Abstract
Objective
To compare the effect initiating different antiretroviral therapy (ART) regimens have on weight, body mass index (BMI), and lean body mass (LBM) and explore how changes in body composition are associated with bone mineral density (BMD).
Methods
A5224s was a substudy of A5202, a prospective trial of 1857 ART-naïve participants randomized to blinded abacavir-lamivudine (ABC/3TC) or tenofovir DF-emtricitabine (TDF/FTC) with open-label efavirenz (EFV) or atazanavir-ritonavir (ATV/r). All subjects underwent dual-energy absorptiometry (DXA) and abdominal CT for body composition. Analyses used 2-sample t-tests and linear regression.
Results
A5224s included 269 subjects: 85% male, 47% white non-Hispanic, median age 38 years, HIV-1 RNA 4.6 log10 copies/mL, and CD4 233 cells/µL. Overall, significant gains occurred in weight, BMI, and LBM at 96 weeks post randomization (all p<0.001). Assignment to ATV/r (vs EFV) resulted in significantly greater weight (mean difference 3.35 kg) and BMI gain (0.88 kg/m2; both p=0.02), but not LBM (0.67 kg; p=0.15), while ABC/3TC and TDF/FTC were not significantly different (p≥0.10). In multivariable analysis, only lower baseline CD4 count and higher HIV-1 RNA were associated with greater increase in weight, BMI, or LBM. In multivariable analyses, increased LBM was associated with an increased hip BMD.
Conclusions
ABC/3TC vs. TDF/FTC did not differ in change in weight, BMI, or LBM; ATV/r vs. EFV resulted in greater weight and BMI gain but not LBM. A positive association between increased LBM and increased hip BMD should be further investigated through prospective interventional studies to verify the impact of increased LBM on hip BMD.
Keywords: antiretroviral therapy, HIV, body composition, body weight, lean body mass, bone mineral density, randomized clinical trial
INTRODUCTION
Body weight is considered a key determinant of bone mineral density, however the body weight component among lean mass, peripheral fat mass or visceral adipose tissue with the greatest impact on bone mass is debated [1, 2]. Lean body mass augments bone mineral density through mechanical load forces and lean body mass is associated with lower risk of bone fractures [3, 4]. Fat mass can have a positive interaction on bone through skeletal loading and adipocyte hormone production, but inflammatory cytokines produced in visceral adipose tissue may exacerbate bone loss [5]. Furthermore, the impact of total fat mass and total lean body mass on bone mineral density may differ by age, sex, race, and skeletal site [6].
Low bone mineral density is reported across multiple cohorts of both men and women with HIV-infection, with a strong association between lower baseline weight and both lower baseline bone mineral density [7, 8] and a greater decline in bone mineral density with antiretroviral therapy (ART) initiation [9–11]. Prior to initiating antiretroviral therapy, individuals with HIV infection have lower bone mineral density than the general population [12]. Lower weight appears to mediate a significant proportion of the bone mineral density differences [13]. The initiation of antiretroviral therapy is often characterized by weight gain [14–17], and it is hypothesized that these changes in weight help to stabilize bone mineral density after the initial loss in bone mineral density observed with ART initiation [13]. Changes in central and peripheral fat with ART initiation and ART regimens are also well-described, however a gain in adiposity could be associated with a myriad of other health problems [18–20]. Despite a strong association between greater muscularity and lower mortality [21, 22], comparisons of the role of individual ART on lean body mass and the contribution of body composition components on bone mineral density have not been well defined.
We have previously presented data on changes after ART initiation in bone mineral density, peripheral fat, and visceral adipose tissue from AIDS Clinical Trials Group A5224s, a substudy of A5202, in which HIV-infected treatment-naïve participants were randomized in a double-blinded fashion to abacavir/lamivudine (ABC/3TC) or tenofovir DF/emtricitabine (TDF/FTC) with open-label efavirenz (EFV) or atazanavir-ritonavir (ATV/r) [20, 23]. Briefly, randomization to TDF/FTC led to a greater decrease in spine and hip bone mineral density, less gain in limb fat, and no significant difference in change in visceral fat compared to ABC/3TC [20, 23]. Assignment to ATV/r led to greater losses in spine but not hip bone mineral density, and was associated with significantly greater increase in limb fat and a trend towards greater increase in visceral fat compared to EFV. Here we compare the changes in weight, body mass index (BMI), and lean body mass between the nucleoside reverse transcriptase inhibitor (NRTI) components and the non-nucleoside reverse transcriptase inhibitor/protease inhibitor (NNRTI/PI) components from A5224s. We also explore the association of changes in BMI, lean body mass, and fat mass with changes in bone mineral density.
METHODS
A5224s was a sub-study of AIDS Clinical Trials Group A5202, in which ART-naïve persons aged ≥16 years and with an HIV-1 RNA load >1000 copies/mL received TDF/FTC or ABC/3TC, with EFV or ATV/r at standard doses. The primary analyses of both A5202 and A5224s have been presented previously [20, 23–26]. Specific A5224s exclusion criteria were uncontrolled thyroid disease or hypogonadism; endocrine diseases, including Cushing’s syndrome, diabetes mellitus, and the use of growth hormone, anabolic steroids, glucocorticoids, or osteoporosis medications (calcium and/or vitamin D were not included); or the intent to start these treatments known to influence bone mineral density. The duration of the study was 96 weeks after the last A5202 participant enrolled.
Any participant enrolling in A5202 at one of the AIDS Clinical Trials Group sites participating in A5224s and meeting criteria for A5224s was eligible to enroll in the sub-study; A5202 randomization was stratified by willingness to enroll into the substudy. Each participant signed a written informed consent before enrollment. The study was approved by the local institutional review board at each site.
At baseline, a complete history was obtained and participants underwent a physical examination, including standardized measurement of height and weight. Substudy evaluations, regardless of antiretroviral treatment status, included whole-body dual-energy absorptiometry (DXA) and site-specific (hip and lumbar spine) bone DXA at baseline and at weeks 24, 48, 96, and every 48 weeks until the end of follow-up, as well as a single-slice abdomen CT scan at the L4–L5 level at baseline and week 96. lean body mass was defined as fat-free, bone-free mass as measured by DXA in the anteroposterior view (using Hologic or Lunar scanners). Hip bone mineral density, lumbar spine bone mineral density (from L1-L4), and limb fat were measured by DXA. Technicians were instructed to scan the same hip of each participant for all bone mineral density measurements and to use the same DXA machine on the same participant throughout the study. CT was used to quantify visceral adipose tissue. All DXAs and CT scans were standardized at the participating sites, then centrally read (Tufts) by blinded personnel.
On 18 February 2008, after a median follow-up of 97 weeks (range 0–124 weeks; Q1–Q3 58–108 weeks), the Data Safety and Monitoring Board recommended unblinding the NRTI component of the study for subjects with screening HIV-1 RNA levels ≥100,000 copies/mL because of excess virological failures associated with ABC/3TC; subjects receiving ABC/3TC were permitted to modify their NRTI regimen [24].
Statistical Analysis
The current study was a post-hoc, exploratory analysis to compare changes from baseline to week 96 in weight, BMI, and lean body mass between pooled, randomized NRTI components (ABC/3TC versus TDF/FTC) and NNRTI/PI components (ATV/r versus EFV). All analyses were initially performed using the intent-to-treat principle based on randomized treatment assignment in which all available data were included and modifications to randomized treatment were ignored; no imputations were made for missing values. Supplemental as-treated analyses were performed in which values were censored after a change in the randomized NRTI component (when comparing NRTI components) or NNRTI/PI component (when comparing NNRTI/PI components). Comparisons used a factorial analysis approach in which, after assessing for treatment effect modification by the other component, the NRTI effect was analyzed by combining EFV and ATV/r arms and vice versa. The assessment of treatment effect modification (interaction) of each ART component with screening HIV-1 RNA stratum (<100,000 or ≥100,000 copies/mL) was pre-specified.
