Abstract
We have shown that experience of transgenic mice harboring familial Alzheimer’s disease (FAD)-linked AβPPswe/PS1ΔE9 in an enriched environment enhances hippocampal neurogenesis and synaptic plasticity and attenuates neuropathology. Nevertheless, the neuronal pathways activated following environmental enrichment underlying this effect are unknown. Using resting-state functional magnetic resonance imaging, we present preliminary evidence to show that transgenic mice, which had been housed in an enriched environment, show increased connectivity between CA1 and cortical areas compared to mice from standard housing. This is the first preliminary demonstration of live-activated neuronal pathways following environmental enrichment in FAD mice. Understanding the activated pathways may unravel the molecular mechanism underlying environmental enrichment-enhanced neuroplasticity in FAD.
Keywords: Alzheimer’s disease, brain plasticity, environmental enrichment, resting state functional MRI
INTRODUCTION
Environmental enrichment has numerous beneficial effects on brain structure and function. Rodents experiencing environmental enrichment exhibit thicker cortex, increased number of synapses and dendritic spines, increased neurogenesis, improved performance in learning and memory tasks, etc. (for review, see [1]). In addition, experience in an enriched environment was shown to attenuate neuropathology in a variety of animal models of neurodegenerative disease, including Alzheimer’s disease (AD) (for review see [2]). We have shown that experience of transgenic mice harboring familial AD (FAD)-linked mutant presenilin-1 and amyloid-β protein precursor (APPswe/PS1ΔE9) in an enriched environment reduced extent of neuropathology, including levels of oligomeric amyloid-β (Aβ), amyloid deposits, and hyperphosphorylated tau in their brains. In addition, these animals exhibited increased levels of hippocampal neurogenesis and synaptic plasticity [3, 4]. Other studies suggest beneficial effects of the experience of mice in an enriched environment, such as reduced amyloidosis [5], enhanced hippocampus-dependent learning and memory [6, 7], and increased neurogenesis [7–9].
Surprisingly, in spite of plethora of studies describing the beneficial effects of environmental enrichment on brain structure and function, information concerning the mechanism by which environmental enrichment exerts its effects is scarce. To gain an insight into the mechanism by which environmental enrichment downregulates neuropathology and upregulates neuroplasticity in the adult brain, we aimed at determining neuronal pathways activated following experience in an enriched environment using resting-state functional magnetic resonance imaging (rsfMRI). rsfMRI has been increasingly used in human cognitive neuroscience to characterize spontaneous neural activity. Human studies have demonstrated that correlations of T2 signal changes between regions during rest produce a consistent set of networks that correspond to the known networks involved in various cognitive, motor, and somatosensory functions. Importantly, these studies have also shown validity under anesthesia and under anesthesia in non-human models including both rats and mice [10]. The advantages of using rsfMRI rather than task-dependent fMRI in the current protocol are significant. First, enrichment is known to affect multiple networks including but not limited to those proposed to underlie memory. Indeed, animals exposed to an enriched environment are believed to promote increased motor, sensory, and cognitive modalities and to reinforce a variety of behaviors including learning, social interactions, physical activity, orientation and exploration (for review, see [2]). Accordingly, the effects of environmental enrichment have been shown to have a positive impact on both morphological and physiological properties in selective regions, including: the auditory cortex [11], the sensorimotor cortex [12–14], visual cortex [15], associative parietal cortex [16], and allocortical regions, such as the hippocampus [3, 4, 8, 16], and increases vasculogenesis [17]. Because of this, virtually every task one could conduct would also be proposed to be affected by enrichment. However, because rsfMRI is not task dependent, there is no such concern here. As it is, our hypothesis is that enrichment has effects on multiple neural networks and that variations in the type of enrichment will alter these patterns or changes relative to standard housing. Second, there is no concern for the effects of anxiety on blood oxygenation level dependent (BOLD) contrast in mice during MRI because they can be anesthetized and importantly, this does not appear to alter the common networks identified by rsfMRI.
