Abstract
Elevated serum levels of a glycoprotein known as chitinase-3-like protein 1 (CHI3L1) have been correlated with poor prognosis and shorter survival of patients with cancer and inflammatory diseases. The biological and physiological functions of CHI3L1 in cancer have not yet been completely elucidated. In this review, we describe the role of CHI3L1 in inducing pro-inflammatory/pro-tumorigenic and angiogenic factors that could promote tumor growth and metastasis.
Keywords: Chitinase-3-like-1 protein, Angiogenesis, Inflammation, Metastasis, Chitin
Introduction
Chitinase-3-like-1 protein, also known as YKL-40, HC-gp39, and breast regression protein 39, is a member of 18-glycosyl hydrolase family [1–3]. Chitinase-3-like protein 1 (CHI3L1) is a 40 kD mammalian glycoprotein. The gene for CHI3L1 is located on chromosome 1q31–32 and consists of 10 axons and spans ~ 8 kB of genomic DNA [4]. The 18-glycosylhydrolase family includes chitinases with and without glycohydrolase enzymatic activity. The only two members with enzymatic activity are chitotriosidase (CHIT1) and acidic mammalian chitinase. The rest of the members in this family bind to chitin or chito-oligosaccharides with high affinity but do not have chitinase/hydrolase activity due to the substitution of an essential glutamic acid with leucine in the chitinase-3-like catalytic domain [5, 6] and thus are known as chitinase-like lectins or chi-lectins. Chitin, a polymer of β1–4 linked N-acetyl-glucosamine, is found in exoskeleton of crustaceans, the lining of digestive tracts in insects, and in the walls of fungi and microfilarial sheaths of parasites [7–13]. Chitin is the second most abundant polysaccharide in nature after cellulose. Although chitin is not found in mammals, several mammalian proteins with homologies to bacterial, fungal, and plant chitinases are expressed in mammals. CHI3L1 also interacts with glycoaminoglycans such as heparin and hyaluronan [3, 14, 15]. Further, CHI3L1 has been reported to bind to collagen type I, II, and III [16].
Although CHI3L1 is not expressed under physiological conditions, an induction of this molecule is observed in patients with inflammatory diseases and cancer. CHI3L1 is normally expressed by many different cell types such as chondrocytes [1], synoviocytes [17], vascular smooth muscle cells [3], macrophages [4], and neutrophils [18]. Additionally, these glycoproteins are known to be expressed in several types of solid tumors that include breast [19], colon [20], kidney [21], small cell lung carcinoma [22],ovarian [23, 24], prostate [25], endometrial [26], malignant melanoma [27], glioblastoma [19], and Hodgkin’s lymphoma [28] (Table 1). Increased CHI3L1 levels have been correlated with poor prognosis and decreased survival rates of cancer patients [19, 20, 23, 29, 30]. Therefore, CHI3L1 may serve as a possible biomarker for cancer.
Table 1.
Expression of CHIL1/YKL-40
| Inflammatory diseases | Cancer | References |
|---|---|---|
| Rheumatoid arthritis | Breast cancer | [19, 56] |
| Osteoarthritis | Colon cancer | [1, 20] |
| Inflammatory bowel disease | Kidney cancer | [5, 21, 57] |
| Sarcoidosis | Small cell carcinoma | [22, 58] |
| Chronic obstructive pulmonary disease | Ovarian cancer | [23, 24, 59] |
| Asthma | Prostate cancer | [25, 60] |
| Atherosclerosis | Endometrial cancer | [26, 61] |
| Type 1 and 2 diabetes | Malignant melanoma | [27, 61] |
| Liver fibrosis | Glioblastoma | [19, 62, 63] |
| Encephalitis | Hodgkin’s lymphoma | [28, 64] |
Although the biological and physiological functions of these highly conserved members in the chitinase family have not been completely elucidated, few studies in literature have explored the roles of these molecules in different disease states. Recently, we, in addition to others, have explored the biological role of one of the members in chi-lectin group, CHI3L1 in inflammatory diseases including cancer [31–34]. CHI3L1 has been shown to regulate cell proliferation and survival [35], function as a growth factor capable of stimulating connective tissue cell growth and endothelial cell migration [21, 36], display mitogenic activity on human skin, lung fibroblasts, and synovial cells [1], inhibit mammary epithelial cell differentiation [35, 37–39], and induce angiogenesis in cancer [40, 41]. Despite these findings, the pathophysiological functions of CHI3L1 are still not fully understood. In our studies using well-characterized DA-3 and 4T1 mouse mammary adenocarcinoma models, we explored the role of CHI3L1 in breast cancer growth and metastasis. Using these tumor models, we have shown that high levels of CHI3L1 are found in circulation that they are produced by both tumor cells and macrophages from tumor bearers, and that they play an important role in tumor growth and metastasis [34].
CHI3L1 and cancer
Numerous studies have correlated high serum levels of CHI3L1 with poor prognosis and survival in a variety of human carcinomas, including breast cancer [42], colorectal cancer [20], ovarian cancer [23], leukemia [29], lymphoma [43], metastatic prostate cancer [44], lung cancer [22], and glioblastoma (GBM) [30]. Elevated serum levels of CHI3L1 have also been related to aggressiveness of metastatic disease [42]. Tissue samples from invasive breast cancers, breast ductal carcinoma in situ (DCIS), and cancer-free reduction mammoplasty were evaluated for CHI3L1 expression. It was shown that increased CHI3L1 levels go hand-in-hand with tumor grade and poor differentiation of cancer cells [45]. Tissue microarray studies correlated CHI3L1 expression with estrogen receptor and progesterone receptor negativity and were positively correlated with Her-2/neu-enriched and basal-like tumors [46]. As it is well known that triple-negative breast tumors are associated with poor survival of breast cancer patients, CHI3L1 could be considered as a prognosticator indicator. Therefore, CHI3L1 may be one of the more promising prognostic markers for cervical adenocarcinoma [31], recurrent breast cancer [47], metastatic breast cancer [42], and advanced breast cancer [48].