Changes from baseline within study arm or regimen component used 1-sample t-tests. Comparisons between regimen components used 2-sample t-tests. There was no evidence of an interaction between the NRTI and NNRTI/PI components on 96 week change in weight, BMI, or lean body mass (all p ≥0.30). Analyses that adjusted for baseline and post-baseline factors and explored associations with baseline and post-baseline factors used linear regression; all multivariable models were adjusted for both ART components. Univariate associations with a p-value <0.20 were included in a multivariable model which utilized backwards selection and only factors with a p-value <0.05 were retained. Analyses were performed using SAS, version 9.1.3 (SAS Institute).
RESULTS
Participant Characteristics
A total of 271 participants from 37 AIDS Clinical Trials Group sites in the United States and Puerto Rico were randomized to receive ART; 2 participants were excluded from the analysis for eligibility violations. Sixty-nine participants were randomized to receive EFV plus TDF/FTC, 70 to EFV plus ABC/3TC, 65 to ATV/r plus TDF/FTC, and 65 to ATV/r plus ABC/3TC. Baseline characteristics are summarized in Table 1 and were balanced across study arms. The median age of the subjects was 38 years, 85% were male, and 47% were white non-Hispanics. The mean weight was 78.0 kg, BMI was 24.9 kg/m2, CD4 cell count was 233 cells/µL, plasma HIV-1 RNA was 4.6 log10 copies/mL, and 80% had an HIV-1 RNA <100,000 copies/mL at study entry.
Table 1.
Baseline characteristics of Study Participants
| Characteristic | EFV + TDF/FTC N=69 |
EFV + ABC/3TC N=70 |
ATV/r + TDF/FTC N=65 |
ATV/r + ABC/3TC N=65 |
Total N=269 |
|---|---|---|---|---|---|
| Age (years) | 39 (10) | 39 (10) | 38 (10) | 37 (10) | 38 (10) |
| 40 (33–44) | 39 (31–46) | 38 (30–44) | 37 (29–43) | 38 (31–44) | |
| Sex | |||||
| Male | 58 (84) | 56 (80) | 56 (86) | 59 (91) | 229 (85) |
| Female | 11 (16) | 14 (20) | 9 (14) | 6 (9) | 40 (15) |
| Race/ethnicity | |||||
| White non-Hispanic | 37 (54) | 34 (49) | 26 (40) | 29 (45) | 126 (47) |
| Black non-Hispanic | 22 (32) | 20 (29) | 21 (32) | 27 (42) | 90 (33) |
| Hispanic | 8 (12) | 14 (20) | 14 (22) | 8 (12) | 44 (16) |
| Other | 2 (<1) | 2 (<1) | 4 (1) | 1 (<1) | 9 (<1) |
| CD4 T-cell count (cells/µL) | 248 (160) | 231 (167) | 226 (142) | 238 (189) | 236 (165) |
| 250 (132–334) | 213 (106–350) | 247 (114–319) | 222 (75–332) | 233 (106–334) | |
| HIV-1 RNA (log10 copies/mL) | 4.6 (0.7) | 4.6 (0.6) | 4.6 (0.7) | 4.7 (0.7) | 4.6 (0.7) |
| 4.7 (4.2–4.9) | 4.7 (4.2–4.9 | 4.5 (4.2–4.9) | 4.6 (4.3–5.1) | 4.6 (4.2–4.9) | |
| HIV-1 RNA (copies/mL) | |||||
| <100,000 copies/mL | 56 (81) | 59 (84) | 52 (80) | 48 (74) | 215 (80) |
| ≥100,000 copies/mL | 13 (19) | 11 (16) | 13 (20) | 17 (26) | 54 (20) |
| Hepatitis C antibody | 5 (7) | 8 (11) | 3 (5) | 7 (11) | 23 (9) |
| Body mass index (kg/m2) | 24.7 (4.0) | 25.5 (4.6) | 26.2 (5.4) | 25.7 (4.5) | 25.5 (4.7) |
| 24.9 (21.6–27.1) | 24.7 (22.6–28.3) | 24.9 (21.8–28.8) | 25.3 (21.8–28.9) | 24.9 (21.8–28.2) | |
| Weight (kg) | 76.2 (15.7) | 76.8 (14.3) | 80.2 (17.1) | 79.1 (15.0) | 78.0 (15.5) |
| 72.6 (64.8–86.9) | 77.6 (67.9–85.3) | 77.0 (68.5–90.5) | 75.7 (67.0–88.4) | 76.2 (66.7–87.0) | |
| Lean body mass (kg) | 53.7 (9.8) | 52.8 (9.1) | 55.5 (9.9) | 56.0 (8.1) | 54.5 (9.3) |
| 53.1 (48.5–61.3) | 54.0 (46.6–58.9) | 55.0 (48.0–60.0) | 56.6 (50.6–61.7) | 54.6 (48.1–61.1) | |
| Limb fat (kg) | 7.7 (3.9) | 8.8 (5.5) | 8.8 (5.5) | 8.1 (5.0) | 8.3 (5.0) |
| 7.3 (4.7–9.4) | 7.8 (4.9–10.5) | 7.4 (5.0–11.6) | 6.8 (4.3–10.5) | 7.4 (4.7–10.1) | |
| Visceral adipose tissue (cm2) | 94.0 (61.5) | 94.5 (53.3) | 93.0 (39.9) | 88.6 (46.9) | 92.6 (51.0) |
| 84.2 (52.0–110.3) | 82.6 (62.8–111.6) | 86.7 (60.2–121.9) | 82.7 (55.2–116.1) | 84.1 (57.2–115.9) | |
| Lumbar spine BMD (g/cm2) | 1.12 (0.17) | 1.10 (0.20) | 1.15 (0.22) | 1.14 (0.17) | 1.13 (0.19) |
| 1.12 (1.00–1.23) | 1.08 (0.97–1.23) | 1.13 (1.03–1.24) | 1.13 (1.04–1.23) | 1.12 (0.99–1.23) | |
| Hip BMD (g/cm2) | 1.00 (0.13) | 1.03 (0.17) | 1.07 (0.18) | 1.06 (0.14) | 1.04 (0.16) |
| 0.99 (0.92–1.07) | 1.02 (0.93–1.11) | 1.05 (0.98–1.18) | 1.02 (0.97–1.13) | 1.02 (0.94–1.11) | |
| Prior bone fracture | 22 (32) | 24 (34) | 18 (28) | 22 (34) | 86 (32) |
Data are shown as mean (standard deviation) and median (interquartile range) or number (frequency). EFV, efavirenz; TDF/FTC, tenofovir-emtricitabine; ABC/3TC, abacavir-lamivudine; ATV/r, atazanavir-ritonavir; BMD, bone mineral density
Sixty-six (25%) of A5224s participants prematurely discontinued study follow-up, 4 (1%) died, and 45% modified the randomized treatment regimen. These details have been previously published [20, 23].
