Abstract
Background
Lung adenocarcinomas can be distinguished by identifying mutated driver oncogenes including EGFR and KRAS. Mutations in EGFR are associated with both an improved survival as well as response to treatment with erlotinib and gefitinib. However, the prognostic significance of KRAS has not been evaluated in large numbers of patients and remains controversial. We examined the association of EGFR and KRAS mutations with survival among patients with advanced lung adenocarcinomas.
Methods
We analyzed data from patients with advanced lung adenocarcinomas and known EGFR and KRAS mutation status evaluated between 2002 and 2009. We collected clinical variables including age, gender, Karnofsky Performance Status, smoking history, and treatment history. Overall survival from diagnosis of advanced disease was analyzed using Kaplan-Meier and Cox proportional hazard methods.
Results
We evaluated 1036 patients, including 610 women (59%) and 344 never-smokers (33%). Patients had a median age of 65 (range, 25–92) and the majority (81%) had a KPS ≥80%. In multivariate analysis, EGFR mutations were associated with a longer overall survival (HR= 0.6, p<0.001) and KRAS mutations with a shorter survival (HR=1.21, p=0.048).
Conclusions
KRAS mutations predict shorter survival for patients with advanced lung adenocarcinomas. The presence of EGFR and KRAS mutations define distinct subsets of patients with lung adenocarcinomas, and should be determined in patients upon diagnosis of advanced disease. Clinical trial reports should include EGFR and KRAS mutation status along with other prognostic factors.
Keywords: non-small cell lung cancer, adenocarcinomas, EGFR, KRAS, survival, prognostic factors
INTRODUCTION
Recent therapeutic advances have highlighted the molecular heterogeneity underlying oncogene-driven lung adenocarcinomas. For example, patients with mutations in the epidermal growth factor receptor (EGFR) make up approximately 10–15% of patients in the United States diagnosed with advanced lung adenocarcinomas. These patients have high rates of radiographic response when treated with erlotinib or gefitinib, tyrosine kinase inhibitors (TKIs) targeting EGFR, and longer progression-free survival compared with patients whose tumors are wild-type for EGFR.1–4
KRAS mutations exist in a larger number of patients, approximately 30% of patients with advanced lung adenocarcinomas in the United States. Efforts to develop effective therapies inhibiting lung cancers with KRAS mutations have been largely unsuccessful, and the prognostic significance of KRAS mutations remains in question. A number of small studies5–9 and one meta-analysis10 have evaluated the prognostic effects of KRAS mutations with conflicting conclusions. However, none of these studies adequately accounted for other prognostic factors, and few of the included patients received the modern chemotherapy regimens now considered standard. We hypothesized that, among patients with stage IV lung adenocarcinomas, EGFR and KRAS mutations would identify patients with different outcomes. Here, we present the largest analysis to date examining a population of patients with advanced lung adenocarcinomas and known EGFR and KRAS mutation status. We report clinical characteristics, treatment histories, and mutation analysis from 1036 patients and investigate their association with survival.
MATERIALS AND METHODS
Study Population
All patients evaluated at Memorial Sloan-Kettering Cancer Center (MSKCC) in the Thoracic Oncology Service clinics between 2002 and 2009 were analyzed. Only those patients with stage IV lung adenocarcinomas (American Joint Committee on Cancer, 7th Edition) whose tumors had undergone routine analysis for EGFR and KRAS mutations were included. Patients with early stage lung adenocarcinoma, who subsequently developed advanced disease, were not included in this analysis. Molecular analysis of all tumors for EGFR began in 2004, so tumors from 2002 and 2003 underwent analyses retrospectively. We obtained permission from the MSKCC Institutional Review Board and Privacy Board for the retrospective chart review.
Mutational Analysis
Genomic DNA was extracted from tumor specimens. EGFR mutations were assessed by polymerase chain reaction (PCR)-based methods that detect exon 19 deletions and exon 21 L858R amino acid substitutions or by mass-spectometry-based genotyping (Sequenom) as described previously.11,12 Testing for these two major EGFR mutations identifies over 90% of patients with sensitizing mutations. KRAS mutations in codons 12 and 13 were assessed by direct sequencing of exon 2 or by mass-spectrometry based genotyping (Sequenome).12,13 Testing for KRAS exon 2 mutations identifies over 95% of lung cancer patients with KRAS mutations.
