Abstract
The T cell Ig and mucin domain 3 (Tim-3) receptor has been implicated as a negative regulator of adaptive immune responses. We have utilized a proteomic strategy to identify novel proteins associated with graft versus host disease (GVHD) after allogeneic hematopoietic cell transplantation (HCT). Mass spectrometry analysis of plasma from subjects with mid-gut and upper-gut GVHD compared with those without GVHD identified increased levels of a protein identified with high confidence as Tim-3. A follow-up validation study using an immunoassay to measure Tim-3 levels in individual plasma samples from 127 patients demonstrated significantly higher plasma Tim-3 concentrations in patients with the more severe mid-gut GVHD, compared with those with upper-gut GVHD (P = .005), patients without GVHD (P = .002), and normal controls (P < .0001). Surface expression of Tim-3 was increased on CD8+ T cells from patients with grade 2 to 4 acute GVHD (P = .01). Mass spectrometry–based profiling of plasma from multiple subjects diagnosed with common diseases provided evidence for restricted release of soluble Tim-3 in the context of GVHD. These findings have mechanistic implications for the development of novel strategies for targeting the Tim-3 immune regulatory pathway as an approach to improving control of GVHD.
Keywords: TIM-3, Proteomics, Marrow transplantation, Hematopoietic cell, transplantation, Graft-versus-host disease
INTRODUCTION
T cell Ig and mucin domain 3 (Tim-3) is a type-1 transmembrane receptor with immunoregulatory properties that suppresses the activation of T helper 1 (TH1) cells on engagement with ligand. Tim-3 is selectively expressed on activated T cells and constitutively expressed on dendritic cells (DCs) and natural killer (NK) cells [1]. Activation of Tim-3 via ligand binding results in decreased T cell proliferation, decreased T cell-mediated cytotoxicity, decreased IFN-γ production, and induction of apoptosis [2–5]. There is also evidence that Tim-3 suppresses antitumor DCs [6]. Interactions between Tim-3 and its ligand galectin-9 (gal-9) are known to play a significant regulatory role in autoimmune disorders, chronic infection, tumor immunity, and transplantation [4–11]. The binding of Tim-3 to gal-9 on activated T cells induces apoptosis and/or blocks T cell effector functions; however, interfering with the interaction between Tim-3 and gal-9 in experimental models can exacerbate acute inflammation [4,12]. The overall mechanisms and pathways by which Tim-3 signals remain unclear.
Acute graft-versus-host disease (GVHD) in experimental models can be modulated and prevented by interventions that facilitate immune regulation. GVHD after allogeneic hematopoietic cell transplantation in humans is a major cause of morbidity and mortality, but clinically significant grades 2 to 4 disease do not occur in all recipients. The frequency of grade 2 to 4 acute GVHD at our center is 71%, and 14% of all patients develop severe grades 3 to 4 disease [13]. Acute GVHD can cause inflammation and injury in the skin, liver, and gastrointestinal mucosa. Acute GVHD of the upper gut causes anorexia, nausea, and vomiting, sometimes accompanied by mild diarrhea and rash, whereas acute GVHD of the mid-gut causes more severe manifestations and is associated with high-volume, protein-losing diarrhea accompanied by abdominal pain and ileus. Upper-gut GVHD can usually be controlled with lower doses of glucocorticoids, including topically active formulations [14,15]. In contrast, mid-gut GVHD usually requires treatment with higher initial glucocorticoid doses, may not respond to initial treatment, and is associated with a higher risk of nonrelapse mortality related to more intense immune suppression and lack of response to treatment [16,17]. In the current era, mortality in patients with acute GVHD is correlated with the severity of gastrointestinal involvement, and liver and skin involvement now play relatively minor roles in affecting outcome [18,19].
Previous clinical studies have identified several plasma cytokines and other proteins associated with T cell activation, inflammation, apoptosis, and tissue injury that are elevated in patients with acute GVHD of skin, liver, and gut including IFNγ, IL-1β, IL-2, IL-2Rα, IL-6, IL-8, IL-12, IL-18, ST2, TNFα, TNFRI, cytokeratin fragment 18 (KRT18), hepatic growth factor (HGF), elafin, and regenerating islet-derived 3-alpha (REG3α) [20–30]. Elafin, a product of keratinocytes, was associated specifically with skin GVHD, and REG3α, a product of Paneth cells, was associated with GVHD of the gut and liver. Paneth cells residing at the bottom of epithelial crypts of small intestinal mucosa secrete alpha-defensins (an important part of the gut mucosal barrier’s antibacterial defense) and interact with epithelial stem cells to regulate proliferation [31,32]. KRT18 is released by apoptotic epithelial cells and has been correlated with response to GVHD treatment [33]. HGF, KRT18, and REG3α levels have been compared in a common cohort of HCT recipients, and all 3 were confirmed to be significantly associated with lower-gut and liver GVHD [34]. As a class, this last group of 4 plasma proteins, elafin, HGF, KRT18, and REG3α, represent markers of tissue injury.
We have undertaken comprehensive proteomic profiling of plasmas to identify potential markers of GVHD. We here report identification of novel form of TIM-3 represented by release of the extracellular domain into the circulation in association with acute GVHD at the presymptomatic stage. This finding was validated by immunoassays of individual samples from a large cohort of patients. We also show that Tim-3 is up-regulated on T cells during acute GVHD, consistent with murine models of GVHD and previous studies demonstrating expression of Tim-3 on activated Th1 effector cells, DC, and NK cells. The unexpected finding of elevated plasma Tim-3 in GVHD patients in the current study suggests that soluble Tim-3, by interfering with the immune regulatory activity of Tim-3 expressed on effector T cells, may play a significant role in the pathogenesis of clinical GVHD.
MATERIALS AND METHODS
Study Population
All patients received a myeloablative conditioning regimen followed by infusion of hematopoietic cells from an HLA-matched related or unrelated donor on day 0 (Table 1). Myeloablative conditioning regimens generally contained high-dose cyclophosphamide with busulfan or 12 Gy to 13.2 Gy total body irradiation (TBI). Recipients received a calcineurin inhibitor plus methotrexate to prevent GVHD. Prophylaxis for infections included low-dose acyclovir, trimethoprim and sulfamethoxazole, or dapsone, an antifungal agent, pre-emptive therapy with ganciclovir for patients with cytomegalovirus (CMV) antigenemia or DNAemia, and antibiotics for patients with neutropenia. Ursodiol was given as prophylaxis against cholestasis [35].
