Abstract
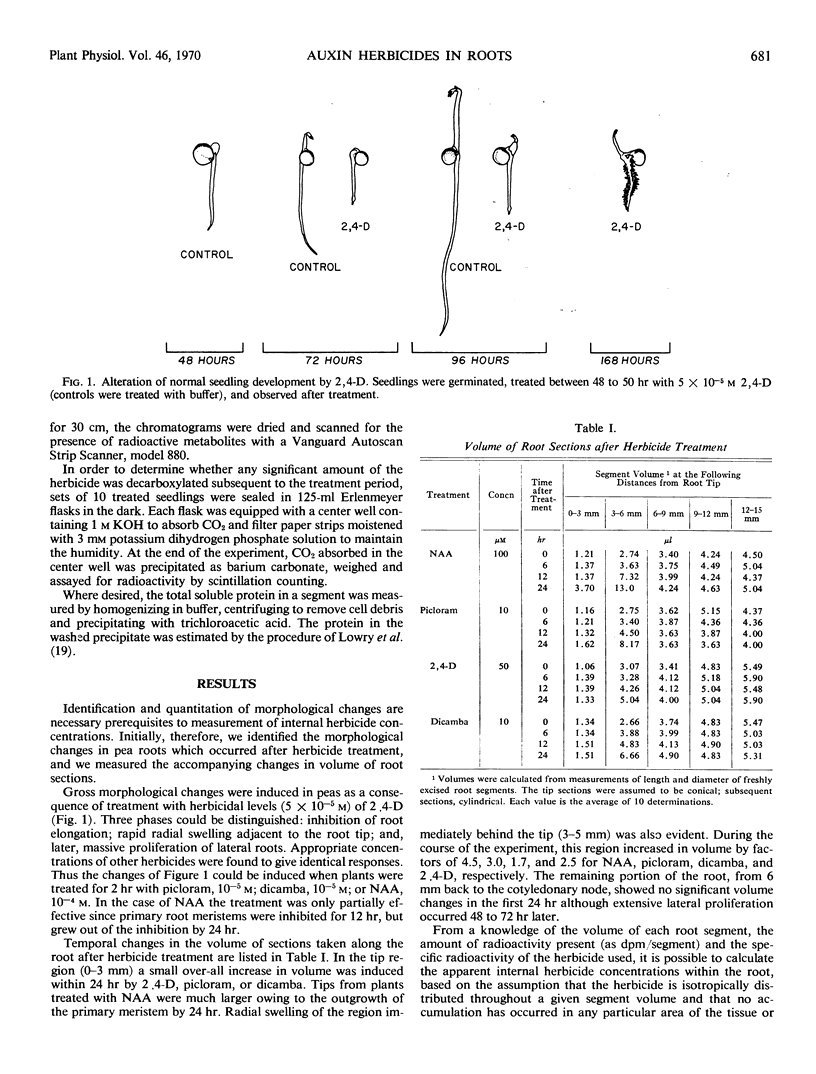
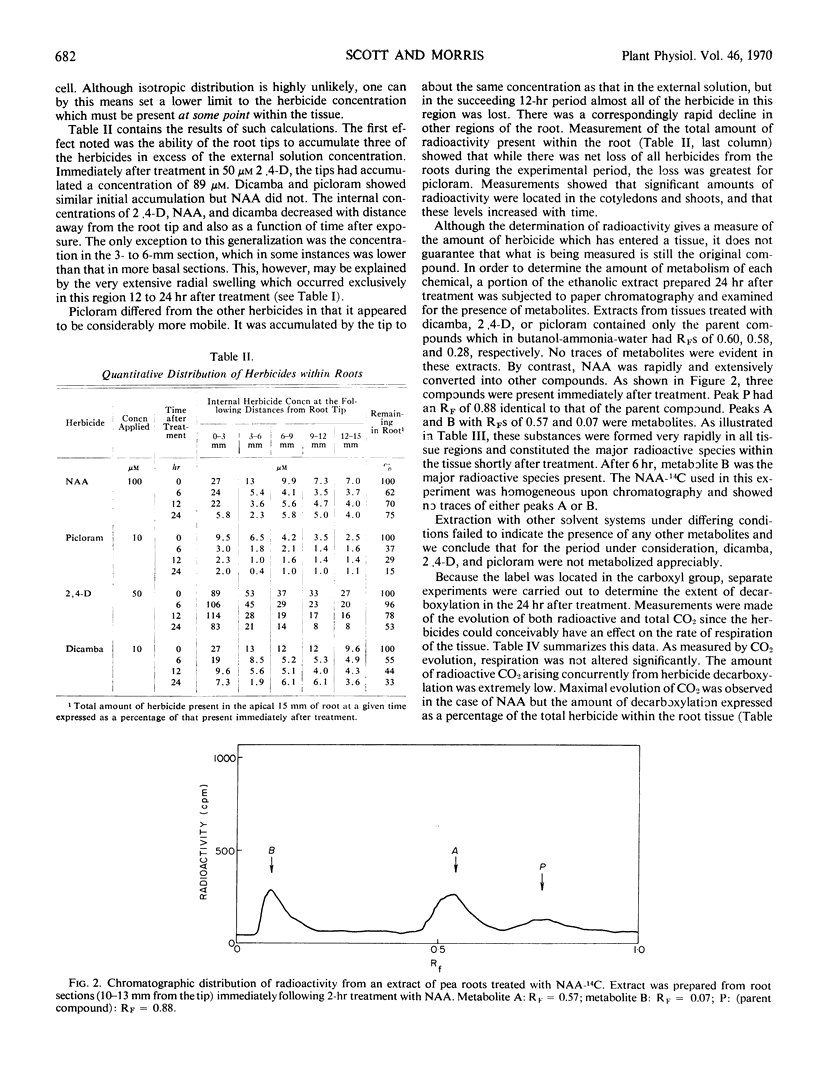
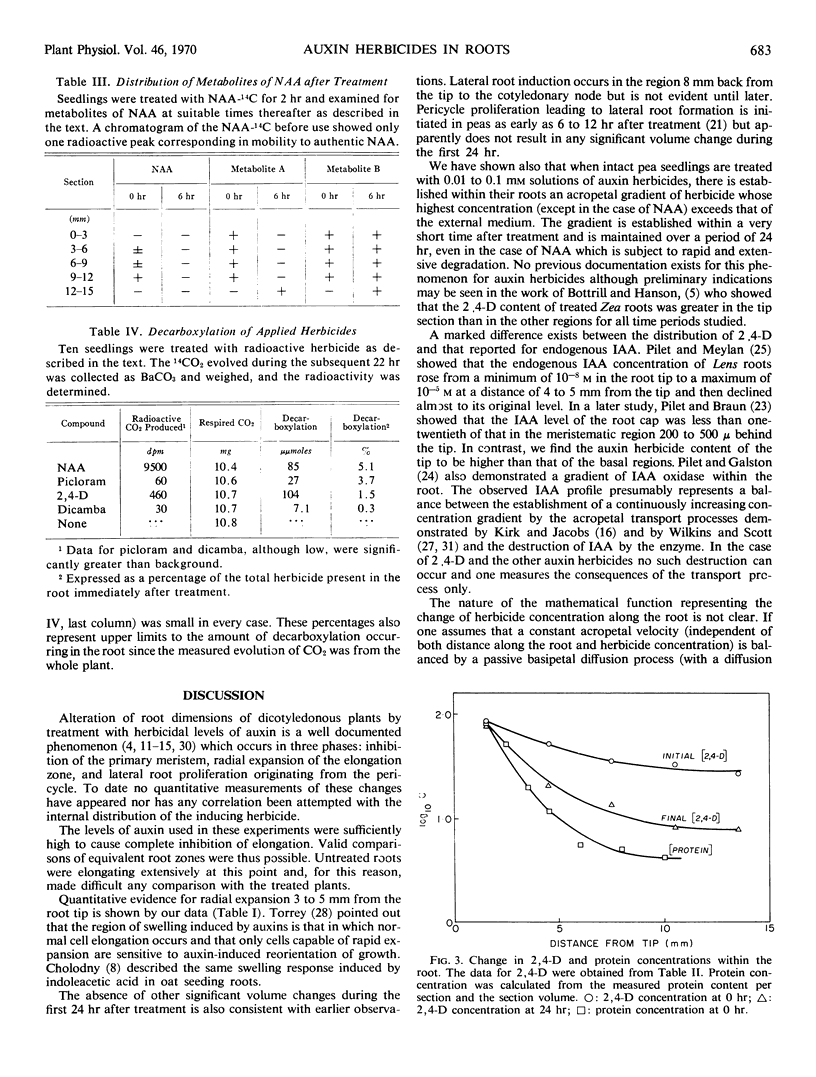
The internal concentrations of four auxin herbicides— 2,4-dichlorophenoxyacetic acid, dicamba, picloram, and naphthaleneacetic acid—were measured in the roots of treated pea seedlings. Intact seedlings were immersed in solutions of labeled herbicides at concentrations sufficient to produce toxic symptoms (inhibition of elongation, radial enlargement, and lateral root proliferation). Measurements of volume and herbicide content of segments taken sequentially along the root showed that an acropetal concentration gradient of each herbicide was established within the root immediately following treatment. Although there was a net loss of herbicide in the following 24 hours, the gradient was maintained. Initially, the concentration of herbicide in the root tips exceeded that in the external medium.