Data Collection
We collected clinical variables for all patients from the medical record, including age, gender, and Karnofsky Performance Status (KPS). We obtained smoking status (never, former, or current cigarette use) using self-reported smoking questionnaires. Never smokers were defined as those people who smoked <100 cigarettes in a lifetime. Current smokers were those who were smoking at the time of diagnosis or quit less than 1 year prior to diagnosis. Patient treatment histories were recorded, including receipt of EGFR-TKIs, platinum-based chemotherapy, or bevacizumab. Patients with unknown treatment histories were excluded from treatment analyses.
Statistical Analysis
Survival was calculated from date of diagnosis of metastatic disease until the date of death. At last available follow-up, all patients still living were censored. Patient demographics, clinical characteristics and treatment histories were compared using a Chi-square test. Overall survival (OS) was estimated using the Kaplan-Meier method and compared across groups using log-rank test (for univariate analysis) and Cox proportion hazard methods (for multivariate analysis).
RESULTS
Clinical Characteristics
Among 1036 patients with advanced lung adenocarcinomas evaluated, 275 tumors (27%) harbored EGFR mutations, 241 tumors (23%) had KRAS mutations, and 520 tumors (50%) were wild-type for both KRAS and EGFR (designated KRAS/EGFR wild-type).
Clinical characteristics were similar across the three sub-groups (Table 1). The median age was 65 years (range, 25–92), and median KPS was 80%. There was variation in gender across mutation sub-groups, with women accounting for 65% of patients with EGFR mutations (179/275, 95% CI, 59–71%), 60% of patients with KRAS mutations (144/241, 95% CI, 53–66%) and 55% of patients without KRAS or EGFR mutations (287/520, 95% CI, 51–60%).
Table 1.
Baseline characteristics of patients with advanced lung adenocarcinomas
| Total (n=1036) | KRAS (+) (n=241) | EGFR (+) (n=275) | KRAS/EGFR WT (n=520) | |
|---|---|---|---|---|
| Women (n, (%)) | 610 (59%) | 144 (60%) | 179 (65%) | 287 (55%) |
|
| ||||
| Age ≥ 70 (n, (%)) | 333 (32%) | 83 (34%) | 80 (29%) | 170 (33%) |
|
| ||||
| Age (median), (range) | 65 (25–92) | 66 (33–87) | 64 (26–89) | 65 (25–92) |
|
| ||||
| KPS (median) | 80% | 80% | 80% | 80% |
|
| ||||
| Smoking history | ||||
| Never | 344 (33%) | 16 (7%) | 166 (60%) | 162 (31%) |
| Current | 197 (19%) | 73 (30%) | 14 (5%) | 110 (21%) |
| Former | 495 (48%) | 152 (63%) | 95 (35%) | 248 (48%) |
Smoking history varied by mutation status. The majority (60%) of patients with EGFR mutations were never-smokers (166/275, 95% CI, 54–66%), while only 7% of patients with KRAS mutations (16/241, 95% CI, 4–11%) and 31% of patients without KRAS or EGFR mutations (162/520, 95% CI, 27–35%) had never smoked cigarettes. On the other hand, 93% of patients with KRAS mutations were current or former smokers (225/241, 95% CI, 89–96%), compared with 69% of patients without KRAS or EGFR mutations (358/520, 95% CI, 65–73%). Forty percent of patients with EGFR mutations (109/275, 95% CI, 34–46%) had smoked cigarettes.