Table 1.
| A Patient and Transplantation Characteristics | |||||||
|---|---|---|---|---|---|---|---|
| Cohort 1, n = 127 |
|||||||
| Discovery Samples, n = 20 |
Validation Samples, n = 127 |
Cohort 2, n = 22 |
|||||
| Upper-gut GVHD, n = 5 |
Mid-gut GVHD, n = 5 |
GVHD− n = 10 | GVHD+ n = 78 | GVHD− n = 49 | GVHD+ n = 14 | GVHD− n = 8 | |
| Patient age, median (range), yr | 42 (18 to 48) | 49 (22 to 59) | 39 (37 to 58) | 43 (18 to 67) | 45 (19 to 65) | 45 (28 to 62) | 39 (30 to 58) |
| Diagnosis | |||||||
| Acute leukemia | 2 | 4 | 6 | 40 | 19 | 9 | 3 |
| MDS | 2 | 1 | 2 | 17 | 14 | 1 | 4 |
| Lymphoma | 0 | 0 | 0 | 7 | 4 | 1 | 1 |
| Chronic leukemia (CLL, CML) | 1 | 0 | 2 | 13 | 11 | 3 | 0 |
| Other | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| Donor type | |||||||
| Related | 3 | 2 | 7 | 33 | 31 | 5 | 3 |
| Unrelated | 2 | 3 | 3 | 45 | 18 | 9 | 5 |
| Sex match | |||||||
| Female to male/Other | 2/3 | 4/1 | 2/8 | 18/60 | 15/34 | 3/11 | 1/7 |
| Graft type | |||||||
| Marrow/Blood/Cord | 0/5 | 0/5 | 1/9/0 | 6/72/0 | 3/46/0 | 2/12/0 | 2/5/1 |
| Conditioning regimen | |||||||
| Myeloablative | 5 | 5 | 10 | 77 | 48 | 7 | 5 |
| TBI >1000 cGy | 1 | 3 | 4 | 32 | 9 | 7 | 3 |
| Reduced intensity | 0 | 0 | 0 | 1 | 1 | 7 | 3 |
| CMV seropositive | |||||||
| Patient/Donor | 3/1 | 4/2 | 2/1 | 41/31 | 18/16 | 9/6 | 3/1 |
| GVHD prophylaxis | |||||||
| CNI + MTX | 4 | 5 | 9 | 72 | 44 | 10 | 7 |
| CNI + MMF | 1 | 0 | 1 | 4 | 2 | 1 | 1 |
| CNI + MTX + Sirolimus | 0 | 0 | 0 | 2 | 3 | 2 | 0 |
| CNI + MTX + Campath | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Acute GVHD onset, median (range), d |
36 (23 to 43) | 43 (23 to 52) | NA | 27 (7 to 64) | NA | 31 (16 to 65) | NA |
| Acute GVHD | |||||||
| Grade 0 | 0 | 0 | 10 | 0 | 49 | 0 | 7 |
| Grade 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Grade 2 | 4 | 0 | 0 | 63 | 0 | 10 | 0 |
| Grade 3 | 1 | 5 | 0 | 13 | 0 | 3 | 0 |
| Grade 4 | 0 | 0 | 0 | 2 | 0 | 1 | 0 |
| Organ-specific acute GVHD* | |||||||
| Mid-gut | 0 | 5 | 0 | 15 | 0 | 3 | 0 |
| Upper-gut | 5 | 0 | 0 | 63 | 0 | 10 | 0 |
| Skin only | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Liver only | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| B Concurrent Involvement of Skin, Liver, and Gut GVHD | ||||||
|---|---|---|---|---|---|---|
| Clinically Significant Grade 2 to 4 Acute GVHD |
||||||
| Skin Only |
Liver Only |
Gut Only |
Gut/ Skin |
Gut/ Liver |
Gut/Liver/ Skin |
|
| GVHD+ cohort 1 | 0 | 0 | 43 | 17 | 7 | 11 |
| GVHD+ cohort 2 | 1 | 0 | 5 | 5 | 1 | 2 |
| GVHD+ discovery samples* |
0 | 0 | 2 | 4 | 2 | 2 |
GVHD indicates graft-versus-host disease; CLL, chronic lymphocytic leukemia; CML, chronic myelogenous leukemia; MDS, myelodysplastic syndrome; TBI, total body irradiation; CNI, calcinuerin inhibitor; MTX, methotrexate.
Grade 2 to 4 acute GVHD.
GVHD indicates graft-versus-host disease.
Cohort 1 GVHD+ cases selected for the initial pooled plasma discovery analysis.
Study Design
Patients were recruited before transplantation, and blood samples were collected and cryopreserved weekly from day 7 through day 35, on days 56 and 80, and at the onset of acute GVHD. Patients who developed clinically significant grades 2 to 4 acute GVHD were declared cases, whereas patients with grades 0 or 1 acute GVHD were declared case-controls. The study was conducted in 3 phases as follows: (1) an initial discovery phase testing pooled plasma samples by mass spectometry; (2) a clinical correlation phase using Luminex-based immunoassays measuring (Luminex Model 200, Luminex Corp., Austin, Tx) Tim-3 in the plasma of individual patients; and (3) an immunophenotype analysis of Tim-3 expression by peripheral blood mononuclear cell (PBMC). The discovery and clinical correlation phases included 127 patients (cohort 1), and the immunophenotype analysis was performed with an independent cohort of 22 patients (cohort 2). The clinical characteristics of cohort 1 and cohort 2 patients are summarized in Table 1. In studies from both cohorts, only plasma and PBMC from GVHD cases collected before beginning immunosuppression therapy were analyzed, whereas samples from case-control patients without clinically significant acute GVHD were matched with cases for time from transplantation. Patients in both cohorts gave informed consent under protocols approved by the Fred Hutchinson Cancer Research Center Institutional Review Board.
Acute Gastrointestinal GVHD
The peak stage of gastrointestinal GVHD, the peak grade of GVHD, and the date of onset of clinical signs and symptoms were independently scored by P.J.M., according to the extent of rash, total serum bilirubin, the presence of upper gastrointestinal symptoms, and daily stool volume [36]. Patients were classified as (1) postallograft controls, with no evidence of acute GVHD in the gut or liver at any time through day 100; (2) mid-gut gastrointestinal GVHD (gut stage 2 to 4; that is, peak diarrhea volumes >1 liter per day or severe abdominal pain); and (3) less severe GVHD, consisting mostly of upper-gut gastrointestinal GVHD (gut stage 1; that is, anorexia, nausea, vomiting, or early satiety with mucosal biopsy positive for GVHD [37] and, if diarrhea was present, peak diarrhea volumes were <1 liter per day.
Collection and Processing of Blood Samples
Patients in cohort 1 had blood samples collected from central venous access catheters into sodium fluoride/potassium oxalate vacutainer and maintained on ice until centrifugation. Plasma was aliquoted in .5 mL tubes for cryopreservation at −80 degrees centigrade within 2 hours of phlebotomy. Patients in cohort 2 had blood samples collected into heparinized tubes; PBMC were isolated by Ficoll-hypaque density centrifugation, resuspended in 10% DMSO and 90% FBS, and cryopreserved in liquid nitrogen. Plasma was collected from blood samples obtained via venipuncture from 48 healthy consenting normal adults who were free of fever or any respiratory or influenza-like symptoms for at least 7 days before phlebotomy, and who had received no nonsteroidal anti-inflammatory drugs, glucocorticoid medication, or antibiotics for at least 48 hours. This normal control cohort included 29 women and 19 men, with a median age of 37 years (range, 23 to 67 years).
