retention interval) repeated measures analysis of variance (RM ANOVA) for discrimination index (F(1,28) = 4.32, P < 0.05) (Fig. 2B) (RM ANOVA was chosen given the within-subjects design of the two behavior tests). Again, this effect cannot be accounted for by differences in acquisition (i.e., time spent exploring both objects during training/habituation) as both wild-type and GABAB(1a)−/− mice exhibited equivalent percentages of time spent exploring each object (F(1,26) = 0.08, ns) (Fig. 2A). Further, we show that mice lacking GABAB(1a) receptors are also impaired in a novel object location task 24 h following training (a 2 [genotype] × 2 [retention interval] RM ANOVA for discrimination index [F(1,28) = 11.09, P < 0.01] [Fig. 2C]). However, consistent with the contextual fear data, GABAB(1a)−/− mice show normal discrimination 2 h after training in both the novel object recognition and location tasks (Fig. 2B,C). These data again suggest that GABAB(1a) receptors are required for the maintenance of discrimination between stimuli, but not the initiation of discrimination.
Figure 2.
Presynaptic GABAB(1a) receptors are required for the maintenance, but not initial encoding, of an object recognition and location memory. (A) Wild-type (WT) and GABAB(1a)−/− mice were habituated to the open field arena and then exposed to two identical objects. Both groups of animals spent equal amounts of time exploring both objects during training. (B) WT and GABAB(1a)−/− mice exhibit equivalently high preference for the novel object when tested for novel object recognition 2 h following the last training session. When tested 24-h post-training, GABAB(1a)−/− mice exhibit impaired discrimination between the novel and familiar object compared to WT animals (P < 0.05). (C) WT and GABAB(1a)−/− mice exhibit equivalently high preference for the object moved to the novel location when tested for novel object location 2 h following the last training session. However, GABAB(1a)−/− mice exhibit impaired discrimination between the novel and familiar object location compared to WT animals (P < 0.05) when tested 24 h following training. Data in B and C are presented using a discrimination index. Asterisks denote a statistically significant difference. (*) P < 0.05, (**) P < 0.01).
Here we have demonstrated that mice lacking presynaptic GABAB(1a) receptors exhibit impaired discrimination of context, novel objects, and object location at 24 h following training, but not 2-h post-training. These data suggest that GABA-mediated presynaptic inhibition is likely involved in the maintenance of a precise memory. However, the loss of GABAB(1a) receptors had no effect on the acquisition or initial consolidation of the precise memory. These results provide novel insight into a potential synaptic mechanism involved in the maintenance of memory specificity.
Previous physiological and behavioral work with GABAB(1a) knockout mice suggested that the loss of presynaptic inhibition shifts the threshold for fear generalization (Shaban et al. 2006). When given a cued fear discrimination task, GABAB(1a) knockout animals trained with a 0.6-mA amplitude footshock were able to discriminate between the CS+ and CS− cues. However, when the shock intensity was increased to 0.9 mA, GABAB(1a)−/− mice generalized fear responses to the CS−, which is in contrast to the WT mice that maintained their discrimination at both shock intensities. Thus, it was concluded that the lack of presynaptic inhibition in GABAB(1a)−/− mice resulted in a shift in the threshold for generalization of fear responses to lower US intensities (Shaban et al. 2006). However, if this were the case, it would be expected that GABAB(1a) knockout animals trained using high shock intensities would fail to discriminate contextual cues at any post-training interval. In the present experiments, we observed a rapid time-dependent decline in memory precision in which GABAB(1a) knockout animals were able to discriminate between contexts at 2-h post-training, but not at 1 or 5 d (note that in WT rodents, this decline in memory precision occurs over 14–36 d [Zhou and Riccio 1996; Wiltgen and Silva 2007]). Furthermore, a similar rapid time-dependent decline in memory precision was observed using nonfear tasks, providing additional support against a threshold shift phenomenon and suggesting a more integral role in regulating the precision of contextual and spatial memories. Taken together, our data suggest that presynaptic inhibition via GABAB(1a) receptors helps maintain the discrimination and/or the precision of the memory, but are not required for the initial encoding of this information.