Treatment History
We reviewed all chemotherapy and targeted agents patients had received, in particular whether patients had been treated with EGFR-TKIs (erlotinib or gefitinib), platinum-based doubled chemotherapy, or anti-angiogenesis antibody bevacizumab (Table 2). Patients whose treatment histories were not available in the medical record were excluded in this analysis. Patients harboring EGFR mutations in their tumors were significantly more likely to have been treated with EGFR-TKIs compared with patients without EGFR mutations (97% vs. 30%, P< 0.001). However, patients with KRAS mutations were equally likely to have received platinum-based chemotherapies as patients without KRAS or EGFR mutations (70% vs. 65%, p=0.13). Patients with KRAS mutations also received bevacizumab at similar frequencies to patients wild-type for KRAS and EGFR (41% vs. 37%, p=0.26).
Table 2.
Summary of treatment history by genotype
| p-values | |||||
|---|---|---|---|---|---|
| Treatment | KRAS (+) (n=241) | EGFR (+) (n=275) | KRAS/EGFR wild-type (WT) (n=520) | EGFR(+)vs. EGFR | KRAS (+)WT vs. KRAS WT |
| Platinum | |||||
| Yes | 152 (63%) | 132 (48%) | 327 (63%) | 0.13 | |
| unknown | 25 (10%) | 29 (11%) | 57 (11%) | ||
|
| |||||
| Bevacizumab | |||||
| Yes | 88 (37%) | 65 (23%) | 190 (37%) | 0.26 | |
| Unknown | 27 (11%) | 39 (14%) | 64 (12%) | ||
|
| |||||
| EGFR-TKI | |||||
| Yes | 44 (18%) | 233 (85%) | 148 (28%) | <0.001 | |
| Unknown | 28 (12%) | 34 (12%) | 84 (16%) | ||
Survival Analysis of Patients According to Mutation Status
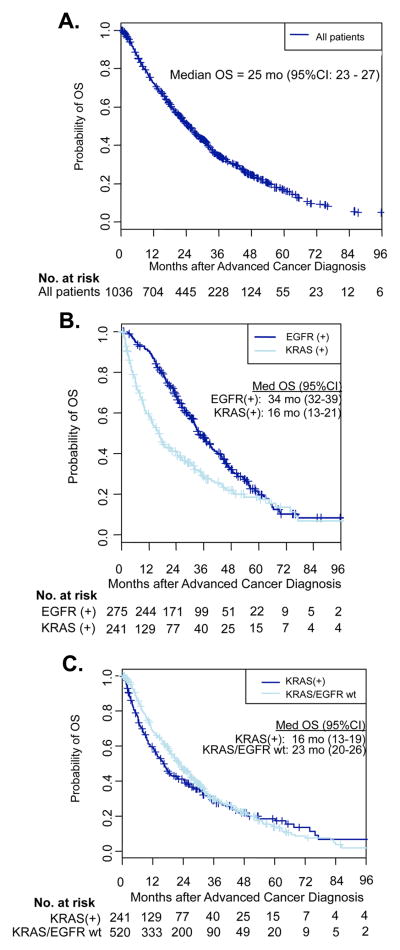
The median follow-up among 326 patients still living with advanced lung adenocarcinoma was 29 months (range, 0.2–108 months). We analyzed OS by genotype (Figure 1). The median OS for all patients was 25 months (95% CI, 23–27). For patients whose tumors displayed KRAS mutations, the median OS was 16 months (95% CI, 13–19), compared with patients whose tumors harbored EGFR mutations with a median OS of 34 months (95% CI, 32–39), and compared with all patients with tumors wild-type for KRAS and EGFR who had a median OS of 23 months (95% CI, 20–26).
Figure 1.
Overall survival (OS) by genotype for patients with advanced lung adenocarcinomas. (A) Kaplan-Meier survival plot of all patients with advanced lung adenocarcinomas. (B) Kaplan-Meier survival plots of EGFR-positive patients and KRAS-positive patients. (C) Kaplan-Meier survival plots of KRAS-positive patients and KRAS/EGFR wild-type (wt) patients.
We evaluated each clinical variable to determine its impact on survival outcomes (Table 3). Gender, KPS and smoking history were all associated with overall survival in univariate analysis. Women lived longer than men (HR= 0.8, p<0.001). Patients with better performance status at diagnosis (KPS 80% or 90%) lived longer than patients with poor baseline KPS (HR= 0.5, p<0.001). Patients who never smoked cigarettes lived longer than patients who smoked (current or former) (HR=0.9, p=0.042). There was also a trend towards improved survival for younger patients (≤70 years of age) compared with patients who were 70 years or older at diagnosis.