Discovery Phase—Quantitative Profiling of Plasma Samples by Mass Spectrometry
A subset of 20 patients from cohort 1 was selected for in-depth proteomic analysis of plasma specimens and consisted of 5 patients with upper-gut GVHD, 5 with mid-gut GVHD, and 10 allograft-matched case-control patients without clinically significant acute GVHD, whose plasma samples had been collected at the same time point after transplantation as samples from patients with upper-gut and mid-gut GVHD, respectively. From each of these patient groups, 100 µL of plasma was removed from thawed aliquots and pooled in a single ampoule, yielding pooled specimens from patients with upper-gut and mid-gut GVHD and their respective allograft case-controls without GVHD. Four independent large-scale quantitative proteomics experiments were performed using a previously described method [7] that employed immunodepletion, isotopic labeling with acrylamide [38], extensive fractionation [39], and high-resolution tandem mass spectrometry. Each experiment compared a 300 µL pool of plasma from 5 upper- or mid-gut cases with a 300 µL pool of 5 controls (equal volume). Pools of case and control plasma samples were immunodepleted of the top 6 most abundant proteins (albumin, IgG, IgA, transferrin, haptoglobin, and anti-trypsin) using a Hu-6 column (4.6 × 250 mm, Agilent Technologies, Santa Clara, California). Columns were equilibrated with buffer A at .5 mL/min for 13 minutes, and pooled plasma samples were injected after filtration through a .22 µm syringe filter. Flow-through fractions were collected for 10 minutes at a flow rate of .5 mL/min buffer A, and stored at −80°C until further use. Immunodepleted samples were concentrated using a Centricon YM-3 device (Millipore Corporation, Billerica, MA) and rediluted in 8 M urea, 30 mM Tris, pH 8.5, .5% OG (octyl-beta-d-glucopyranoside, Roche Diagnostics). Samples were reduced with DTT and isotopic labeling of cysteine residues of intact proteins was performed. Pools received either the light acrylamide isotope (C12, Sigma-Aldrich) or the heavy 1,2,3-C13-acrylamide isotope (C13, Cambridge Isotope Laboratories). Alkylation with acrylamide was performed for 1 hour at room temperature. For each of the 4 experiments, a case pool was then mixed with a corresponding control pool for further analysis.
In each experiment, the combined isotopically labeled case-control samples were separated by an automated online 2D HPLC system (Shimadzu). The combined labeled plasma samples were separated in the first dimension by anion exchange chromatography (Poros HQ/10, 10 × 100 mm, Applied Biosystems) using an 8-step elution (0 to 1000 mM NaCl) at .8 mL/min. Fractions from each of the 8 anion-exchange separation elution steps were automatically transferred onto a reversed-phase column (PorosR2/10, 4.6 × 100 mm, Applied Biosystems) for a second dimension of separation. A 25-minute gradient elution (5% to 95% mobile phase B) was used at 2.4 mL/min. Mobile phase A for anion-exchange chromatography consisted of 20 mM Tris (Sigma-Aldrich), 6% isopropanol (Fisher Scientific), and 4 M urea, pH 8.5, and mobile phase B was the same composition and pH as mobile phase A with 1 M NaCl (Fisher Scientific) added. Mobile phase A for reversed-phase chromatography consisted of 95% water, 5% acetonitrile, and .1% TFA (Supelco), and mobile phase B consisted of 90% acetonitrile, 10% water, and .1% TFA. In-solution digestion was performed with lyophilized aliquots from the reversed-phase (second dimension) fractionation step. Proteins were resuspended in .25 M urea containing 50 mM ammonium bicarbonate and 4% acetonitirle and then digested overnight at 37°C with modified trypsin (Promega). The digestion was stopped by addition of 10% formic acid. Aliquots were subjected to mass spectrometry shotgun analysis. Ninety-six fractions were analyzed for each experiment using an LTQ-Orbitrap (Thermo) mass spectrometer coupled with a NanoLC-1D (Eksigent). Liquid chromatography separation was performed on a 25-cm column (Picofrit 75 µm i.d., New Objectives, packed in-house with Magic C18 resin) using a 90-minute linear gradient from 5% to 40% of acetonitirle in .1% formic acid at 300 nL/min for shotgun analysis. Spectra were acquired in data-dependent mode over m/z range 400 to 1800, and included selection of the 5 most abundant doubly or triply charged ions of each MS spectrum for MS/MS analysis. Mass spectrometer parameters included capillary voltage of 2.0 kV, capillary temperature of 200°C, resolution of 60,000, and target value of 1,000,000.
Analysis of Mass Spectrometry Discovery Data
Mass spectra were searched using Mascot against the human International Protein Index (IPI) database (v. 3.13). Quantitative information was extracted from acrylamide labeled peptides using an in-house script (Q3) for peptides with minimum PeptideProphet = .75, expect score <.10, and maximum fractional delta mass = 20 ppm [38]. Also, because the label orientation was reversed in some experiments, we additionally required that any quantified peptide had to be observed in both isotopic states at least once in at least 1 experiment. This criterion removes spurious ratios that arise because of misidentification. Proteins with ProteinProphet scores >.90 were aligned by their protein group number to identify master groups of indistinguishable proteins. The master group ratio for each experiment was set to the geometric mean of the corresponding peptide ratios, which were logarithmically transformed and median centered. Proteins that had become defunct in IPI or were immunodepleted were removed from the analysis. Mean log2 ratios and moderated P values for groups of experiments were computed using the LIMMA package from bioconductor.org and weighted using the number of quantitated events (peptides) [40].
Clinical Correlation Phase—Luminex Microbead Assay
A sandwich ELISA method was used to screen commercially available capture/detection matched pair antibodies to develop optimal assay characteristics for quantitation of human Tim-3 in plasma from patients in cohort 1. Recombinant human Tim-3:Fc chimera (R&D Systems #2365-TM, Minneapolis, MN) at a concentration of 100 ng/mL was used as a positive control for testing various capture/detection antibody combinations. Our optimal ELISA used an affinity-purified monoclonal capture antibody against human Tim-3 (R&D Systems #MAB2365) and a biotinylated polyclonal detection antibody (R&D Systems #BAF2365). We next conjugated this capture antibody to Luminex microbeads (BioRad, Hercules, CA) and tested the operating characteristics of the microbead assay using the recombinant human Tim-3:Fc spiked into assay buffer at 100 ng/mL as the high standard and diluted serially to .1 pg/mL.
Clinical Correlation Phase—Immunophenotyping and Flow Cytometry
Cryopreserved PBMC from cohort 2 patients were thawed and subjected to cell surface staining using fluorochrome-labeled anti-human monoclonal antibodies CD3 FITC, CD8β APC, CD4 APC-Cy7 (BD Biosciences, Franklin Lakes, NJ), Tim-3 PE, CD19 PerCP-Cy5.5, CD56 PE-Cy7, and CD14 Pacific Blue (Biolegend, San Diego, CA). Isotype control antibodies included IgG1κ FITC, IgG1κ PE, IgG1κ PerCP-Cy5.5, IgG1κ PE-Cy7, IgG1κ APC-Cy7 (BD Biosciences), and IgG2aκ APC and IgG1κ Pacific Blue (Biolegend). Flow cytometry data were collected on a BD LSRII (BD Biosciences) and analyzed using CellQuest Pro (BD Biosciences) software.
Statistical Analyses
All Tim-3 levels were log transformed for statistical analysis. For the presentation in Figure 1, the sample most proximal to onset of GVHD treatment was used for GVHD cases. For mid-gut cases, the median day of this sample was day -3 (range, −15 to 0) before GVHD clinical onset; for upper-gut cases the median day of this sample was day -2 (range, −10 to 1) before onset. Thirteen mid-gut cases and 46 upper-gut cases were time-matched to a no-GVHD control sample. Case and control groups were compared by paired t-test. Mid-gut cases and upper-gut cases, and GVHD cases and normals were compared by 2-sample t-test. The multivariate analysis in Table 3 includes all 303 samples from 127 cases and controls. Generalized estimating equation methods using an exchangeable correlation structure were used to account for varying numbers of samples per subject. GVHD samples were defined as any sample taken within 15 days before the onset of treatment for GVHD. Models were also adjusted for time since HCT, using linear, quadratic, and cubic terms. The regression effects on the log scale were transformed by antilog to effect ratios, which represent multiplicative effects of covariates on Tim-3 plasma levels. Tim-3 cellular expression in Table 4 was also analyzed by generalized estimating equation methods using exchangeable correlation. Logistic regression was used to perform a receiver-operating characteristic (ROC) analysis of Tim-3 as a predictor of GVHD, using day 21 ± 3 days post-transplantation as a land-mark time. Samples from 64 patients without a history of acute GVHD were available in this window; 7 subsequently developed mid-gut GVHD and 24 subsequently developed upper-gut GVHD. Percentages and absolute numbers of cells expressing surface Tim-3 were compared by 2-sample t-test using Satterwaite’s correction for unequal variance.
