Abstract
A nuclear mutation of Glycine max (soybean) segregates 1:2:1 in regard to chlorophyll content. The heterozygous (LG) leaf blade contains about one-half the pigment content of the wild type (DG) per gram fresh weight. A lethal yellow (LY) type contains about 1 to 2% of the DG leaf pigment values. The chlorophyll a/b ratio in the LG is about 5 compared to about 2 in the DG. Protein/leaf values are lower in the LG and LY types when compared to DG. The LG plastid lamellae contain more protein/chlorophyll, cytochromes/chlorophyll, and quinones/chlorophyll than the DG. P700/chlorophyll values are similar in the DG and LG types.
The chlorophyll-depleted LG and LY types had less total acyl lipids per leaf weight when compared to the DG type. Similar amounts of sulfolipid and phosphatidyl glycerol per protein residue weight were found in the LG and DG plastids; however, the monogalactosyl and digalactosyl diglycerides were reduced in the LG paralleling the chlorophyll depletion.
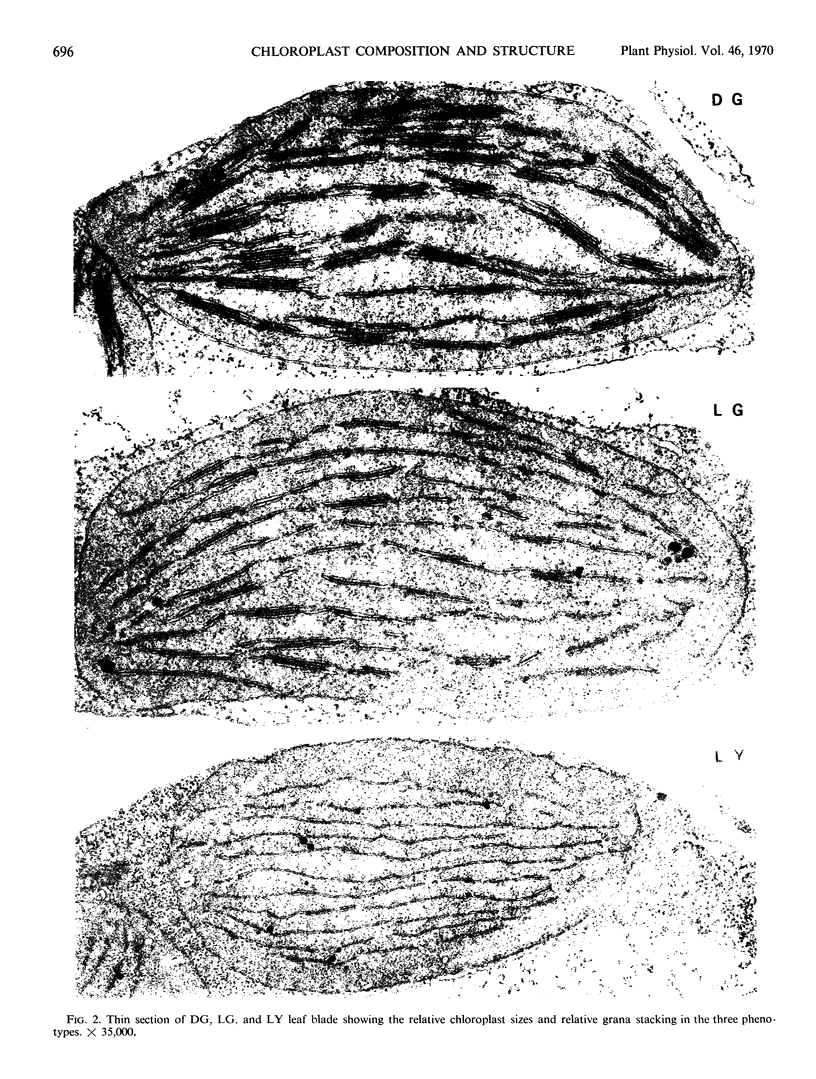
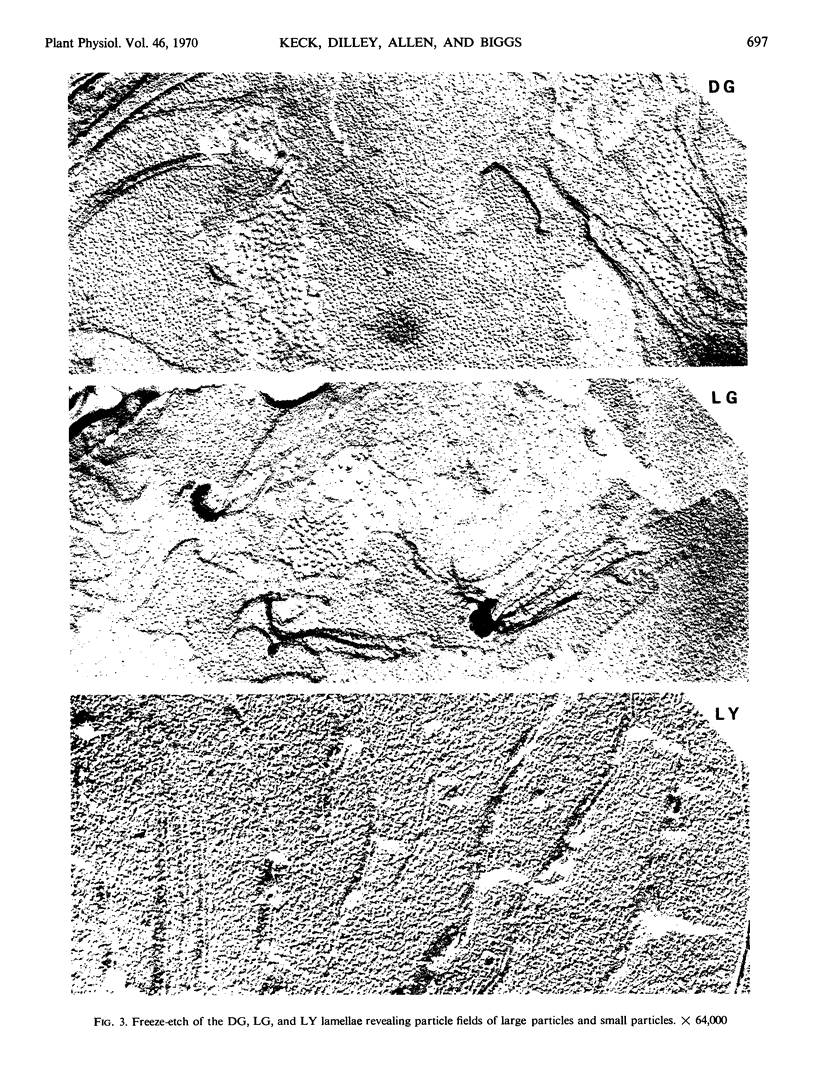
Thin sections of leaf tissue show similar-sized LG and DG plastids but reduced grana formation in the LG. The LY has very few grana and very small grana compared to either DG or LG. The two characteristic particles revealed in higher plant chloroplasts by freeze-etching are about 15% smaller in the LG compared to the DG plants.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLAAUW-JANSEN G., KOMEN J. G., THOMAS J. B. On the relation between the formation of assimilatory pigments and the rate of photosynthesis in etiolated oat seedlings. Biochim Biophys Acta. 1950 Apr;5(2):179–185. [PubMed] [Google Scholar]
