Abstract
The lack of relevant animal models is the major bottleneck for understanding human immunology and immunopathology. In the last few years, a novel model of humanized mouse has been successfully employed to investigate some of the most critical questions in human immunology. We have set up and tested in our laboratory the latest technology for generating mice with a human immune system by reconstituting newborn immunodeficient mice with human fetal liver-derived hematopoietic stem cells. These humanized mice have been deemed most competent as human models in a thorough comparative study with other humanized mouse technologies. Lymphocytes in these mice are of human origin while other hematopoietic cells are chimeric, partly of mouse and partly of human origin. We demonstrate that human CD8 T lymphocytes in humanized mice are fully responsive to our novel cell-based secreted heat shock protein gp96HIV-Ig vaccine. We also show that the gp96HIV-Ig vaccine induces powerful mucosal immune responses in the rectum and the vagina, which are thought to be required for protection from HIV infection. We posit the hypothesis that vaccine approaches tested in humanized mouse models can generate data rapidly, economically and with great flexibility (genetic manipulations are possible), to be subsequently tested in larger nonhuman primate models and humans.
Keywords: Humanized immunity, mice, Vaccine, gp96 chaperone
Introduction
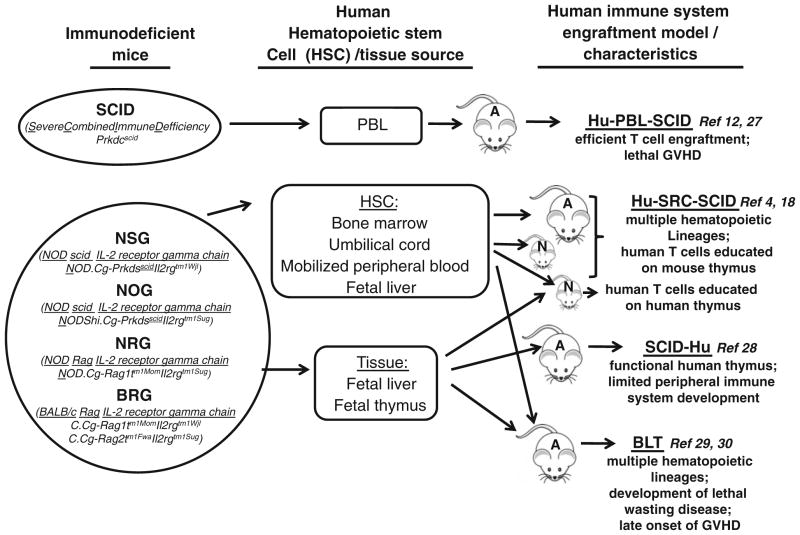
The development of novel techniques and systems to study biological processes in humans, both in an in vitro and in vivo setting, is always in high demand. Small animal models are the most efficient method of studying human afflictions; however, many aspects of mammalian biological systems, including immune systems, are species specific. Moreover, rodents are refractory to certain human-specific infectious agents, for example they are unable to support productive HIV infection, even when made to express human co-receptors for the virus [1–3]. Over the past two decades, the construction of humanized animal models through the transplantation and engraftment of human tissues or progenitor cells into immunocompromized mouse strains has allowed for the development of a reconstituted human tissue scaffold in a small animal system [4]. In recent years, the technology of constructing chimeric mice with a humanized immune system has markedly improved [5]. Early versions of humanized mice, CB-17 SCID mice which lack both T and B lymphocytes, supported productive HIV infection and allowed investigators to begin to address important questions in HIV biology in vivo [6–10]. The implantation of uneducated human immune cells and associated tissues provided the basis for the SCID-hu Thy/Liv and hu-PBL-SCID models [11, 12]. Engraftment efficiency of these tissues was further improved through the integration of the nonobese diabetic (NOD) mutation leading to the creation of NOD/SCID, and models, which further minimized the response of the murine innate immune system [13–15]. The IL-2R-common gamma chain is required for high affinity ligand binding and signaling through multiple cytokine receptors, including those for IL-2, IL-4, IL-7, IL-9, IL-15 and IL-21 [16]. Immunodeficient mice bearing a targeted mutation within the IL2R gene support higher levels of human hematolymphoid engraftment than all previous immunodeficient mouse strains and permit the engraftment of a functional human immune system [15, 17–24]. These models marked an important advancement: the use of human CD34+ hematopoietic stem cells (HSC). Human cord blood or fetal liver CD34+ HSC had been used to reconstitute and mice, resulting in higher levels of sustained human immune cell engraftment [18, 21, 25, 26]. In summary, today there are 3 main immunodeficient mouse strains that are used for creating humanized mice: NOD.Cg-PrkdcscidIL-2Rtm1Wjl (NSG mice) [20], NODShi.Cg-PrkdcscidIL-2Rtm1Sug (NOG mice) [18] and strains based on C;129S4-Rag2tm1FlvIL-2Rtm1Flv as well as BALB/c-Rag1−/− or BALB/c-Rag2−/− strains of mice (BRG mice) [21, 25] (Fig. 1). Recent evidence shows that these mouse models differ in their ability to support the engraftment of a functional human immune system. Overall, NSG and NRG mice support higher levels of human HSC engraftment and T- and B-cell development than do BRG mice [25]. There are four different technological approaches for the engraftment of a functional human immune system in the above-mentioned immunodeficient mouse strains, each with distinct advantages and caveats, discussed elsewhere [5, 27–30] and Fig. 1.