To demonstrate that enrichment does in fact alter the strength of correlations between regions and determine the neural pathways activated following experience of FAD mice in an enriched environment, male APPswe/PS1ΔE9 mice were either placed in an enriched environment right after weaning (n = 3) or maintained in standard housing conditions (n = 3), as previously described [3, 4]. Two months later, mice were anesthetized and brain activity was analyzed by rsfMRI using Bruker 9.4Tesla 30 cm bore small animal MR system (Bruker BioSpin MRI, Ettlingen, Germany) with Paravision 4.0 software capable of both standard structural and echo planer imaging.
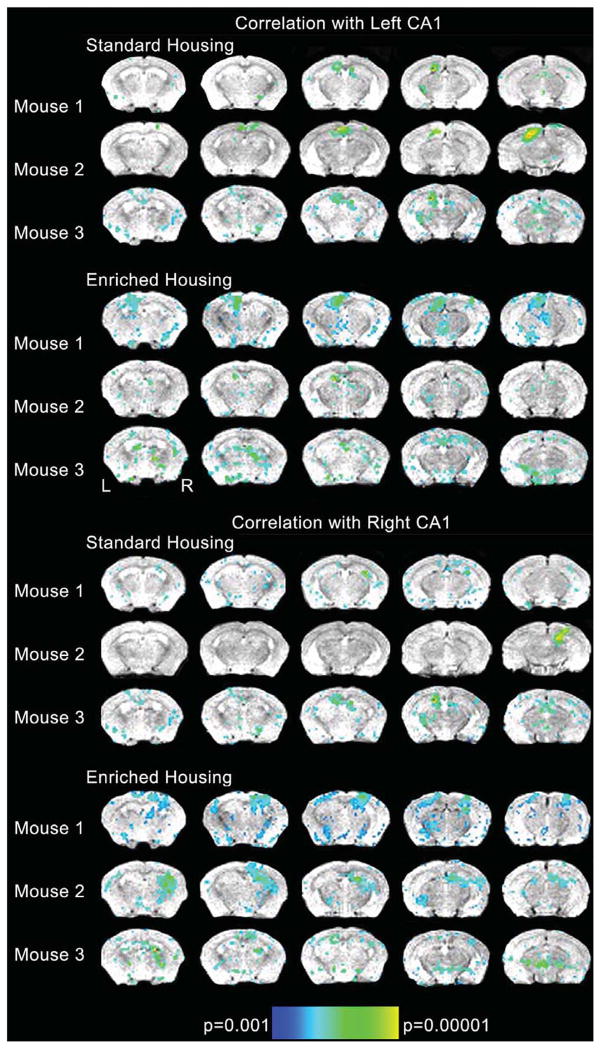
Here we show a widespread network of increased connectivity in those mice that had experienced an enriched environment when compared to standard housing mice (Fig. 1). Regions that correlated with the extracted time-course from left CA1 and right CA1 for each individual animal (each row represents a separate animal) are presented on the top and bottom of Fig. 1. First, the total number of significant regions that correlated with left and right CA1 were calculated. This provides simply a general analysis of the total volume of correlated voxels. For the standard housing mice, an average of 362 voxels correlated with the averaged ideal time-course from left CA1 (for each mouse: 186 voxels, 341 voxels, 560 voxels) whereas on average 377 voxels correlated with the time-course from right CA1 (for each mouse: 212, 306, 612 voxels). In contrast and as presented in Fig. 1, the three mice that experienced an enriched environment showed greater activation overall with an average of 808 voxels correlating with left CA1 (742, 911, and 1070 voxels) and 1260 with right CA1 (1008, 1237, 1536 voxels). Detailed results are presented in Tables 1 and 2, showing the specific brain regions that showed significant correlations with either left CA1 (Table 1) or right CA1 (Table 2) and specifies which hemisphere (or both) that correlated with either left or right CA1. It is important to note that for inclusion in the Tables, regions had to be identified as present in at least two mice to be included. As can be seen by reviewing Tables 1 and 2, the enriched mice all showed significantly more regions, which were correlated with the time-course from CA1 than the mice from standard housing. These regions go beyond what would be expected for memory networks themselves. As would be expected within the resting state design, there is excellent concordance between regions that correlate with the left CA1 seed voxels and those which correlate with the right CA1 seeds within mice. Of note, it is not the case that enrichment itself simply increases the low frequency correlations across all regions. Both groups of mice show strong connectivity between the seed voxels and areas within primary and secondary auditory cortex and visual cortex. The divergence between groups based on housing type occurs in additional regions within the hippocampal formation (i.e., dorsal hippocampal commissure, dentate gyrus), thalamus (i.e., post thalamic nuclear group), and other association cortices (i.e., temporal association cortex, parietal association cortex).