- Bishop N. I. THE REACTIVITY OF A NATURALLY OCCURRING QUINONE (Q-255) IN PHOTOCHEMICAL REACTIONS OF ISOLATED CHLOROPLASTS. Proc Natl Acad Sci U S A. 1959 Dec;45(12):1696–1702. doi: 10.1073/pnas.45.12.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman N. K., Highkin H. R. Studies on a barley mutant lacking chlorophyll b. I. Photochemical activity of isolated chloroplasts. Biochim Biophys Acta. 1966 Oct 10;126(2):189–199. doi: 10.1016/0926-6585(66)90054-9. [DOI] [PubMed] [Google Scholar]
- Branton D., Park R. B. Subunits in chloroplast lamellae. J Ultrastruct Res. 1967 Aug;19(3):283–303. doi: 10.1016/s0022-5320(67)80222-3. [DOI] [PubMed] [Google Scholar]
- Crane F. L. Internal Distribution of Coenzyme Q in Higher Plants. Plant Physiol. 1959 Mar;34(2):128–131. doi: 10.1104/pp.34.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DILLEY R. A., CRANE F. L. A specific assay for tocopherols in plant tissue. Anal Biochem. 1963 Jun;5:531–541. doi: 10.1016/0003-2697(63)90073-3. [DOI] [PubMed] [Google Scholar]
- DILLEY R. A. THIN-LAYER CHROMATOGRAPHY OF NATURALLY OCCURRING QUINONES AND HYDROQUINONES. Anal Biochem. 1964 Feb;7:240–246. doi: 10.1016/0003-2697(64)90234-9. [DOI] [PubMed] [Google Scholar]
- Dilley R. A., Crane F. L. Light-Dependent Conversions of Endogenous alpha-Tocopherylquinone and Plastoquinone-D in Spinacia Oleracea Chloroplasts. Plant Physiol. 1964 Jan;39(1):33–36. doi: 10.1104/pp.39.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough U. W., Armstrong J. J., Levine R. P. Photosynthetic Properties of ac-31, a Mutant Strain of Chlamydomonas reinhardi Devoid of Chloroplast Membrane Stacking. Plant Physiol. 1969 Jul;44(7):1001–1012. doi: 10.1104/pp.44.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highkin H. R., Boardman N. K., Goodchild D. J. Photosynthetic Studies on a Pea-mutant Deficient in Chlorophyll. Plant Physiol. 1969 Sep;44(9):1310–1320. doi: 10.1104/pp.44.9.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KROGMANN D. W. A requirement for plastoquinone in photosynthetic phosphorylation. Biochem Biophys Res Commun. 1961 Mar 24;4:275–277. doi: 10.1016/0006-291x(61)90233-9. [DOI] [PubMed] [Google Scholar]