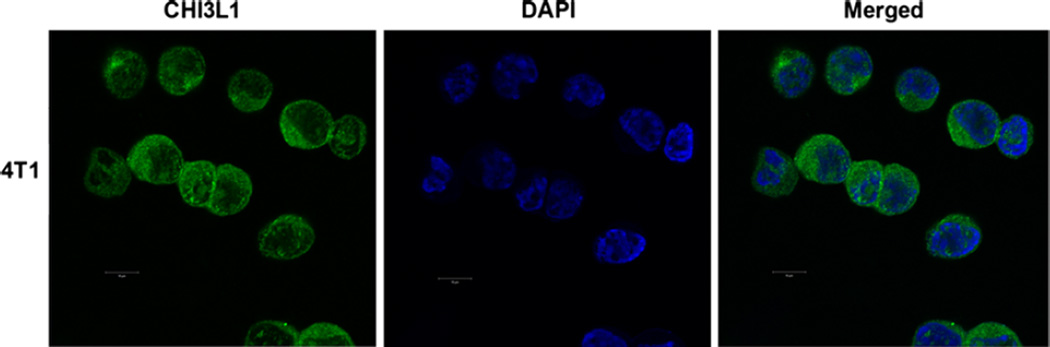
4T1 mammary tumor model used in our studies closely mimics human breast cancer in that it exhibits spontaneous metastasis to the lung, bone, and liver. We have shown that both 4T1 and DA-3 mouse mammary tumors used in our studies express CHI3L1 at the mRNA [34] and protein levels (Fig. 1). Overexpression of CHI3L1 in MD MBA-231, U87, and HCT116 cancer cells resulted in larger tumor volume and shorter survival rates compared to the controls [41, 49, 50]. These studies suggest that CHI3L1 may play a crucial role in tumor progression.
Fig. 1.
CHI3L1 is expressed in 4T1 mammary tumor cells. CHI3L1 expression in 4T1 mammary tumor cell was determined by immunofluorescence and visualized by confocal microscopy, magnification = ×60
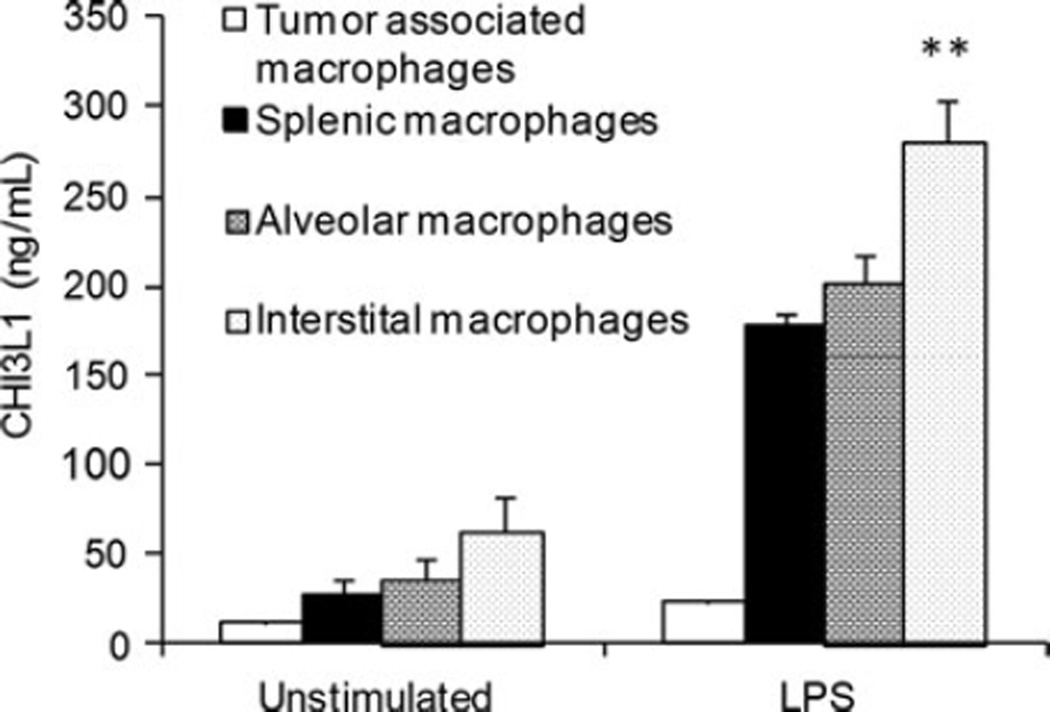
Tumor-derived and tumor-induced molecules by the stromal compartment are known to play a role in tumor progression. CHI3L1 is expressed in solid tumors as well as in the stromal cell compartment. CHI3L1 is difficult to be detected under normal conditions. Although not seen in monocytes, CHI3L1 expression can be observed in late-stage macrophage differentiation [51]. Analysis of macrophages from normal and 4T1 mammary tumor bearers revealed significantly higher CHI3L1 expression in tumor bearers [34]. Macrophages from various tissues including the tumor, spleen, and the lungs were compared for CHI3L1 expression. CHI3L1 was expressed at higher levels in all of these macrophages from tumor bearers relative to the macrophages from normal mice (Fig. 2). Physiological concentrations of CHI3L1 were reported to promote proliferation of various cell types including chondrocytes, vascular endothelial cells, fibroblasts, and ductal epithelial cells [14, 35]. Although the biological function of CHI3L1 has not yet been elucidated, it is possible that the increased expression of CHI3L1 in mammary tumor bearers may induce proliferation of tumor cells. CHI3L1 can also play a role in tumor invasion/metastasis [52], suppression of host immune responses [34], inhibition of tumor cell apoptosis [53], and induction of chemotaxis [41, 50]. CHI3L1 was shown to promote two major signaling pathways associated with mitogenesis and anti-apoptosis. Mitogen-activated protein kinase and phosphoinositide kinase-3 (PI-3 K) signaling cascade were shown to control mitogenesis in fibroblasts, colonic epithelial cells, and cell survival of macrophages [35, 53–55].
