Abstract
Endothelial progenitor cells (EPCs) may contribute to neurovascular repair after stroke and neurodegeneration. A key step in this process should involve adhesive interactions between EPCs and the targeted cerebral endothelium. Here, we tested the hypothesis that reactive astrocytes may play a critical role in enhancing adhesive interactions and transmigration of EPCs across cerebral endothelial cells. Transiently seeding EPCs onto a monolayer of RBE.4 rat brain endothelial cells resulted in a time-dependent adherence between the two cell types. Blocking β2 integrins on EPCs or blocking the receptor for advanced glycation endproducts (RAGE) on endothelial cells significantly decreased EPC-endothelial adherence. Next, we tested whether reactive astrocytes can enhance this process by growing EPCs, brain endothelial cells and astrocytes together in a transwell co-culture system. The presence of reactive astrocytes in the lower chamber significantly promoted adherence between EPCs and endothelial cells in the upper chamber. This process involved the release of soluble HMGB1 from reactive astrocytes that then upregulated endothelial expression of RAGE via Egr1 signaling. Directly adding HMGB1 to the transwell system also promoted EPC-endothelial adhesion and accelerated EPC transmigration into the lower chamber. These initial findings provide proof-of-concept that reactive astrocytes promote crosstalk between cerebral endothelium and EPCs. Further investigation of this phenomenon may lead to a better understanding of cell-cell interactions required for neurovascular recovery after stroke.
Keywords: Endothelial progenitor cells, high-mobility group box 1 (HMGB1), receptor for advanced glycation end product (RAGE), reactive astrocytes
1. Introduction
For many years, CNS was considered to be an isolated and immune-privileged organ. However, it is now recognized that systemic responses in blood contribute to bi-directional signaling with the CNS after injury. Integrated responses in neurons, glia, brain blood vessels, and circulating peripheral cells all contribute to the pathogenesis of injury and mechanisms of repair after stroke and trauma.1
A specific set of peripheral cells that have been implicated comprise the population of circulating endothelial progenitor cells (EPCs). EPCs have been viewed as adult stem cells that can play an important role in tissue vascularization and endothelium homeostasis after CNS damage and neurodegeneration.2, 3 EPCs are highly migratory and may be attracted to injured or diseased brain areas, where they are thought to mediate angiogenesis, vasculogenesis and neurovascular tissue repair.4, 5 The underlying mechanisms are not fully understood, and many potential mediators continue to be investigated, including “homing signals” such as stromal cell-derived factor-1 (SDF-1) 6 and “cell-cell interactive signals” such as β1- and β2- integrins.7, 8 However, what tends to be missing from these previous models is the consideration of cross-talk signals from other cells in the brain.
We recently showed that, by acting as sensors of neurovascular injury, astrocytes could augment EPC-mediated neurovascular remodeling after stroke and brain injury.9, 10 But what are the molecular mechanisms that underlie this ability of astrocytes to promote EPC function during CNS repair? In the present study, we examined the hypothesis that reactive astrocytes can release HMGB1, a member of the damage-associated-molecular-pattern (DAMP) family of proteins that subsequently enhances adhesive interactions and increases transmigration of EPCs across targeted brain endothelium.
2. Methods
All experiments were performed following an institutionally approved protocol in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.1 Chemicals
Rat recombinant IL-1β and human recombinant HMGB1 were purchased from Sigma-aldrich
2.2 Antibodies
CD34, Flk-1 (VEGFR2) and Egr1 antibodies, control goat IgG and mouse IgG were purchased from Santa Cruz Biotechnology. RAGE antibody was purchased from R&D systems. HMGB1 antibody (Abcam) and integrins antibodies (CD11a, CD11b, and CD18, BD biosciences) were purchased from each company. Antibodies against RAGE, CD11a, CD11b, and CD18 have neutralizing capacity.
2.3 Cell cultures
2.3.1 Rat brain endothelial cells
A rat brain microendothelial cell line, RBE.4 were maintained in EBM-2 containingEGM-2MV SingleQuots kit onto collagen-coated 25 cm2 flasks at a density of 2×105 cells/cm2 incubated in a 5% CO2 incubator at 37°C. RBE.4 was dissociated by trypsinization and then reseeded on collagen I-coated 24 well plates for cell adhesion assay.
2.3.2 Rat endothelial progenitor cells
Spleens were used for the obtaining of EPCs as described as before.11 For each independent experiment, spleens from 11–12 weeks old Sprague-Dawley (SD) rats were kept in PBS solution. Under the hood, spleens were mechanically minced, placed at 37°C for 15 min in EPCs lysis buffer and run through a 40-μm nylon membrane to obtain cell suspension. Mononuclear cells (MNCs) were obtained by density gradient centrifugation with Ficoll-Paque Plus (Amersham Biosciences Corp). Isolated MNCs were shortly washed with red blood cells lysis solution and gently washed twice with complete growth media EGM-2MV (Lonza). MNCs were finally resuspended in EGM-2MV and 3 × 107 MNCs per well were seeded on collagen I-coated six-well plates (Becton Dickinson Labware) and incubated in a 5% CO2 incubator at 37°C. Under daily observation, first media change was performed 3–4 days after plating. Early EPCs were used for each independent experiment between 5–7 days after seeding.
2.3.3 Primary astrocyte cultures
Primary astrocyte cultures were prepared from cerebral cortices of 2-day-old neonatal Sprague-Dawley rats as described as before.12 Briefly, dissociated cortical cells were suspended in Dulbecco's modified Eagle medium (NBM, Life Technology) containing 25 mM glucose, 4 mM glutamine, 1 mM sodium pyruvate, and 10% fetal bovine serum and plated on uncoated 25 cm2 flasks at a density of 6×105 cells/cm2. Monolayers of type 1 astrocytes were obtained 12–14 days after plating. Non-astrocytic cells such as microglia and neurons were detached from the flasks by shaking and removed by changing the medium. Astrocytes were dissociated by trypsinization and then reseeded on uncoated 6- and 24-well plates at a density of 1×105 cells/cm2.