Change in Weight
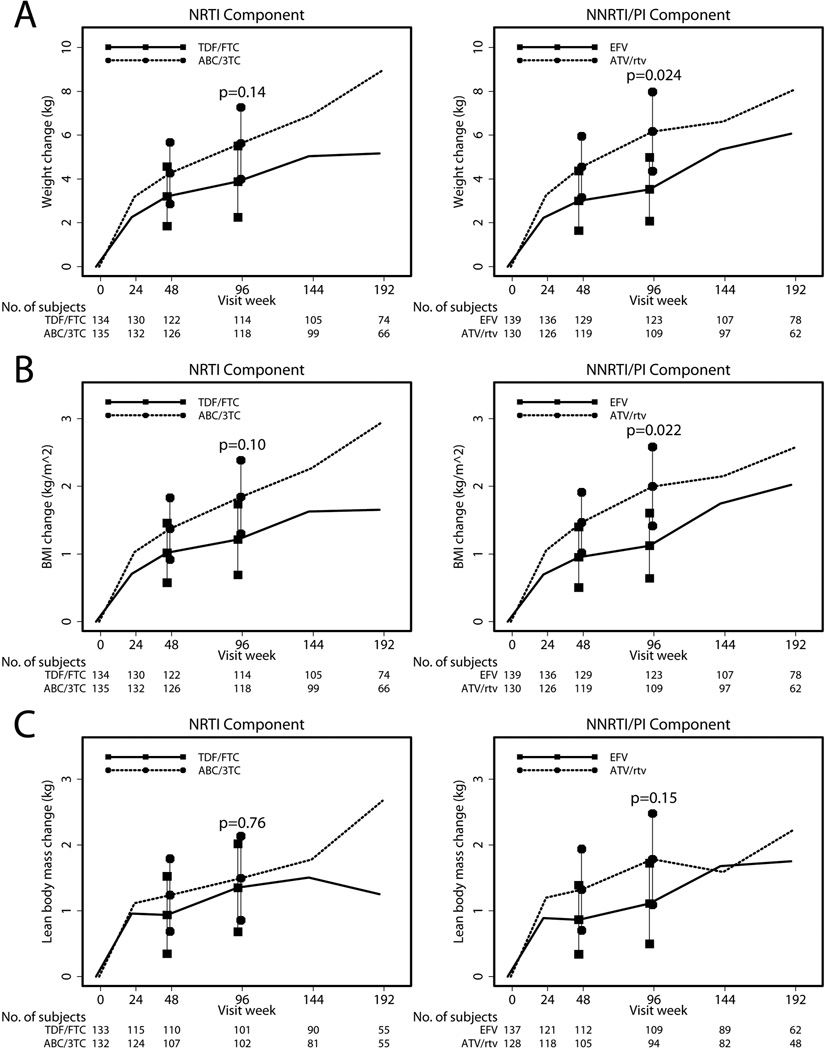
Among all participants, weight increased from baseline by a mean of 4.8 kg at week 96 (p<0.001). The mean changes in weight for each study arm are shown in Figure 1A. Although ABC/3TC had a trend towards greater weight gain compared to TDF/FTC by intent-to-treat analyses at week 96, this difference was not statistically significant (Figure 1A). Results in the as-treated analysis were similar (Δ=2.00 kg; 95% CI −0.94, 5.06 kg; p=0.21). ATV/r assignment resulted in significantly greater weight gain in both intent-to-treat (Figure 1A) and as-treated analyses (Δ=3.24 kg; 95% CI 0.13, 6.36 kg; p=0.04) compared to EFV.
Figure 1.
Absolute changes in total weight, body mass index, and lean body mass by treatment arms. Mean and 95% confidence intervals are represented by symbols and error bars; P value from comparison between arms at 96 weeks; TDF/FTC, tenofovir-emtricitabine; ABC/3TC, abacavir-lamivudine; EFV, efavirenz; ATV/r, atazanavir-ritonavir. A. Changes in total weight between the nucleoside reverse transcriptase inhibitor (NRTI) and non-nucleoside reverse transcriptase inhibitor/protease inhibitor (NNRTI/PI) components. B. Changes in body mass index between NRTI and NNRTI/PI components. C. Changes in lean body mass between NRTI and NNRTI/PI components.
Change in BMI
Among all participants, BMI increased by a mean of 1.5 kg/m2 at week 96 (p<0.001). The mean changes in BMI across study arms are shown in Figure 1B. No significant differences in BMI were detected between ABC/3TC and TDF/FTC by intent-to-treat (Figure 1B) or as-treated analyses (Δ=0.53 kg/m2; 95% CI −0.25, 1.31 kg/m2; p=0.18). Participants randomized to ATV/r experienced a 0.88 kg/m2 greater increase in BMI compared to EFV in the intent-to-treat analysis (Figure 1B). BMI increase was also higher in the ATV/r compared to EFV by as-treated analysis (Δ=0.90; 95% CI 0.10, 1.70 kg/m2; p=0.028).
Change in Lean Body Mass
Across all treatment arms, lean body mass increased significantly by a mean 1.4 kg at week 96 (p<0.001). Mean changes in lean body mass across study arms are shown in Figure 1C. No significant differences in lean body mass gain were seen between ABC/3TC and TDF/FTC by intent-to-treat (Figure 1C) or as-treated analyses (Δ=−0.03 kg; 95% CI −1.09, 1.03 kg; p=0.96). In comparison to those receiving EFV, participants randomized to ATV/r did not have a significantly different lean body mass change by intent-to-treat analysis (Figure 1C) but the difference did approach statistical significance by as-treated analysis (Δ=1.03 kg; 95% CI −0.01, 2.07 kg; p=0.051).
A pre-specified intent-to-treat subgroup analysis detected a significant interaction between the NNRTI/PI component and screening HIV-1 RNA stratum (p=0.053), indicating that the treatment effect differed by RNA level. Participants with screening HIV-1 RNA ≥100,000 copies/mL had a significantly greater mean gain in lean body mass with ATV/r (n=38) compared to EFV (n=43; Δ=1.75 kg; 95% CI 0.18, 3.33; p=0.029). Differences between ATV/r (n=56) and EFV (n=66) in lean body mass gain were not seen among participants with HIV-1 RNA <100,000 copies/mL (Δ=−0.06 kg; 95% CI −1.15, 1.03 kg; p=0.91).
Baseline Associations with Change in Total Body Mass, BMI, and Lean Body Mass
In both univariate and multivariable analyses of variables associated with body composition change, higher baseline HIV-1 RNA level and lower CD4 count were associated with a greater gain in total body mass, BMI, and lean body mass at week 96 after adjusting for treatment arm (Table 2).
Table 2.
