Summary
Treatment with cabozantinib, an inhibitor of MET and VEGFR2 signaling, has demonstrated clinical benefit in early trials in men with metastatic prostate cancer. Preclinical evidence suggests that cabozantinib can kill cancer cell seeds while disrupting angiogenesis and stromal cells in the metastatic soil.
In this issue of Clinical Cancer Research, Dai and colleagues provide evidence that the tyrosine kinase inhibitor cabozantinib impacts both cancer cells and microenvironment in bone metastasis models of prostate cancer [1]. Cabozantinib is an orally bioavailable inhibitor of MET, VEGFR2, RET, and several other tyrosine kinases. The early clinical experience with cabozantinib in patients with metastatic castration-resistant prostate cancer (mCRPC) demonstrated marked improvements in bone scans in subjects with bone metastases [2]. The bone scan improvement was not limited to an improvement in imaging; subjects reported clinical benefits with improved pain perception and decreased narcotic pain medication requirements. Bone scans are an indirect measure of tumor activity because they detect deposition of the technetium tracer by osteoblasts. It is unclear whether cabozantinib leads to clinical benefit by impacting prostate cancer cell viability, osteoblast viability, perfusion of bone tumor deposits, or a combination of effects.
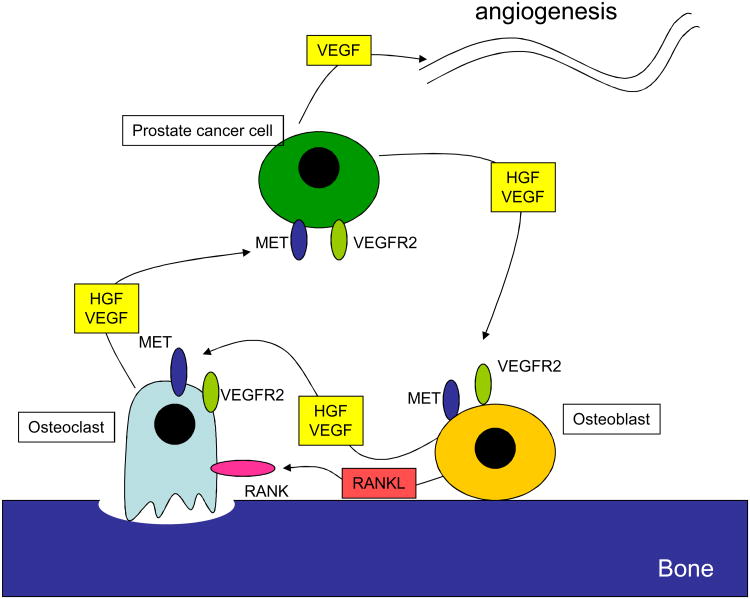
MET and VEGFR2 signaling pathways are active in both bone turnover and prostate cancer progression (Figure 1) (reviewed in [3]). Bone remodeling is a continuous process balancing bone-forming activity by osteoblasts with the resorptive activity of osteoclasts. The balance of bone turnover is mediated by receptor activator of nuclear factor-kappa-B (RANK) signaling between osteoblasts and osteoclasts. Additionally, MET and VEGFR and their respective ligands, hepatocyte growth factor (HGF) and VEGF, are expressed by both osteoblasts and osteoclasts. HGF/MET and VEGF/VEGFR signaling mediate both autocrine and paracrine roles in the normal activity and survival of osteoblasts and osteoclasts. MET and VEGFR signaling also play important roles in prostate cancer progression and bone metastasis. Increased MET and HGF expression in prostate cancer cells correlate with disease recurrence and metastasis, with highest levels in bone metastases. VEGFR signaling is critical for angiogenesis, a key step in tumor growth. Higher VEGFR2 levels are expressed in high-grade prostate cancers. Prostate cancer cells also express VEGF, unlike their benign counterparts. Higher levels of VEGF are independent predictors of worse overall survival (OS) in men with mCRPC. The activities of MET and VEGFR signaling in bone turnover and metastasis provide a strong rationale for dual inhibition of these pathways as a therapeutic strategy in men with mCRPC.