Table 3.
Clinical variables and EGFR/KRAS mutations associated with overall survival (univariate analysis)
| N | Median Survival (months) | HR (95% CI) | p-value | |
|---|---|---|---|---|
| Female vs. | 610 (59%) | 28.4 (25.3–31.4) | 0.78 (0.67–0.9) | <0.001 |
| Male | 426 (41%) | 19.3 (17.4–23.3) | 1 | |
|
| ||||
| Age <70 vs. | 703 (68%) | 26.4 (23.6–28.9) | 0.87 (0.74–1.0) | 0.068 |
| Age ≥70 | 333 (32%) | 22.9 (19.3–26.6) | 1 | |
|
| ||||
| KPS 80–90% vs. | 842 (81%) | 27.5 (25.3–31.0) | 0.53 (0.44–0.63) | <0.001 |
| ≤70% | 194 (19%) | 13.2 (10.1–17.2) | 1 | |
|
| ||||
| Never-Smoker vs. | 344 (33%) | 30.4 (25.3–32.6) | 0.85 (0.73–0.99) | 0.042 |
| Current/Former | 692 (67%) | 22.1 (19.0–25.5) | 1 | |
|
| ||||
| EGFR (+) vs. | 275 (27%) | 33.7 (31.6–39.0) | 0.63 (0.53– 0.74) | <0.001 |
| EGFR wild-type | 761 (73%) | 20.7 (18.5–23.0) | 1 | |
|
| ||||
| KRAS (+) vs. | 241 (23%) | 16.3 (13.0–19.2) | 1.73 (1.41–2.14) | <0.001 |
| EGFR(+) | 275 (27%) | 33.7 (31.6–39.0) | 1 | |
|
| ||||
| KRAS (+) vs. | 241(23%) | 16.3 (13.0–19.2) | 1.13 (0.94–1.35) | 0.17 |
| EGFR/KRAS wild-type | 520 (50%) | 22.8 (20.4–26.4) | 1 | |
Mutation status varied with overall survival in univariate analysis (Table 3). Patients with EGFR mutations in their tumors lived longer than patients without EGFR mutations (HR=0.6, p <0.001). Patients with KRAS mutations in their tumors had significantly worse survival compared to patients with EGFR mutations (HR=1.7, p<0.001), and a trend towards shorter survival compared to patients whose tumors were wild-type for KRAS and EGFR (HR 1.13, p=0.17).
In multivariate analysis, female sex (HR =0.8, p< 0.001) and higher performance status (HR=0.5, p<0.001) remained associated with favorable survival outcomes. Likewise, the presence of an EGFR mutation remained strongly associated with favorable survival compared with patients who were wild-type for EGFR (HR=0.6, p< 0.001). After adjusting for age, gender, performance status and the presence of KRAS and EGFR mutations, the presence of a KRAS mutation became more strongly associated with shorter survival compared with those patients who were KRAS/EGFR wild-type (HR=1.21,p=0.048) (Table 4).
Table 4.
Clinical variables and EGFR/KRAS mutations associated with overall survival (multivariate analysis)
| HR (95% CI) | p-value | |
|---|---|---|
| Female vs. | 0.78 (0.67–0.90) | <0.001 |
| Male | 1 | |
|
| ||
| Age* | 1.15 (1.6–1.25) | 0.001 |
| 1 | ||
|
| ||
| KPS 80 –90% vs. | 0.54 (0.45–0.64) | <0.001 |
| ≤70% | 1 | |
|
| ||
| Current/Former vs. | 1.00 (0.85–1.18) | >0.99 |
| Never-Smoker | 1 | |
|
| ||
| EGFR (+) vs. | 0.62 (0.52–0.74) | <0.001 |
| EGFR wild-type | 1 | |
|
| ||
| KRAS (+) vs | 1.85 (1.47–2.32) | <0.001 |
| EGFR(+) | 1 | |
|
| ||
| KRAS (+) vs. | 1.21 (1.01–1.46) | 0.048 |
| EGFR/KRAS wild-type | 1 | |
the effect of age is constant until the age of 70 and linear thereafter. Therefore, HR=1.15 corresponds to an increase of 15% in the risk of death per 5 years of age, for patients 70 years and old.