Univariate and Multivariable Linear Regression to Assess the Association Between Baseline Factors and Change in Measures of Body Mass, Adjusted for Treatment Arm
| Univariate Analyses | Multivariable Analyses | ||||
|---|---|---|---|---|---|
| Endpoint | Baseline Covariate | Estimate (95% CI) | P | Estimate (95% CI) | P |
| Change in Body Weight (kg) | Male | 1.63 (−1.59, 4.85) | 0.32 | ||
| Age* | −0.09 (−0.21, 0.02) | 0.12 | |||
| Race/Ethnicity (vs White non-Hispanic) | 0.83 | ||||
| Black non-Hispanic | 0.92 (−1.71, 3.54) | ||||
| Hispanic | 1.19 (−2.21, 4.59) | ||||
| Other | −0.78 (−7.22, 5.67) | ||||
| Baseline HIV-1 RNA† | 4.39 (2.75, 6.03) | <0.001 | 2.94 (1.19, 4.70) | 0.001 | |
| Baseline CD4 count§ | −0.91 (−1.24, −0.58) | <0.001 | −0.65 (−1.00, −0.29) | <0.001 | |
| Baseline BMI¶ | −0.07 (−0.33, 0.18) | 0.57 | |||
| Change in BMI (kg/m2) | Male | 0.32 (−0.74, 1.37) | 0.55 | ||
| Age* | −0.03 (−0.07, 0.01) | 0.12 | |||
| Race/Ethnicity (vs White non-Hispanic) | 0.69 | ||||
| Black non-Hispanic | 0.36 (−0.50, 1.21) | ||||
| Hispanic | 0.56 (−0.55, 1.67) | ||||
| Other | −0.26 (−2.36, 1.85) | ||||
| Baseline HIV-1 RNA† | 1.44 (0.91, 1.98) | <0.001 | 0.97 (0.40, 1.54) | 0.001 | |
| Baseline CD4 count§ | −0.30 (−0.41, −0.19) | <0.001 | −0.21 (−0.33, −0.09) | <0.001 | |
| Change in Lean Body Mass (kg) | Male | 0.81 (−0.47, 2.08) | 0.21 | ||
| Age* | −0.03 (−0.08, 0.02) | 0.20 | |||
| Race/Ethnicity (vs White non-Hispanic) | 0.15 | ||||
| Black non-Hispanic | 0.58 (−0.47, 1.63) | ||||
| Hispanic | 1.47 (0.15, 2.80) | ||||
| Other | 1.22 (−1.75, 4.19) | ||||
| Baseline HIV-1 RNA† | 1.55 (0.87, 2.23) | <0.001 | 0.76 (0.05, 1.46) | 0.035 | |
| Baseline CD4 count§ | −0.43 (−0.56, −0.31) | <0.001 | −0.37 (−0.51, −0.23) | <0.001 | |
| Baseline BMI¶ | −0.04 (−0.14, 0.06) | 0.41 | |||
CI, confidence interval; BMI, body mass index;
per 1 year older;
per log10 copies/mL higher;
per 50 cells/mm3 higher;
per 1 kg/m2 higher
Multivariable Linear Regression Analyses
Univariate and multivariable analyses assessed baseline and post-baseline factors associations with week 96 change in hip and lumbar spine bone mineral density. Compared to TDF/FTC, assignment to ABC/3TC was associated with less percent loss in hip bone mineral density from week 0 to week 96 (mean Δ 1.35; 95% CI 0.18, 2.53; p=0.02; results previously presented [23]). The change in hip bone mineral density between ATV/r and EFV arms was not statistically significant (mean Δ −0.31; 95% CI −1.50, 0.89; p=0.61). For hip bone mineral density, in addition to the significant TDF/FTC effect, lower baseline weight, higher increase in CD4 count over 96 weeks, lesser increase in lean body mass at 96 weeks, and history of fracture were independently and significantly associated with less increase in hip bone mineral density at 96 weeks after adjusting for treatment arm.
Compared to TDF/FTC, assignment to ABC/3TC was associated with less percent loss in lumbar spine bone mineral density from week 0 to 96 (mean Δ 2.00; 95% CI 0.66, 3.33; p=0.004) while ATV/r was associated with significantly greater percent loss in lumbar spine bone mineral density compared to EFV (mean Δ −1.46; −2.82, −0.10; p=0.035; results previously presented [23]). In multivariable analyses, higher baseline HIV-1 RNA, lower baseline CD4 count, and lack of HIV-1 RNA suppression <50 copies/mL at week 96 were independently and significantly associated with a less increase in lumbar spine bone mineral density at 96 weeks after adjusting for treatment arm (Table 3).
Table 3.
Linear Regression Identifying Significant Variables in BMD Change with Antiretroviral Initiation, Adjusted for Treatment Arm
| Univariate Analyses | Multivariable Analyses | |||
|---|---|---|---|---|
| Covariate | Estimate (95% CI) | P value | Estimate (95% CI) | P value |
| Hip BMD (% change week 0 to 96) | ||||
| Male | 0.00 (−1.63, 1.63) | 1.00 | ||
| Age* | −0.05 (−0.11, 0.02) | 0.15 | ||
| Race/Ethnicity (vs White non-Hispanic) | 0.75 | |||
| Black non-Hispanic | 0.43 (−0.93, 1.80) | |||
| Hispanic | 0.90 (−0.90, 2.70) | |||
| Other | −0.39 (−4.21, 3.44) | |||
| Baseline HIV-1 RNA† | −0.53 (−1.44, 0.37) | 0.24 | ||
| 96 week HIV-1 RNA suppression§ | −2.30 (−4.00, −0.60) | 0.008 | ||
| Baseline CD4 count¶ | 0.06 (−0.12, 0.25) | 0.51 | ||
| 96 week CD4 count change¶ | −0.20 (−0.37, −0.03) | 0.020 | −0.24 (−0.40, −0.08) | 0.004 |
| Hepatitis C antibody | −0.60 (−2.89, 1.70) | 0.61 | ||
| History of fracture | −1.42 (−2.65, −0.19) | 0.024 | −1.60 (−2.78, −0.41) | 0.008 |
| Baseline weight‡ | 0.05 (0.01, 0.10) | 0.013 | 0.07 (0.03, 0.11) | 0.001 |
| 96 week weight change‡ | 0.08 (0.00, 0.15) | 0.040 | ||
| Baseline BMI** | 0.16 (0.02, 0.29) | 0.021 | ||
| 96 week BMI change** | 0.24 (0.02, 0.46) | 0.031 | ||
| Baseline lean body mass‡ | 0.02 (−0.04, 0.09) | 0.50 | ||
| 96 week lean body mass change‡ | 0.25 (0.08, 0.43) | 0.005 | 0.28 (0.11, 0.45) | 0.001 |
| Baseline limb fat‡ | 0.17 (0.04, 0.29) | 0.008 | ||
| 96 week limb fat change‡ | 0.19 (−0.05, 0.42) | 0.12 | ||
| Baseline visceral abdominal fat†† | 0.00 (−0.01, 0.01) | 0.65 | ||
| 96 week visceral abdominal fat change†† | 0.02 (0.00, 0.04) | 0.044 | ||
| Lumbar Spine BMD (% change week 0–96) | ||||
| Male | 0.41 (−1.45, 2.27) | 0.66 | ||
| Age* | −0.04 (−0.11, 0.03 | 0.25 | ||
| Race/ethnicity (vs White non-Hispanic) | 0.73 | |||
| Black non-Hispanic | 0.23 (−1.35, 1.82) | |||
| Hispanic | −0.78 (−2.78, 1.21) | |||
| Other | −1.47 (−5.88, 2.94) | |||
| Baseline HIV-1 RNA† | −2.00 (−3.00, −1.01) | <0.001 | −1.22 (−2.25, −0.19) | 0.021 |
| 96 week HIV-1 RNA suppression§ | 1.96 (−0.02, 3.93) | 0.052 | 2.19 (0.38, 4.00) | 0.018 |
| Baseline CD4 count ¶ | 0.48 (0.28, 0.68) | <0.001 | 0.34 (0.13, 0.55) | 0.002 |
| 96 week CD4 count change¶ | −0.16 (−0.35, 0.04) | 0.11 | ||
| Hepatitis C antibody | 0.44 (−2.21, 3.09) | 0.74 | ||
| History of fracture | 0.11 (−1.32, 1.55) | 0.87 | ||
| Baseline weight‡ | 0.02 (−0.03, 0.07) | 0.45 | ||
| 96 week weight change‡ | −0.14 (−0.23, −0.06) | 0.001 | ||
| Baseline BMI** | 0.01 (−0.14, 0.16) | 0.90 | ||
| 96 week BMI change** | −0.42 (−0.67, −0.17) | 0.001 | ||
| Baseline lean body mass‡ | 0.03 (−0.04, 0.11) | 0.38 | ||
| 96 week lean body mass change‡ | −0.39 (−0.59, −0.19) | <0.001 | ||
| Baseline limb fat‡ | 0.00 (−0.14, 0.15) | 0.97 | ||
| 96 week limb fat change‡ | −0.27 (−0.54, −0.00) | 0.048 | ||
| Baseline visceral abdominal fat†† | 0.00 (−0.01, 0.01) | 0.97 | ||
| 96 week visceral abdominal fat change†† | −0.02 (−0.04, −0.00) | 0.030 | ||
For multivariate analyses, only those with p <0.05 and antiretroviral therapy arm (regardless of p value) are reported; BMD, bone mineral density; CI, confidence interval; BMI, body mass index; ABC/3TC, abacavir-lamivudine; TDF/FTC, tenofovir-emtricitabine; ATV/r, atazanavir-ritonavir; EFV, efavirenz;
per 1 year older;
per log10 copies/mL higher;
< 50 copies/mL;
per 50 cells/µL higher;
per 1 kg higher;
per 1 kg/m2 higher;
per 1 cm2 higher
DISCUSSION
Our study presents the first longitudinal assessment of changes in lean body mass after the initiation of antiretroviral therapy and the first longitudinal assessment of body fat, visceral fat, and lean body mass on the change on bone density with current first-line antiretroviral therapy initiation. In the setting of a large, randomized trial of antiretroviral initiation among treatment-naïve subjects, we demonstrated an increase in body weight and BMI across all treatment arms, consistent with prior studies [14–16, 18]. A significantly greater gain in total body mass and BMI was observed in the ATV/r arm compared to the EFV arm. Lower baseline CD4 count and higher HIV-1 RNA had a strong association with a positive gain in total body mass, BMI and lean body mass. These findings likely reflect HIV disease severity and cachexia prior to ART initiation and the return to health phenomenon in patients with more advanced disease.
