Introduction
Previous studies revealed gender-specific differences in the incidence and development of cardiovascular diseases, including hypertension, atherosclerosis, heart failure, and the corresponding myocardial remodeling.1,2 Most epidemiological studies have shown a remarkable advantage of premenopausal women (characterized by high serum oestrogen levels) compared with men of the same age groups regarding cardiovascular morbidity and mortality. Recent observations, however, also indicate gender-specific disadvantages for females in specific cases of ischemic heart disease.3 Complex gender-specific differences in the pathophysiology of cardiovascular disorders merit further in-depth studies.
The key hormone, which is responsible for differences in the regulation of vascular function in males and females, is oestrogen. Thus, most of the experimental studies have focused on the potential mechanisms by which oestrogen affords mostly beneficial effects on cardiovascular functions. In addition to oestrogen, progesterone may also contribute to gender-specific differences in the regulation of vascular functions in females; its effects, however, remain controversial.4 Testosterone, on the other hand, was shown to have negative effects on blood pressure and cardiovascular morbidity and mortality.2
Vascular effects of oestrogen
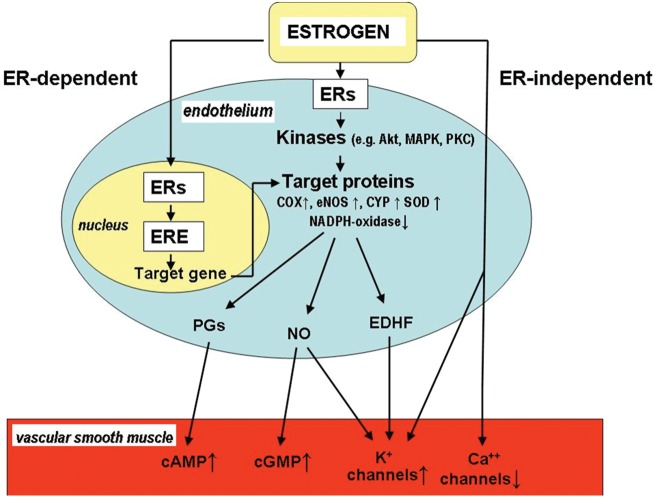
The effects of oestrogen include genomic vs. non-genomic regulation; oestrogen receptor alpha- and beta-dependent vs. oestrogen receptor-independent pathways, specific signal transduction cascades, especially those involving protein kinase B (Akt) and mitogen-activated protein kinase, as well as their downstream targets, such as nitric oxide synthase (NOS), cyclooxygenase (COX), cytochrome P450 (CYP), neutrophil nicotinamide adenine dinucleotide phosphate oxidase, and superoxide dismutase (Figure 1).
Figure 1.
Proposed mechanisms by which oestrogen is able to modulate the regulation of vasomotor function (modified after Huang and Kaley2). EC, endothelial cell; ER, oestrogen receptor; ERE, oestrogen response element; Akt, protein kinase B; MAPK, mitogen-activated protein kinase; PK, protein kinases; COX, cyclooxigenase; eNOS, endothelial nitric oxide/NO/synthase; SOD, superoxide dismutase; CYP, cytochrome P450; PG, prostaglandin; EDHF, endothelium-derived hyperpolarizing factor; VSM, vascular smooth muscle. The contribution of these mechanisms can be modified by the presence of various diseases and age.
Having considered the essential role of the microvessels in the control of total peripheral vascular resistance in vivo, oestrogen-related regulation of microvascular function and blood pressure is highlighted.
Attention is focused on the effects of oestrogen on pressure-dependent (myogenic) and flow/shear stress-dependent vasomotor mechanisms of arterioles, both of which contribute significantly to the control of local blood flow and peripheral resistance via alterations in the release of endothelial mediators, such as NO, prostaglandins (PGs), and endothelium-derived hyperpolarizing factor (EDHF), as shown by several seminal papers of Huang et al.2,5–10.