DISCUSSION
With the identification of EGFR mutations and ALK rearrangements, and a renewed interest in KRAS mutations, our understanding of the heterogeneity of lung adenocarcinomas has changed dramatically since 2004. Here, we present outcomes of patients with advanced lung adenocarcinomas and known KRAS and EGFR mutation status. In our analyses, we confirm the known prognostic effects of sex, age, performance status, smoking history, and EGFR mutations.14–16 In this, the largest collection of patients with advanced lung adenocarcinomas and KRAS mutations from one institution, we also demonstrate that the presence of a KRAS mutation is an independent factor associated with shorter survival, HR=1.21 (p=0.048). The negative prognostic effect of KRAS mutations persists in our analyses despite the fact that patients with KRAS mutations were equally likely to have received platinum-based chemotherapy and bevacizumab as patients whose tumors were wild-type for KRAS and EGFR. We conclude that patients with KRAS mutations have shorter survival due to inherent differences in the tumor biology of KRAS-driven lung cancer, rather than differences in life-lengthening therapies (platinum-based chemotherapy17 and bevacizumab18) that patients received.
Although the presence of KRAS mutations in NSCLC tumors was well-established almost two decades before the discovery of EGFR,19 there is no analogous targeted therapy available for patients with KRAS mutations. Likewise, conclusions about the prognostic or predictive value of KRAS mutations remain uncertain. Slebos et al. first reported the prognostic importance of KRAS mutations in lung cancer in 1990, when they studied tumors from 69 patients who had undergone complete lung tumor resection. After adjustments for sex, smoking, and tumor stage, the presence of a KRAS mutation was the single most important factor identifying patients with shorter disease-free survival (p=0.038), overall survival (p=0.002) and death due to cancer (p<0.001).5 Mitsudomi et al. subsequently reported that KRAS mutations were also associated with shortened survival in 45 patients with advanced lung cancer (p=0.0103).6
Further investigations of the prognostic significance of KRAS mutations in NSCLC have been limited to additional small studies. Mascaux et al. compiled 28 studies (3620 patients) to determine the prognostic value of KRAS in a meta-analysis.10 Among studies evaluating only lung adenocarcinomas, the presence of a KRAS mutation was associated with worse survival, HR=1.59 (95% CI, 1.26–2.02). This univariate analysis of aggregate data combined patients of all stages, combinations of curative and palliative treatment modalities, as well as a variety of techniques used to identify KRAS mutations in tumor tissue, all of which limited the strength and generalizability of its conclusions. Since other prognostic variables were not available for many of the studies included in the meta-analysis, the authors were unable to perform a multivariate analysis to exclude the effects of such important variables as sex, stage, smoking history, and performance status.
In contrast to conventional wisdom and prior studies20, our analysis did not find smoking status to be an independent variable associated with prognosis by multivariate analysis. Our results are best explained by a recent publication in this journal which explored the relationship between patient smoking history, driver mutations (EGFR, KRAS and ALK) and prognosis.21 Paik et al. found distinct frequencies of EGFR, KRAS and ALK mutations among never-smokers and current/former smokers with advanced lung adenocarcinoma. While prognosis differed between never-smokers and current/smokers, and between patients separated by genotype in both groups, there were no difference in survival between never-smokers and current/former smokers who harbored a given mutation in multivariate analysis. Paik et al. concluded that distinct mutation profiles drive prognostic differences observed among never-smokers and current/former smokers. Therefore, we would expect the impact of smoking on survival to diminish in significance in a multivariate analysis which accounts also for EGFR and KRAS mutations, as ours did.