- Keck R. W., Dilley R. A., Ke B. Photochemical characteristics in a soybean mutant. Plant Physiol. 1970 Nov;46(5):699–704. doi: 10.1104/pp.46.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MOLLENHAUER H. H. PLASTIC EMBEDDING MIXTURES FOR USE IN ELECTRON MICROSCOPY. Stain Technol. 1964 Mar;39:111–114. [PubMed] [Google Scholar]
- Moor H., Mühlethaler K. FINE STRUCTURE IN FROZEN-ETCHED YEAST CELLS. J Cell Biol. 1963 Jun 1;17(3):609–628. doi: 10.1083/jcb.17.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rntzen C. J., Dilley R. A., Crane F. L. A comparison of chloroplast membrane surfaces visualized by freeze-etch and negative staining techniques; and ultrastructural characterization of membrane fractions obtained from digitonin-treated spinach chloroplasts. J Cell Biol. 1969 Oct;43(1):16–31. doi: 10.1083/jcb.43.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMILLIE R. M., LEVINE R. P. THE PHOTOSYNTHETIC ELECTRON TRANSPORT CHAIN OF CHLAMYDOMONAS REINHARDI. II. COMPONENTS OF THE TRIPHOSPHOPYRIDINE NUCLEOTIDE-REDUCTIVE PATHWAY IN WILD-TYPE AND MUTANT STRAINS. J Biol Chem. 1963 Dec;238:4058–4062. [PubMed] [Google Scholar]
- Schmid G. H., Gaffron H. Light metabolism and chloroplast structure in chlorophyll-deficient tobacco mutants. J Gen Physiol. 1967 Jan;50(3):563–582. doi: 10.1085/jgp.50.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichler-Zallen D. The effect of manganese on chloroplast structure and photosynthetic ability of Chlamydomonas reinhardi. Plant Physiol. 1969 May;44(5):701–710. doi: 10.1104/pp.44.5.701. [DOI] [PMC free article] [PubMed] [Google Scholar]