The rapid decline in memory precision observed in the present study may be the result of disruption of consolidation specifically within hippocampal circuits. This is because we observe a loss of memory precision in both the contextual fear task as well as novel object recognition and novel object location tasks in GABAB(1a) knockout mice. Both of these novel object tasks are independent of fear responses and rely heavily on hippocampal functioning (Best and Orr 1973; Lorenzini et al. 1996; Ambrogi Lorenzini et al. 1997; Baarendse et al. 2007). Moreover, dysfunction of presynaptic inhibition and synaptic potentiation within the mossy fiber pathway and Schaffer commissural pathway is well documented in these mice (Vigot et al. 2006; Guetg et al. 2009; Gassmann and Bettler 2012), as is the importance of the mossy fiber pathway in memory precision (Ruediger et al. 2011; Jasnow et al. 2012). Thus, the loss of context specificity over time may result from a decay of the hippocampal memory trace (Rudy 2005; Biedenkapp and Rudy 2007). In other words, the quality of the contextual memory decays, while the fear or object trace remains strong, and becomes reactivated by novel contextual stimuli. GABAB(1a) receptors regulating presynaptic inhibition may be necessary within the hippocampus for retaining information about context specificity. The lack of presynaptic inhibition within hippocampal circuits in GABAB(1a) knockout mice would then cause context specificity to rapidly decay, as we observed in the present study. Indeed, recent work demonstrates that feed-forward inhibition within the hippocampus is necessary for maintaining contextually precise memories (Ruediger et al. 2011). Specifically, it was discovered that a learning event, such as context fear conditioning and spatial learning, results in an increase in mossy fiber filopodial contacts onto both interneurons (feed-forward inhibition) and excitatory pyramidal neurons (feed-forward excitation) within the CA3 region of the hippocampus. Increased feed-forward inhibition was associated with expression of a contextually precise memory and the time-dependent decline in feed-forward inhibitory synapses was correlated with the time-dependent increase in fear generalization (i.e., a loss of memory precision). A lack of inhibition on feed-forward excitatory contacts may result in enhanced excitatory drive within the hippocampus and negate the effects of feed-forward inhibition within the CA3 region of the hippocampus, resulting in a rapid decline of memory precision.
Although data from the current study suggest that the GABA-mediated loss of precision may be due to hippocampal dysfunction, we cannot rule out the possibility that our behavioral results are due to the loss of GABA receptors in other neural structures. Because the experimental animals used in the current study expressed a global knock out of the GABAB(1a) receptor, the memory impairments may be the result of disruption of other neural structures known to be involved in the consolidation and expression of a context fear memory (e.g., the amygdala and prefrontal cortex). However, we found the same loss of precision in the novel object/location procedures, which are spatial tasks dependent upon the hippocampus and less so on the amygdala. These data suggest that the loss of memory precision in these tasks was unlikely due to impaired amygdala functioning. Alternatively, the loss of precision may be due to impaired prefrontal cortex functioning or impaired hippocampal–prefrontal cortex interaction. Additional experiments, which focally delete or block GABAB(1a) receptors, will help determine the precise location and neural circuit necessary for regulating memory precision. However, given the pattern of behavioral results we found in the present experiments, we would expect disruption of GABAB(1a) receptors within the hippocampus to cause a similar time-dependent loss of memory precision.
The current data provide a potential mechanism for the maintenance of memory precision prior to the systems consolidation of that memory. Hippocampal-dependent memory, such as memory for context, undergoes a transfer from short-term hippocampal stores to more long-term cortical stores, a process known as systems consolidation (Kim and Fanselow 1992; Anagnostaras et al. 2001; Wiltgen 2006; Jasnow et al. 2012). It has been proposed that this transfer involves a transformation of the original memory trace from a context-specific hippocampal-dependent memory to a hippocampal-independent cortical memory that lacks context specificity (Winocur et al. 2009). However, prior to this systems consolidation, the mechanisms that maintain context-specificity are not well understood. Data from the current study, along with Ruediger et al.’s (2011) study, provide a potential synaptic mechanism that may, in part, maintain context-specificity when the memory is still hippocampus-dependent. The rapid time-dependent loss of memory precision observed in the present study suggests that presynaptic inhibition may play a key role in preserving or maintaining the precision of a particular memory trace before that memory trace undergoes systems consolidation.
In conclusion, GABAB(1a) receptors appear to be required for the maintenance of a contextually precise memory, but not for the initial encoding of that memory. Mice lacking these presynaptic receptors demonstrated an initially precise contextual and spatial memory that rapidly degraded within 24 h. These findings provide novel insight into one of the synaptic mechanisms underlying the preservation of memory precision and provide a potential mechanism for the transformation of initially precise contextual memories into those that lack context specificity.
Acknowledgments
We thank Dr. Bernhard Bettler and Dr. Martin Gassmann from the Pharmazentrum, Department of Clinical-Biological Sciences, University of Basel for the generous donation of the GABAB(1a) knockout and wild-type mice. This work was partially supported through a Farris Family Foundation Grant.