Fig. 1.
Regions that correlated with the extracted time-course from left CA1 and right CA1 for each individual animal. Spin echo anatomical reference T1 weighted images were acquired in the coronal plane with the following parameters: field of view (FOV) = 25.6 × 25.6 mm2, matrix = 128 × 128, slice thickness = 0.5 mm, gap = 0.0 mm, repetition time (TR) = 4000 ms, echo time (TE) = 28.3 ms, number of averages (NEX) = 1, number of slices = 27. Based upon the T1 sequence 5 coronal slices covering the hippocampal formation were placed in reference to the high resolution anatomical images. Blood oxygenation level dependent (BOLD) data were acquired using a gradient echo echo planar imaging (GE-EPI) sequence with the following parameters: FOV = 25.6×25.6 mm2, matrix = 128 × 128, slice thickness = 0.5 mm, gap = 0.0 mm, repetition time (TR) = 1000 ms, echo time (TE) = 10 ms, number of averages (NEX) = 1 for a spatial resolution of 0.2 × 0.2 × 0.5 mm3. Please note that following the EPI sequence, a second high resolution dataset was acquired with the same parameters as above with the exception of slice prescription and number of slices which matched the EPI prescription. This was collected to allow better co-registration with the high resolution dataset given the limited spatial coverage of the EPI. Data was first converted from Paravision format to Analyze using the Bruker2Analyze Toolkit associated with MRIcro (http://www.pvconv.sourceforge.net). Data were preprocessed using the SPMMouse toolbox within SPM5 [18] with corrections for high frequency oscillations expected with cardiac output and respiration, and co-registration with the high resolution anatomical images. The EPI data were first realigned to the first volume acquired using a least squares method and rigid body transformation. The data were also smoothed with a Gaussian kernel of 0.2 mm3. Three seed voxels were placed in both the left and right CA1 region identified on the high resolution images. The timecourse from seed voxel each region of interest was extracted and averaged with the other two seed voxel timecourses from that cerebral hemisphere resulting in one average timecourse for left and one average timecourse for right CA1 for each of the six mice. This averaged time-course was then correlated with all other voxels in the brain. We applied a threshold of p < 0.001 and a cluster threshold of 6 voxels without additional correction for multiple comparisons to each individual dataset. Correlation analyses were carried out between the seed reference and the whole brain for each animal in a voxel-wise manner. To improve normality, a Fisher’s z-transform was applied. From these, the individual z value was submitted to a random effects one-sample t test for each animal. A false discovery rate was applied to reduce errors associated with multiple comparisons. Significance for each animal was set at p(FDR) < 0.05.
Table 1.