Fig. 2.
Macrophages from mammary tumor-bearing mice express higher levels of CHI3L1. Supernatants from unstimulated and LPS-stimulated tumor-associated, splenic alveolar, and interstitial were analyzed by ELISA, p ≤ 0.01 with N = 10 mice/group
CHI3L1 deficient mice were shown to have decreased metastases to the lungs in a melanoma model [33]. For breast tumors to seed and establish colonies in the lung, it is vital that the pulmonary microenvironment is able to support the growth of invading tumor cells. We assessed the role of CHI3L1 in promoting the growth of metastatic mammary tumor cells. CHI3L1 was found to induce pro-inflammatory mediators that could support the growth of newly immigrated 4T1 tumor cells. In determining which cells in the lungs contribute to increased CHI3L1 production, we found that highest levels were produced by interstitial macrophages followed by alveolar macrophages. Alveolar epithelial cells were also found to contribute to CHI3L1 levels seen in tumor bearers (unpublished data). The up-regulated CHI3L1 expression in the pulmonary microenvironment could play a role in supporting infiltrating breast cancer cells.
A robust host immune response is vital to control tumor growth. We and others previously determined the role of CHI3L1 in immune responses [34, 53]. It is well established that breast cancer patients are often immunosuppressed with decreased circulating levels of IFN-γ. In vitro treatment of T cells with CHI3L1 resulted in decreased IFN-γ production, a cytokine important for anti-proliferative effect on tumor cells [34]. More significantly, CHI3L1 deficient mice were shown to have higher levels of IFN-γ in allergen-sensitized mice [53]. Thus, CHI3L1 has adverse effects on the host as it promotes tumor growth via its proliferative role while exhibiting anti-immune effects through inhibition of IFN-γ production.
CHI3L1 and inflammation
Serum levels of CHI3L1 are increased during inflammatory conditions including rheumatoid arthritis [56], osteoarthritis [1], inflammatory bowel disease [54, 57], sarcoidosis [58], chronic obstructive pulmonary disease [59], asthma [60], atherosclerosis [61], Type 1 and Type 2 diabetes[61], liver fibrosis [62, 63], and encephalitis [64] (Table 1). Studies indicate that CHI3L1 is one of the chitinases associated with the development of inflammatory conditions in mucosal tissues [32, 65–67]. Eurich et al. reported that CHI3L1 plays a unique role during the development of intestinal inflammation. CHI3L1 was induced in both colonic lamina propria macrophages and colonic epithelial cells during intestinal inflammation and in patients with inflammatory bowel disease [68].
It is well established that chronic inflammation is a key factor in cancer development and metastasis [69]. CHI3L1 is recognized as a pro-inflammatory factor and has been reported to induce chemokines such as IL-8 from tumor cells [41, 70, 71] and CCL2 from colonic epithelial cells, macrophages, and synovial cells [34, 54, 72]. CCL2 and IL-8 expression was increased in mice bearing mammary tumors [34, 73]. As pro-inflammatory IL-8 and CCL2 are molecules with chemotactic functions that promote tumor growth [74], we determined whether CHI3L1 induces these molecules. Our studies verify findings of others in that CHI3L1 was found to induce the production of both CCL2 and IL-8 by macrophages and epithelial cells [34, 54, 75]. Furthermore, in vitro silencing of CHI3L1 in macrophages by siRNA decreased the production of CCL2 and IL-8 while in vivo treatment with chitin microparticles, the substrate for CHI3L1, significantly reduced not only CHI3L1 expression but also the expression of pro-inflammatory chemokines [34].
During inflammatory conditions, the expression of CHI3L1 in pathogenic macrophages is significantly elevated in the inflamed tissues [76]. Lee et al. [53] reported that CHI3L1 is prominently expressed in the ova-sensitized inflammatory lungs of mice. CHI3L1 deficient mice had significantly diminished antigen-induced TH2 responses as well as IL-13-induced tissue inflammation and fibrosis. These authors also demonstrated that CHI3L1 plays a role in antigen sensitization, dendritic cell accumulation/activation, and induction of alternatively activated macrophages. These studies suggest that CHI3L1 plays a pathogenic role in inflammatory conditions. However, additional studies are needed to fully understand its role in inflammation.