Fig. 1.
Different approaches for creating humanized mice. Different strains of immunodeficient mice :SCID (Severe Combined Immune Defficiency, Prkdcscid), NSG (NOD/scid IL-2 receptor gamma chain knock out, NOD.Cg-PrkdsscidIl2rgtm1Wjl), NOG (NOD/scid IL-2 receptor gamma chain knock out, NOD/Shi.Cg-Prkdsscid Il2rgtm1Sug), NRG (NOD/Rag IL-2 receptor gamma chain knock out, NOD.Cg-Rag1tm1MomIl2rgtm1Sug) and BRG (BALB/c Rag IL-2 receptor gamma chain knock out, C.Cg-Rag1tm1MomIl2rgtm1Wjl and C.Cg-Rag2tm1FwaIl2rgtm1Sug) were engrafted with human hematopoietic cells (HSC) and/or immune tissues to establish four different immune system engraftment models. 1. Hu-PBL-SCID: engraftment of human peripheral blood leukocytes (PBL) into SCID mouse. 2. Hu-SRC-SCID: human stem repopulating cell model, engraftment of human HSC derived from bone marrow, umbilical cord blood, fetal liver or mobilized peripheral blood into adult or neonatal NSG, NOG, NRG and BRG mice. 3. SCID-Hu: implantation of human fetal liver and thymus fragments under the renal capsule of SCID mice. 4. BLT: bone marrow, liver, thymus humanized mouse model is established by implantation of human fetal liver and thymus fragments under the renal capsule of sublethally irradiated immunodeficient mice (NSG) accompanied by intravenous injection of autologous fetal liver HSC
We have set up and tested in our laboratory the latest technology for generating mice with a human immune system by reconstituting newborn immunodeficient mice (NSG) with human fetal liver-derived hematopoietic stem cells. To overcome the problems associated with low HSC engraftment, we developed an effective conditioning regimen in recipient mice that includes a combination of total body irradiation (1 Gy) with an antibody regiment capable of selective depletion of endogenous mouse HSC (anti-mouse c-kit antibody). In this model, we have achieved robust repopulation of mouse lymphoid tissues with human immune cells and have generated robust cellular immune responses systemically and in mucosal compartments. Furthermore, recently we modified the model by implanting human fetal thymic tissue subcutaneously accompanied by intrahepatic injection of human autologous fetal liver HSC. We believe this improved humanized mouse model will allow us to study questions surrounding innovative vaccines and their protective effects not readily approachable through human studies.
Generation of humanized mice
Breeding and purification of human CD34+ HSC
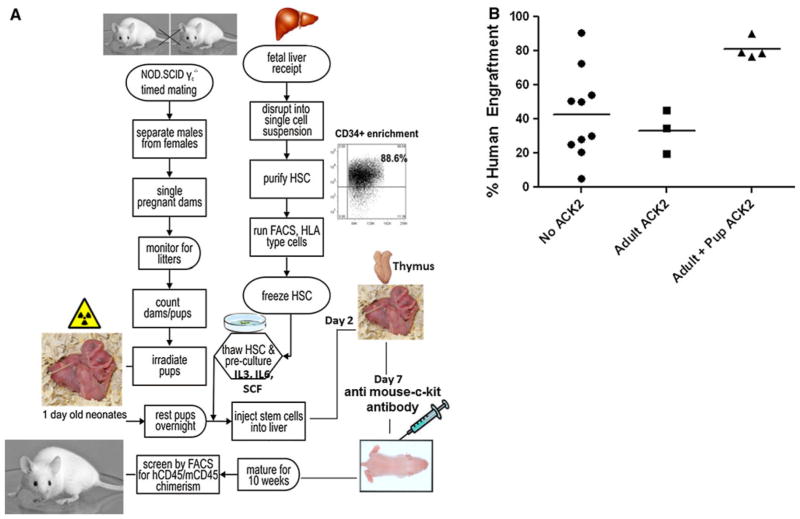
The construction of humanized mice, as shown in Figs. 1 and 2, is a very complex process and requires a breeding colony of mice, a source of fetal tissue, purification of fetal CD34+ HSC, an irradiator and the surgical expertise necessary for proper implantation of human fetal thymus. We have established our own protocol for the generation of humanized mice (Fig. 1). In brief, mice are bred in two parallel cycles that are offset by 7 days. During the breeding cycle, mice are placed in timed matings on day 0 and separated on day 4; pregnant mice are segregated to single cages on day 18 and monitored through day 23 for litters.
Fig. 2.
Generation of humanized mice and breeding schedule. a mice are bred in two parallel cycles. Fetal liver tissue is processed into single cell suspensions that are frozen for later pre-culture with cytokines and transplant into neonates. One-day-old mice are irradiated (sublethal, 1 Gy, whole body irradiation) and returned to dams for 24 h at which time they are transplanted with 106–2 × 106 pre-cultured HSC intrahepatically (i.h.). At day 2, subcutaneous implantation of autologous human thymic tissue (1–2 mm3 fragments) is performed. At week 1 and 5, mice are injected with 100 g anti-mouse c-kit antibody (ACK2) i.p. Ten weeks later, mice are screened for human/mouse chimerism. b Comparison of human engraftment levels in mice that received antimouse c-kit antibody (ACK2) as adult (5 weeks old mice) or as a pups and adult (1 and 5 weeks old) with control mice (no antibody treatment)
Human fetal liver and thymus from elective terminations, 12–23 weeks of gestational age (Advanced Bioscience Resources, Alameda, CA), are acquired on a fee for service basis, and the tissue is delivered approximately every 14 days. Upon receipt, liver tissue is digested with collagenase to make single cell suspensions. After density gradient centrifugation, CD34+ cells are enriched using immunomagnetic beads according to the manufacturer’s instructions (CD34+ selection kit; Stem Cell Technologies, Vancouver, Canada). Purity of isolated CD34+ HSC is evaluated by flow cytometry at >90 %. Purified cells are aliquoted and frozen for later HLA typing and transplantation into neonatal mice. HSC designated for transplant are thawed on day 21 of the breeding cycle and placed into culture containing 10 % human serum, IL-3, IL-6 and SCF (10 ng/ml) for 3 days. On the day of transplant, cells are harvested from culture and washed in HBSS to remove traces of serum and exogenous cytokine.