Regions with suprathreshold correlations with seed voxels in the left CA1
| Functional brain area | Structure | Correlations with Left CA1 Seed Voxel
|
|||||
|---|---|---|---|---|---|---|---|
| Standard housing
|
Enriched housing
|
||||||
| Mouse 1 | Mouse 2 | Mouse 3 | Mouse 1 | Mouse 2 | Mouse 3 | ||
| Association cortex | Temporal association cortex | R | R | L | R+L | ||
| Medial parietal association cortex | R+L | R | R+L | ||||
| Association/Limbic system | Retrosplenial dysgranular cortex | R | R+L | R+L | R | R+L | |
| Limbic system | Field CA2 of hippocampus | R+L | R+L | R+L | R | R+L | |
| Field CA3 of hippocampus | R+L | R+L | R | R+L | R+L | ||
| Cingulum | L | R | L | ||||
| Dentate gyrus | L | R | R+L | R+L | R | R+L | |
| Dorsal hippocampal commissure | R | R | |||||
| Entorhinal cortex | R+L | R+L | R | R | |||
| Fimbria of hippocampus | L | L | R | R | |||
| Molecular layer of dentate gyrus | R | R+L | |||||
| Fasciculus retroflexus | L | L | R | ||||
| Perirhinal cortex | L | R+L | R | ||||
| Radiatum layer of hippocampus | R+L | R | R+L | ||||
| Motor system | Caudate putamen | R+L | R | R | R | R+L | |
| Visual system | Dorsal lateral geniculate nucleus | R+L | L | R+L | R | R+L | |
| Primary visual cortex | R+L | R+L | L | R+L | R | R+L | |
| Secondary visual cortex | R+L | R+L | R+L | L | R | R | |
| Post pretectal nucleus | R | L | R+L | ||||
| Precommissural nucleus | R | L | |||||
| Somatosensory system | Primary somatosensory cortex | L | |||||
| Primary somatosensory cortex barrel field | R+L | L | R+L | ||||
| Post thalamic nuclear group | R+L | R | R | ||||
| Auditory system | Primary auditory cortex | L | R+L | L | R+L | R+L | |
| Secondary auditory cortex | R+L | R+L | R+L | R | R+L | ||
Table 2.
Regions with suprathreshold correlations with seed voxels in the right CA1
| Functional brain area | Structure | Correlations with Left CA1 Seed Voxel
|
|||||
|---|---|---|---|---|---|---|---|
| Standard housing
|
Enriched housing
|
||||||
| Mouse 1 | Mouse 2 | Mouse 3 | Mouse 1 | Mouse 2 | Mouse 3 | ||
| Association cortex | Temporal association cortex | R | R+L | R | |||
| Medial parietal association cortex | R | L | |||||
| Association/Limbic system | Retrosplenial dysgranular cortex | R+L | R+L | R+L | L | ||
| Limbic system | Field CA2 of hippocampus | R | R+L | R+L | L | R+L | |
| Field CA3 of hippocampus | R+L | R+L | R+L | ||||
| Cingulum | L | R+L | L | L | R | R | |
| Dentate gyrus | R+L | L | R+L | R+L | R+L | R+L | |
| Dorsal hippocampal commissure | L | L | R | R+L | |||
| Entorhinal cortex | R | R+L | |||||
| Fimbria of hippocampus | R | R+L | |||||
| Molecular layer of dentate gyrus | L | L | R | ||||
| Fasciculus retroflexus | L | R | R+L | ||||
| Perirhinal cortex | R+L | R+L | |||||
| Radiatum layer of hippocampus | L | L | L | ||||
| Motor system | Caudate putamen | R+L | R | R | L | ||
| Visual system | Dorsal lateral geniculate nucleus | L | L | R | R | ||
| Primary visual cortex | L | R+L | R+L | R+L | |||
| Secondary visual cortex | R | R+L | R+L | R | R | ||
| Post pretectal nucleus | L | ||||||
| Precommissural nucleus | R | L | R | ||||
| Somatosensory system | Primary somatosensory cortex | L | L | ||||
| Primary somatosensory cortex barrel field | R+L | R | |||||
| Post thalamic nuclear group | R+L | R | R | ||||
| Auditory system | Primary auditory cortex | R+L | R+L | L | L | ||
| Secondary auditory cortex | R | R | L | R+L | R+L | ||
The data above provides preliminary evidence that enrichment leads to an increase not only in the magnitude of correlations between regions for the enriched mice but also a significant increase in the regions that are associated with the time-course extracted from the resting state data. But, beyond simply showing an overall increase in connectivity between regions within animals that experienced an enriched environment, the data suggest specificity of these effects. Of particular interest is the similarity between groups within primary and secondary sensory areas. This is counter to one of our original hypotheses that the enrichment would increase connectivity between these regions because of increased sensory stimulation. But then again, these regions are not normally associated (at least in human data) with hippocampal function unless one examines acquisition of material rather than recollection of material. The divergence between groups especially in terms of correlations during the resting state with higher order association areas is likely the most significant finding suggesting that enrichment does alter networks that have both feedback and feedforward connections to the hippocampal formation. These findings are suggestive of an interpretation that enrichment alters the neuronal synchrony across cortical and subcortical areas in the brain. The hippocampal formation and cortex are the most affected brain regions in AD. Nevertheless, these regions in particular are capable of exhibiting environment-induced plasticity throughout adult life. It is also important to note that conclusion is supported by five out of six of the animals scanned. The exception to this pattern was Mouse 2 in the enriched housing group. This animal showed much lower correlations across the hippocampal network. This is important to note not only because this animal deviated from the general pattern of findings but also because it highlights the need to examine individual differences between animals and the need to quantify behavior within housing environments.