In the present study, we demonstrated an average increase in lean body mass among all participants by 96 weeks, with no significant difference between NRTIs but a trend towards greater gain in those assigned to ATV/r compared to EFV. Prior studies of ART initiation or change in ART found an increase in lean body mass when using older treatment regimens (primarily zidovudine or stavudine based) [14, 16, 27, 28] despite the potential for the thymidine NRTIs to induce mitochondrial toxicity in the muscle tissue [29, 30]. Our randomized study reports an increase in lean body mass for the first time with contemporary first-line ART regimens [31]. Observational cohorts including both ART-treated and ART-naïve populations demonstrate stable [32] or increased lean body mass over time, particularly among those on ART [33–35]. However, these findings have not been consistent across observational cohorts as other studies have demonstrated a decrease in lean body mass [36, 37].
Low bone mineral density and its resultant bone fractures are more prevalent in HIV-infected subjects on ART compared to HIV-uninfected populations [38]. The etiology of low bone mineral density is unclear but is likely multifactorial. In cross-sectional and longitudinal data of older, HIV-uninfected individuals (primarily women), greater lean body mass and fat mass are associated with greater bone mineral density [1, 6, 39]. Furthermore, cross-sectional studies suggest that total body mass may be one of the most significant determinants of bone mineral density of HIV-infected persons [9, 10, 13]. A cross-sectional study of 221 HIV-infected men (85% on ART) found that weight, lean body mass, total fat mass, and limb fat were significantly higher among men with normal bone mineral density; older age, lower lean body mass, and greater stavudine exposure were independently associated with lower bone mineral density in multivariate regression [40]. A recent publication from the Women’s Interagency HIV Study cohort (83% on ART) measuring change in bone mineral density over a 5 year period found that among both HIV-infected and uninfected women, higher lean body mass was associated with increased bone mineral density at the lumbar spine, total hip, and femoral neck and that higher total body fat was associated with increased bone mineral density at the total hip and femoral neck [41].
Consistent with these studies, we demonstrate for the first time in a randomized ART-initiation study that the increase in lean body mass over 96 weeks was associated with an increase in hip bone mineral density. Surprisingly, we found that increased lean body mass was associated with greater bone loss at the lumbar spine, although this association was not seen in the multivariable analyses. Furthermore, increased visceral fat over 96 weeks was associated with increased bone mineral density at the hip but associated with decreased bone mineral density at the lumbar spine. The association of visceral fat on hip bone mineral density that we observed may be the result of the mechanical loading effect. Indeed, other studies have demonstrated an increased hip bone mineral density among both men and women with central obesity [42–44]. Similarly, these studies and others found no correlation or a negative correlation between direct or surrogate markers (waist circumference) of visceral adipose tissue and lumbar spine bone mineral density [44–46]. The negative association of adipose tissue with lumbar spine bone mineral density is hypothesized to be the result of pro-inflammatory cytokines [47].
As demonstrated in the Table 3 univariate analyses, week 96 changes in weight, BMI, and lean body mass were significantly associated with week 96 changes in both hip and lumbar spine bone mineral density. Furthermore, randomization to TDF/FTC led to a greater percent decrease in both hip and lumbar spine bone mineral density at 96 weeks compared to ABC/3TC, and ATV/r led a greater percent decrease in lumbar spine bone mineral density change at 96 weeks compared to EFV (previously published [23]). Because of these findings and the significant difference between ATV/r and EFV on week 96 change in weight and BMI presented here, we feel that the effect of the NNRTI/PI component on lumbar spine bone mineral density change may be mediated through changes in weight, BMI, lean body mass or another factor associated with both weight and bone mineral density change. In addition to the body composition factors presented here, additional metabolic and HIV-related factors could be incorporated using structural equation models or causal mediation analysis to fully assess direct and indirect effects of regimen components.
The study has several limitations. First, the duration of follow-up for bone endpoints was relatively short and the impact of ART or body composition changes on bone mineral density could take several years. Second, the study population was relatively young for bone measures and results may not be applicable to older HIV-infected populations. Third, assignment of ATV/r versus EFV was not blinded and changes in the NRTI backbone occurred relatively frequently. However, the intent-to-treat results were consistent with the as-treated results, suggesting that changes in the backbone regimens do not explain our results. The A5224s study did not collect smoking, alcohol, menopause status, or physical activity data which could affect body composition measures, but it is likely that these were evenly distributed at baseline between treatment arms given the randomized study design. Finally, a large number of analyses were performed without adjustment, increasing the probability of committing one or more type I errors, and therefore results should be interpreted with caution. However, this was an exploratory analysis and it will be important for our findings to be confirmed in other studies.
In summary, our study shows that assignment to ATV/r leads to greater gain in body weight and BMI than EFV. Although overall gain in lean body mass was observed, there were no significant differences in lean body mass gain between NRTI or NNRTI/PI components. Furthermore, we found both an independent effect of NRTIs and a positive association of increased lean body mass with change in hip bone mineral density. These findings support the role of lifestyle interventions such as resistance exercise and nutrition to increase lean mass in order to potentially attenuate the initial decline in bone mineral density observed with ART initiation. Prospective studies are needed to assess the role of such lifestyle interventions.
Acknowledgements
The project described was supported by Award Numbers U01AI068636, AI068634, AI38855 from the National Institute of Allergy and Infectious Diseases, UL1 RR 025005 from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health supported by National Institute of Mental Health (NIMH), National Institute of Dental and Craniofacial Research (NIDCR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health. GlaxoSmithKline and Gilead funded the cost of the DEXA scans. Study medications were provided by Abbott Pharmaceuticals, Bristol-Myers Squibb, Gilead Sciences, and GlaxoSmithKline.