Figure 1.
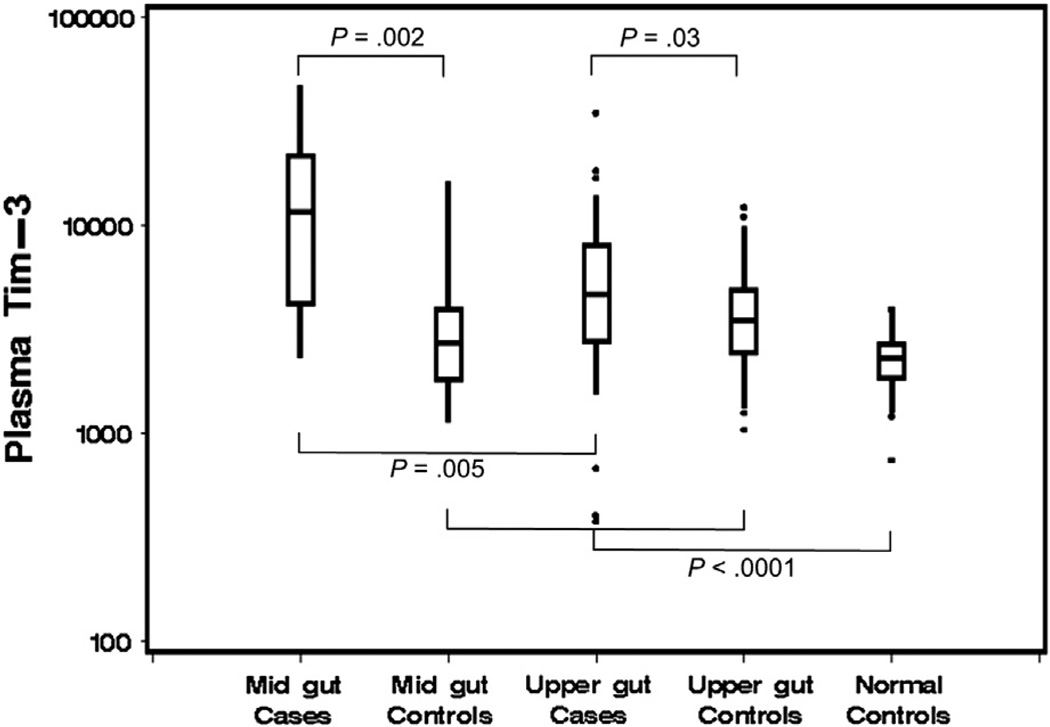
Levels of Tim-3 in pg/mL were measured in 185 individual plasma samples. These included 78 samples from cohort 1 cases with mid-gut (n = 15) or upper-gut (n = 63) acute GVHD, 59 individual plasma samples from 37 case-controls matched with cases for time post-transplant, and 48 samples from normal healthy controls. Boxes represent 25th, 50th, and 75th percentiles; whiskers indicate 5th and 95th percentiles.
Table 3.
Multivariate Analysis of Clinical Variables Associated with Soluble Tim-3 Plasma Levels*
| Characteristic | Effect Ratio (95% CI) | P Value |
|---|---|---|
| Sample characteristics | ||
| Upper-gut GVHD† | 1.04 (.80 to 1.33) | .78 |
| Mid-gut GVHD† | 1.98 (1.20 to 3.29) | .008 |
| Patient pretransplantation characteristics | ||
| CMV seropositive pretransplantation | 1.32 (1.02 to 1.72) | .04 |
| Donor CMV seropositive | 1.17 (.92 to 1.50) | .21 |
| Unrelated donor | 1.35 (1.02 to 1.78) | .04 |
| Mismatched donor | 1.09 (.76 to 1.57) | .63 |
| Age (per decade) | 1.00 (.98 to 1.03) | .75 |
| Female | .94 (.72 to 1.23) | .66 |
| Female to male D/R | .86 (.62 to 1.20) | .37 |
| PBSC | .93 (.56 to 1.56) | .79 |
| TBI (>1200 cGy) | 1.14 (.88 to 1.47) | .33 |
GVHD indicates graft-versus-host disease; CMV, cytomegalovirus; PBSC, peripheral blood stem cells; TBI, total body irradiation; D/R, donor/recipient.
Multivariate analysis of soluble Tim-3 plasma levels in 303 samples collected from 127 patients before initiation of treatment for acute GVHD. Data were analyzed after log transformation of Tim-3 levels. The “effect ratio” is the antilog of the estimated difference in means on the log scale, and represents a multiplicative effect of covariates on Tim-3 levels. Models were also adjusted for time since HCT (linear, quadratic, and cubic terms).
Defined as any sample within 15 days of onset of treatment for GVHD and compared with grade 0 to 1 acute GVHD.
Table 4.
Transplantation Characteristics Associated with Tim-3 Cellular Expression*
| Clinical Variables | CD3+ T cells |
CD4+ T cells |
CD8+ T cells |
CD19+ B cells |
CD56+ NK cells |
|---|---|---|---|---|---|
| Age, patient† | .58 | .90 | .83 | .60 | P = .002 |
| CMV serostatus, patient‡ |
P = .01 | P = .01 | .30 | P = .02 | .83 |
Univariate associations of the percentage of Tim-3+ blood mononuclear cells in GVHD+ cases and GVHD− case-controls studied during the first 100 days after allogeneic HCT (P values and direction of association). Study includes 22 HCT patients (14 acute GVHD+ cases and 8 GVHD− case-controls) and 61 patient samples (32 cases and 29 case-controls). Samples from case and case-control patients were matched for time post transplantation: median 30 (range, 20 to 55) days and 32 (range, 25 to 36) days, respectively (P = .97). No significant effect was observed for sex, sex match, donor type (related, unrelated), HLA match, donor CMV serostatus, HSC source (blood, marrow), and TBI >1000 cGy.
Increasing patient age by decade.
CMV serostatus (positive patient, negative donor) pretransplantation.
RESULTS
Acute GVHD
Seventy-eight of the 127 patients in cohort 1 developed clinically significant grades 2 to 4 acute GVHD (grade 2, n = 63; grade 3, n = 13; grade 4, n = 2), and 49 patients had no acute GVHD. Fifteen (19%) of the 78 had more severe mid-gut GVHD; 63 (81%) had less severe upper-gut GVHD (Table 1). The median day of onset of grades 2 to 4 acute GVHD was day 26 (range, 7 to 64) after HCT. Fourteen (64%) of the 22 patients in cohort 2 developed grades 2 to 4 acute GVHD (grade 2, n = 10; grade 3, n = 3; grade 4, n = 1). Aliquots of selected cohort 1 plasma samples collected at time points closest to the clinical onset of GVHD were pooled. One pool included samples collected from 5 patients at a median of 2 days (range, 1 to 3) before the onset of upper-gut GVHD, and the other pool included samples collected from 5 patients at a median of 6 days (range, 2 to 7) before the onset of mid-gut GVHD. Similarly, aliquots of selected cohort 1 plasma samples collected 1 to 2 weeks before the clinical onset of GVHD were pooled. One pool included samples collected from 5 patients at a median of 10 days (range, 8 to 13) before the onset of upper-gut GVHD, and the other pool included samples collected from 5 patients at a median of 10 days (range, 8 to 14) before the onset of mid-gut GVHD.