Isolated donor CD34+ HSC are assessed for purity as well as for their potential to proliferate and differentiate into progenitor cells using CFU and LTC-IC assays in addition to being HLA typed. Briefly, cells are plated in methylcellulose media supplemented with SCF, GM-CSF, IL-3 and EPO, at various cell concentrations, in triplicate. After 14 days, colonies are identified and analyzed. Because no established references could be found for progenitor counts from fetal liver cells, we compared our colony counts to known counts for CD34+ bone marrow. None of our tissues gave rise to colonies in numbers significantly different than those for bone marrow.
Transplantation of human CD34+ HSC and characterization of human immune reconstitution
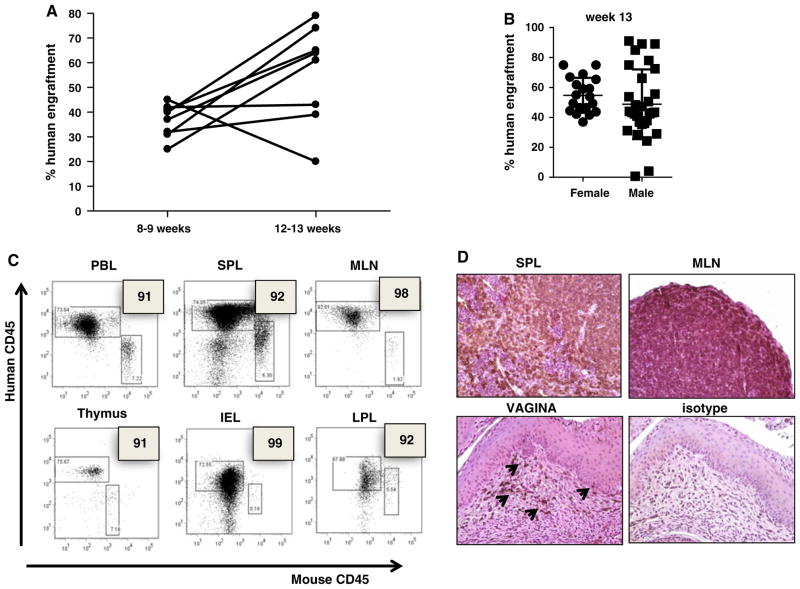
Newborn 1-day-old mice are irradiated with a single sublethal dose of 1 Gy whole body irradiation and then housed with dams for 24 h (Fig. 2a). Transplantation in mice older than 4 days is difficult because the liver is more difficult to visualize through the skin as the mouse ages. Importantly, it has been demonstrated that newborn mice (less than 3 days) support higher transplant efficiency [17, 21, 25, 31, 32]. Allowing the mice to recover from irradiation for 1 day, they are injected with 1–2 × 106 pre-cultured HSC, intrahepatically in a volume of 30 μl. The pups are immediately returned to their dams and monitored for the next few hours. At day 7, mice are injected i.p. with 100 g of anti-c-kit antibody (ACK2) (a generous gift from Dr. I. Weissman). It has been shown that administration of ACK2 leads to the transient removal of more than 98 % of endogenous HSCs in immunodeficient mice [33]. We reasoned that human HSC engraftment might be limited by the occupancy of appropriate niches by endogenous, mouse HSC and that the specific removal of host HCS could significantly improve engraftment. The pups are allowed to nurse until weaning at 28 days. At week 5, mice are again injected i.p. with 100 μg of ACK2. Weaned mice are further allowed 2–3 weeks of maturation before being screened for initial levels of human/mouse chimerism. Mice 8–12 weeks old are bled, and heparinized peripheral blood is treated with red cell lysis buffer and analyzed for relative percentages of murine and human CD45+ cells by flow cytometry. Once human CD45+ cells are detected, analysis is extended to include human CD3, CD4, CD8, CD56, CD11c, CD19, HLA-DR and CD123 on all blood collections. A human CD45+ level of >2 % was considered positive. Reconstituted mice became candidates for functional analyses of the immune system when at least several members of the cohort showed measurable levels of engrafted cells in circulation (≤30 %). Mice that received antibody as a pup and adult had significantly higher engraftment than the mice that received the ACK2 treatment when adults (week 5) (Fig. 2b). All mice survived the ACK2 treatment with no signs of distress. Consistent with prior reports [4, 10, 11, 19, 34], mice generated in our hands demonstrated a high proportion of human CD45+ cells among the leukocyte population in the peripheral blood (85 % human to 15 % murine, range 98/15–55/35) that continued to increase overtime; remaining stable for at least 1 year (Fig. 3a, b). mice, which lack NK cell activity, have been reported to support improved human immune engraftment compared to that of NOD/SCID mice [17, 20]. Interestingly, human HSC engraftment was more efficient in female NSG recipient mice than in male (Fig. 3b). Similar observations have been reported by other investigators [34, 35]. Furthermore, we found that hu-mice demonstrate a high level engraftment of human CD45+ cells not only in their peripheral blood but also in the peripheral tissues: spleen, mesenteric lymph nodes, gut and the reproductive tract mucosal tissue (lamina propria and intraepithelial compartment) (Fig. 3c, d).