CHI3L1 and angiogenesis
CHI3L1 expression is known to play an important role in tumor growth through increased angiogenesis and invasiveness [40, 50, 52]. A role for CHI3L1 in angiogenesis has been suggested since CHI3L1 was reported to promote adhesion and migration of vascular endothelial cells [38, 40, 41]. To evaluate whether CHI3L1 possesses angiogenic activity, Shao et al. and Kawada et al. engineered breast cancer cell lines MDA-MB231 and colon cancer cell lines HCT-116 and SW480 to express CHI3L1 and xenotrans-planted these cells into nude mice. A fourfold increase in tumor volume with augmented blood vasculature was found in mice implanted with CHI3L1 overexpressing tumor cells compared to control cells [40, 41]. In other cancer models, implantation of CHI3L1 shRNA silence U87 glioblastoma cells into mice resulted in considerable suppression of tumor angiogenesis with decreased vessel density compared to the controls [50].
Further studies were done to determine whether CHI3L1-induced endothelial cell angiogenic responses were VEGF-dependent. It was found that these responses were VEGF-independent as an anti-VEGF neutralizing antibody failed to impede CHI3L1-induced migration and tube formation of human microvascular endothelial cells (HMVECs) [49, 50]. However, a regulatory role of CHI3L1 in VEGF production was found in studies demonstrating a reduction in VEGF when CHI3L1 expression was inhibited via shRNA in U87 brain tumor cells [50]. In contrast, long-term blockade of VEGF induced the expression of CHI3L1 [77], indicating a compensative role for CHI3L1 when VEGF is lacking in tumor cells. CHI3L1 neutralized conditioned medium from MF-63 and U87 cells showed decreased endothelial tube formation indicating decreased angiogenesis [49]. Further, the same investigators showed that CHI3L1 induced activation of the VEGF receptor 2 (Flk-1/KDR) and the downstream intracellular signaling is abolished by neutralizing anti-CHI3L1 antibodies.
It is well established that angiogenic factors in tumor bearers could be derived not only from the tumor but also from stromal cells in the tumor microenvironment. Kawada et al. [41] demonstrated that CHI3L1 expression in colon cancer cells promoted the chemotaxis of macrophages and angiogenesis. In our studies, macrophages from mammary tumor-bearing mice were found to express higher levels of CHI3L1 [34]. In vitro treatment of macrophages with recombinant murine CHI3L1 resulted in significantly increased secretion of angiogenic chemokines, CCL2, and IL-8 [34, 53, 54, 75]. These data imply a significant role for host-derived CHI3L1 in enhancing angiogenic factors.
CHI3L1 and tissue remodeling
Under physiological conditions, CHI3L1 plays a role in mammary gland involution. Extensive tissue remodeling was found to occur after cessation of lactation, and CHI3L1 levels were significantly increased during mammary tissue regression [37, 78]. Using an in vitro model mimicking mammary tissue regression in vivo, Scully et al. [37] found that CHI3L1 suppressed E-cadherin but increased matrix metalloproteinase-9 (MMP-9) and cell motility. MMP-9 is well known to play a vital role during tumor cell invasion. Tumor cell invasiveness is a complex multistep process that involves cell detachment, adhesion to extracellular matrix (ECM), proteolysis of the ECM, and migration of tumor cells through the disrupted matrix [79]. MMP-9 is a well-known matrix metalloproteinase associated with ECM remodeling and tumor infiltration [80, 81]. We have shown that elevated levels of MMP-9 are associated with breast cancer progression and that GM-CSF is one of the tumor-derived factors that induce MMP-9 [82]. Other studies have reported that expression levels of MMP-9 are regulated by CHI3L1 [35]. Our findings concur with those of others as we have shown that MMP-9 is also induced by CHI3L1 in macrophages while silencing CHI3L1 gene in tumor bearer’s macrophages decreased MMP-9 production. These studies indicate a role for CHI3L1 in tissue remodeling [34].
Blocking of CHI3L1
Lee et al. found that in vivo administration of anti-CHI3L1 neutralizing antibodies ameliorates pulmonary inflammation while Mizoguchi reported a significant decrease in inflammation in DSS-induced colitis model. On the other hand, using a neutralizing anti-CHI3L1 antibody, Faibish et al. [49] showed a dose-dependent decrease in HMVEC migration and tube formation. Tumor angiogenesis in U87 glioblastoma xenotransplanted mice was also shown to be abrogated by treatment with neutralizing anti-CHI3L1 antibodies.
Chitin, a nontoxic, nonallergenic, and biodegradable compound with powerful immune effects, is known to bind to chitinases including CHI3L1. Shibata et al. [83] established that chitin microparticles (<10 µm) induce a TH1 type of immune response with increased IFN-γ production in allergen-sensitized mice. Using chitin microparticles to neutralize the effect of CHI3L1, we [34] reported a significant decrease in tumor growth and metastasis. More importantly, the levels of anti-tumorigenic cytokine IFN-γ were significantly increased in in vivo chitin microparticles-treated mice. It was also shown that the levels of CHI3L1 and pro-inflammatory/metastatic molecules CCL2, IL-8, and MMP-9 were significantly decreased in circulation and in the pulmonary microenvironment. Na-gatani et al. [84] reported similar findings using chitin microparticles in acute colitis model and found alleviation of disease with significant decrease in inflammation. Thus, CHI3L1 may prove to be a therapeutic target for inflammatory diseases and cancer.
Conclusions
CHI3L1 plays a vital role in inflammatory conditions and tumor growth by inducing pro-angiogenic/pro-tumorigenic factors. Understanding the biological and physiological functions of CHI3L1 is crucial for the development of novel therapeutic agents for inflammatory diseases and cancer.