Discovery of Elevated Tim-3 Levels in Acute Gut GVHD
Mass spectrometry identified several tryptic peptides spanning from amino acids 56 to 142 in the extracellular domain of Tim-3. Quantitative data derived from a cysteine-containing peptide spanning amino acids 56 to 69 yielded increased ratios in pooled plasma samples from patients before the onset of acute gut GVHD compared with case matched controls, based on differential isotopic labeling. Tim-3 plasma levels were higher in patients with mid-gut compared with upper-gut GVHD, and higher in the samples collected closest to GVHD onset compared with samples collected earlier (Table 2). The Tim-3 case/control ratios in pooled mid-gut samples closest to GVHD onset and earlier were 4.32 and 3.19 (P < .0001), respectively. The Tim-3 case/control ratios in pooled upper-gut samples closest to GVHD onset and earlier were 2.58 (P = .05) and 1.02 (P = not significant), respectively. In separate studies, we have performed in-depth quantitative plasma proteome profiling of samples collected from subjects who were diagnosed with lung, colon, and breast cancer and subjects who were diagnosed with coronary heart disease and stroke. None of these samples yielded increased levels of circulating TIM-3 (data not shown), suggestive of restricted release of soluble TIM-3 in the context of GVHD.
Table 2.
Ratio of Soluble Tim-3 in Pooled Case and Case-Control Plasma Samples*
| Plasma Sample | Upper Gut |
Mid-Gut |
||
|---|---|---|---|---|
| Ratio (P)* | Day (range)† | Ratio (P)* | Day (range)† | |
| Closest to GVHD symptom onset |
2.58 (.0489) | 6 (2 to 7) | 4.32 (<.0001) | 6 (2 to 7) |
| Earlier before onset |
1.02 (ns) | 10 (8 to 3) | 3.19 (<.0001) | 10 (8 to 14) |
GVHD indicates graft-versus-host disease.
Ratio of soluble Tim-3 in pooled case samples compared with control samples determined by mass spectometry. P value represents cumulative differences in labeled peptide intensities between cases and controls based on t-test.
Median day (range) of sample collection before onset of grades 2 to 4 acute GVHD.
Validation of Plasma Tim-3 Association with Acute GVHD by Immunoassay
Tim-3 levels were measured by immunoassay in 185 individual plasma samples selected for validation testing. These included samples from 78 cohort 1 cases with clinically significant GVHD, 59 samples from 37 GVHD− case-controls, and 48 samples from normal healthy controls. Significantly higher Tim-3 levels were found in plasma of patients with acute gastrointestinal GVHD characterized by more severe mid-gut involvement (median 11,550 pg/mL) compared with (1) patients with the less severe GVHD characterized mostly by upper-gut involvement (median, 4670 pg/mL; P = .005); and (2) time-matched case-controls (median, 2710 pg/mL; P = .002) (Figure 1). Plasma levels of Tim-3 were also higher in the samples of less severe upper-gut GVHD compared with their time-matched case-controls (medians, 4670 versus 3460 pg/mL; P = .03). Tim-3 plasma levels were also significantly higher in GVHD-free case-control samples compared with normal healthy controls (medians, 3275 versus 2285 pg/mL; P < .0001).
Clinical variables previously associated with risk of acute GVHD were included in a multivariate analysis to determine any association of these variables with Tim-3 plasma levels. Donor type unrelated compared with related had an effect ratio (ER) of 1.35 (P = .04) and a positive CMV serology test pretransplantation in the patient had an ER of 1.32 (P = .04) (Table 3). The ER for mid-gut GVHD in the multivariate model was 1.98 (P = .008), whereas the ER for upper-gut GVHD was not statistically significant (ER = 1.04, P = .78). Patient age, donor CMV serostatus pretransplantation, HLA mismatch, sex, a female donor for a male recipient, and blood as a stem cell source had no statistically significant association with Tim-3 plasma levels in this model.