Oestrogen-modulated pressure-dependent (myogenic) vasomotor mechanisms
Although the myogenic response of arterioles is generated in the vascular smooth muscle, some studies have demonstrated that they may be modulated by the endothelium.11 This myogenic response has also been shown to be influenced by oestrogen through NO- and PG-mediated dilator mechanisms. These effects elicit a lower basal tone of microvessels2; therefore, they appear to be beneficial in hypertension and various other cardiovascular disorders.
Oestrogen-enhanced flow-mediated dilation
Flow/shear stress-mediated dilations of arterioles—another major regulatory mechanism of the arteriolar (and venular) tone—are mediated mainly by endothelium-derived factors, such as NO, vasodilator prostanoids (PGE2, PGI2), and EDHF.2 These mechanisms also show strong gender-specific characteristics, as they are more pronounced in females. Oestrogen has been demonstrated to enhance the production and release of NO, vasodilator PGs, and EDHF. Human observations using post-ischemic brachial artery reactivity testing—so called ‘flow-mediated dilation’, ‘FMD’—showed that it is enhanced in pre-menopausal females compared with males.12,13 This enhancement is seen throughout the premenopausal period and it deteriorates with menopause. In addition, in post-menopausal women, FMD improved upon oestrogen supplementation.14,15
Oestrogen vs. NO
NO-mediated endothelium-dependent vasodilatation
Previous animal experiments showed that in isolated gracilis muscle arterioles (active diameter is ∼60 µm, whereas the passive diameter is ∼110 µm)—vessels that are true resistance vessels—agonist-induced NO-mediated arteriolar dilations are greater in female than in male rats. Correspondingly, dilations to stepwise increases in perfusate flow from 0 to 25 µm/min were significantly greater in arterioles of female rats and ovariectomized rats with oestrogen replacement (OVE) (by 20%) than in male and ovariectomized female (OVX) rats. Calculation of wall shear stress (WSS) revealed that the maintained WSS was significantly lower in arterioles of female than in those of male rats (∼20 vs. ∼35 dyn/cm2). After indomethacin pretreatment, N-omega-nitro-l-arginine methyl ester (L-NAME; 10−4 M), an inhibitor of NOS eliminated flow-dependent dilation in arterioles of male and OVX rats, but only attenuated (by ∼50%) the responses in arterioles of female and OVE rats. In vessels of these latter two groups of rats, the remaining flow-induced dilation was completely eliminated by administration of 10−5 M bovine haemoglobin (Hb) or 10−3 M L-NAME.
The greater flow/shear stress-induced dilation of arterioles of female rats indicates a gender difference in the regulation of WSS, which is likely to be due to the greater release of NO in female vessels requiring the chronic presence of oestrogen. These findings suggest an important role for oestrogen in the regulation of peripheral resistance in females.5
Indeed, it was found that in OVX female spontaneously hypertensive rats (SHRs), oestrogen replacement [50 µg/kg subcutaneous 17β-oestradiol (β-E2) benzoate every 48 h] moderated the dysfunction of arterioles by preserving NO synthesis. It was concluded that oestrogen in female SHR is responsible for the preservation of NO synthesis in skeletal muscle arterioles, resulting in a greater modulation of pressure-induced myogenic tone than in male SHR and in the maintenance of NO-mediated dilations.6