Fig. 3.
Human immune reconstitution in mice after fetal liver CD34+ hematopoietic cell engraftment. a Chimerism (% human engraftment) is determined with anti-human and anti-mouse CD45 at weeks 8–12,12–13, a and b. Representative flow cytometry dot plots of chimerism in several compartments: PBL, spleen, mesenteric lymph nodes (MLN), thymus, gut intraepithelial compartment (IEL) and lamina propria (LPL). c Immunohistochemical analysis of human CD45 expression in the spleen (SPL), mesenteric lymph nodes (MLN) and vaginal tissue
Recently, we have established the novel protocol of combined intrahepatic injection of human fetal liver HSC at day 2 and subcutaneous implantation of autologous human thymic tissue at day 4 (Figs. 1, 2). We have observed that subcutaneous co-transplant of human fetal thymus with autologous HSC increases human immune reconstitution, particularly T regulatory cells. The implantation of human fetal thymic and liver tissues accompanied by i.v. injections of human fetal HSC offers a number of reported advantages, including the robust levels of human chimerism, development of functional T cells and education of T cell progenitors on autologous human thymic epithelium [36, 37].
T, B, NK cell and DC reconstitution
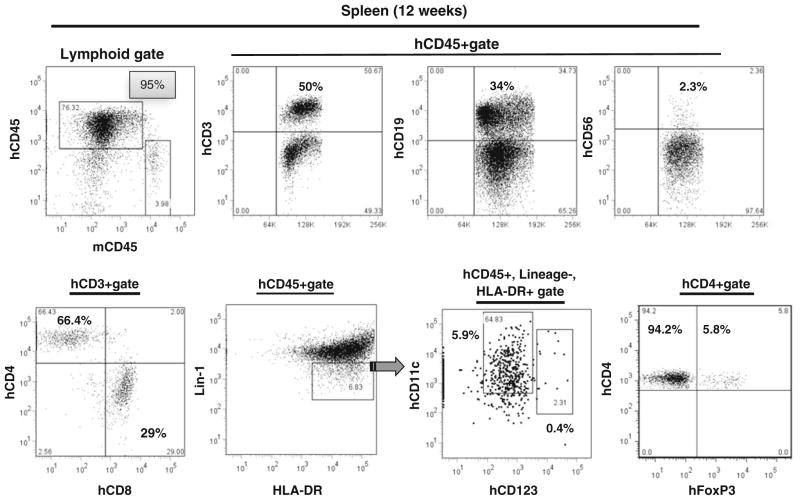
We next identified human T cells, B cells, NK cells, dendritic cells (DC) and T regulatory cells (Treg) in humanized mice, using polychromatic flow cytometry with the gating scheme shown in Fig. 4, depicting a representative sample of spleen leukocytes from a 12-week-old humanized mouse. As shown in Fig. 4, the majority of human CD45+ lymphocytes in the spleen were T cells (~50 %), followed by B cells (30 %), NK cells (1.2 %) and DC (1 %). Most of the CD3+ cells were CD4+ T cells. Intracellular staining for Foxp3 revealed the presence of Treg cells. As demonstrated in Fig. 4, both CD11c+ myeloid DCs (mDC) and CD123+ plasmocytoid DCs (pDC) could be identified among human CD45+, lineage-negative (CD16−, CD3−, CD19−), HLA-DR+ cells. 1 % of human CD45+ cells in the blood were mDC, whereas pDC represented 0.2 % of human CD45+ cells. Similarly, greater numbers of mDCs than pDCs have been noted in human peripheral blood [38, 39].
Fig. 4.
T-cell, B-cell, NK cell and DC reconstitution of 12 weeks old hu-mouse was sacrificed, and human cells were identified in blood by flow cytometry following staining with anti-mouse CD45 and antihuman CD45, CD3, CD4, CD8, CD19, CD56, Lin1, HLA-DR, CD123, CD25 and Foxp3 antibody. mDC are identified as CD11c+ and pDC as CD123+ cells among human CD45+, lin-1 (lineage-1) negative (CD16−, CD3−, CD19−), HLA-DR+ cells. Numbers in the panels represent the percentage of cells contained in the indicated gates
Specific T cell immune response
Human CTL in humanized mice are cross-primed, expand and migrate to mucosal sites in response to allogeneic 293-gp96HIV-gag-Ig vaccination. Over the last two decades, Dr. Podack and his group have developed an innovative vaccine approach based on a genetically modified form of the endoplasmic reticulum (ER) chaperone gp96, the fusion protein gp96-Ig. The gp96-Ig vaccine is composed of live, but replication incompetent cells, containing the antigens of interest and secreting gp96-Ig, which has been introduced into the cell by transfection and G418-selection [40–48]. Secreted gp96-Ig chaperones peptides (gp96pep-Ig) come into ER via the TAP transporter [49]. Gp96 also chaperones proteins synthesized in the ER of the vaccine cells [50], including peptides and proteins derived from infectious agents or tumor antigens. In murine studies, nonhuman primates and in cancer patients, we have shown that gp96-Ig-based vaccines generate CD8 CTL responses to gp96-Ig-chaperoned, antigenic peptides derived from SIV or HIV and to other test antigens. Immune responses ensue systemically and are especially powerful in the intestinal, rectal and vaginal mucosa [40, 47, 51].