References
- Ambrogi Lorenzini CG, Baldi E, Bucherelli C, Sacchetti B, Tassoni G 1997. Role of ventral hippocampus in acquisition, consolidation and retrieval of rat's passive avoidance response memory trace. Brain Res 768: 242–248 [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Gale GD, Fanselow MS 2001. Hippocampus and contextual fear conditioning: Recent controversies and advances. Hippocampus 11: 8–17 [DOI] [PubMed] [Google Scholar]
- Baarendse PJJ, van Grootheest G, Jansen RF, Pieneman AW, Ögren SO, Verhage M, Stiedl O 2007. Differential involvement of the dorsal hippocampus in passive avoidance in C57bl/6J and DBA/2J mice. Hippocampus 18: 11–19 [DOI] [PubMed] [Google Scholar]
- Best PJ, Orr J 1973. Effects of hippocampal lesions on passive avoidance and taste aversion conditioning. Physiol Behav 10: 193–196 [DOI] [PubMed] [Google Scholar]
- Biedenkapp JC, Rudy JW 2007. Context preexposure prevents forgetting of a contextual fear memory: Implication for regional changes in brain activation patterns associated with recent and remote memory tests. Learn Mem 14: 200–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann M, Bettler B 2012. Regulation of neuronal GABAB receptor functions by subunit composition. Nat Rev Neurosci 13: 380–394 [DOI] [PubMed] [Google Scholar]
- Guetg N, Seddik R, Vigot R, Turecek R, Gassmann M, Vogt KE, Brauner-Osborne H, Shigemoto R, Kretz O, Frotscher M, et al. 2009. The GABAB1a isoform mediates heterosynaptic depression at hippocampal mossy fiber synapses. J Neurosci 29: 1414–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson LH, Kelly PH, Bettler B, Kaupmann K, Cryan JF 2007. Specific roles of GABAB(1) receptor isoforms in cognition. Behav Brain Res 181: 158–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasnow AM, Cullen PK, Riccio DC 2012. Remembering another aspect of forgetting. Front Psychol 3: 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS 1992. Modality-specific retrograde amnesia of fear. Science 256: 675–677 [DOI] [PubMed] [Google Scholar]
- Kulik A, Vida I, Luján R, Haas CA, López-Bendito G, Shigemoto R, Frotscher M 2003. Subcellular localization of metabotropic GABAB receptor subunits GABAB1a/b and GABAB2 in the rat hippocampus. J Neurosci 23: 11026–11035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Biggs AL, Rabin SJ, Cornwell BR, Alvarez RP, Pine DS, Grillon C 2008. Generalization of conditioned fear-potentiated startle in humans: Experimental validation and clinical relevance. Behav Res Ther 46: 678–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzini CA, Baldi E, Bucherelli C, Sacchetti B, Tassoni G 1996. Role of dorsal hippocampus in acquisition, consolidation and retrieval of rat's passive avoidance response: A tetrodotoxin functional inactivation study. Brain Res 730: 32–39 [DOI] [PubMed] [Google Scholar]
- Riccio DC, Richardson R, Ebner DL 1984. Memory retrieval deficits based upon altered contextual cues: A paradox. Psychol Bull 96: 152–165 [PubMed] [Google Scholar]
- Rudy JW 2005. Prefrontal cortex and the organization of recent and remote memories: An alternative view. Learn Mem 12: 445–446 [DOI] [PubMed] [Google Scholar]
- Ruediger S, Vittori C, Bednarek E, Genoud C, Strata P, Sacchetti B, Caroni P 2011. Learning-related feedforward inhibitory connectivity growth required for memory precision. Nature 473: 514–518 [DOI] [PubMed] [Google Scholar]
- Shaban H, Humeau Y, Herry C, Cassasus G, Shigemoto R, Ciocchi S, Barbieri S, van der Putten H, Kaupmann K, Bettler B, et al. 2006. Generalization of amygdala LTP and conditioned fear in the absence of presynaptic inhibition. Nat Neurosci 9: 1028–1035 [DOI] [PubMed] [Google Scholar]
- Stefanko DP, Barrett RM, Ly AR, Reolon GK, Wood MA 2009. Modulation of long- term memory for object recognition via HDAC inhibition. Proc Natl Acad Sci 106: 9447–9452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigot R, Barbieri S, Bräuner-Osborne H, Turecek R, Shigemoto R, Zhang Y-P, Luján R, Jacobson LH, Biermann B, Fritschy J-M 2006. Differential compartmentalization and distinct functions of GABAB receptor variants. Neuron 50: 589–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltgen BJ 2006. Context fear learning in the absence of the hippocampus. J Neurosci 26: 5484–5491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltgen BJ, Silva AJ 2007. Memory for context becomes less specific with time. Learn Mem 14: 313–317 [DOI] [PubMed] [Google Scholar]
- Winocur G, Frankland PW, Sekeres M, Fogel S, Moscovitch M 2009. Changes in context-specificity during memory reconsolidation: Selective effects of hippocampal lesions. Learn Mem 16: 722–729 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Riccio DC 1996. Manipulation of components of context: The context shift effect and forgetting of stimulus attributes. Learn Motiv 27: 400–407 [DOI] [PubMed] [Google Scholar]