To further evaluate the effect of elevated Tim-3 levels on risk of gut GVHD, we performed a ROC analysis using day 21 ± 3 days posttransplantation as a landmark time and identified 64 patients with samples collected in that window (excluding samples obtained from patients after the onset of GVHD). Seven of the 64 patients subsequently developed mid-gut GVHD, 24 subsequently developed upper-gut GVHD, and 33 did not develop GVHD. An ROC analysis of Tim-3 plasma levels as a predictor of any GVHD (n = 31) versus control (n = 33) had an area under the ROC curve of .59, P = .17. An ROC analysis of Tim-3 as a predictor of mid-gut GVHD (n = 7) versus all others (n = 57) had an area under ROC curve of .79, P =.003. Because of the limited number of mid-gut cases, it was not possible to perform multivariate analysis accounting for other GVHD risk factors. Nonetheless, Tim-3 was the strongest univariate risk factor.
Expression of Tim-3 on Peripheral Blood Mononuclear Cells of Patients with Acute GVHD
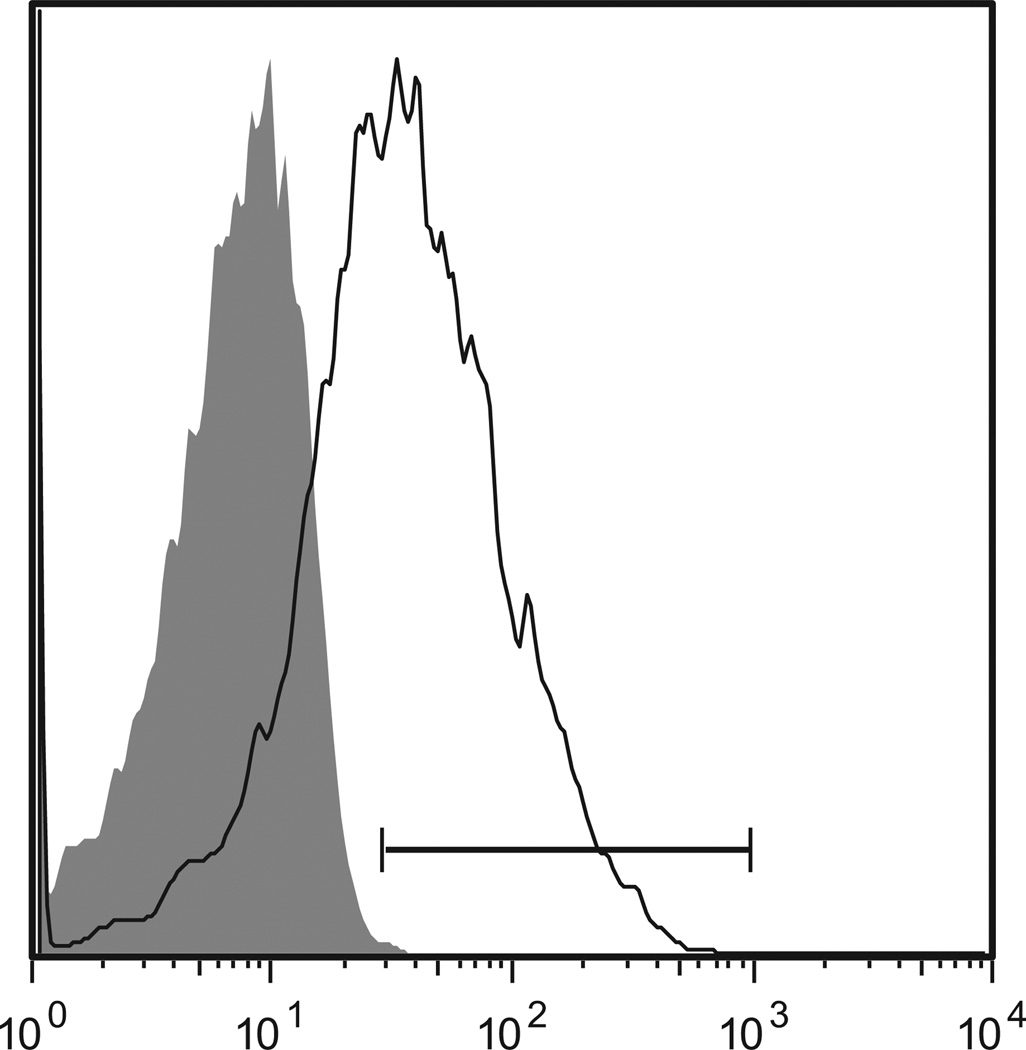
Further validation was undertaken with an independent cohort of 22 patients (cohort 2). Fourteen of 22 patients developed clinically significant grades 2 to 4 acute GVHD (grade 2, n = 10; grade 3, n = 3; grade 4, n = 1), including 3 with mid-gut, 9 with upper-gut, and 2 with skin-only acute GVHD (Table 1). The median GVHD onset was day 31 (range, 16 to 65) after transplantation. Blood samples from cases were collected at a median of 4 (range, 0 to 10) days before GVHD onset and at a median of 30 (range, 20 to 55) days after HCT. Blood samples from time-matched case-controls were collected at a median of 32 (range, 25 to 36) days after HCT. Expression of Tim-3 on PBMC from cases was determined by flow cytometry and compared with case-controls. A representative histogram illustrating Tim-3 expression on gated CD8+CD3+ PBMC from a patient 2 days before the onset of clinically significant acute mid-gut GVHD is shown in Figure 2.
Figure 2.
FACS histogram for expression of Tim-3 on gated CD8+ T cells from a patient with clinically significant acute GVHD (PT no. 0728). The shaded area represents the staining profile for an isotype-negative control antibody, and the horizontal line represents the gating used to define positive expression of Tim-3 (57.5%). The x-axis represents fluorescent intensity and the y-axis represent number of events.
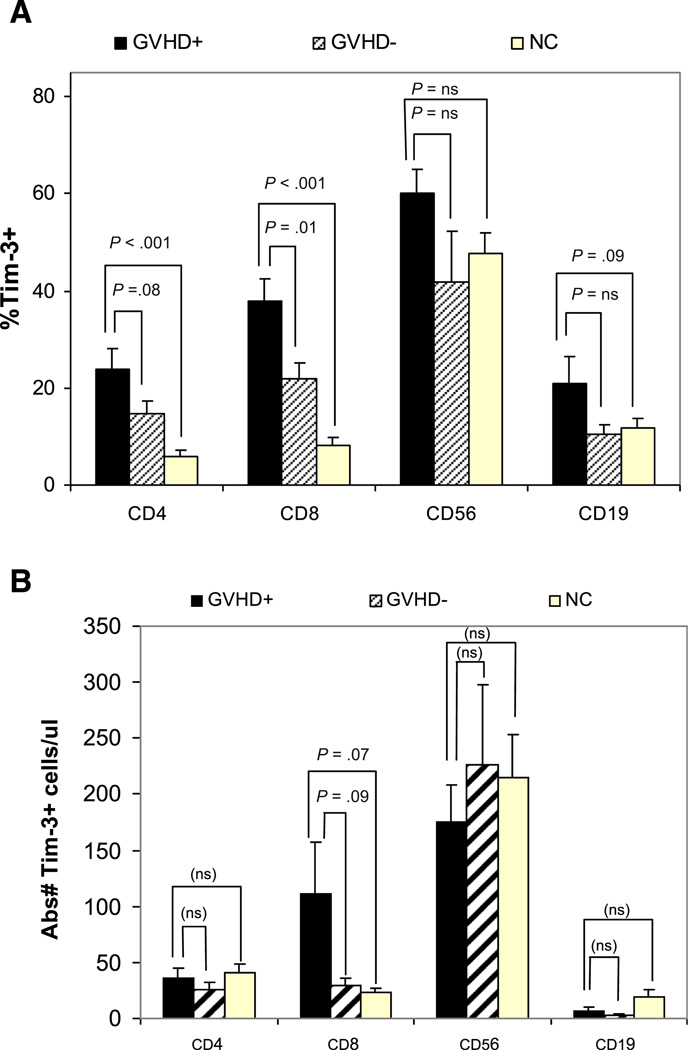
The percentage of Tim-3+/CD8+T cells in acute GVHD cases was significantly higher compared with case-controls (38 ± 5% versus 22 ± 3%, P = .01) (Figure 3A), and the percentage of Tim-3+/CD4+ T cells tended to be higher in acute GVHD cases compared with case-controls, but with only marginal (24 ± 4% versus 15 ± 3%, P = .08). We also found a nonsignificant trend for a higher percentage of Tim-3+/CD19+ B cells in acute GVHD cases compared with both case-controls (21 ± 6% versus 10 ± 2%, P = .11) and normal healthy controls (21 ± 6% versus 12 ± 2%, P =.09). The results showed a nonsignificant trend for higher absolute numbers of Tim-3+/CD8+ T cells in cases compared with case-controls, with no significant difference in the absolute number of Tim-3+/CD4+ T cells and Tim-3+/CD19+ B cells in cases compared with case-controls (Figure 3B). No statistically significant differences were observed in the percentage or absolute number of Tim-3+/CD56+ NK cells in cases, case-controls, and normal controls. The expression of Tim-3 on CD14+ monocytes was uniformly high in cases, case-controls, and normal controls ranging between 94 ± 2% and 98 ± .4% (data not shown).
Figure 3.
Tim-3 cell-surface expression measured as percentage (%) (Figure 3A) and absolute cell counts (Figure 3B) for Tim-3+ CD14+ monocytes, CD4+ and CD8+ T cells, CD56+ natural killer cells and CD19+ B cells. Bar graphs represent Tim-3 expression on cells from patients with clinically significant acute GVHD (GVHD+, shaded black), patients who remained GVHD-free for at least the first 100 days posttransplant (GVHD -, shaded stripe) and cells from healthy normal controls (NC, shaded yellow).
A univariate analysis of clinical variables that might affect Tim-3 expression in cells was performed for patient age, sex, sex mismatch, CMV pretransplantation serostatus, donor type, HLA match, and use of high-dose TBI (>1000 cGy) in the pretransplantation conditioning. Tim-3 expression on CD4+ T cells and CD19+ B cells were significantly higher in CMV-seropositive patients (P =.01 and P =.02, respectively), and Tim-3 expression on CD56+ NK cells was significantly higher in older patients (P = .002) (Table 4).