2.3.4 Primary monocytes/macrophages cultures
Monocytes/macrophages were isolated from rat peritoneal cavity. After collecting cells from the cavity, 1 × 105 cells per well were seeded on non-coated six-well plates and grown in RPMI medium 1640 containing 10% FBS, 1% penicillin/streptomycin. Twenty four hours later, attached cells were used as a mix of monocytes/macrophages.
2.4 EPCs labeling
Cells were incubated with 5 μg/ml 1, 1 -dioctadecyl-3, 3, 3, 3-tetramethylin- docarbocyanine perchlorate (DiI) -labeled acetylated low density lipoprotein (ac-LDL; Molecular Probes) at 37°C for 120 min in EGM-2 MV.
2.5 Monocytes/macrophages labeling
Cells were incubated with 3 μM calcein AM at 37°C for 30 min in RPMI medium 1640 containing 10% FBS, 1% penicillin/streptomycin.
2.6 Cell adhesion assay
After 24 hours incubation with or without IL-1β (1, 10, and 100 ng/ml), ac-LDL labeled EPCs (1 × 104 cells/well) were coincubated with RBE.4 monolayer at 37°C for 10, 30, 60 and 90 min. Calcein AM-labeled monocytes/macrophages (1 × 105 cells/well) were coincubated with RBE.4 monolayer 37°C for 20 min. Then, adhered EPCs or monocytes/macrophages were fixed with 4% paraformaldehyde for 10 min and were washed three times with ice-cold phosphate-buffered saline (PBS) to remove non adherent cells. The numbers of adhered cells were quantified by counting the number of ac-LDL positive cells or calcein AM positive cells.
2.7 In vitro trans-endothelial migration assay
Rat brain endothelial cells (RBE.4) (1×105 cells/well) were seeded on polycarbonate membrane (3-μm pore filters, Corning Costar) coated with collagen I to obtain confluent endothelial monolayers. Ac LDL-labeled EPCs (1×105 cells/well) were placed in the upper chamber on top of the RBE.4 monolayer. The chambers were placed in a 24-well culture plate containing IL-1β (1 ng/ml) or recombinant HMGB1 (10 ng/ml) or rat cortical astrocytes. After 24 h of incubation at 37°C, the lower side of the filter was washed with PBS and fixed with 4% PFA. Labeled EPCs migrating into the lower chamber were directly counted in 4 random microscopic fields.
2.8 Western Blot Analysis
Cultures were rinsed twice with PBS and cell membrane extracts were collected according to the method of ProteoExtract Kit (Calbiochem). Samples were heated with equal volumes of SDS sample buffer (Novex) and 10% 2-mercaptoethanol at 95°C for 5 min, and then each sample was loaded onto 4–20% Tris-glycine gels. After electorophoresis and transferring to polyvinylidene difluoride membranes (Novex), the membranes were blocked in Tris-buffered saline containing 0.1% Tween 20 and 0.2% I-block (Tropix) for 60 min at 4°C. Membranes were then incubated overnight at 4°C with primary antibodies. After incubation with peroxidase-conjugated secondary antibodies, chemiluminescence was enhanced (GE Healthcare). Optical density was assessed using the NIH Image analysis software.
2.9 Immunohistochemistry
Cells were washed with ice-cold PBS (pH 7.4), followed by 4% paraformaldehyde for 10 min. After being washed three times in PBS containing 0.1% Triton X-100, they were incubated with 3% bovine serum albumin (BSA) in PBS for 1 h. Then cells were incubated with primary antibodies at 4°C overnight. After washing with PBS, they were incubated with secondary antibodies (1:400; Jackson ImmunoResearch) for 1 h at room temperature. Finally, nuclei were counterstained with 4,6-diamidino-2-phenylindole (DAPI), and coverslips were placed. Immunostaining was analyzed with a fluorescence microscope (Olympus BX51) interfaced with a digital charge-coupled device camera and an image analysis system.
2.10 Egr1 siRNA transfection in rat brain endothelial cells
Control siRNA and Egr1 siRNA were obtained from Santa Cruz Biotechnology. Control siRNA (sc37007) consists of a scrambled sequence that will not lead to the specific degradation of any known cellular mRNA. Egr1 siRNA (sc270177) is a pool of 3 target-specific 19–25nt siRNAs designed to knock down gene expression. The sequences for rat Egr1 siRNAs are designed as followed; Sequence 1: 5’-CAGCGCUUUCAAUCCUCAAtt-3’, Sequence 2: 5’- CCAUGAUCCCUGACUAUCUtt-3’, Sequence 3: 5’-CUCCACUAUCCACUAUCAAtt-3’. siRNAs were prepared according to the transfection protocol for cell cultures from Santa Cruz Biotechnology. Briefly, siRNA transfection reagent mixture of 1 ml (Transfection reagent, sc-29528, Transfection medium, sc36868) was co-incubated with RBE.4 for 6 hours in a 5% CO2 incubator at 37°C, and then same amount of EBM-2 1% FBS was added. An additional incubation was performed for 18 hours, and then the procedure for conditioned media was carried on.
2.11 Statistical analysis
All of experiments were blinded and performed in duplicate, repeated 3–5 times independently. GraphPad Prism 5 was used overall statistical analysis in this study. Results were expressed as mean±SD. When only two groups were compared, Student’s t-test was used. Multiple comparisons were evaluated by Tukey’s test after one-way ANOVA or two-way ANOVA. P<0.05 was considered to be statistically significant.