Potential Conflicts of Interest: Eric S. Daar has received grant support from Abbott, Gilead, Merck, Pfizer and ViiV as well as been consultant/advisor for Bristol Myers Squibb, Gilead, Merck, ViiV and Janssen. Grace A McComsey has served as a scientific advisor or speaker for Bristol Myers Squibb, GlaxoSmithKline, Janssen, Merck, and Gilead Sciences, has received research grants from Bristol Myers Squibb, GlaxoSmithKline, and Gilead Sciences, and is currently serving as the Data Safety and Monitoring Board Chair for a Pfizer-sponsored study. Kathleen Melbourne is an employee of Gilead Sciences. Belinda Ha is an employee of ViiV Healthcare/ GlaskoSmithKline. Paul E. Sax is a consultant for Abbott, Bristol- Myers Squibb, Gilead, GlaxoSmithKline, Merck, Janssen, and receives grant support from Bristol- Myers Squibb, Gilead, Merck, and GlaxoSmithKline. Pablo Tebas has served as a consultant for Merck, is currently serving on a Data Safety and Monitoring Board for Cytheris and on an adjudication committee for GlaxoSmithKline. Camlin Tierney is a member of a Data Safety and Monitoring Board for a Tibotec/Janssen hepatitis C drug.
Acknowledgements: G.A.M developed and led the study protocol. P.E.S, E.S.D., P.T., B.H., K.M., N.J., D.K., and C.T. assisted with study development and implementation. D.K. and C.T. conducted the data analysis. K.M.E. assisted in the data analysis plan and wrote the first manuscript draft. All authors reviewed and edited the manuscript.
Acknowledgement Appendix for A5224s
Sadia Shaik, M.D. and Ruben Lopez, M.D.- Harbor-UCLA Medical Center (Site 603) CTU Grant #:AI0694241,UL1-RR033176
Susan L. Koletar, MD and Diane Gochnour, RN- The Ohio State University Medical Center (Site 2301) CTU Grant # AI069474
Geyoul Kim, RN and Mark Rodriguez, RN- Washington University (Site 2101) CTU Grant #:U01AI069495; GCRC Grant: UL1 RR024992
Elizabeth Lindsey, RN and Tamara James, BS - Alabama Therapeutics CRS (Site 5801) CTU Grant #: U01 AI069452
Ann C. Collier, MD and Jeffrey Schouten, MD, JD- University of Washington (Site 1401) CTU Grant #: AI069434; UL1 RR025014
Jorge L. Santana Bagur, MD and Santiago Marrero,MD- Puerto Rico-AIDS Clinical Trials Unit (Site 5401) CTU Grant # 5 U0I AI069415-03
Jenifer Baer, RN, BSN and Carl Fichtenbaum, MD- University of Cincinnati (Site 2401) CTU Grant # AI069513
Patricia Walton, BSN, RN and Barbara Philpotts, BSN, RN- Case Western Reserve (Site 2501) CTU Grant #: AI69501
Princy Kumar, M.D. and Joseph Timpone, M.D.- Georgetown University (Site 1008) CTU Grant#: AIDS Clinical Trials Group grant # 5U01AI069494
Donna Pittard, RN, BSN and David Curri,n RN- University of North Carolina (Site 3201) CTU Grant #: 5-U01 AI069423-03; UNC CFAR #: P30 AI050410(-11); UNC CTRC #: UL 1RR 025747
Julie Hoffman, R.N. and Edward Seefried, R.N.- San Diego Medical Center UC (Site 701) CTU Grant # AI69432
Susan Swindells, MBBS and Frances Van Meter, APRN- University of Nebraska (Site 1505) CTU Grant #: AI 27661
Deborah McMahon, MD and Barbara Rutecki, MSN, MPH, CRNP- University of Pittsburgh (Site 1001) CTU Grant #: 1 U01 AI069494-01
Michael P. Dube, M.D. and Martha Greenwald, R.N., M.S.N- Indiana University (Site 2601) CTU Grant #: 5U01AI025859; GCRC #: M01 RR00750
Ilene Wiggins, RN, and Eric Zimmerman, RN- Johns Hopkins University (Site 201) CTU Grant #: AI27668; CTSA Grant # UL1 RR025005
Judith. Aberg, M.D. and Margarita Vasquez R.N.- New York University/NYC HHC at Bellevue Hospital Center (Site 401) CTU Grant #: AI27665, New grant number: AI069532
Martin McCarter and M. Graham Ray, R.N., M.S.N. - Colorado AIDS Clinical Trials Unit, (Site 6101) CTU Grant # AI69450; RR025780
Mamta Jain, MD -PI and Tianna Petersen, MS- University of Texas Southwestern Medical Center (Site 3751) CTU Grant #: 3U01AI046376-05S4
Emily Stumm, BS and Pablo Tebas MD- University of Pennsylvania, Philadelphia (Site 6201) CTU Grant #: P30-AI0450008-11; CFAR Grant #: UO1-AI069467-04
Mary Albrecht, MD and Neah Kim, NP- Beth Israel Deaconess (Partners/Harvard) CRS (Site 103) CTU Grant # U01 AI069472-04
Paul Edward Sax, M.D. and Joanne Delaney RN- Brigham and Women's Hospital (Site 107) CTU Grant # UOI AI 069472
Christine Hurley, RN and Roberto Corales, DO- AIDS Care (Site 1108) CTU Grant #: U01AI069511-02 (as of 2/12/08); GCRC: UL1 RR 024160
Keith Henry, MD and Bette Bordenave, RN- Hennepin County Medical Center (Site 1502) CTU Grant #: N01 AI72626
Wendy Armstrong, MD and Ericka R. Patrick, RN, MSN, CCRC- Emory University HIV/AIDS Clinical Trails Unit (Site 5802) CTU Grant #: UO1Al69418-01/CFAR Grant Number: P30Al050409
Jane Reid, RNC, MS and Mary Adams RN, MPh- University of Rochester (Site 1101) CTU Grant #: U01AI069511-02 (as of 2/12/08); GCRC: UL1 RR 024160
Gene D. Morse, Pharm.D., FCCP, BCPS- SUNY - Buffalo, Erie County Medical Ctr. (Site 1102) CTU Grant # AI27658
Michael P. Dube, M.D. and Martha Greenwald, R.N., M.S.N- Wishard Memorial Hospital Indiana University (Site 2603) CTU Grant #: 5U01AI025859; GCRC #: M01 RR00750
Kimberly Y. Smith, MD, MPH and Joan A. Swiatek, APN- Rush University Medical Center (Site 2702) CTU Grant #: U01 AI069471
Nancy Hanks, RN, and Debra Ogata-Arakaki, RN, -University of Hawaii at Manoa, Leahi Hospital (Site 5201) CTU Grant # AI34853
Ardis Moe, MD and Maria Palmer, PA-C- UCLA Medical Center (Site 601) CTU Grant # 1U01AI069424-01
Jeffery Meier, M.D. and Jack T. Stapleton, M.D. - University of Iowa Hospitals and Clinics (Site 1504) CTU Grant #: UL1RR024979
Gary Matthew Cox, MD and Martha Silberman, RN- Duke University Medical Center Adult CRS (Site 1601) CTU Grant # 5U01 AI069 484-02
2705 - Cook County Hospital
Gerianne Casey, RN and William O’Brien, MD-University of Texas, Galveston (Site 6301) CTU Grant # AI32782
Valery Hughes, FNP and Todd Stroberg, RN- Cornell CRS (Site 7803, 7804) – CTU Grant#: U01 AI069419; CTSC #: UL1 RR024996
Nyef El-Daher, MD -McCree McCuller Wellness Center at the Connection (Site 1107) CTU Grant #: U01AI069511-02 (as of 2/12/08); GCRC: UL1 RR 024160
Rebecca J. Basham, B.S. and Husamettin Erdem, M.D.-Vanderbilt Therapeutics CRS (Site 3652) CTU Grant #: AI46339-01; MO1 RR 00095
References
- 1.Liu-Ambrose T, Kravetsky L, Bailey D, Sherar L, Mundt C, Baxter-Jones A, et al. Change in lean body mass is a major determinant of change in areal bone mineral density of the proximal femur: a 12-year observational study. Calcif Tissue Int. 2006;79:145–151. doi: 10.1007/s00223-006-0098-z. [DOI] [PubMed] [Google Scholar]