The potential importance of the preservation of NO-mediated regulation of vasomotor tone is exampled by investigation of flow/WSS-induced dilations. It was found that oestrogen preserves the NO-mediated portion of flow/shear stress-induced dilation in female SHR resulting in a lower maintained WSS in female than in male SHR. Because WSS correlates with the power loss in the circulatory system, one can hypothesize that the lower WSS may contribute to the mechanisms by which oestrogen lowers systemic blood pressure and the incidence of cardiovascular diseases, such as heart failure and stroke in women.7
Oestrogen vs. genomic modulation of vasomotor responses
Studies showed that in gracilis muscle arterioles isolated from male SHR incubation with 10−9 M 17 β-E2 for 16–18 h, basal diameter of arterioles was significantly increased (by ∼10%), and flow-induced dilation was significantly enhanced resulting in a lowered WSS (∼60.0 vs. ∼32 dyn/cm2). Also, vasoconstrictions to the calcium ionophore A23187 were reversed to dilations (approximately −20–+19 µm), and constrictions to norepinephrine were significantly attenuated (−31 to −21 µm). These improvements in dilation were eliminated by ICI 182780 (10−7 M), an oestrogen receptor antagonist; 5,6-dichloro-1-beta-d-ribofuranosylbenzimidazole (10−5 M), a transcription inhibitor; or N-omega-nitro-l-arginine methyl ester (10−4 M), an inhibitor of NOS. In contrast, they were not affected by aminoguanidine (5 × 10−5 M), a specific inhibitor of inducible NOS. Also, arteriolar responses were not altered by incubation with 17α-E2. Thus, the overall conclusion from these studies is that oestrogen, via a receptor-mediated pathway, up-regulates endothelial NOS (eNOS) gene expression, leading to increased NO production, and restores the regulation of WSS in arterioles of male SHR.8 Similar mechanism likely exists in humans, as well. Oestrogen administration, for example, has been demonstrated to suppress asymmetrical dimethylarginine (ADMA)—an intrinsic inhibitor of NOS—in female patients with impaired endothelial function due to polycystic ovary syndrome.16 In these women, the outcome of the oestrogen treatment was remarkably improved vasomotor regulation.
It is suggested that one of the mechanism by which ADMA causes arteriolar dysfunction and increased vascular tone is via activating the local renin-angiotensin system, release of angiotensin II, consequent activation of NAD(P)H oxidase, and production of superoxide leading to a decreased bioavailability of NO. These processes result in diminished flow-induced dilation.17
Oestrogen vs. endothelial PGs vs. EDHF
It has been shown that NO-mediated vasomotor responses are impaired in many cardiovascular diseases,18 but the effects are different in males and females.19
The underlying mechanisms for these observations were addressed in experimental studies. Flow-induced dilation of gracilis muscle arterioles was examined in both genders of control rats and rats chronically treated with inhibitor of NOS. After a 4-week treatment, systolic blood pressure was significantly increased compared with control, whereas the plasma concentration of nitrate/nitrite was significantly reduced in treated animals. Interestingly, flow-induced dilation was comparable in arterioles of control and L-NAME-treated rats but was significantly greater in female than in male rats. L-NAME + indomethacin, which abolished flow-induced dilation in arterioles of male control rats, inhibited the dilation by only ∼75% in female control rats. The residual portion of the response was eliminated by additional administration of miconazole, an inhibitor of CYP. Indomethacin did not affect the dilation in female L-NAME-treated rats but completely inhibited the response in male L-NAME-treated rats. The indomethacin-insensitive flow-induced dilation in female L-NAME-treated arterioles was abolished by miconazole, 6-2-proparglyoxyphenyl-hexanoic acid, or charybdotoxin.