3. Results
3.1 EPC-endothelial adherence is mediated via β2-integrin-RAGE interactions
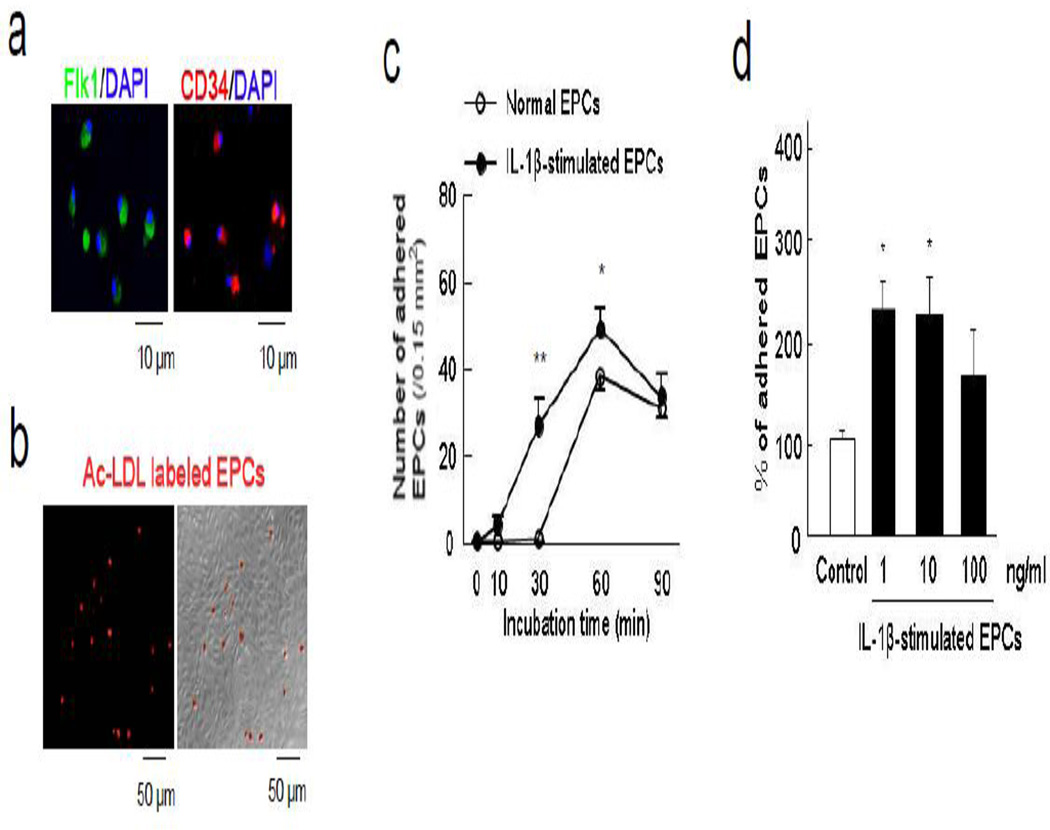
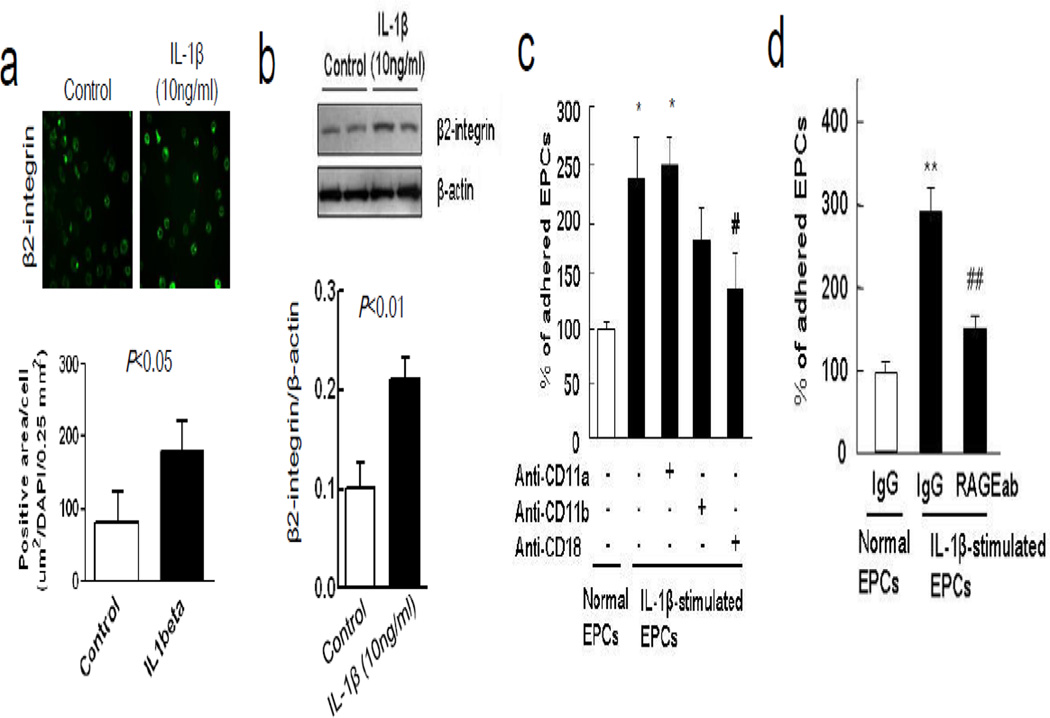
After isolation from rat spleen, early EPCs were positive for representative markers including Flk1 and CD34 at 5 days after seeding (Figure 1a). Adhesion of diI-ac-LDL-labeled EPCs was assessed by counting labeled cells that remain attached after a transient co-incubation onto a monolayer of RBE.4 rat brain endothelial cells (Figure 1b-c). When EPCs were stimulated with low concentrations of IL-1β for 24 hours prior to incubation, adherence was significantly increased (Figure 1c-d). Immunocytochemistry and western blots suggested that the increased adherence may be due to an IL-1β-induced upregulation of β2-integrin expression in EPCs (Figure 2a-b). Blockade with anti-CD18 (β2-integrin) antibodies in EPCs decreased their adhesion to the endothelial monolayer (Figure 2c). Conversely, adding an anti-RAGE antibody onto the RBE.4 endothelial cells also decreased EPC adhesion (Figure 2d). Taken together, these data suggest that RAGE on endothelial cells binds β2-integrin on EPCs, thus facilitating their adhesive interactions.
Figure 1.
IL-1β-stimulated EPCs was enhanced the adhesive interaction to brain endothelial cells. (a) Early EPCs (5 days after seeding) were identified by representative markers including Flk1 and CD34. (b) Ac-LDL labeled EPCs adhered to rat brain endothelial cells (RBE.4). (c) The number of attached EPCs to RBE.4 monolayer was increased in a time-dependent manner and IL-1β (10 ng/ml)-stimulated EPCs showed more adherent ability to endothelial monolayer. *P<0.05, **P<0.01 vs normal EPCs. (d) Low concentration of IL-1β (1 and 10 ng/ml) stimulated EPCs and significantly augmented their adhesive interaction with endothelium. *P<0.05 vs normal EPCs.