Acknowledgments
The authors would like to thank Dr. Yoshimi Shibata for providing us the chitin microparticles used in these studies. This work was supported by different National Institutes of Health grants NIH R15 CA135513-01 and R15 CA135513-01-OS1.
Biography

Ramon Garcia-Areas Vijaya Iragavarapu-Charyulu Stephania Libreros
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Hakala BE, White C, Recklies AD. Human cartilage gp-39, a major secretory product of articular chondrocytes and synovial cells, is a mammalian member of a chitinase protein family. J Biol Chem. 1993;268(34):25803–25810. [PubMed] [Google Scholar]
- 2.Morrison BW, Leder P. neu and ras initiate murine mammary tumors that share genetic markers generally absent in c-myc and int-2-initiated tumors. Oncogene. 1994;9(12):3417–3426. [PubMed] [Google Scholar]
- 3.Shackelton LM, Mann DM, Millis AJ. Identification of a 38-kDa heparin-binding glycoprotein (gp38 k) in differentiating vascular smooth muscle cells as a member of a group of proteins associated with tissue remodeling. J Biol Chem. 1995;270(22):13076–13083. doi: 10.1074/jbc.270.22.13076. [DOI] [PubMed] [Google Scholar]
- 4.Rehli M, Krause SW, Andreesen R. Molecular characterization of the gene for human cartilage gp-39 (CHI3L1), a member of the chitinase protein family and marker for late stages of macrophage differentiation. Genomics. 1997;43(2):221–225. doi: 10.1006/geno.1997.4778. [DOI] [PubMed] [Google Scholar]
- 5.Renkema GH, et al. Chitotriosidase, a chitinase, and the 39-kDa human cartilage glycoprotein, a chitin-binding lectin, are homologues of family 18 glycosyl hydrolases secreted by human macrophages. Eur J Biochem. 1998;251(1–2):504–509. doi: 10.1046/j.1432-1327.1998.2510504.x. [DOI] [PubMed] [Google Scholar]
- 6.Fusetti F, et al. Crystal structure and carbohydrate-binding properties of the human cartilage glycoprotein-39. J Biol Chem. 2003;278(39):37753–37760. doi: 10.1074/jbc.M303137200. [DOI] [PubMed] [Google Scholar]
- 7.Shibata Y, et al. Oral administration of chitin down-regulates serum IgE levels and lung eosinophilia in the allergic mouse. J Immunol. 2000;164(3):1314–1321. doi: 10.4049/jimmunol.164.3.1314. [DOI] [PubMed] [Google Scholar]
- 8.Boot RG, et al. Identification of a novel acidic mammalian chitinase distinct from chitotriosidase. J Biol Chem. 2001;276(9):6770–6778. doi: 10.1074/jbc.M009886200. [DOI] [PubMed] [Google Scholar]
- 9.Araujo AC, Souto-Padron T, de Souza W. Cytochemical localization of carbohydrate residues in microfilariae of Wuchereria bancrofti and Brugia malayi. J Histochem Cytochem. 1993;41(4):571–578. doi: 10.1177/41.4.8450196. [DOI] [PubMed] [Google Scholar]
- 10.Debono M, Gordee RS. Antibiotics that inhibit fungal cell wall development. Annu Rev Microbiol. 1994;48:471–497. doi: 10.1146/annurev.mi.48.100194.002351. [DOI] [PubMed] [Google Scholar]
- 11.Fuhrman JA, Piessens WF. Chitin synthesis and sheath morphogenesis in Brugia malayi microfilariae. Mol Biochem Parasitol. 1985;17(1):93–104. doi: 10.1016/0166-6851(85)90130-6. [DOI] [PubMed] [Google Scholar]
- 12.Neville AC, Parry DA, Woodhead-Galloway J. The chitin crystallite in arthropod cuticle. J Cell Sci. 1976;21(1):73–82. doi: 10.1242/jcs.21.1.73. [DOI] [PubMed] [Google Scholar]
- 13.Shahabuddin M, Vinetz JM. Chitinases of human parasites and their implications as antiparasitic targets. EXS. 1999;87:223–234. doi: 10.1007/978-3-0348-8757-1_16. [DOI] [PubMed] [Google Scholar]
- 14.Coffman FD. Chitinase 3-Like-1 (CHI3L1): a putative disease marker at the interface of proteomics and glycomics. Crit Rev Clin Lab Sci. 2008;45(6):531–562. doi: 10.1080/10408360802334743. [DOI] [PubMed] [Google Scholar]
- 15.Houston DR, et al. Structure and ligand-induced conformational change of the 39-kDa glycoprotein from human articular chondrocytes. J Biol Chem. 2003;278(32):30206–30212. doi: 10.1074/jbc.M303371200. [DOI] [PubMed] [Google Scholar]
- 16.Bigg HF, et al. The mammalian chitinase-like lectin, YKL-40, binds specifically to type I collagen and modulates the rate of type I collagen fibril formation. J Biol Chem. 2006;281(30):21082–21095. doi: 10.1074/jbc.M601153200. [DOI] [PubMed] [Google Scholar]
- 17.Nyirkos P, Golds EE. Human synovial cells secrete a 39 kDa protein similar to a bovine mammary protein expressed during the non-lactating period. Biochem J. 1990;269(1):265–268. doi: 10.1042/bj2690265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kzhyshkowska J, Gratchev A, Goerdt S. Human chitinases and chitinase-like proteins as indicators for inflammation and cancer. Biomark Insights. 2007;2:128–146. [PMC free article] [PubMed] [Google Scholar]
- 19.Johansen JS, et al. High serum YKL-40 levels in patients with primary breast cancer is related to short recurrence free survival. Breast Cancer Res Treat. 2003;80(1):15–21. doi: 10.1023/A:1024431000710. [DOI] [PubMed] [Google Scholar]