Figure 1.
MET and VEGFR signaling in prostate cancer metastasis to bone. Prostate cancer cells, osteoblasts, and osteoclasts express the ligands and receptors HGF/MET and VEGF/VEGFR2, which may thus mediate autocrine and paracrine signaling in the bone metastatic niche. The balance of bone turnover between osteoblasts and osteoclasts is also mediated by RANK and RANK ligand (RANKL) activity. Expression of VEGF may also cause angiogenesis by signaling to VEGFRs on endothelial cells. Dual inhibition of MET and VEGFR2 pathways by cabozantinib may disrupt these signals, affecting prostate cancer “seed” and bone microenvironment “soil.”
Inhibition of either MET or VEGF/angiogenesis pathways in men with mCRPC has been evaluated in clinical trials (reviewed in [3]). Four phase III clinical trials targeted angiogenesis with bevacizumab, sunitinib, lenalidomide, or aflibercept; none prolonged OS. A fifth phase III study of the agent tasquinimod is ongoing. A phase II randomized trial evaluating the HGF inhibitor rilotumumab did not improve OS.
Dual inhibition of MET and VEGFR2 with cabozantinib has shown promise in mCRPC and other malignancies [2]. Cabozantinib is FDA-approved for advanced medullary thyroid cancer (MTC), due in part to its inhibition of RET, which is frequently mutated in MTC [4]. In a randomized discontinuation study involving subjects with mCRPC, cabozantinib (100mg daily) was associated with 5% objective response rate at 12 weeks (soft tissue lesions measured by RECIST) and 68% rate of bone scan improvement, including complete resolution in 12%. Bone pain was improved in 67% of evaluable subjects with a decrease in narcotic use in 56% [2]. PSA changes were often discordant with bone scan responses. Dose reductions occurred in 62% of subjects due to adverse events. A dose-ranging study of cabozantinib in mCRPC identified a lower dose (40mg daily) with similar activity but improved tolerability [5]. The effects of cabozantinib on OS and pain improvement in men with mCRPC are being evaluated in two current phase III clinical trials (COMET-1 and COMET-2).
In this issue, Dai and colleagues evaluated whether cabozantinib affects prostate cancer cells, bone stroma, or both, using established cell lines and in vivo models of metastatic progression. Cabozantinib diminished viability of prostate cancer and pre-osteoblastic cell lines, and inhibited differentiation of pre-osteoclast cells, consistent with the important roles of MET and VEGFR2 in the normal activity and survival of the three cell types that interact in bone metastases. Cabozantinib affects the intended molecular targets, with decreased phosphorylation of MET and VEGFR2. In mouse models, cabozantinib had differential effects on tumor growth depending on the prostate cancer cell line and the site of tumor growth. Cabozantinib inhibited intratibial metastasis of prostate cancer cell lines that produce osteoblastic or mixed blastic/lytic lesions, but did not affect the intratibial growth of a prostate cancer cell line that produces osteolytic metastases (PC-3). Cabozantinib did inhibit growth of the same PC-3 cell line when implanted subcutaneously, however, suggesting that the drug's activity is context-dependent. Together, these studies suggest that the clinical activity of cabozantinib may result from both inhibiting the seed and disrupting the soil of Paget's classic “seed and soil” hypothesis of metastasis.
These data add to our understanding of metastasis and raise important questions about the role of cabozantinib in the management of mCRPC. First, context of tumor type and metastatic niche are critical. The same cell line (PC-3) grew tumors in bone and soft tissue, but cabozantinib only decreased tumor-associated vasculature and inhibited growth in the subcutaneous tumors. Because bone is a prevascular tissue, bone metastases may be less dependent on angiogenesis for nutrient supply, compared with subcutaneous tumors. Thus, some subcutaneous tumors may be more susceptible to antiangiogenic therapies. These studies highlight the need for accurate modeling of the heterotypic interactions between tumor cells and stroma.