Thus, an augmented release of endothelial PGs accounts for the preserved flow-induced dilation in arterioles of male rats, whereas a metabolite of CYP (presumably EDHF) is responsible for the maintenance of flow-induced dilation in female rats, suggesting important differences in the adaptation of the endothelium of arterioles from male and female rats to the lack of NO synthesis.9,20
To investigate the role of oestrogen in flow-induced dilation in NO deficiency, this response was examined in isolated gracilis arterioles of OVX and OVE rats. Both groups of rats were treated chronically with N-omega-nitro-l-arginine methyl ester. Plasma concentration of NO2/NO3 was reduced in both groups. Plasma concentration of oestradiol was lower in OVX than in OVE rats. Flow-induced dilations were similar in vessels of the two groups; calculated WSS and basal tone were significantly greater in OVX vs. OVE rats. Indomethacin did not affect flow-induced dilation in vessels from OVE rats but abolished dilation in vessels from OVX rats. Valeryl salicylate (COX-1 inhibitor) or NS-398 (COX-2 inhibitor) inhibited flow-induced dilation by ∼50%, whereas their simultaneous administration eliminated the response in arterioles from OVX rats. In vessels from OVE rats, miconazole (inhibitor of CYP/epoxygenase) or charybdotoxin (a blocker of Ca2+-dependent K+-channels) eliminated this dilation. Thus, in NO deficiency, PGs derived from both COX isoforms mediate flow-induced dilations in gracilis arterioles of OVX rats. Oestrogen replacement switches the mediation, showing dependence on EDHF in the arterioles of OVE rats.9
Flow-induced dilation was examined in isolated coronary arteries of eNOS knockout mice (eNOS-KO) and wild-type (WT) mice. The basal tone of arteries (percentage of passive diameter) was significantly greater in eNOS-KO than in WT mice; their flow-induced dilations, however, were similar. Endothelial removal eliminated the dilations in vessels of both strains of mice. In arteries of WT mice, L-NAME (10−4 M) or indomethacin (10−5 M) alone, inhibited flow-induced dilation by ∼50%, whereas their simultaneous administration abolished the responses. In arteries of eNOS-KO mice, flow-induced dilation was inhibited by ∼40% with L-NAME. The residual portion (60%) of the response was eliminated by the additional administration of indomethacin. 7-Nitroindazole [a specific inhibitor of neuronal NOS (nNOS), 10−4 M] attenuated flow-induced dilation by ∼40% in arteries of eNOS-KO mice, but did not affect responses in those of WT mice. 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (an inhibitor of guanylate cyclase, 3 × 10−5 M) inhibited the L-NAME/7-nitroindazole-sensitive portion of the responses in arteries of eNOS-KO mice. Immunohistochemical evidence confirms the presence of nNOS in the arterial endothelium of eNOS-KO mice. In conclusion, nNOS-derived NO, via activation of cGMP, together with PGs, maintains flow-induced dilation in coronary arteries of male eNOS-KO mice.10
The above-mentioned ideas and mechanisms are summarized in Figure 1.
Controversial issues and unresolved problems
Animal studies suggested beneficial cardiovascular effects of oestrogen. Consequently, human clinical trials with post-menopausal hormone replacement therapy (HRT) were initiated. The clinical studies with HRT, however, did not fulfil the hopes evoked by experimental observations.21 The timing of initiation of HRT after the onset of menopause may determine the risk of coronary heart disease (CHD). A subsequent analysis of the women's health initiative data showed that the CHD risk was decreased when HRT was started earlier (within 10 years after the menopause).22 Therefore, several additional factors have to be considered, e.g. the onset and duration of HRT, the addition of other hormones, e.g. progesterone, or combination therapies with selective oestrogen receptor modulators. A better selection of the target population (younger women with low oestrogen levels, without enhanced risk for oestrogen—associated side effects) would also help to assess better the efficacy of HRT. The use of polyphenolic phytoestrogens has also been suggested, as they show some of the beneficial effects of oestrogen without causing significant side effects. To settle these important issues, further extensive experimental studies and human clinical trials are needed.
Conclusions
In summary, experimental data, obtained so far, suggest complex gender-specific differences between the regulation of vasomotor function of microvessels of female and males. All these experimental findings contribute to the understanding of human epidemiological observations showing significant gender-specific differences in the epidemiology, pathophysiology, clinical features, and prognosis and treatment success of cardiovascular diseases between women and men. Analysis of the current literature suggests that gender differences concerning the coronary circulation appear to be especially pronounced.23 More importantly, the gender-specific differences demand the need to develop different therapeutic and preventive measures for women and men, for which further research has to be conducted to clarify the underlying mechanisms.
Funding
Hungarian National Science Research Fund (OTKA) K 108444, Developing Competitiveness of Universities in the South Transdanubian Region (SROP-4.2.2.A-11/1/KONV-2012–0024, SROP-4.2.2.A-11/1/KONV-2012–0024, American Heart Association, FA, 0855910D; NIH PO-1 HL-430243.