The sheer magnitude of this effect raises questions about the specific mechanism that underlies this observation with alternatives ranging from vascular effects which would theoretically alter not only neuronal activity but also the T2 signal and therefore increased correlations over time, metabolic demand from increased motor, sensory, and cognitive activity, or potentially additional interpretations including alterations in neurotransmitter function. Additional questions related to individual differences between mice, collection of behavioral data in terms of how often components within the enriched environment were used, and correlations between imaging and behavior need to be investigated to fully understand and quantify the effects of enrichment and neuronal function. The data observed in the current study also raise the need to examine the specific components of enrichment and identify how each component affects the resting state networks. For the present preliminary data and proposed studies, we are in specific reference to low frequency fluctuations in T2* signal of less than 0.1 Hz. Although an independent components analysis would be preferred, we limited our analysis here to correlations conducted using seed voxels in straital regions and conducted this on each of the 6 mice separately. This approach was taken for two reasons: first, we only acquired 50 volumes of data on one enriched mouse and one mouse in standard housing reducing the statistical power in these two mice compared with 100 volumes on the remaining mice and second, because of limited spatial coverage for the fMRI study (2.5 mm). Beyond limited spatial coverage, the effects of both partial volume and low signal to noise must be acknowledged. As with all fMRI studies, but even more so for resting state fMRI studies in small animals, we risk not identifying all potential findings due to low signal to noise and partial volume effects. Both of these are compounded by the limited spatial coverage, non-isotropic voxels, and sluggish BOLD response.
It is important to note that the limited sample size restricts the conclusions that can be made, and further studies using fMRI for the detection of neural connectivity following environmental enrichment are warranted. Nevertheless, these data do provide preliminary evidence that specific neuronal pathways are activated in the hippocampus of FAD mice following environmental enrichment.
Acknowledgments
This research was supported by The Alzheimer’s Association Young Investigator Award (OL), The Illinois Department of Public Health Research Award (OL), and NIA 1RC1AG036208-01 ARRA (OL and DML). The authors thank Ms. Zhang and Dr. Sangram S. Sisodia for providing the transgenic mice and helping with the inter-institutional logistics.
Footnotes
The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the Alzheimer’s Association, Illinois Department of Public Health, Department of Veterans Affairs, United States Government, University of Illinois at Chicago, Scott and White Healthcare or Texas A & M Health Sciences. All studies using mice were performed in accordance with standards and approval from the IACUC committee of the University of Illinois at Chicago and the University of Chicago, Chicago, IL.
Authors’ disclosures available online (http://www.jalz.com/disclosures/view.php?id=1351).