One limitation of our study was that the patients who underwent mutation testing during this time frame represented a select population with distinct clinical features—they were younger, had a better performance status, and were more likely to be women than the average person with lung cancer. The frequency of patients with EGFR mutations was also greater than typically reported in United States populations, as would be expected with the described clinical characteristics. As a consequence of this enrichment of favorable clinical characteristics, the survival outcomes for the total population (median OS of 25 months) were longer than those generally seen in unselected patients with advanced lung adenocarcinomas. While our data describes a “healthier” cross-section of the total lung cancer population, we demonstrate here that patients whose tumors harbor KRAS mutations have comparatively worse survival, which suggests that the disparity between mutation sub-groups may even be more pronounced in a broader population of patients with lung adenocarcinoma.
Furthermore, we believe enrichment for favorable clinical characteristics among our patients also explains why the association between KRAS mutations and survival was not significant in univariate analysis, but in multivariate analysis became more strongly associated with shorter survival. That is, after adjusting for the favorable effects of younger age, better performance status and female gender, the true association between the presence of KRAS mutations and shorter survival became apparent.
Our study was also limited by the absence of ALK rearrangements from the molecular analysis. All patients included in this analysis were diagnosed and had molecular analysis prior to 2009, when ALK FISH analysis became a common practice for all patients without EGFR and KRAS mutations, and well before the 2011 FDA approval of crizotinib and the companion diagnostic test for ALK rearrangements. Thus, we estimate 2–10% of patients labeled KRAS/EGFR wild-type in our analysis would have had ALK rearrangements.22–23 While the identification of these patients may have affected the survival analysis for KRAS/EGFR wild-type patients, it would not have impacted our findings in patients whose tumors had KRAS mutations. Patients with ALK rearrangements have been shown to have similar response rates to platinum-based chemotherapy and no difference in overall survival when compared to patients without ALK rearrangements.22 Other known molecular subsets of lung adenocarcinoma, such as tumors with BRAF,24 HER2, and NRAS mutations represent even smaller proportions of cases than EML4-ALK, and were even less likely to be confounding factors in this analyses.
In conclusion, we report here that the presence of a KRAS mutation is a poor prognostic factor for patients with lung adenocarcinomas. Since patients with KRAS mutations have a distinct clinical course which results in shorter survival, they should be evaluated separately in clinical trials. We recommend including KRAS testing in upfront mutation analyses along with EGFR and EML4-ALK in order to prospectively identify these patients in the clinic.
Footnotes
Financial Disclosures: Dr. Johnson: consultant to Genentech; research funding from Novartis; spouse employed as Governmental Affairs lobbyist for Astellas/OSI. Dr. Pao: consultant to Molecular MD, AstraZeneca, Bristol-Myers Squibb, Symphony Evolution, Clovis Oncology; research funding from Enzon, Xcovery, AstraZeneca, Symphogen; rights to EGFR T790M testing licensed to Molecular MD by Dr. Pao. Dr. Kris: consultant to Pfizer, Inc., Boehringer Ingelheim, Genentech. Dr. Riely: consultant to Chugai, Tragara, Ariad, Daiichi, Novartis; research funding from Pfizer, GSK, Chugai, Novartis.
Drs. Sima, Chaft, Paik and Ladanyi have no financial disclosures.