Conflict of interest: none declared.
Acknowledgements
The help of Márta Balaskó in writing this review is greatly appreciated and acknowledged.
References
- 1.Sytkowski PA, D'Agostino RB, Belanger A, Kannel WB. Sex and time trends in cardiovascular disease incidence and mortality: the Framingham Heart Study, 1950–1989. Am J Epidemiol. 1996;143:338–350. doi: 10.1093/oxfordjournals.aje.a008748. doi:10.1093/oxfordjournals.aje.a008748. [DOI] [PubMed] [Google Scholar]
- 2.Huang A, Kaley G. Gender-specific regulation of cardiovascular function: estrogen as a key player. Microcirculation. 2004;11:9–38. doi: 10.1080/10739680490266162. doi:10.1080/10739680490266162. [DOI] [PubMed] [Google Scholar]
- 3.Vaccarino V. Ischemic heart disease in women: many questions, few facts. Circ Cardiovasc Qual Outcomes. 2010;3:111–115. doi: 10.1161/CIRCOUTCOMES.109.925313. doi:10.1161/CIRCOUTCOMES.109.925313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wakatsuki A, Okatani Y, Ikenoue N, Fukaya T. Effect of medroxyprogesterone acetate on endothelium-dependent vasodilation in postmenopausal women receiving estrogen. Circulation. 2001;104:1773–1778. doi: 10.1161/hc4001.097035. doi:10.1161/hc4001.097035. [DOI] [PubMed] [Google Scholar]
- 5.Huang A, Sun D, Koller A, Kaley G. Gender difference in flow-induced dilation and regulation of shear stress: role of estrogen and nitric oxide. Am J Physiol. 1998;275:R1571–R1577. doi: 10.1152/ajpregu.1998.275.5.R1571. [DOI] [PubMed] [Google Scholar]
- 6.Huang A, Sun D, Kaley G, Koller A. Estrogen maintains nitric oxide synthesis in arterioles of female hypertensive rats. Hypertension. 1997;29:1351–1356. doi: 10.1161/01.hyp.29.6.1351. doi:10.1161/01.HYP.29.6.1351. [DOI] [PubMed] [Google Scholar]
- 7.Huang A, Sun D, Kaley G, Koller A. Estrogen preserves regulation of shear stress by nitric oxide in arterioles of female hypertensive rats. J Hypertens. 1998;31:309–314. doi: 10.1161/01.hyp.31.1.309. doi:10.1161/01.HYP.31.1.309. [DOI] [PubMed] [Google Scholar]
- 8.Huang A, Sun D, Koller A, Kaley G. 17beta-estradiol restores endothelial nitric oxide release to shear stress in arterioles of male hypertensive rats. Circulation. 2000;101:94–100. doi: 10.1161/01.cir.101.1.94. doi:10.1161/01.CIR.101.1.94. [DOI] [PubMed] [Google Scholar]
- 9.Huang A, Wu Y, Sun D, Koller A, Kaley G. Effect of estrogen on flow-induced dilation in NO deficiency: role of prostaglandins and EDHF. J Appl Physiol. 2001;91:2561–2566. doi: 10.1152/jappl.2001.91.6.2561. [DOI] [PubMed] [Google Scholar]
- 10.Huang A, Sun D, Shesely EG, Levee EM, Koller A, Kaley G. Neuronal NOS-dependent dilation to flow in coronary arteries of male eNOS-KO mice. Am J Physiol Heart Circ Physiol. 2002;282:H429–H436. doi: 10.1152/ajpheart.00501.2001. [DOI] [PubMed] [Google Scholar]
- 11.Huang A, Sun D, Koller A. Endothelial dysfunction augments myogenic arteriolar constriction. Hypertension. 1993;22:913–921. doi: 10.1161/01.hyp.22.6.913. doi:10.1161/01.HYP.22.6.913. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto M, Akishita M, Eto M, Ishikawa M, Kozaki K, Toba K, Sagara Y, Taketani Y, Orimo H, Ouchi Y. Modulation of endothelium-dependent flow-mediated dilatation of the brachial artery by sex and menstrual cycle. Circulation. 1995;92:3431–3435. doi: 10.1161/01.cir.92.12.3431. doi:10.1161/01.CIR.92.12.3431. [DOI] [PubMed] [Google Scholar]