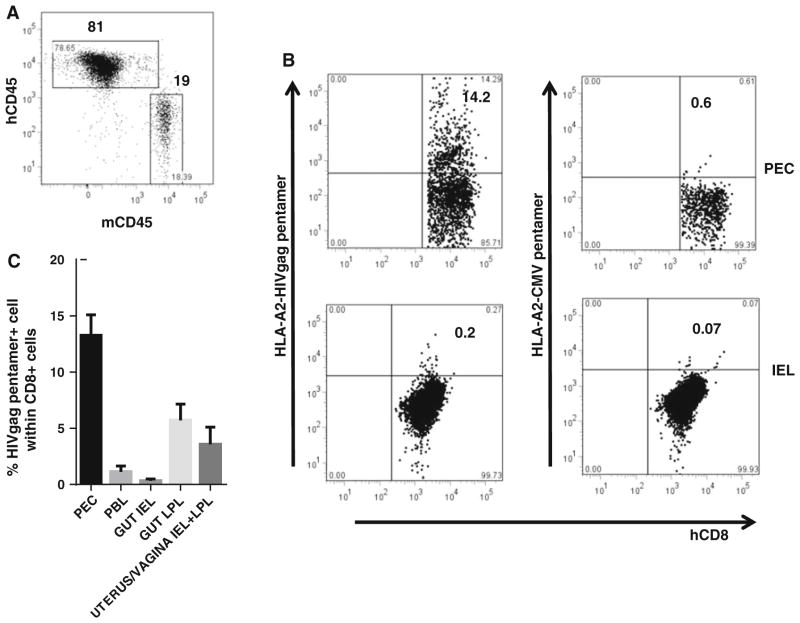
To determine HIV-gp96-Ig cross-priming with human immune cells, we used humanized mice generated from NSG mice transplanted with HLA A2+ human fetal liver CD34+ cells, with ~80 % chimerism for the human CD45 molecule (Fig. 5a). As vaccine cells, we used allogeneic 293-gp96HIV-gag-Ig cells secreting 1 μg gp96HIVgag-Ig in 24 h. The cells were injected i.p. on day 0 and 14 into 12-week-old mice. The HIV structural protein Gag contains the peptide SLYNVATL, which is presented by HLA A2. Gag-specific CD8 CTL in HLA A2+ humanized mice can be detected with HLA A2-gag-tetramers or pentamers. As negative control for specificity, HLA A2-CMV pentamers were used. The mice were analyzed 5 days after the second vaccination to determine the frequency of gag-specific CD8 CTL at various sites. Human gag-specific CD8 cells expanded from undetectable to 14 % of all CD8 cells after two immunizations (Fig. 5b) in the peritoneal cavity; the assay is specific because HLA A2-pentamer carrying CMV peptides did not detect any CD8 T cells. Importantly, high frequencies of gag-specific CD8 CTL were also found after vaccination in the intestinal/vaginal lamina propria (LPL) and among intestinal/vaginal intraepithelial lymphocytes (IEL), in mesenteric lymph nodes (MLN), spleen (SP) and peripheral blood (PBL) (Fig. 5c). These data show for the first time that human CD8 CTL in humanized mice are generated by cross-presentation induced by gp96HIVgag-Ig, which depends on HLA A2+ APC (DC)—CD8 interaction and priming and NK help. The data also show a normal trafficking pattern of human CD8 CTL to the mucosa. Therefore, human cells in humanized mice are able to receive the proper signals and programming instructions for migration within the murine environment and exhibit proper homing behavior to mucosal sites including epithelia (IEL).
Fig. 5.
Expansion of human CD8 CTL in hu-mice to 293-gp96HIVgag-Ig vaccination. were transplanted with HLA-A2+ human fetal liver CD34+ cells. The vaccine cells, 293-gp96HIVgag (secreting 1 μg gp96 HIVgag in 24 h), were injected i.p on days 0 and 14 in 12-week-old mice and analyzed 5 days later gating on hu-CD45+ cells. a Chimerism in blood. b HLA A2-gag-pentamer+ cells in the peritoneal cavity (PEC) and within intraepithelial lymphocytes (IEL). HLA A2 CMV pentamers as specificity control. c Expansion and trafficking of HLA A2-gag-pentamer+ cells to various sites. PEC peritoneal exudates cells, SP spleen, PBL peripheral blood lymphocytes, MLN mesenteric lymph node, LPL lamina propria lymphocytes, IEL intraepithelial lymphocytes
In summary
Humanized mice have the advantage of providing a small animal model with these associated benefits: they are economical, offer greater flexibility, are susceptible to genetic manipulations and experimental protocols not ethical in humans. Using defined HLA types for reconstitution of immunodeficient mice, epitope-specific responses can be measured using HLA tetramers/pentamers.
To further overcome the limitations of humanized mice vis-à-vis the murine microenvironment, treatment for humanized mice with antimouse c-kit antibody (ACK2) enhanced the human HSC engraftment.