References
- 1.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 2.Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 3.Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004;101(36):13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 5.Slebos RJ, Kibbelaar RE, Dalesio O, et al. K-ras oncogene activation as a prognostic marker in adenocarcinoma of the lung. N Engl J Med. 1990;323(9):561–565. doi: 10.1056/NEJM199008303230902. [DOI] [PubMed] [Google Scholar]
- 6.Mitsudomi T, Steinberg SM, Oie HK, et al. ras gene mutations in non-small cell lung cancers are associated with shortened survival irrespective of treatment intent. Cancer Res. 1991;51(18):4999–5002. [PubMed] [Google Scholar]
- 7.Zhu CQ, da Cunha Santos G, Ding K, et al. Role of KRAS and EGFR as biomarkers of response to erlotinib in National Cancer Institute of Canada Clinical Trials Group Study BR.21. J Clin Oncol. 2008;26(26):4268–4275. doi: 10.1200/JCO.2007.14.8924. [DOI] [PubMed] [Google Scholar]
- 8.Tsao MS, Aviel-Ronen S, Ding K, et al. Prognostic and predictive importance of p53 and RAS for adjuvant chemotherapy in non small-cell lung cancer. J Clin Oncol. 2007;25(33):5240–5247. doi: 10.1200/JCO.2007.12.6953. [DOI] [PubMed] [Google Scholar]
- 9.Eberhard DA, Johnson BE, Amler LC, et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol. 2005;23(25):5900–5909. doi: 10.1200/JCO.2005.02.857. [DOI] [PubMed] [Google Scholar]
- 10.Mascaux C, Iannino N, Martin B, et al. The role of RAS oncogene in survival of patients with lung cancer: a systematic review of the literature with meta-analysis. British Journal of Cancer. 2005;92(1):131–139. doi: 10.1038/sj.bjc.6602258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan Q, Pao W, Ladanyi M. Rapid polymerase chain reaction-based detection of epidermal growth factor receptor gene mutations in lung adenocarcinomas. J Mol Diagn. 2005;7(3):396–403. doi: 10.1016/S1525-1578(10)60569-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chitale D, Gong Y, Taylor BS, et al. An integrated genomic analysis of lung cancer reveals loss of DUSP4 in EGFR-mutant tumors. Oncogene. 2009;28(31):2773–2783. doi: 10.1038/onc.2009.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pao W, Wang TY, Riely GJ, et al. KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS Med. 2005;2(1):e17. doi: 10.1371/journal.pmed.0020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paesmans M, Sculier JP, Libert P, et al. Prognostic factors for survival in advanced non-small-cell lung cancer: univariate and multivariate analyses including recursive partitioning and amalgamation algorithms in 1,052 patients. The European Lung Cancer Working Party. J Clin Oncol. 1995;13(5):1221–1230. doi: 10.1200/JCO.1995.13.5.1221. [DOI] [PubMed] [Google Scholar]
- 15.O'Connell JP, Kris MG, Gralla RJ, et al. Frequency and prognostic importance of pretreatment clinical characteristics in patients with advanced non-small-cell lung cancer treated with combination chemotherapy. J Clin Oncol. 1986;4(11):1604–1614. doi: 10.1200/JCO.1986.4.11.1604. [DOI] [PubMed] [Google Scholar]
- 16.Janjigian YY, McDonnell K, Kris MG, et al. Pack-years of cigarette smoking as a prognostic factor in patients with stage IIIB/IV nonsmall cell lung cancer. Cancer. 2010;116(3):670–675. doi: 10.1002/cncr.24813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spiro SG, Rudd RM, Souhami RL, et al. Chemotherapy versus supportive care in advanced non-small cell lung cancer: improved survival without detriment to quality of life. Thorax. 2004;59(10):828–836. doi: 10.1136/thx.2003.020164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 19.Rodenhuis S, van de Wetering ML, Mooi WJ, Evers SG, van Zandwijk N, Bos JL. Mutational activation of the K-ras oncogene. N Engl J Med. 1987;317(15):929–935. doi: 10.1056/NEJM198710083171504. A possible pathogenetic factor in adenocarcinoma of the lung. [DOI] [PubMed] [Google Scholar]
- 20.Janjigian YY, McDonnell K, Kris MG, et al. Pack-years of cigarette smoking as a prognostic factor in patients with stage IIIB/IV nonsmall cell lung cancer. Cancer. 2010;116:670–675. doi: 10.1002/cncr.24813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paik PK, Johnson ML, D'Angelo SP, et al. Driver mutations determine survival in smokers and never-smokers with stage IIIB/IV lung adenocarcinomas. Cancer. 2012 doi: 10.1002/cncr.27637. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27(26):4247–4253. doi: 10.1200/JCO.2009.22.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448(7153):561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 24.Paik PK, Arcila ME, Fara M, et al. Clinical characteristics of patients with lung adenocarcinomas harboring BRAF mutations. J Clin Oncol. 2011;29(15):2046–2051. doi: 10.1200/JCO.2010.33.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]