- 13.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24:471–476. doi: 10.1016/0735-1097(94)90305-0. doi:10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 14.Kawano H, Motoyama T, Kugiyama K, Hirashima O, Ohgushi M, Fujii H, Ogawa H, Yasue H. Gender difference in improvement of endothelium-dependent vasodilation after estrogen supplementation. J Am Coll Cardiol. 1997;30:914–919. doi: 10.1016/s0735-1097(97)00234-9. doi:10.1016/S0735-1097(97)00234-9. [DOI] [PubMed] [Google Scholar]
- 15.Gerhard M, Walsh BW, Tawakol A, Haley EA, Creager SJ, Seely EW, Ganz P, Creager MA. Estradiol therapy combined with progesterone and endothelium-dependent vasodilation in postmenopausal women. Circulation. 1998;98:1158–1163. doi: 10.1161/01.cir.98.12.1158. doi:10.1161/01.CIR.98.12.1158. [DOI] [PubMed] [Google Scholar]
- 16.Charitidou C, Farmakiotis D, Zournatzi V, Pidonia I, Pegiou T, Karamanis N, Hatzistilianou M, Katsikis I, Panidis D. The administration of estrogens, combined with anti-androgens, has beneficial effects on the hormonal features and asymmetric dimethyl-arginine levels, in women with the polycystic ovary syndrome. Atherosclerosis. 2008;196:958–965. doi: 10.1016/j.atherosclerosis.2007.03.002. doi:10.1016/j.atherosclerosis.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Veresh Z, Racz A, Lotz G, Koller A. ADMA impairs nitric oxide-mediated arteriolar function due to increased superoxide production by angiotensin II-NAD(P)H oxidase pathway. Hypertension. 2008;52:960–966. doi: 10.1161/HYPERTENSIONAHA.108.116731. doi:10.1161/HYPERTENSIONAHA.108.116731. [DOI] [PubMed] [Google Scholar]
- 18.Calver A, Collier J, Moncada S, Vallance P. Effect of local intra-arterial NG-monomethyl-L-arginine in patients with hypertension: the nitric oxide dilator mechanism appears abnormal. J Hypertens. 1992;10:1025–1031. doi:10.1097/00004872-199209000-00017. [PubMed] [Google Scholar]
- 19.Thompson J, Khalil RA. Gender differences in the regulation of vascular tone. Clin Exp Pharmacol Physiol. 2003;30:1–15. doi: 10.1046/j.1440-1681.2003.03790.x. doi:10.1046/j.1440-1681.2003.03790.x. [DOI] [PubMed] [Google Scholar]
- 20.Wu Y, Huang A, Sun D, Falck JR, Koller A, Kaley G. Gender-specific compensation for the lack of NO in the mediation of flow-induced arteriolar dilation. Am J Physiol Heart Circ Physiol. 2001;280:H2456–H2461. doi: 10.1152/ajpheart.2001.280.6.H2456. [DOI] [PubMed] [Google Scholar]
- 21.Writing Group for the Women's Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. doi:10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 22.Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, Ko M, LaCroix AZ, Margolis KL, Stefanick ML. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465–1477. doi: 10.1001/jama.297.13.1465. doi:10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 23.Blum A, Blum N. Coronary artery disease: are men and women created equal? Gender Med. 2009;6:410–418. doi: 10.1016/j.genm.2009.09.005. doi:10.1016/j.genm.2009.09.005. [DOI] [PubMed] [Google Scholar]