Figure 2.
IL-1β enhanced adhesive interaction between β2-integrin in EPCs and endothelium RAGE. (a) Immunocytochemistry showed enhancement of β2-integrin expression in EPCs after treatment with IL-1β (10 ng/ml). (b) Western blot analysis confirmed accumulation of β2-integrin in EPCs membrane. (c) Blocking with anti-CD18 (β2-integrin) antibody in EPCs significantly decreased their adhesion, but anti-CD11a blockade had no effects. *P<0.05 vs normal EPCs adherence, #P<0.05 vs IL-1β-stimulated EPCs adherence. These data support the idea that β2-integrin on EPCs are involved in adhesion. (d) Conversely, blocking with an anti-RAGE antibody in RBE.4 also decreased EPC adhesion. **P<0.01 vs normal EPCs adherence, ##P<0.01 vs IL-1β-stimulated EPCs adherence.
3.2 Reactive astrocytes enhance EPC adherence via HMGB1-induced upregulation of endothelial RAGE
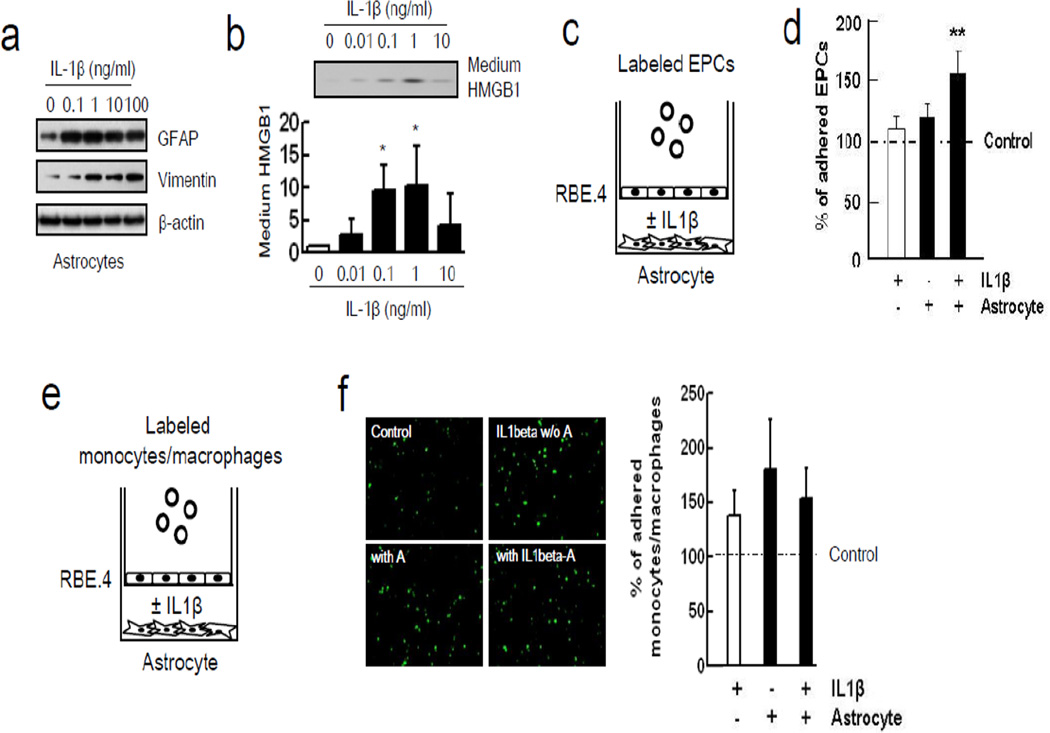
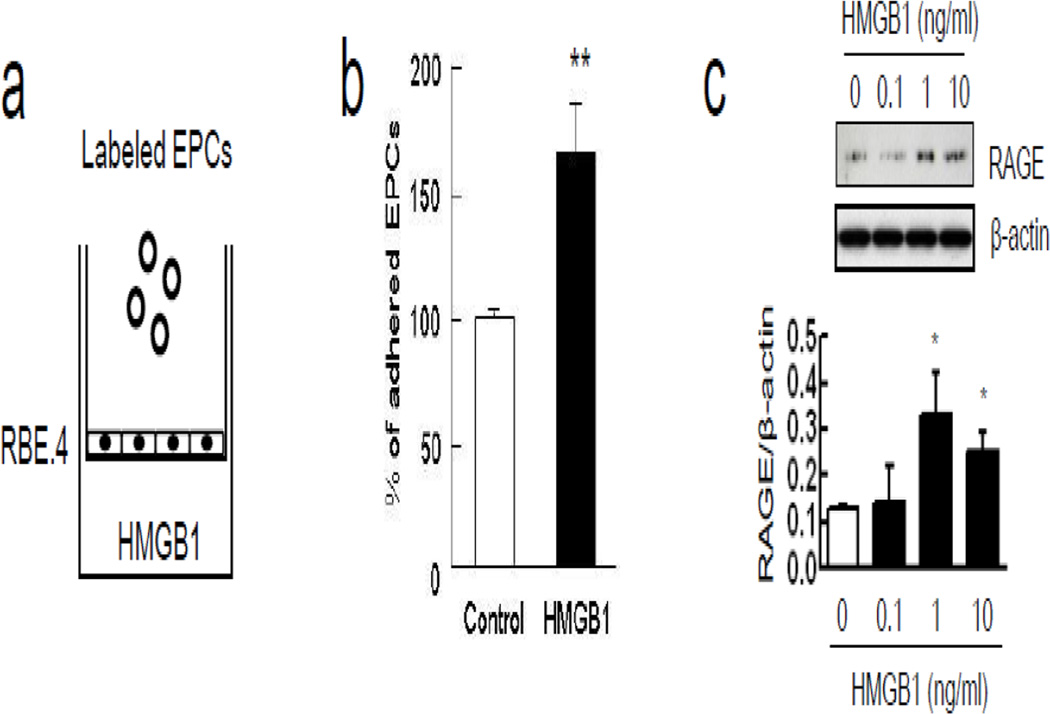
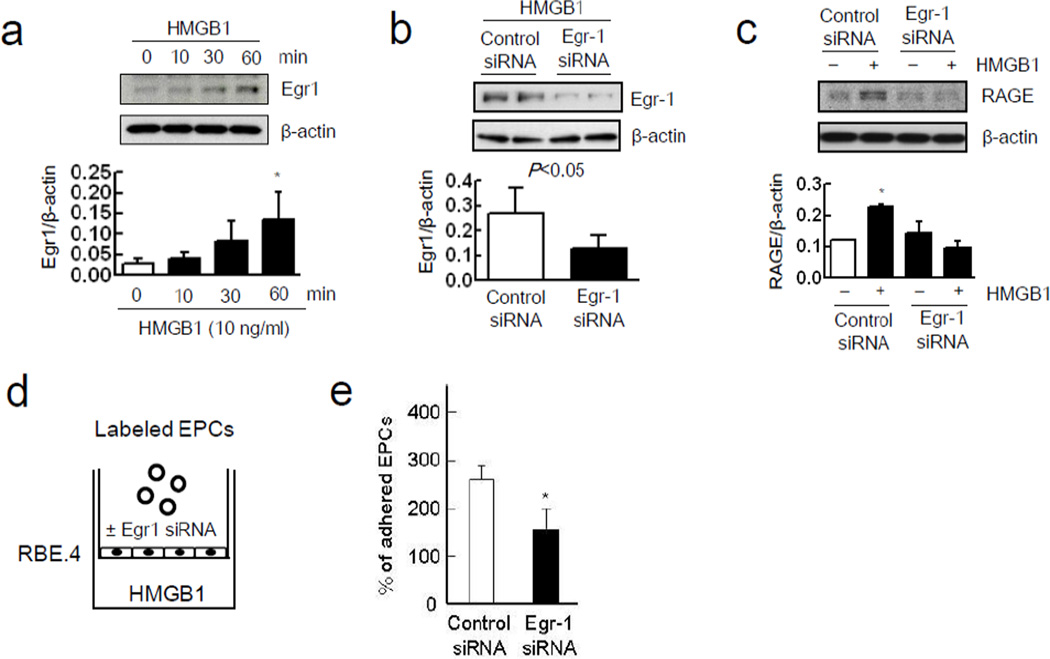
Astrocytes were stimulated with IL-1β, and as expected, this standard method yielded reactive astrocytes with elevated expression of GFAP and vimentin (Figure 3a), and promoted the release of HMGB1 into the conditioned media (Figure 3b). Quantitation revealed that IL-1β-stimulated astrocytes released approximately 1–2 ng/ml of HMGB1. To ascertain whether reactive astrocytes can augment adhesion of EPCs onto RBE.4 endothelial cells, a triple-culture was performed with a transwell co-culture system (Figure 3c). When reactive astrocytes were grown in the lower chamber, adhesion of EPCs onto endothelial cells was significantly increased in the upper chamber (Figure 3d). To check the specificity of this phenomenon, monocytes/macrophages were also used in a triple-culture model (Figure 3e). IL-1β itself enhanced adhesive interactions between monocytes/macrophages on endothelial cells but cell adherence was not significantly augmented by the presence of IL-1β-stimulated astrocytes in the lower chamber (Figure 3f). Next, the role of astrocytic HMGB1 was tested by removing astrocytes from the transwell system completely and adding exogenous HMGB1 (0.1, 1, 10 ng/ml) into the lower chamber instead (Figure 4a). This range of exogenous HMGB1 was chosen based on the levels of HMGB1 released by