Allogeneic HIV-gag vaccine cells via gp96HIV-gag-Ig secretion in the murine environment induce gag-antigen cross-presentation by human HLA A2+ APC to prime the expansion of human HLA A2 restricted gag-specific CD8 CTL (Fig. 5). This evidence validates the humanized mouse model as an excellent system for testing novel vaccines.
The hu-mice studies described here will allow a comparison of the macaque model with the humanized mouse model in order to evaluate which model better reflects human HIV pathogenesis and vaccine responses.
Acknowledgments
The work is supported by the NIAID R33 AI 073234, NCATS NIH UL1TR000460 and 1KL2TR000461, Miami-CFAR and NIH P30A1073961, Biopsychosocial Research Training In Immunology and AIDS 5T32MH018917-22, National Cancer Institute, Center for Cancer Research and support from the Alliance for Cancer Gene Therapy (ACGT), New York.
Footnotes
Conflict of interest Dr. E. R. Podack and the University of Miami have financial interest and hold equity in a commercial enterprise developing this vaccine technology.
References
- 1.Browning J, Horner JW, Pettoello-Mantovani M, Raker C, Yurasov S, DePinho RA, et al. Mice transgenic for human CD4 and CCR5 are susceptible to HIV infection. Proc Natl Acad Sci USA. 1997;94(26):14637–41. doi: 10.1073/pnas.94.26.14637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lores P, Boucher V, Mackay C, Pla M, Von Boehmer H, Jami J, et al. Expression of human CD4 in transgenic mice does not confer sensitivity to human immunodeficiency virus infection. AIDS Res Hum Retroviruses. 1992;8(12):2063–71. doi: 10.1089/aid.1992.8.2063. [DOI] [PubMed] [Google Scholar]
- 3.Sawada S, Gowrishankar K, Kitamura R, Suzuki M, Suzuki G, Tahara S, et al. Disturbed CD4+ T cell homeostasis and in vitro HIV-1 susceptibility in transgenic mice expressing T cell line-tropic HIV-1 receptors. J Exp Med. 1998;187(9):1439–49. doi: 10.1084/jem.187.9.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brehm MA, Cuthbert A, Yang C, Miller DM, DiIorio P, Laning J, et al. Parameters for establishing humanized mouse models to study human immunity: analysis of human hematopoietic stem cell engraftment in three immunodeficient strains of mice bearing the IL2rgamma(null) mutation. Clin Immunol. 2010;135(1):84–98. doi: 10.1016/j.clim.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shultz LD, Brehm MA, Garcia-Martinez JV, Greiner DL. Humanized mice for immune system investigation: progress, promise and challenges. Nat Rev Immunol. 2012;12(11):786–98. doi: 10.1038/nri3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gorantla S, Santos K, Meyer V, Dewhurst S, Bowers WJ, Federoff HJ, et al. Human dendritic cells transduced with herpes simplex virus amplicons encoding human immunodeficiency virus type 1 (HIV-1) gp120 elicit adaptive immune responses from human cells engrafted into NOD/SCID mice and confer partial protection against HIV-1 challenge. J Virol. 2005;79(4):2124–32. doi: 10.1128/JVI.79.4.2124-2132.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakata H, Maeda K, Miyakawa T, Shibayama S, Matsuo M, Takaoka Y, et al. Potent anti-R5 human immunodeficiency virus type 1 effects of a CCR5 antagonist, AK602/ONO4128/ GW873140, in a novel human peripheral blood mononuclear cell nonobese diabetic-SCID, interleukin-2 receptor gamma-chain-knocked-out AIDS mouse model. J Virol. 2005;79(4):2087–96. doi: 10.1128/JVI.79.4.2087-2096.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKinney DM, Lewinsohn DA, Riddell SR, Greenberg PD, Mosier DE. The antiviral activity of HIV-specific CD8+ CTL clones is limited by elimination due to encounter with HIV-infected targets. J Immunol. 1999;163(2):861–7. [PubMed] [Google Scholar]
- 9.Mosier DE, Gulizia RJ, Baird SM, Wilson DB, Spector DH, Spector SA. Human immunodeficiency virus infection of human-PBL-SCID mice. Science. 1991;251(4995):791–4. doi: 10.1126/science.1990441. [DOI] [PubMed] [Google Scholar]
- 10.Namikawa R, Kaneshima H, Lieberman M, Weissman IL, McCune JM. Infection of the SCID-hu mouse by HIV-1. Science. 1988;242(4886):1684–6. doi: 10.1126/science.3201256. [DOI] [PubMed] [Google Scholar]
- 11.Carballido JM, Namikawa R, Carballido-Perrig N, Antonenko S, Roncarolo MG, de Vries JE. Generation of primary antigen-specific human T- and B-cell responses in immunocompetent SCID-hu mice. Nat Med. 2000;6(1):103–6. doi: 10.1038/71434. [DOI] [PubMed] [Google Scholar]
- 12.Mosier DE, Gulizia RJ, Baird SM, Wilson DB. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature. 1988;335(6187):256–9. doi: 10.1038/335256a0. [DOI] [PubMed] [Google Scholar]
- 13.Shultz LD, Schweitzer PA, Christianson SW, Gott B, Schweitzer IB, Tennent B, et al. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J Immunol. 1995;154(1):180–91. [PubMed] [Google Scholar]
- 14.Hesselton RM, Greiner DL, Mordes JP, Rajan TV, Sullivan JL, Shultz LD. High levels of human peripheral blood mononuclear cell engraftment and enhanced susceptibility to human immunodeficiency virus type 1 infection in NOD/LtSz-scid/scid mice. J Infect Dis. 1995;172(4):974–82. doi: 10.1093/infdis/172.4.974. [DOI] [PubMed] [Google Scholar]