DISCUSSION
The principal finding in this study was the discovery that plasma levels of the soluble extracellular domain of Tim-3 were elevated in patients with clinically significant acute GVHD and that higher levels of Tim-3 were associated with the more severe mid-gut GVHD as compared with less severe upper-gut GVHD and the absence of GVHD. The presence of Tim-3 in these plasma samples was initially detected by mass spectometry that identified several tryptic peptides corresponding to the extracellular domain of Tim-3, a member of the T cell Ig and mucin-domain (Tim) family of type I glycoproteins. The mature Tim-3 molecule consists of a distal Ig variable region (IgV)-like extracellular domain, a proximal mucin-like extracellular domain, a transmembrane region, and an intracellular domain containing a tyrosine phosphorylation motif [41]. The full-length that underwent translatation protein consists of 288 amino acids with an extracellular domain of 171 amino acids.
After the discovery of soluble Tim-3 in pooled plasma samples by mass spectrometry, validation of the Tim-3 association with acute GVHD was accomplished in a 2-step process.
First, a microbead assay for TIM-3 was developed with a dynamic range of 5 to 10,000 pg/mL, specific for TIM-3, and with no detectable cross-reactivity with other analytes. TIM-3 levels were then measured in 303 individual plasma samples from all 127 cohort 1 patients. Significantly increased Tim-3 plasma levels were found in cohort 1 cases with grades 2 to 4 acute GVHD overall, with significantly higher plasma levels found in patients with the more severe mid-gut compared with upper-gut acute GVHD. In addition, an ROC analysis of Tim-3 as a predictor of mid-gut GVHD compared with all others had an area under ROC curve of .79 (P = .003). In a second independent validation, staining for Tim-3 expression on PBMC from an independent cohort of patients demonstrated a significantly higher percentage of Tim-3+/CD8+ T cells in cases with clinically significant grades 2 to 4 compared with case-controls with grades 0 to 1 acute GVHD. We also observed trends for higher percentages of Tim-3+/CD4+ T cells and Tim-3+/CD19+ B cells in patients with GVHD compared with those without clinically significant GVHD. Tim-3 expression on T cells from normal healthy controls was low, but Tim-3 expression on NK cells and monocytes was high, both in patients overall and in normal controls, as reported previously [42].
The mechanism by which Tim-3 is released from cells is not known. The presence of soluble Tim-3 identified in the plasma of these GVHD patients could represent an actively secreted slice variant, a cleaved product, or the passive release of a soluble fragment from apoptotic cells. Using PCR primers for the full Tim-3 gene and putative splice variant, Geng et al. found expression of the full Tim-3 transcript in spleen, thymus, and bone marrow of normal mice, but the splice variant was expressed only in splenocytes [43]. After in vitro activation of splenocytes, expression of both the full-length Tim-3 message and the splice variant were increased. Supernates of transfected CHO cells were analyzed by Western blots and a ~24 kDa protein was identified that bound anti-Tim-3 antibody. The size of the secreted protein was consistent with the predicted size of the glycosylated splice variant, demonstrating that the splice variant encodes a soluble form of Tim-3 [43].
Expression of cellular Tim-3 is highly variable. Expression on normal human blood T cells has been reported as low or undetectable, but Tim-3 is constitutively expressed on cells of the innate immune system including NK cells, monocytes, and DC [5,42,44]. Hastings et al. found Tim-3 expression on 1% to 2% of CD8+ blood T cells, no detectable expression on CD4+ blood T cells, but approximately 10% of CD4+ T cells in human lymph node were Tim-3+ [5]. Studies in mice have also found distinct differences in Tim-3 expression in adaptive and innate immune cells [8,45]. Low levels of Tim-3 expression, <5%, were found on CD4+ and CD8+ splenic T cells. Tim-3 expression on activated CD4+ T cells was found to be limited to the Th1 and Th17 subsets [5,46–49].
Previous studies have demonstrated that Tim-3 plays a significant role in regulation of the immune response, autoimmunity, and transplantation including murine GVHD. T cell receptor-mediated activation induces significant upregulation of Tim-3 expression in both CD4+ and CD8+ T cells [46]. Human Tim-3−CD4+ T cells isolated from human lymph node proliferate vigorously and secrete cytokines when stimulated ex vivo with plate-bound anti-CD3/CD28 antibodies, but Tim-3+CD4+ T cells do not. Increased production of IFNγ, by stimulated Tim-3+CD4+ T cells occurs, however, when Tim-3 blocking antibodies are added or when Tim-3 expression is knocked down with siRNA [50]. Other experiments have demonstrated that the interaction of Tim-3 with Gal-9 expressed on accessory cells suppresses Th1 responses and facilitates the development of peripheral tolerance, whereas T cell activation in Tim-3 KO (Tim-3−/−) mice and in mice given a Tim-3 fusion protein, leads to uncontrolled Th1 responses and resistance to peripheral tolerance [4,48,49]. The administration of anti-Tim-3 antibody in an EAE induction model exacerbates the intensity of disease. Defective expression of Tim-3 occurs in patients with autoimmune disease, suggesting that dysfunction in the Tim-3 immune regulatory pathway may result in persistent autoimmune reactivity [50,51]. Ndhlovu et al. showed that human NK cells transcribe the highest amounts of Tim-3 among all lymphocytes and in contrast to T cells Tim-3 expression by NK cells corresponds with functional maturation [52]. Exposure of NK cells to Tim-3 antibodies, however, suppresses NK cell-mediated cytotoxicity, indicating that NK cells like T cells are subject to negative regulation in the presence of Tim-3 ligand.
Studies by Oikawa et al. in a murine model of acute GVHD found marked up-regulation of Tim-3 expression on splenic and hepatic CD4+ and CD8+ T cells, dendritic cells, and macrophages [8]. The most prominent increase was noted among hepatic CD8+ T cells. Administration of anti-Tim-3 antibody increased the severity of acute GVHD, suggesting that blocking the interaction between Tim-3 and its ligand interfered with Tim-3–mediated modulation of T cell effector activity, resulting in a stronger and more pathogenic GVHD reaction. These same investigators also used a Tim-3/IgH fusion protein to demonstrate up-regulation of Tim-3 ligand in splenic T cells, DCs, and macrophages, but not hepatic lymphocytes during GVHD, suggesting that dysregulation of the Tim-3 pathway in this model contributes to severity of hepatic acute GVHD [8]. Veenstra et al. have recently confirmed and extended the findings of Oikawa et al. in experiments using Tim-3−/− donor T cells or a Tim-3-Ig fusion protein in an H2-incompatible GVHD model [11]. The blocking or absence of Tim-3 increased lethality; however, GVHD lethality was significantly reduced in Gal-9 Tg recipients expressing the Tim-3 ligand Gal-9 in B220+, CD11 b+, and CD11c+ splenic APC compared with wild-type recipients. These Tim-3 fusion protein experiments suggest that circulating Tim-3 can inhibit Tim-3 regulatory activity and thereby allow unrestrained expansion of Th1 effector cells, an increase in inflammation, and exacerbation of GVHD [43,48,49]. Reduction of Tim-3 plasma concentrations, on the other hand, might provide a therapeutic target for limiting GVHD activity and promoting the development of tolerance.
In summary, concentrations of the soluble extracellular domain of Tim-3 are increased in the blood of patients before clinical onset of acute GVHD, and levels correlate with the severity of gut GVHD. We speculate that plasma Tim-3 may exacerbate the severity of acute GVHD by blocking the interaction between Tim-3 and its ligand, thereby abrogating the regulatory activity of the Tim-3 pathway. Mechanistic studies are needed to better understand the biology of Tim-3 in the alloimmune reaction and to determine whether the increased plasma concentrations of Tim-3 associated with acute GVHD represent dysregulation of a pathway that, under optimal conditions, can mitigate the inflammation and tissue injury associated with acute GVHD. Better understanding of cellular Tim-3—mediated immune regulation and the possible proinflammatory effect of soluble Tim-3 may provide the insight and rationale for novel therapies targeted to enhance the ability of Tim-3 to down-regulate the effector component of the alloimmune response and facilitate induction of tolerance.