stimulated astrocytes. HMGB1 directly promoted adhesion between EPCs and the RBE.4 endothelial cells in the upper chamber (Figure 4b). This effect may be mediated in part by the ability of HMGB1 to upregulate RAGE levels in the endothelial monolayer over time (Figure 4c). HMGB1-induced upregulation of endothelial RAGE was dependent on Egr1, which is known to activate key promoter regions on the RAGE gene (Figure 5a). When siRNA was used to suppress Egr1 signaling (Figure 5b), the ability of HMGB1 to upregulate RAGE levels on RBE.4 brain endothelial cells was significantly reduced (Figure 5c). Consistent with this suppression of endothelial RAGE, siRNA against Egr1 significantly decreased EPC adherence on endothelial monolayers after HMGB1 stimulation (Figure 5d-e).
Figure 3.
Reactive astrocytic HMGB1 enhanced EPCs adherence to RBE.4 monolayer. (a) Western blot analysis showed that IL-1β increased reactive astrocytes markers (GFAP and vimentin) in a concentration-dependent manner. (b) Lower concentration of IL-1β (0.1 and 1 ng/ml) induced the release of HMGB1 in astrocytes as described as before.29 *P<0.05 vs IL1β 0 ng/ml. (c) Co-culture between rat cortical astrocytes in the lower chamber and rat brain endothelial cells in the upper chamber was performed for 24 hours. Then, labeled EPCs were added into the upper chamber for 45 min. (d) IL-1β (1 ng/ml)-stimulated reactive astrocytes enhanced EPCs adherence to RBE.4 monolayer. **P<0.01 vs control group. (e) Calcein AM-labeled monocytes/macrophages were added into the upper chamber for 20 min. (f) IL-1β (1 ng/ml) itself increased adhesive interaction of monocytes/macrophages. IL-1β-stimulated reactive astrocytes (IL-1β-A) did not enhance monocytes/macrophages adherence to RBE.4 monolayer. A; astrocytes.
Figure 4.
Soluble HMGB1 promoted EPCs adherence through up-regulation of endothelium RAGE expression. (a) RBE.4 monolayer was prepared onto collagen I-coated transwell and HMGB1 (10 ng/ml) was added into the lower chamber for 24 hours. (b) HMGB1 significantly induced more adhesive interaction between EPCs and endothelium. **P<0.01 vs control group. (c) Treatment with HMGB1 in the lower chamber increased endothelium RAGE expression in a concentration-dependent manner. *P<0.05 vs HMGB1 0 ng/ml.
Figure 5.