References
- 1.Markham JA, Greenough WT. Experience-driven brain plasticity: Beyond the synapse. Neuron Glia Biol. 2004;1:351–363. doi: 10.1017/s1740925x05000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lazarov O, Larson J. Environmental enrichment: From mouse AD model to AD therapy. Chapter XI. Nova Science Publishers, Inc; 2007. pp. 303–328. [Google Scholar]
- 3.Lazarov O, Robinson J, Tang YP, Hairston IS, Korade-Mirnics Z, Lee VM, Hersh LB, Sapolsky RM, Mirnics K, Sisodia SS. Environmental enrichment reduces Abeta levels and amyloid deposition in transgenic mice. Cell. 2005;120:701–713. doi: 10.1016/j.cell.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 4.Hu YS, Xu P, Pigino G, Brady ST, Larson J, Lazarov O. Complex environment experience rescues impaired neurogenesis, enhances synaptic plasticity, and attenuates neuropathology in familial Alzheimer’s disease-linked APPswe/PS1{Delta}E9 mice. FASEB J. 2010;24:1667–1681. doi: 10.1096/fj.09-136945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ambree O, Leimer U, Herring A, Gortz N, Sachser N, Heneka MT, Paulus W, Keyvani K. Reduction of amyloid angiopathy and Abeta plaque burden after enriched housing in TgCRND8 mice: Involvement of multiple pathways. Am J Pathol. 2006;169:544–552. doi: 10.2353/ajpath.2006.051107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costa DA, Cracchiolo JR, Bachstetter AD, Hughes TF, Bales KR, Paul SM, Mervis RF, Arendash GW, Potter H. Enrichment improves cognition in AD mice by amyloid-related and unrelated mechanisms. Neurobiol Aging. 2007;28:831–844. doi: 10.1016/j.neurobiolaging.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Wolf SA, Kronenberg G, Lehmann K, Blankenship A, Overall R, Staufenbiel M, Kempermann G. Cognitive and physical activity differently modulate disease progression in the amyloid precursor protein (APP)-23 model of Alzheimer’s disease. Biol Psychiatry. 2006;60:1314–1323. doi: 10.1016/j.biopsych.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci. 1998;18:3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kempermann G, Gast D, Gage FH. Neuroplasticity in old age: Sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann Neurol. 2002;52:135–143. doi: 10.1002/ana.10262. [DOI] [PubMed] [Google Scholar]
- 10.Jonckers E, Van Audekerke J, De Visscher G, Van der Linden A, Verhoye M. Functional connectivity fMRI of the rodent brain: Comparison of functional connectivity networks in rat and mouse. PLoS One. 2011;6:e18876. doi: 10.1371/journal.pone.0018876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engineer ND, Percaccio CR, Pandya PK, Moucha R, Rathbun DL, Kilgard MP. Environmental enrichment improves response strength, threshold, selectivity, and latency of auditory cortex neurons. J Neurophysiol. 2004;92:73–82. doi: 10.1152/jn.00059.2004. [DOI] [PubMed] [Google Scholar]
- 12.Coq JO, Xerri C. Sensorimotor experience modulates age-dependent alterations of the forepaw representation in the rat primary somatosensory cortex. Neuroscience. 2001;104:705–715. doi: 10.1016/s0306-4522(01)00123-3. [DOI] [PubMed] [Google Scholar]
- 13.Coq JO, Xerri C. Environmental enrichment alters organizational features of the forepaw representation in the primary somatosensory cortex of adult rats. Exp Brain Res. 1998;121:191–204. doi: 10.1007/s002210050452. [DOI] [PubMed] [Google Scholar]
- 14.Godde B, Berkefeld T, David-Jurgens M, Dinse HR. Age-related changes in primary somatosensory cortex of rats: Evidence for parallel degenerative and plastic-adaptive processes. Neurosci Biobehav Rev. 2002;26:743–752. doi: 10.1016/s0149-7634(02)00061-1. [DOI] [PubMed] [Google Scholar]
- 15.Beaulieu C, Cynader M. Effect of the richness of the environment on neurons in cat visual cortex. II. Spatial and temporal frequency characteristics. Brain Res Dev Brain Res. 1990;53:82–88. doi: 10.1016/0165-3806(90)90126-j. [DOI] [PubMed] [Google Scholar]
- 16.Frick KM, Stearns NA, Pan JY, Berger-Sweeney J. Effects of environmental enrichment on spatial memory and neurochemistry in middle-aged mice. Learn Mem. 2003;10:187–198. doi: 10.1101/lm.50703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swain RA, Harris AB, Wiener EC, Dutka MV, Morris HD, Theien BE, Konda S, Engberg K, Lauterbur PC, Greenough WT. Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience. 2003;117:1037–1046. doi: 10.1016/s0306-4522(02)00664-4. [DOI] [PubMed] [Google Scholar]
- 18.Sawiak SJ, Wood NI, Williams GB, Morton AJ, Carpenter TA. Voxel-based morphometry in the R6/2 transgenic mouse reveals differences between genotypes not seen with manual 2D morphometry. Neurobiol Dis. 2009;33:20–27. doi: 10.1016/j.nbd.2008.09.016. [DOI] [PubMed] [Google Scholar]