- 2.Macdonald HM, New SA, Campbell MK, Reid DM. Influence of weight and weight change on bone loss in perimenopausal and early postmenopausal Scottish women. Osteoporos Int. 2005;16:163–171. doi: 10.1007/s00198-004-1657-7. [DOI] [PubMed] [Google Scholar]
- 3.Ilich-Ernst J, Brownbill RA, Ludemann MA, Fu R. Critical factors for bone health in women across the age span: how important is muscle mass? Medscape Womens Health. 2002;7:2. [PubMed] [Google Scholar]
- 4.Zhang Z, Shen X, Zhang H, Li S, Zhou H, Wu X, et al. The relationship between body composition and fracture risk using the FRAX model in central south Chinese postmenopausal women. Clin Endocrinol (Oxf) 2012 doi: 10.1111/j.1365-2265.2012.04399.x. [DOI] [PubMed] [Google Scholar]
- 5.Russell M, Mendes N, Miller KK, Rosen CJ, Lee H, Klibanski A, et al. Visceral fat is a negative predictor of bone density measures in obese adolescent girls. The Journal of clinical endocrinology and metabolism. 2010;95:1247–1255. doi: 10.1210/jc.2009-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gnudi S, Sitta E, Fiumi N. Relationship between body composition and bone mineral density in women with and without osteoporosis: relative contribution of lean and fat mass. J Bone Miner Metab. 2007;25:326–332. doi: 10.1007/s00774-007-0758-8. [DOI] [PubMed] [Google Scholar]
- 7.Mulligan K, Harris DR, Emmanuel P, Fielding RA, Worrell C, Kapogiannis BG, et al. Low Bone Mass in Behaviorally HIV-Infected Young Men on Antiretroviral Therapy: Adolescent Trials Network Study 021B. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012;55:461–468. doi: 10.1093/cid/cis455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haskelberg H, Hoy JF, Amin J, Ebeling PR, Emery S, Carr A, et al. Changes in Bone Turnover and Bone Loss in HIV-Infected Patients Changing Treatment to Tenofovir-Emtricitabine or Abacavir-Lamivudine. PloS one. 2012;7:e38377. doi: 10.1371/journal.pone.0038377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma A, Cohen HW, Freeman R, Santoro N, Schoenbaum EE. Prospective evaluation of bone mineral density among middle-aged HIV-infected and uninfected women: Association between methadone use and bone loss. Maturitas. 2011;70:295–301. doi: 10.1016/j.maturitas.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dolan SE, Kanter JR, Grinspoon S. Longitudinal analysis of bone density in human immunodeficiency virus-infected women. The Journal of clinical endocrinology and metabolism. 2006;91:2938–2945. doi: 10.1210/jc.2006-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolland MJ, Wang TK, Grey A, Gamble GD, Reid IR. Stable bone density in HAART-treated individuals with HIV: a meta-analysis. The Journal of clinical endocrinology and metabolism. 2011;96:2721–2731. doi: 10.1210/jc.2011-0591. [DOI] [PubMed] [Google Scholar]
- 12.Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS. 2006;20:2165–2174. doi: 10.1097/QAD.0b013e32801022eb. [DOI] [PubMed] [Google Scholar]
- 13.Bolland MJ, Grey AB, Gamble GD, Reid IR. CLINICAL Review # : low body weight mediates the relationship between HIV infection and low bone mineraldensity: a meta-analysis. The Journal of clinical endocrinology and metabolism. 2007;92:4522–4528. doi: 10.1210/jc.2007-1660. [DOI] [PubMed] [Google Scholar]
- 14.Mallon PW, Miller J, Cooper DA, Carr A. Prospective evaluation of the effects of antiretroviral therapy on body composition in HIV-1-infected men starting therapy. AIDS. 2003;17:971–979. doi: 10.1097/00002030-200305020-00005. [DOI] [PubMed] [Google Scholar]
- 15.Silva M, Skolnik PR, Gorbach SL, Spiegelman D, Wilson IB, Fernandez-DiFranco MG, et al. The effect of protease inhibitors on weight and body composition in HIV-infected patients. AIDS. 1998;12:1645–1651. doi: 10.1097/00002030-199813000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Shikuma CM, Zackin R, Sattler F, Mildvan D, Nyangweso P, Alston B, et al. Changes in weight and lean body mass during highly active antiretroviral therapy. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2004;39:1223–1230. doi: 10.1086/424665. [DOI] [PubMed] [Google Scholar]
- 17.Gupta V, Biswas A, Sharma SK. Metabolic and body composition changes after six months of highly active antiretroviral therapy in northern Indian patients. Int J STD AIDS. 2011;22:46–49. doi: 10.1258/ijsa.2010.010193. [DOI] [PubMed] [Google Scholar]
- 18.Shlay JC, Sharma S, Peng G, Gibert CL, Grunfeld C. The effect of individual antiretroviral drugs on body composition in HIV-infected persons initiating highly active antiretroviral therapy. Journal of acquired immune deficiency syndromes. 2009;51:298–304. doi: 10.1097/QAI.0b013e3181aa1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mulligan K, Parker RA, Komarow L, Grinspoon SK, Tebas P, Robbins GK, et al. Mixed patterns of changes in central and peripheral fat following initiation of antiretroviral therapy in a randomized trial. Journal of acquired immune deficiency syndromes. 2006;41:590–597. doi: 10.1097/01.qai.0000214811.72916.67. [DOI] [PubMed] [Google Scholar]
- 20.McComsey GA, Kitch D, Sax PE, Tebas P, Tierney C, Jahed NC, et al. Peripheral and central fat changes in subjects randomized to abacavir-lamivudine or tenofovir-emtricitabine with atazanavir-ritonavir or efavirenz: ACTG Study A5224s. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;53:185–196. doi: 10.1093/cid/cir324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toss F, Wiklund P, Nordstrom P, Nordstrom A. Body composition and mortality risk in later life. Age and ageing. 2012 doi: 10.1093/ageing/afs087. [DOI] [PubMed] [Google Scholar]