Second, not all bone metastases are created equal. Prostate cancer can produce osteoblastic, osteolytic or mixed blastic/lytic lesions. Could the nature of the bone metastases predict susceptibility to dual VEGFR/MET inhibition? Cell lines that create predominantly osteolytic metastases (like PC-3) may release more growth factors from the bony matrix and thereby have less dependence on angiogenesis for establishment of viable tumors. The therapeutic implication is that such tumors may be less susceptible to antiangiogenic therapy and by extension, less sensitive to dual pathway inhibition of therapies like cabozantinib. Since we know from large clinical trials that inhibition of just one pathway does not improve OS, perhaps we can use these observations to identify predictive biomarkers (i.e., denoting predominance of osteoblastic lesions) that can improve patient selection for cabozantinib therapy.
Third, inhibition of two important targets may be insufficient to diminish overall tumor burden in patients. Much like PC-3 response to cabozantinib is dependent on whether the tumor is in bone or soft tissue, men with CRPC metastases to both soft tissue and bone may require co-administration of multiple targeted therapies that can disrupt different kinds of soil.
Lastly, the promise of cabozantinib may lie in its ability to target both seed and soil. By inhibiting prostate cancer cell growth and inducing apoptosis, cabozantinib may be tumoricidal. By disrupting vasculature or affecting viability and activity of osteoblasts and osteoclasts, cabozantinib may be tumoristatic. The clinical evidence of rapid improvement in bone scans but discordant PSA response in some subjects with mCRPC suggests that the initial effect may relate to altered perfusion of bone metastases. The discordance between PSA and bone scan response in some patients suggests that cabozantinib may be tumoricidal in some tumors but tumoristatic in others. In conclusion, the compelling preclinical observations by Dai et al. provide mechanism(s) for the clinical activity of cabozantinib in mCPRC. Their observations may also inform future development of cabozantinib including the rational selection of combination therapies.
Acknowledgments
Supported by: the Department of Defense Prostate Cancer Research Program under Award W81XWH-09-1-0471 and a Conquer Cancer Foundation Career Development Award (R. J. Lee); by National Institutes of Health Midcareer Investigator Award No. 5K24CA121990 (M. R. Smith); and competitive research awards from the Prostate Cancer Foundation.
Footnotes
Conflicts of Interest: RJL: research support from Exelixis, Inc. MRS: research support from Exelixis, Inc. and consultant for Exelixis, Inc.
References
- 1.Dai J, Zhang H, Karatsinides A, Keller JM, Kozloff KM, Aftab DT, et al. Cabozantinib inhibits prostate cancer growth and prevents tumor-induced bone lesions. Clin Cancer Res. doi: 10.1158/1078-0432.CCR-13-0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith DC, Smith MR, Sweeney C, Elfiky AA, Logothetis C, Corn PG, et al. Cabozantinib in Patients With Advanced Prostate Cancer: Results of a Phase II Randomized Discontinuation Trial. J Clin Oncol. 2013;31:412–9. doi: 10.1200/JCO.2012.45.0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee RJ, Smith MR. Targeting MET and vascular endothelial growth factor receptor signaling in castration-resistant prostate cancer. Cancer J. 2013;19:90–8. doi: 10.1097/PPO.0b013e318281e280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elisei R, Schlumberger MJ, Muller SP, Schoffski P, Brose MS, Shah MH, et al. Cabozantinib in Progressive Medullary Thyroid Cancer. J Clin Oncol. 2013;31:3639–3646. doi: 10.1200/JCO.2012.48.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee RJ, Saylor PJ, Michaelson MD, Rothenberg SM, Smas ME, Miyamoto DT, et al. A dose-ranging study of cabozantinib in men with castration-resistant prostate cancer and bone metastases. Clin Cancer Res. 2013;19:3088–3094. doi: 10.1158/1078-0432.CCR-13-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]