Egr1 signaling pathway supports endothelium RAGE expression and EPCs adherence. (a) Western blot analysis showed that HMGB1 (10 ng/ml) significantly increased Egr-1 expression in RBE.4 in a time-dependent manner. *P<0.05 vs HMGB1 0 min. (b) Egr-1 siRNA transfection successfully reduced Egr-1 expression induced by HMGB1 (10 ng/ml) after 60 minutes. (c) Rat brain endothelium RAGE expression 24 hours after HMGB1 (10 ng/ml) stimulation significantly attenuated by Egr-1 siRNA transfection. *P<0.05 vs control siRNA without HMGB1 stimulation. (d) RBE.4 monolayer treated with Egr-1 siRNA or control siRNA was prepared onto collagen I-coated transwell. Recombinant HMGB1 was added into the lower chamber for 24 hours. Then, labeled EPCs were added into the upper chamber for 45 min. (e) Egr-1 siRNA transfection significantly reduced EPCs adherence rate. *P<0.05 vs control siRNA-treated group.
3.3 Reactive astrocytes enhance EPC transmigration
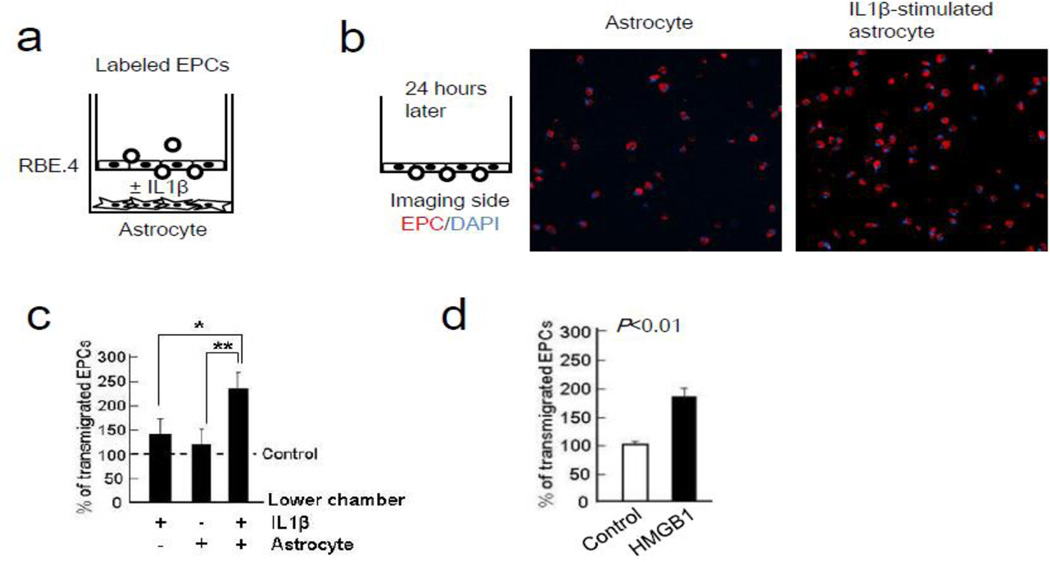
After EPCs adhere onto endothelial cells, they may also transmigrate. Hence, we asked whether reactive astrocytes could also promote EPC transmigration in our transwell culture system (Figure 6a). When IL-1β-stimulated reactive astrocytes were present in the lower chamber, diI-ac-LDL-labeled EPCs were able to adhere and then after 24 hrs, transmigrate across the RBE.4 brain endothelial monolayer growing in the upper chamber (Figure 6b-c). In the absence of astrocytes, EPC transmigration across the endothelial monolayer was also induced by directly adding exogenous HMGB1 into the empty lower chamber (Figure 6d).
Figure 6.
HMGB1 induced trans-endothelial migration of early EPCs. (a) Rat brain endothelial cells (RBE.4) monolayer was prepared onto the upper chamber. After adding recombinant IL-1β protein in rat cortical astrocytes in the lower chamber, labeled EPCs migration was allowed for 24 hours. (b) Representative images of the trans-endothelial migration of diI-ac LDL labeled EPCs (red). Nuclear are indicated by DAPI. (c) IL-1β-stimulated astrocytes strongly attracted EPCs compared with normal astrocytes or IL-1β. *P<0.05, **P<0.01 (d) Data analysis showed that HMGB1 (10 ng/ml) itself enhanced trans-endothelial migration in early EPCs. *P<0.05.
4. Discussion
Our study provides in vitro proof-of-concept that reactive astrocytes play a key role in promoting EPC-brain endothelial adherence and transmigration. Our overall findings demonstrate that (i) β2 integrin-RAGE interactions, (ii) reactive astrocytes release HMGB1 that promotes EPCs adherence but not monocytes/macrophages adherence by upregulating RAGE receptors on brain endothelial cells, and (iii) after adherence, HMGB1 also promotes EPC transmigration across brain endothelial monolayers. Hence, EPC targeting in the brain may require a well-regulated set of cell-cell signals at the neurovascular interface.
EPCs are well known to play a major role in the pathology of diverse vascular disorders, including traumatic brain injury and stroke. The beneficial effects of EPCs have been described in rat models of focal cerebral ischemia.4, 13 In humans, circulating levels of EPCs tend to track the temporal profile of recovery from 7 to 14 days after stroke onset.14, 15 More recently, soluble factors secreted from EPCs such as VEGF, FGF-beta, and PDGF-BB may be considered as positive mediators to promote angiogenesis and neurogenesis after stroke.16 Although the therapeutic potential of EPCs in stroke has been suggested, it remains unclear how EPCs target the central nervous system. Our present findings may now provide a mechanism that directly links central crosstalk between reactive astrocytes and brain endothelial cells with a peripheral response in the systemic EPC response. Further dissection of these molecular pathways may lead to therapeutic approaches to potentially amplify these potentially beneficial EPC responses in damaged or diseased brain.