- 15.van Rijn RS, Simonetti ER, Hagenbeek A, Hogenes MC, de Weger RA, Canningavan Dijk MR, et al. A new xenograft model for graft-versus-host disease by intravenous transfer of human peripheral blood mononuclear cells in RAG2−/− gammac−/− double-mutant mice. Blood. 2003;102(7):2522–31. doi: 10.1182/blood-2002-10-3241. [DOI] [PubMed] [Google Scholar]
- 16.Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nat Rev Immunol. 2009;9(7):480–90. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishikawa F, Yasukawa M, Lyons B, Yoshida S, Miyamoto T, Yoshimoto G, et al. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor gamma chain(null) mice. Blood. 2005;106(5):1565–73. doi: 10.1182/blood-2005-02-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, et al. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100(9):3175–82. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- 19.Legrand N, Weijer K, Spits H. Experimental models to study development and function of the human immune system in vivo. J Immunol. 2006;176(4):2053–8. doi: 10.4049/jimmunol.176.4.2053. [DOI] [PubMed] [Google Scholar]
- 20.Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, et al. Human lymphoid and myeloid cell development in NOD/ LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174(10):6477–89. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 21.Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti JC, Lanzavecchia A, et al. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304(5667):104–7. doi: 10.1126/science.1093933. [DOI] [PubMed] [Google Scholar]
- 22.Brehm MA, Shultz LD, Greiner DL. Humanized mouse models to study human diseases. Curr Opin Endocrinol Diabetes Obes. 2010;17(2):120–5. doi: 10.1097/MED.0b013e328337282f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stoddart CA, Maidji E, Galkina SA, Kosikova G, Rivera JM, Moreno ME, et al. Superior human leukocyte reconstitution and susceptibility to vaginal HIV transmission in humanized NOD-scid IL-2Rgamma(−/−) (NSG) BLT mice. Virology. 2011;417(1):154–60. doi: 10.1016/j.virol.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.King M, Pearson T, Shultz LD, Leif J, Bottino R, Trucco M, et al. A new Hu-PBL model for the study of human islet alloreactivity based on NOD-scid mice bearing a targeted mutation in the IL-2 receptor gamma chain gene. Clin Immunol. 2008;126(3):303–14. doi: 10.1016/j.clim.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Brehm MA, Cuthbert A, Yang C, Miller DM, DiIorio P, Laning J, et al. Parameters for establishing humanized mouse models to study human immunity: analysis of human hematopoietic stem cell engraftment in three immunodeficient strains of mice bearing the IL2rgamma(null) mutation. Clin Immunol. 2010;135(1):84–98. doi: 10.1016/j.clim.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang Q, Zhang L, Wang R, Jeffrey J, Washburn ML, Brouwer D, et al. FoxP3+CD4+regulatory T cells play an important role in acute HIV-1 infection in humanized Rag2−/− gammaC−/− mice in vivo. Blood. 2008;112(7):2858–68. doi: 10.1182/blood-2008-03-145946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.King MA, Covassin L, Brehm MA, Racki W, Pearson T, Leif J, et al. Human peripheral blood leucocyte non-obese diabetic-severe combined immunodeficiency interleukin-2 receptor gamma chain gene mouse model of xenogeneic graft-versus-host-like disease and the role of host major histocompatibility complex. Clin Exp Immunol. 2009;157(1):104–18. doi: 10.1111/j.1365-2249.2009.03933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7(2):118–30. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- 29.McCune JM, Namikawa R, Kaneshima H, Shultz LD, Lieberman M, Weissman IL. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988;241(4873):1632–9. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- 30.Covassin L, Jangalwe S, Jouvet N, Laning J, Burzenski L, Shultz LD, et al. Human immune system development and survival of non-obese diabetic (NOD)-scid IL2rgamma(null) (NSG) mice engrafted with human thymus and autologous haematopoietic stem cells. Clin Exp Immunol. 2013;174(3):372–88. doi: 10.1111/cei.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gimeno R, Weijer K, Voordouw A, Uittenbogaart CH, Legrand N, Alves NL, et al. Monitoring the effect of gene silencing by RNA interference in human CD34+ cells injected into newborn RAG2−/− gammac−/− mice: functional inactivation of p53 in developing T cells. Blood. 2004;104(13):3886–93. doi: 10.1182/blood-2004-02-0656. [DOI] [PubMed] [Google Scholar]