- 22.Scherzer R, Heymsfield SB, Lee D, Powderly WG, Tien PC, Bacchetti P, et al. Decreased limb muscle and increased central adiposity are associated with 5-year all-cause mortality in HIV infection. AIDS. 2011;25:1405–1414. doi: 10.1097/QAD.0b013e32834884e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McComsey GA, Kitch D, Daar ES, Tierney C, Jahed NC, Tebas P, et al. Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: Aids Clinical Trials Group A5224s, a substudy of ACTG A5202. J Infect Dis. 2011;203:1791–1801. doi: 10.1093/infdis/jir188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sax PE, Tierney C, Collier AC, Fischl MA, Mollan K, Peeples L, et al. Abacavir-lamivudine versus tenofovir-emtricitabine for initial HIV-1 therapy. The New England journal of medicine. 2009;361:2230–2240. doi: 10.1056/NEJMoa0906768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daar ES, Tierney C, Fischl MA, Sax PE, Mollan K, Budhathoki C, et al. Atazanavir plus ritonavir or efavirenz as part of a 3-drug regimen for initial treatment of HIV-1. Annals of internal medicine. 2011;154:445–456. doi: 10.1059/0003-4819-154-7-201104050-00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sax PE, Tierney C, Collier AC, Daar ES, Mollan K, Budhathoki C, et al. Abacavir/lamivudine versus tenofovir DF/emtricitabine as part of combination regimens for initial treatment of HIV: final results. J Infect Dis. 2011;204:1191–1201. doi: 10.1093/infdis/jir505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson V, Medard B, Taseera K, Chakera AJ, Andia I, Emenyonu N, et al. Regional anthropometry changes in antiretroviral-naive persons initiating a Zidovudine-containing regimen in Mbarara, Uganda. AIDS research and human retroviruses. 2011;27:785–791. doi: 10.1089/aid.2010.0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dube MP, Qian D, Edmondson-Melancon H, Sattler FR, Goodwin D, Martinez C, et al. Prospective, intensive study of metabolic changes associated with 48 weeks of amprenavir-based antiretroviral therapy. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2002;35:475–481. doi: 10.1086/341489. [DOI] [PubMed] [Google Scholar]
- 29.McComsey GA, Paulsen DM, Lonergan JT, Hessenthaler SM, Hoppel CL, Williams VC, et al. Improvements in lipoatrophy, mitochondrial DNA levels and fat apoptosis after replacing stavudine with abacavir or zidovudine. AIDS. 2005;19:15–23. doi: 10.1097/00002030-200501030-00002. [DOI] [PubMed] [Google Scholar]
- 30.McComsey GA, Walker UA. Role of mitochondria in HIV lipoatrophy: insight into pathogenesis and potential therapies. Mitochondrion. 2004;4:111–118. doi: 10.1016/j.mito.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 31.Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Department of Health and Human Services; [accessed: 10/12/2012]. Panel on Antiretroviral Guidelines for Adults and Adolescents. Available at: http://aidsinfo.nih.gov/guidelines. Section. [Google Scholar]
- 32.Yarasheski KE, Scherzer R, Kotler DP, Dobs AS, Tien PC, Lewis CE, et al. Age-related skeletal muscle decline is similar in HIV-infected and uninfected individuals. The journals of gerontology. Series A, Biological sciences and medical sciences. 2011;66:332–340. doi: 10.1093/gerona/glq228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDermott AY, Terrin N, Wanke C, Skinner S, Tchetgen E, Shevitz AH. CD4+ cell count, viral load, and highly active antiretroviral therapy use are independent predictors of body composition alterations in HIV-infected adults: a longitudinal study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2005;41:1662–1670. doi: 10.1086/498022. [DOI] [PubMed] [Google Scholar]
- 34.McDermott AY, Shevitz A, Knox T, Roubenoff R, Kehayias J, Gorbach S. Effect of highly active antiretroviral therapy on fat, lean, and bone mass in HIV-seropositive men and women. Am J Clin Nutr. 2001;74:679–686. doi: 10.1093/ajcn/74.5.679. [DOI] [PubMed] [Google Scholar]
- 35.Bolland MJ, Grey A, Horne AM, Briggs SE, Thomas MG, Ellis-Pegler RB, et al. Stable bone mineral density over 6 years in HIV-infected men treated with highly active antiretroviral therapy (HAART) Clin Endocrinol (Oxf) 2012;76:643–648. doi: 10.1111/j.1365-2265.2011.04274.x. [DOI] [PubMed] [Google Scholar]
- 36.Kolta S, Flandre P, Van PN, Cohen-Codar I, Valantin MA, Pintado C, et al. Fat tissue distribution changes in HIV-infected patients treated with lopinavir/ritonavir. Results of the MONARK trial. Curr HIV Res. 2011;9:31–39. doi: 10.2174/157016211794582687. [DOI] [PubMed] [Google Scholar]
- 37.Boyd MA, Carr A, Ruxrungtham K, Srasuebkul P, Bien D, Law M, et al. Changes in body composition and mitochondrial nucleic acid content in patients switched from failed nucleoside analogue therapy to ritonavir-boosted indinavir and efavirenz. J Infect Dis. 2006;194:642–650. doi: 10.1086/505709. [DOI] [PubMed] [Google Scholar]
- 38.McComsey GA, Tebas P, Shane E, Yin MT, Overton ET, Huang JS, et al. Bone disease in HIV infection: a practical review and recommendations for HIV care providers. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2010;51:937–946. doi: 10.1086/656412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ho-Pham LT, Nguyen ND, Lai TQ, Nguyen TV. Contributions of lean mass and fat mass to bone mineral density: a study in postmenopausal women. BMC Musculoskelet Disord. 2010;11:59. doi: 10.1186/1471-2474-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carr A, Miller J, Eisman JA, Cooper DA. Osteopenia in HIV-infected men: association with asymptomatic lactic acidemia and lower weight pre-antiretroviral therapy. AIDS. 2001;15:703–709. doi: 10.1097/00002030-200104130-00005. [DOI] [PubMed] [Google Scholar]
- 41.Sharma A, Tian F, Yin MT, Keller MJ, Cohen M, Tien PC. Association of Regional Body Composition with Bone Mineral Density in HIV-infected and Uninfected Women: Women's Interagency HIV Study. Journal of acquired immune deficiency syndromes. 2012 doi: 10.1097/QAI.0b013e31826cba6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kinjo M, Setoguchi S, Solomon DH. Bone mineral density in adults with the metabolic syndrome: analysis in a population-based U.S. sample. The Journal of clinical endocrinology and metabolism. 2007;92:4161–4164. doi: 10.1210/jc.2007-0757. [DOI] [PubMed] [Google Scholar]
- 43.Szulc P, Varennes A, Delmas PD, Goudable J, Chapurlat R. Men with metabolic syndrome have lower bone mineral density but lower fracture risk--the MINOS study. J Bone Miner Res. 2010;25:1446–1454. doi: 10.1002/jbmr.13. [DOI] [PubMed] [Google Scholar]
- 44.von Muhlen D, Safii S, Jassal SK, Svartberg J, Barrett-Connor E. Associations between the metabolic syndrome and bone health in older men and women: the Rancho Bernardo Study. Osteoporos Int. 2007;18:1337–1344. doi: 10.1007/s00198-007-0385-1. [DOI] [PubMed] [Google Scholar]
- 45.Jankowska EA, Rogucka E, Medras M. Are general obesity and visceral adiposity in men linked to reduced bone mineral content resulting from normal ageing? A population-based study. Andrologia. 2001;33:384–389. doi: 10.1046/j.1439-0272.2001.00469.x. [DOI] [PubMed] [Google Scholar]
- 46.Blaauw R, Albertse EC, Hough S. Body fat distribution as a risk factor for osteoporosis. S Afr Med J. 1996;86:1081–1084. [PubMed] [Google Scholar]
- 47.Sheu Y, Cauley JA. The role of bone marrow and visceral fat on bone metabolism. Curr Osteoporos Rep. 2011;9:67–75. doi: 10.1007/s11914-011-0051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]