- 20.Cintin C, et al. Serum YKL-40 and colorectal cancer. Br J Cancer. 1999;79(9–10):1494–1499. doi: 10.1038/sj.bjc.6690238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johansen JS. Studies on serum YKL-40 as a biomarker in diseases with inflammation, tissue remodelling, fibroses and cancer. Dan Med Bull. 2006;53(2):172–209. [PubMed] [Google Scholar]
- 22.Johansen JS, et al. High serum YKL-40 level in patients with small cell lung cancer is related to early death. Lung Cancer. 2004;46(3):333–340. doi: 10.1016/j.lungcan.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Hogdall EV, et al. High plasma YKL-40 level in patients with ovarian cancer stage III is related to shorter survival. Oncol Rep. 2003;10(5):1535–1538. [PubMed] [Google Scholar]
- 24.Dupont J, et al. Early detection and prognosis of ovarian cancer using serum YKL-40. J Clin Oncol. 2004;22(16):3330–3339. doi: 10.1200/JCO.2004.09.112. [DOI] [PubMed] [Google Scholar]
- 25.Brasso K, et al. Prognostic value of PINP, bone alkaline phosphatase, CTX-I, and YKL-40 in patients with metastatic prostate carcinoma. Prostate. 2006;66(5):503–513. doi: 10.1002/pros.20311. [DOI] [PubMed] [Google Scholar]
- 26.Diefenbach CS, et al. Preoperative serum YKL-40 is a marker for detection and prognosis of endometrial cancer. Gynecol Oncol. 2007;104(2):435–442. doi: 10.1016/j.ygyno.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt H, et al. Elevated serum level of YKL-40 is an independent prognostic factor for poor survival in patients with metastatic melanoma. Cancer. 2006;106(5):1130–1139. doi: 10.1002/cncr.21678. [DOI] [PubMed] [Google Scholar]
- 28.Biggar RJ, et al. Serum YKL-40 and interleukin 6 levels in Hodgkin lymphoma. Clin Cancer Res. 2008;14(21):6974–6978. doi: 10.1158/1078-0432.CCR-08-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bergmann OJ, et al. High serum concentration of YKL-40 is associated with short survival in patients with acute myeloid leukemia. Clin Cancer Res. 2005;11(24 Pt 1):8644–8652. doi: 10.1158/1078-0432.CCR-05-1317. [DOI] [PubMed] [Google Scholar]
- 30.Pelloski CE, et al. YKL-40 expression is associated with poorer response to radiation and shorter overall survival in glioblastoma. Clin Cancer Res. 2005;11(9):3326–3334. doi: 10.1158/1078-0432.CCR-04-1765. [DOI] [PubMed] [Google Scholar]
- 31.Mitsuhashi A, et al. Serum YKL-40 as a marker for cervical adenocarcinoma. Ann Oncol. 2009;20(1):71–77. doi: 10.1093/annonc/mdn552. [DOI] [PubMed] [Google Scholar]
- 32.Mizoguchi A, Mizoguchi E. Inflammatory bowel disease, past, present and future: lessons from animal models. J Gastroenterol. 2008;43(1):1–17. doi: 10.1007/s00535-007-2111-3. [DOI] [PubMed] [Google Scholar]
- 33.He CH, et al. Chitinase 3-like 1 regulates cellular and tissue responses via IL-13 receptor alpha2. Cell Rep. 2013;4(4):830–841. doi: 10.1016/j.celrep.2013.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Libreros S, et al. Induction of proinflammatory mediators by CHI3L1 is reduced by chitin treatment: Decreased tumor metastasis in a breast cancer model. Int J Cancer. 2011;131(2):377–386. doi: 10.1002/ijc.26379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Recklies AD, White C, Ling H. The chitinase 3-like protein human cartilage glycoprotein 39 (HC-gp39) stimulates proliferation of human connective-tissue cells and activates both extracellular signal-regulated kinase- and protein kinase B-mediated signalling pathways. Biochem J. 2002;365(Pt 1):119–126. doi: 10.1042/BJ20020075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johansen JS, Jensen HS, Price PA. A new biochemical marker for joint injury. Analysis of YKL-40 in serum and synovial fluid. Br J Rheumatol. 1993;32(11):949–955. doi: 10.1093/rheumatology/32.11.949. [DOI] [PubMed] [Google Scholar]
- 37.Scully S, et al. Inhibitory activity of YKL-40 in mammary epithelial cell differentiation and polarization induced by lactogenic hormones: a role in mammary tissue involution. PLoS ONE. 2011;6(10):e25819. doi: 10.1371/journal.pone.0025819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malinda KM, et al. Gp38 k, a protein synthesized by vascular smooth muscle cells, stimulates directional migration of human umbilical vein endothelial cells. Exp Cell Res. 1999;250(1):168–173. doi: 10.1006/excr.1999.4511. [DOI] [PubMed] [Google Scholar]
- 39.De Ceuninck F, et al. YKL-40 (cartilage gp-39) induces proliferative events in cultured chondrocytes and synoviocytes and increases glycosaminoglycan synthesis in chondrocytes. Biochem Biophys Res Commun. 2001;285(4):926–931. doi: 10.1006/bbrc.2001.5253. [DOI] [PubMed] [Google Scholar]