RAGE expression usually is low under normal condition but enhanced expression of RAGE has been observed in diabetic vasculature and other inflammatory diseases.17–19 RAGE is not only involved in the regulation of cellular activation, but also functions as an adhesion receptor on endothelial cells. Enhancement of RAGE expression can promote the recruitment of leukocytes across endothelial barriers through its interaction with myeloid cells expressing the β2-integrin macrophage receptor 1 (Mac-1).20 In a static in vitro assay, RAGE was found to bind to the I-domain of Mac-1 with Kd 80–100 nM.21 Here, our findings may provide another example of how upregulations in brain endothelial RAGE may bind with β2-integrin in EPCs, thus promoting their central accumulation.
In this study, our findings suggest that the ability of reactive astrocytes to promote EPC response may be due to the release of HMGB1. As a damage-associated-molecular-pattern (DAMP) mediator, HMGB1 is traditionally known as a nuclear and cellular danger signal.22 Large elevations in HMGB1 can be deleterious after stroke and brain injury.23 However, more recently, it is recognized that HMGB1 can exert different functions depending on its cellular localization. When large amounts are passively released from damaged cells, HMGB1 worsens neuroinflammation and brain injury.23 But when lower levels of HMGB1 are actively secreted from reactive astrocytes, beneficial actions can be detected.9 Upon release, extracellular HMGB1 binds to its putative receptors (RAGE, TLR2, TLR4), and induces series of signaling pathways in response to the original damage. Recently, we showed that suppressing HMGB1 in reactive astrocytes might worsen neurovascular recovery after focal ischemia in mice.9, 24 The present study may provide a specific mechanism for this phenomenon that directly links the reactive astrocyte and endothelial cells within the neurovascular unit to a pro-recovery EPC response.
Taken together, our findings support a novel mechanism whereby reactive astrocytes release HMGB1 that upregulates RAGE receptors on brain endothelial cells, thus augmenting β2-integrin-mediated EPC adhesion and transmigration. However, there are several caveats worth keeping in mind. First, we mainly focus on EPCs and we could not detect clear differences in monocytes/macrophages. Recent study demonstrates that TLR4 signaling stimulated by lipopolysaccharide increased microvascular CD200 expression, a cell surface glycoprotein which induces immunosuppressive activity, and CD200 receptor agonist inhibited macrophages interaction to endothelial cells. 25 HMGB1 can also stimulate TLR4 in endothelial cells thus the attachment of monocytes/macrophages may not be augmented through CD200 signaling. Nevertheless, how all blood cells interact as they move toward a diseased neurovascular unit remains to be elucidated. Second, other adherence factors and receptors may also contribute. For example, HMGB1 is known to increase expression of vascular cell-adhesion molecule 1 (VCAM-1), intercellular adhesion molecule 1 (ICAM-1) and endothelial-cell selectin.22, 26 Our focus was on RAGE, but how multiple signals coordinate with multiple receptors in EPCs and endothelium will be a very complicated system that must be further investigated. Third, . Fourth, our in vitro model is limited in its ability to fully replicate in vivo pathophysiology. For example, our models use standard neonatal and very young cell cultures. Burt of course, disease tends to occur in aged CNS. How our hypothesis of endothelial RAGE and β2-integrin in EPCs contribute to neurovascular remodeling after stroke should be eventually addressed with in vivo studies. Finally, our endpoints are all cell-based and not functional. Whether EPCs has specific phenotype for transmigration is unclear. In addition, during transmigration, EPCs may be stimulated with many factors from both the brain parenchyma and circulating factors in blood thus the phenotype might be changed after transmigration. Future studies should be required to determine the functional sequelae of EPCs that have been amplified by astrocyte-endothelial crosstalk.
5. Conclusion
The concept of the neurovascular unit, i.e. cell-cell signaling between neuronal, glial and vascular compartments, has provided a powerful framework for investigating the mechanisms of acute injury and delayed recovery after stroke, brain injury and neurodegeneration.27, 28 This study suggests that signaling at the neurovascular interface may also play a key role in promoting EPC-mediated peripheral responses. By upregulating RAGE on affected endothelial cells, reactive astrocytes may augment EPC adherence and transmigration. This phenomenon may comprise a novel mechanism whereby crosstalk between reactive astrocytes and cerebral endothelium augments EPC responses for neurovascular recovery in damaged or diseased brain. Further studies are warranted to dissect the in vivo consequences of this phenomenon in animal models of stroke, brain injury and neurodegeneration.
Highlights.
EPC-endothelial adherence is mediated via β2-integrin-RAGE interactions
Reactive astrocytes enhance EPC adherence via HMGB1-induced upregulation of endothelial RAGE
Reactive astrocytes enhance EPC transmigration
Astrocytic HMGB1 promotes EPC-mediated vascular repair process during brain remodeling
Acknowledgement
Supported in part by Rappaport Foundation and NIH (R01-NS76694, R01-NS65089, K99-NS80991).
List of abbreviations
- EPCs
endothelial progenitor cells
- RAGE
Receptor for advanced glycation endproducts
- TLR
toll like receptor
- HMGB1
high-mobility group box 1
- CD
cluster of differentiation
- DiI ac LDL
1, 1-dioctadecyl-3, 3, 3, 3-tetramethylin- docarbocyanine perchlorate-labeled acetylated low density lipoprotein
- PBS
phosphate-buffered saline
- DAPI
4,6-diamidino-2-phenylindole
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: KH – study design, experiments, grant support, manuscript writing
LDP – experiments
KA – study design, grant support, manuscript writing
EHL – study design, grant support, manuscript writing
Disclosure of potential conflicts of interest: The authors declare they have no competing financial interest.