- 32.Zhang L, Su L. HIV-1 immunopathogenesis in humanized mouse models. Cell Mol Immunol. 2012;9(3):237–44. doi: 10.1038/cmi.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Czechowicz A, Kraft D, Weissman IL, Bhattacharya D. Efficient transplantation via antibody-based clearance of hematopoietic stem cell niches. Science. 2007;318(5854):1296–9. doi: 10.1126/science.1149726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Notta F, Doulatov S, Dick JE. Engraftment of human hematopoietic stem cells is more efficient in female NOD/SCID/IL-2Rgc-null recipients. Blood. 2010;115(18):3704–7. doi: 10.1182/blood-2009-10-249326. [DOI] [PubMed] [Google Scholar]
- 35.McDermott SP, Eppert K, Lechman ER, Doedens M, Dick JE. Comparison of human cord blood engraftment between immunocompromised mouse strains. Blood. 2010;116(2):193–200. doi: 10.1182/blood-2010-02-271841. [DOI] [PubMed] [Google Scholar]
- 36.Wege AK, Melkus MW, Denton PW, Estes JD, Garcia JV. Functional and phenotypic characterization of the humanized BLT mouse model. Curr Top Microbiol Immunol. 2008;324:149–65. doi: 10.1007/978-3-540-75647-7_10. [DOI] [PubMed] [Google Scholar]
- 37.Joo SY, Chung YS, Choi B, Kim M, Kim JH, Jun TG, et al. Systemic human T cell developmental processes in humanized mice cotransplanted with human fetal thymus/liver tissue and hematopoietic stem cells. Transplantation. 2012;94(11):1095–102. doi: 10.1097/TP.0b013e318270f392. [DOI] [PubMed] [Google Scholar]
- 38.Giannelli S, Taddeo A, Presicce P, Villa ML, Della Bella S. A six-color flow cytometric assay for the analysis of peripheral blood dendritic cells. Cytometry B Clin Cytom. 2008;74(6):349–55. doi: 10.1002/cyto.b.20434. [DOI] [PubMed] [Google Scholar]
- 39.Berges BK, Akkina SR, Remling L, Akkina R. Humanized Rag2(−/−) gammac(−/−) (RAG-hu) mice can sustain long-term chronic HIV-1 infection lasting more than a year. Virology. 2010;397(1):100–3. doi: 10.1016/j.virol.2009.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strbo N, Pahwa S, Kolber MA, Gonzalez L, Fisher E, Podack ER. Cell-secreted Gp96-Ig-peptide complexes induce lamina propria and intraepithelial CD8+ cytotoxic T lymphocytes in the intestinal mucosa. Mucosal Immunol. 2010;3(2):182–92. doi: 10.1038/mi.2009.127. [DOI] [PubMed] [Google Scholar]
- 41.Schreiber TH, Deyev VV, Rosenblatt JD, Podack ER. Tumor-induced suppression of CTL expansion and subjugation by gp96-Ig vaccination. Cancer Res. 2009;69(5):2026–33. doi: 10.1158/0008-5472.CAN-08-3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oizumi S, Deyev V, Yamazaki K, Schreiber T, Strbo N, Rosenblatt J, et al. Surmounting tumor-induced immune suppression by frequent vaccination or immunization in the absence of B cells. J Immunother. 2008;31(4):394–401. doi: 10.1097/CJI.0b013e31816bc74d. [DOI] [PubMed] [Google Scholar]
- 43.Oizumi S, Strbo N, Pahwa S, Deyev V, Podack ER. Molecular and cellular requirements for enhanced antigen cross-presentation to CD8 cytotoxic T lymphocytes. J Immunol. 2007;179(4):2310–7. doi: 10.4049/jimmunol.179.4.2310. [DOI] [PubMed] [Google Scholar]
- 44.Strbo N, Oizumi S, Sotosek-Tokmadzic V, Podack ER. Perforin is required for innate and adaptive immunity induced by heat shock protein gp96. Immunity. 2003;18(3):381–90. doi: 10.1016/s1074-7613(03)00056-6. [DOI] [PubMed] [Google Scholar]
- 45.Yamazaki K, Nguyen T, Podack ER. Cutting edge: tumor secreted heat shock-fusion protein elicits CD8 cells for rejection. J Immunol. 1999;163(10):5178–82. [PubMed] [Google Scholar]
- 46.Podack ER, Raez LE. Allogeneic tumor-cell-based vaccines secreting endoplasmic reticulum chaperone gp96. Expert Opin Biol Ther. 2007;7(11):1679–88. doi: 10.1517/14712598.7.11.1679. [DOI] [PubMed] [Google Scholar]
- 47.Strbo N, Vaccari M, Pahwa S, Kolber MA, Fisher E, Gonzalez L, et al. Gp96 SIV Ig immunization induces potent polyepitope specific, multifunctional memory responses in rectal and vaginal mucosa. Vaccine. 2011;29(14):2619–25. doi: 10.1016/j.vaccine.2011.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Strbo N, Vaccari M, Pahwa S, Kolber MA, Doster MN, Fisher E, et al. Cutting edge: novel vaccination modality provides significant protection against mucosal infection by highly pathogenic simian immunodeficiency virus. J Immunol. 2013;190(6):2495–9. doi: 10.4049/jimmunol.1202655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kropp LE, Garg M, Binder RJ. Ovalbumin-derived precursor peptides are transferred sequentially from gp96 and calreticulin to MHC class I in the endoplasmic reticulum. J Immunol. 2010;184(10):5619–27. doi: 10.4049/jimmunol.0902368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Staron M, Yang Y, Liu B, Li J, Shen Y, Zuniga-Pflucker JC, et al. gp96, an endoplasmic reticulum master chaperone for integrins and Toll-like receptors, selectively regulates early T and B lymphopoiesis. Blood. 2010;115(12):2380–90. doi: 10.1182/blood-2009-07-233031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strbo N, Pahwa S, Kolber MA, Gonzalez L, Fisher E, Podack ER. Cell-secreted Gp96-Ig-peptide complexes induce lamina propria and intraepithelial CD8+ cytotoxic T lymphocytes in the intestinal mucosa. Mucosal Immunol. 2010;3(2):182–92. doi: 10.1038/mi.2009.127. [DOI] [PubMed] [Google Scholar]