- 40.Shao R, et al. YKL-40, a secreted glycoprotein, promotes tumor angiogenesis. Oncogene. 2009;28(50):4456–4468. doi: 10.1038/onc.2009.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kawada M, et al. Chitinase 3-like 1 promotes macrophage recruitment and angiogenesis in colorectal cancer. Oncogene. 2012;31(26):3111–3123. doi: 10.1038/onc.2011.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jensen BV, Johansen JS, Price PA. High levels of serum HER-2/neu and YKL-40 independently reflect aggressiveness of metastatic breast cancer. Clin Cancer Res. 2003;9(12):4423–4434. [PubMed] [Google Scholar]
- 43.Hottinger AF, et al. YKL-40 and MMP-9 as serum markers for patients with primary central nervous system lymphoma. Ann Neurol. 2011;70(1):163–169. doi: 10.1002/ana.22360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brasso K, Iversen P. Prostatic cancer 2006–status and new challenges. The Danish society of urology. Ugeskr Laeger. 2006;168(12):1243. [PubMed] [Google Scholar]
- 45.Shao R, et al. Breast cancer expression of YKL-40 correlates with tumour grade, poor differentiation, and other cancer markers. Br J Cancer. 2011;105(8):1203–1209. doi: 10.1038/bjc.2011.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kang EJ, et al. YKL-40 expression could be a poor prognostic marker in the breast cancer tissue. Tumour Biol. 2013 doi: 10.1007/s13277-013-1036-0. [DOI] [PubMed] [Google Scholar]
- 47.Johansen JS, et al. Serum YKL-40: a new potential marker of prognosis and location of metastases of patients with recurrent breast cancer. Eur J Cancer. 1995;31A(9):1437–1442. doi: 10.1016/0959-8049(95)00196-p. [DOI] [PubMed] [Google Scholar]
- 48.Coskun U, et al. Locally advanced breast carcinoma treated with neoadjuvant chemotherapy: are the changes in serum levels of YKL-40, MMP-2 and MMP-9 correlated with tumor response? Neoplasma. 2007;54(4):348–352. [PubMed] [Google Scholar]
- 49.Faibish M, et al. A YKL-40-neutralizing antibody blocks tumor angiogenesis and progression: a potential therapeutic agent in cancers. Mol Cancer Ther. 2011;10(5):742–751. doi: 10.1158/1535-7163.MCT-10-0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Francescone RA, et al. Role of YKL-40 in the angiogenesis, radioresistance, and progression of glioblastoma. J Biol Chem. 2011;286(17):15332–15343. doi: 10.1074/jbc.M110.212514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krause SW, et al. Differential screening identifies genetic markers of monocyte to macrophage maturation. J Leukoc Biol. 1996;60(4):540–545. doi: 10.1002/jlb.60.4.540. [DOI] [PubMed] [Google Scholar]
- 52.Nigro JM, et al. Integrated array-comparative genomic hybridization and expression array profiles identify clinically relevant molecular subtypes of glioblastoma. Cancer Res. 2005;65(5):1678–1686. doi: 10.1158/0008-5472.CAN-04-2921. [DOI] [PubMed] [Google Scholar]
- 53.Lee CG, et al. Role of breast regression protein 39 (BRP-39)/chitinase 3-like-1 in Th2 and IL-13-induced tissue responses and apoptosis. J Exp Med. 2009;206(5):1149–1166. doi: 10.1084/jem.20081271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mizoguchi E. Chitinase 3-like-1 exacerbates intestinal inflammation by enhancing bacterial adhesion and invasion in colonic epithelial cells. Gastroenterology. 2006;130(2):398–411. doi: 10.1053/j.gastro.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 55.Pearson G, et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22(2):153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 56.Johansen JS, et al. Serum YKL-40 concentrations in patients with rheumatoid arthritis: relation to disease activity. Rheumatology (Oxford) 1999;38(7):618–626. doi: 10.1093/rheumatology/38.7.618. [DOI] [PubMed] [Google Scholar]
- 57.Vind I, et al. Serum YKL-40, a potential new marker of disease activity in patients with inflammatory bowel disease. Scand J Gastroenterol. 2003;38(6):599–605. doi: 10.1080/00365520310000537. [DOI] [PubMed] [Google Scholar]
- 58.Johansen JS, et al. Increased serum YKL-40 in patients with pulmonary sarcoidosis a potential marker of disease activity? Respir Med. 2005;99(4):396–402. doi: 10.1016/j.rmed.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 59.Sakazaki Y, et al. Overexpression of chitinase 3-like 1/YKL-40 in lung-specific IL-18-transgenic mice, smokers and COPD. PLoS One. 2011;6(9):e24177. doi: 10.1371/journal.pone.0024177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Elias JA, et al. Chitinases and chitinase-like proteins in T(H)2 inflammation and asthma. J Allergy Clin Immunol. 2005;116(3):497–500. doi: 10.1016/j.jaci.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 61.Rathcke CN, Vestergaard H. YKL-40, a new inflammatory marker with relation to insulin resistance and with a role in endothelial dysfunction and atherosclerosis. Inflamm Res. 2006;55(6):221–227. doi: 10.1007/s00011-006-0076-y. [DOI] [PubMed] [Google Scholar]