In support of the contention that toxic symptoms due to herbicide treatment are caused by the presence of unmetabolized chemical at the site of action, it was found that metabolism was negligible for all herbicides except naphthaleneacetic acid.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreae W. A., Van Ysselstein M. W. Studies on 3-Indoleacetic Acid Metabolism. V. Effect of Calcium Ions on 3-indoleacetic Acid Uptake and Metabolism by Pea Roots. Plant Physiol. 1960 Mar;35(2):220–224. doi: 10.1104/pp.35.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreae W. A., Van Ysselstein M. W. Studies on 3-Indoleacetic Acid Metabolism. VI. 3-Indoleacetic Acid Uptake and Metabolism by Pea Roots and Epicotyls. Plant Physiol. 1960 Mar;35(2):225–232. doi: 10.1104/pp.35.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eames A. J. Histological Effects of Treatments with Growth-regulating Substances of the 2,4-D Group. Science. 1949 Sep 2;110(2853):235–236. doi: 10.1126/science.110.2853.235. [DOI] [PubMed] [Google Scholar]
- Kirk S. C., Jacobs W. P. Polar Movement of Indole-3-acetic Acid-C in Roots of Lens and Phaseolus. Plant Physiol. 1968 May;43(5):675–682. doi: 10.1104/pp.43.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liao S. H., Hamilton R. H. Intracellular localization of growth hormones in plants. Science. 1966 Feb 18;151(3712):822–823. doi: 10.1126/science.151.3712.822. [DOI] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Sabnis D. D., Hirshberg G., Jacobs W. P. Radioautographic analysis of the distribution of label from h-indoleacetic Acid supplied to isolated coleus internodes. Plant Physiol. 1969 Jan;44(1):27–36. doi: 10.1104/pp.44.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins M. B., Scott T. K. Auxin transport in roots. Nature. 1968 Sep 28;219(5161):1388–1389. doi: 10.1038/2191388a0. [DOI] [PubMed] [Google Scholar]
- Zwar J. A., Brown R. Distribution of labelled plant growth regulators within cells. Nature. 1968 Nov 2;220(5166):500–501. doi: 10.1038/220500a0. [DOI] [PubMed] [Google Scholar]