References
- 1.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rouhl RP, van Oostenbrugge RJ, Damoiseaux J, et al. Endothelial progenitor cell research in stroke: a potential shift in pathophysiological and therapeutical concepts. Stroke. 2008;39:2158–2165. doi: 10.1161/STROKEAHA.107.507251. [DOI] [PubMed] [Google Scholar]
- 3.Nan Z, Grande A, Sanberg CD, et al. Infusion of human umbilical cord blood ameliorates neurologic deficits in rats with hemorrhagic brain injury. Ann N Y Acad Sci. 2005;1049:84–96. doi: 10.1196/annals.1334.009. [DOI] [PubMed] [Google Scholar]
- 4.Fan Y, Shen F, Frenzel T, et al. Endothelial progenitor cell transplantation improves long-term stroke outcome in mice. Ann Neurol. 2010;67:488–497. doi: 10.1002/ana.21919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sobrino T, Hurtado O, Moro MA, et al. The increase of circulating endothelial progenitor cells after acute ischemic stroke is associated with good outcome. Stroke. 2007;38:2759–2764. doi: 10.1161/STROKEAHA.107.484386. [DOI] [PubMed] [Google Scholar]
- 6.Lapidot T. Mechanism of human stem cell migration and repopulation of NOD/SCID and B2mnull NOD/SCID mice. The role of SDF-1/CXCR4 interactions. Ann N Y Acad Sci. 2001;938:83–95. doi: 10.1111/j.1749-6632.2001.tb03577.x. [DOI] [PubMed] [Google Scholar]
- 7.Shyu WC, Lin SZ, Chiang MF, et al. Intracerebral peripheral blood stem cell (CD34+) implantation induces neuroplasticity by enhancing beta1 integrin-mediated angiogenesis in chronic stroke rats. J Neurosci. 2006;26:3444–3453. doi: 10.1523/JNEUROSCI.5165-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chavakis E, Aicher A, Heeschen C, et al. Role of beta2-integrins for homing and neovascularization capacity of endothelial progenitor cells. J Exp Med. 2005;201:63–72. doi: 10.1084/jem.20041402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayakawa K, Pham LD, Katusic ZS, et al. Astrocytic high-mobility group box 1 promotes endothelial progenitor cell-mediated neurovascular remodeling during stroke recovery. Proc Natl Acad Sci U S A. 2012;109:7505–7510. doi: 10.1073/pnas.1121146109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayakawa K, Miyamoto N, Seo JH, et al. High-mobility group box 1 from reactive astrocytes enhances the accumulation of endothelial progenitor cells in damaged white matter. J Neurochem. 2012 doi: 10.1111/jnc.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosell A, Arai K, Lok J, et al. Interleukin-1beta augments angiogenic responses of murine endothelial progenitor cells in vitro. J Cereb Blood Flow Metab. 2009;29:933–943. doi: 10.1038/jcbfm.2009.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arai K, Lee SR, Lo EH. Essential role for ERK mitogen-activated protein kinase in matrix metalloproteinase-9 regulation in rat cortical astrocytes. Glia. 2003;43:254–264. doi: 10.1002/glia.10255. [DOI] [PubMed] [Google Scholar]
- 13.Taguchi A, Soma T, Tanaka H, et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114:330–338. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Navarro-Sobrino M, Rosell A, Hernandez-Guillamon M, et al. Mobilization, endothelial differentiation and functional capacity of endothelial progenitor cells after ischemic stroke. Microvasc Res. 2010;80:317–323. doi: 10.1016/j.mvr.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 15.Navarro-Sobrino M, Rosell A, Hernandez-Guillamon M, et al. A large screening of angiogenesis biomarkers and their association with neurological outcome after ischemic stroke. Atherosclerosis. 2011;216:205–211. doi: 10.1016/j.atherosclerosis.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 16.Rosell A, Morancho A, Navarro-Sobrino M, et al. Factors secreted by endothelial progenitor cells enhance neurorepair responses after cerebral ischemia in mice. PLoS One. 2013;8:e73244. doi: 10.1371/journal.pone.0073244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huttunen HJ, Kuja-Panula J, Rauvala H. Receptor for advanced glycation end products (RAGE) signaling induces CREB-dependent chromogranin expression during neuronal differentiation. J Biol Chem. 2002;277:38635–38646. doi: 10.1074/jbc.M202515200. [DOI] [PubMed] [Google Scholar]
- 18.Hori O, Brett J, Slattery T, et al. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J Biol Chem. 1995;270:25752–25761. doi: 10.1074/jbc.270.43.25752. [DOI] [PubMed] [Google Scholar]
- 19.Bierhaus A, Humpert PM, Morcos M, et al. Understanding RAGE, the receptor for advanced glycation end products. J Mol Med. 2005;83:876–886. doi: 10.1007/s00109-005-0688-7. [DOI] [PubMed] [Google Scholar]
- 20.Rouhiainen A, Kuja-Panula J, Wilkman E, et al. Regulation of monocyte migration by amphoterin (HMGB1) Blood. 2004;104:1174–1182. doi: 10.1182/blood-2003-10-3536. [DOI] [PubMed] [Google Scholar]
- 21.Chavakis T, Bierhaus A, Al-Fakhri N, et al. The pattern recognition receptor (RAGE) is a counterreceptor for leukocyte integrins: a novel pathway for inflammatory cell recruitment. J Exp Med. 2003;198:1507–1515. doi: 10.1084/jem.20030800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 23.Kim JB, Sig Choi J, Yu YM, et al. HMGB1, a novel cytokine-like mediator linking acute neuronal death and delayed neuroinflammation in the postischemic brain. J Neurosci. 2006;26:6413–6421. doi: 10.1523/JNEUROSCI.3815-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayakawa K, Nakano T, Irie K, et al. Inhibition of reactive astrocytes with fluorocitrate retards neurovascular remodeling and recovery after focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2010;30:871–882. doi: 10.1038/jcbfm.2009.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ko YC, Chien HF, Jiang-Shieh YF, et al. Endothelial CD200 is heterogeneously distributed, regulated and involved in immune cell-endothelium interactions. J Anat. 2009;214:183–195. doi: 10.1111/j.1469-7580.2008.00986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fiuza C, Bustin M, Talwar S, et al. Inflammation-promoting activity of HMGB1 on human microvascular endothelial cells. Blood. 2003;101:2652–2660. doi: 10.1182/blood-2002-05-1300. [DOI] [PubMed] [Google Scholar]
- 27.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 28.Zlokovic BV. Neurodegeneration and the neurovascular unit. Nat Med. 2010;16:1370–1371. doi: 10.1038/nm1210-1370. [DOI] [PubMed] [Google Scholar]
- 29.Hayakawa K, Arai K, Lo EH. Role of ERK map kinase and CRM1 in IL-1beta-stimulated release of HMGB1 from cortical astrocytes. Glia. 2010;58:1007–1015. doi: 10.1002/glia.20982. [DOI] [PMC free article] [PubMed] [Google Scholar]