- 62.Johansen JS, et al. Plasma YKL-40: a new potential marker of fibrosis in patients with alcoholic cirrhosis? Scand J Gastroenterol. 1997;32(6):582–590. doi: 10.3109/00365529709025104. [DOI] [PubMed] [Google Scholar]
- 63.Johansen JS, et al. Serum YKL-40 is increased in patients with hepatic fibrosis. J Hepatol. 2000;32(6):911–920. doi: 10.1016/s0168-8278(00)80095-1. [DOI] [PubMed] [Google Scholar]
- 64.Bonneh-Barkay D, et al. YKL-40, a marker of simian immunodeficiency virus encephalitis, modulates the biological activity of basic fibroblast growth factor. Am J Pathol. 2008;173(1):130–143. doi: 10.2353/ajpath.2008.080045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chupp GL, et al. A chitinase-like protein in the lung and circulation of patients with severe asthma. N Engl J Med. 2007;357(20):2016–2027. doi: 10.1056/NEJMoa073600. [DOI] [PubMed] [Google Scholar]
- 66.Ober C, et al. Effect of variation in CHI3L1 on serum YKL-40 level, risk of asthma, and lung function. N Engl J Med. 2008;358(16):1682–1691. doi: 10.1056/NEJMoa0708801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Johansen JS, et al. Serum YKL-40, a new prognostic biomarker in cancer patients? Cancer Epidemiol Biomarkers Prev. 2006;15(2):194–202. doi: 10.1158/1055-9965.EPI-05-0011. [DOI] [PubMed] [Google Scholar]
- 68.Eurich K, et al. Potential role of chitinase 3-like-1 in inflammation-associated carcinogenic changes of epithelial cells. World J Gastroenterol. 2009;15(42):5249–5259. doi: 10.3748/wjg.15.5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tang H, et al. YKL-40 induces IL-8 expression from bronchial epithelium via MAPK (JNK and ERK) and NF-kappaB pathways, causing bronchial smooth muscle proliferation and migration. J Immunol. 2013;190(1):438–446. doi: 10.4049/jimmunol.1201827. [DOI] [PubMed] [Google Scholar]
- 71.Qin W, et al. Increased expression of the inflammatory protein YKL-40 in precancers of the breast. Int J Cancer. 2007;121(7):1536–1542. doi: 10.1002/ijc.22881. [DOI] [PubMed] [Google Scholar]
- 72.Kawada M, et al. Chitinase 3-like-1 enhances bacterial adhesion to colonic epithelial cells through the interaction with bacterial chitin-binding protein. Lab Invest. 2008;88(8):883–895. doi: 10.1038/labinvest.2008.47. [DOI] [PubMed] [Google Scholar]
- 73.Owen JL, et al. Expression of the inflammatory chemokines CCL2, CCL5 and CXCL2 and the receptors CCR1–3 and CXCR2 in T lymphocytes from mammary tumor-bearing mice. Cell Immunol. 2011;270(2):172–182. doi: 10.1016/j.cellimm.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lazennec G, Richmond A. Chemokines and chemokine receptors: new insights into cancer-related inflammation. Trends Mol Med. 2010;16(3):133–144. doi: 10.1016/j.molmed.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Letuve S, et al. YKL-40 is elevated in patients with chronic obstructive pulmonary disease and activates alveolar macrophages. J Immunol. 2008;181(7):5167–5173. doi: 10.4049/jimmunol.181.7.5167. [DOI] [PubMed] [Google Scholar]
- 76.Aguilera B, et al. Transglycosidase activity of chitotriosidase: improved enzymatic assay for the human macrophage chitinase. J Biol Chem. 2003;278(42):40911–40916. doi: 10.1074/jbc.M301804200. [DOI] [PubMed] [Google Scholar]
- 77.Saidi A, et al. Experimental anti-angiogenesis causes upregulation of genes associated with poor survival in glioblastoma. Int J Cancer. 2008;122(10):2187–2198. doi: 10.1002/ijc.23313. [DOI] [PubMed] [Google Scholar]
- 78.Hughes K, et al. Conditional deletion of Stat3 in mammary epithelium impairs the acute phase response and modulates immune cell numbers during post-lactational regression. J Pathol. 2012;227(1):106–117. doi: 10.1002/path.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Werb Z, et al. Matrix-degrading proteases and angiogenesis during development and tumor formation. Apmis. 1999;107(1):11–18. doi: 10.1111/j.1699-0463.1999.tb01521.x. [DOI] [PubMed] [Google Scholar]
- 80.Coussens LM, et al. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103(3):481–490. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295(5564):2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 82.Owen JL, et al. Up-regulation of matrix metalloproteinase-9 in T lymphocytes of mammary tumor bearers: role of vascular endothelial growth factor. J Immunol. 2003;171(8):4340–4351. doi: 10.4049/jimmunol.171.8.4340. [DOI] [PubMed] [Google Scholar]
- 83.Shibata Y, Metzger WJ, Myrvik QN. Chitin particle-induced cell-mediated immunity is inhibited by soluble mannan: mannose receptor-mediated phagocytosis initiates IL-12 production. J Immunol. 1997;159(5):2462–2467. [PubMed] [Google Scholar]
- 84.Nagatani K, et al. Chitin microparticles for the control of intestinal inflammation. Inflamm Bowel Dis. 2012;18(9):1698–1710. doi: 10.1002/ibd.22874. [DOI] [PMC free article] [PubMed] [Google Scholar]