ACKNOWLEDGMENTS
Financial disclosure: This work was supported by grants from the National Institutes of Health (NIH) AI33484, CA18029, CA15704 and HL094260.
Footnotes
Conflict of interest statement: There are no conflicts of interest to report.
Authorship statement J.A.H., G.B.M., and S.M.H. designed, organized, and supervised the research, and wrote the paper. C.B., A.C., B.M.G., M.J., R.L.L., L.T., C.H.W., and Q.Z. performed experiments and analyzed data. B.E.S. analyzed data, provided advice, and edited the paper. P.J.M. provided clinical data and edited the paper.
REFERENCES
- 1.Kuchroo VK, Dardalhon V, Xiao S, Anderson AC. New roles for TIM family members in immune regulation. Nat Rev Immunol. 2008;8:577–580. doi: 10.1038/nri2366. [DOI] [PubMed] [Google Scholar]
- 2.Sehrawat S, Reddy PB, Rajasagi N, et al. Galectin-9/TIM-3 interaction regulates virus-specific primary and memory CD8 T cell response. PLoS Pathog. 2010;6:e1000882. doi: 10.1371/journal.ppat.1000882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sehrawat S, Suryawanshi A, Hirashima M, Rouse BT. Role of Tim-3/galectin-9 inhibitory interaction in viral-induced immunopathology: Shifting the balance toward regulators. J Immunol. 2009;182:3191–3201. doi: 10.4049/jimmunol.0803673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu C, Anderson AC, Schubart A, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6:1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 5.Hastings WD, Anderson DE, Kassam N, et al. TIM-3 is expressed on activated human CD4+ T cells and regulates Th1 and Th17 cytokines. Eur J Immunol. 2009;39:2492–2501. doi: 10.1002/eji.200939274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiba S, Baghdadi M, Akiba H, et al. Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nat Immunol. 2012;13:832–842. doi: 10.1038/ni.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katayama H, Paczesny S, Prentice R, et al. Application of serum proteomics to the Women’s Health Initiative conjugated equine estrogens trial reveals a multitude of effects relevant to clinical findings. Genome Med. 2009;1:47. doi: 10.1186/gm47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oikawa T, Kamimura Y, Akiba H, et al. Preferential involvement of Tim-3 in the regulation of hepatic CD8+ T cells in murine acute graft-versus-host disease. J Immunol. 2006;177:4281–4287. doi: 10.4049/jimmunol.177.7.4281. [DOI] [PubMed] [Google Scholar]
- 9.Hafler DA, Kuchroo V. TIMs: Central regulators of immune responses. J Exp Med. 2008;205:2699–2701. doi: 10.1084/jem.20082429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ngiow SF, Teng MW, Smyth MJ. Prospects for TIM3-targeted antitumor immunotherapy. Cancer Res. 2011;71:6567–6571. doi: 10.1158/0008-5472.CAN-11-1487. [DOI] [PubMed] [Google Scholar]
- 11.Veenstra RG, Taylor PA, Panoskaltsis-Mortari A, et al. Contrasting acute graft-versus-host-disease effects of Tim-3/galectin-9 pathway blockade dependent upon the presence of donor regulatory T cells. Blood. 2012;120:682–690. doi: 10.1182/blood-2011-10-387977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kashio Y, Nakamura K, Abedin MJ, et al. Galectin-9 induces apoptosis through the calcium-calpain-caspase-1 pathway. J Immunol. 2003;170:3631–3636. doi: 10.4049/jimmunol.170.7.3631. [DOI] [PubMed] [Google Scholar]
- 13.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363:2091–2101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mielcarek M, Storer BE, Boeckh M, et al. Initial therapy of acute graft-versus-host disease with low-dose prednisone does not compromise patient outcomes. Blood. 2009;113:2888–2894. doi: 10.1182/blood-2008-07-168401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hockenbery DM, Cruickshank S, Rodell TC, et al. A randomized, placebo-controlled trial of oral beclomethasone dipropionate as a prednisone-sparing therapy for gastrointestinal graft-versus-host disease. Blood. 2007;109:4557–4563. doi: 10.1182/blood-2006-05-021139. [DOI] [PubMed] [Google Scholar]
- 16.Hsu KC, Gooley T, Malkki M, et al. KIR ligands and prediction of relapse after unrelated donor hematopoietic cell transplantation for hematologic malignancy. Biol Blood Marrow Transplant. 2006;12:828–836. doi: 10.1016/j.bbmt.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Kreisel W, Dahlberg M, Bertz H, et al. Endoscopic diagnosis of acute intestinal GVHD following allogeneic hematopoietic SCT: A retrospective analysis in 175 patients. Bone Marrow Transplant. 2012;47:430–438. doi: 10.1038/bmt.2011.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cahn JY, Klein JP, Lee SJ, et al. Prospective evaluation of 2 acute graft-versus-host (GVHD) grading systems: A joint Societe Francaise de Greffe de Moelle et Therapie Cellulaire (SFGM-TC), Dana Farber Cancer Institute (DFCI), and International Bone Marrow Transplant Registry (IBMTR) prospective study. Blood. 2005;106:1495–1500. doi: 10.1182/blood-2004-11-4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leisenring WM, Martin PJ, Petersdorf EW, et al. An acute graft-versus-host disease activity index to predict survival after hematopoietic cell transplantation with myeloablative conditioning regimens. Blood. 2006;108:749–755. doi: 10.1182/blood-2006-01-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foley R, Couban S, Walker I, et al. Monitoring soluble interleukin-2 receptor levels in related and unrelated donor allogenic bone marrow transplantation. Bone Marrow Transplant. 1998;21:769–773. doi: 10.1038/sj.bmt.1701163. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura H, Komatsu K, Ayaki M, et al. Serum levels of soluble IL-2 receptor, IL-12, IL-18, and IFN-gamma in patients with acute graft-versus-host disease after allogeneic bone marrow transplantation. J Allergy Clin Immunol. 2000;106:S45–S50. doi: 10.1067/mai.2000.106774. [DOI] [PubMed] [Google Scholar]
- 22.Shaiegan M, Iravani M, Babaee GR, Ghavamzadeh A. Effect of IL-18 and sIL2R on aGVHD occurrence after hematopoietic stem cell transplantation in some Iranian patients. Transpl Immunol. 2006;15:223–227. doi: 10.1016/j.trim.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Paczesny S, Krijanovski OI, Braun TM, et al. A biomarker panel for acute graft-versus-host disease. Blood. 2009;113:273–278. doi: 10.1182/blood-2008-07-167098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holler E, Kolb HJ, Möller A, et al. Increased serum levels of tumor necrosis factor a precede major complications of bone marrow transplantation. Blood. 1990;75:1011–1016. [PubMed] [Google Scholar]
- 25.Miyamoto T, Akashi K, Hayashi S, et al. Serum concentration of the soluble interleukin-2 receptor for monitoring acute graft-versus-host disease. Bone Marrow Transplant. 1996;17:185–190. [PubMed] [Google Scholar]
- 26.Or R, Kalinkovich A, Nagler A, et al. Soluble tumor necrosis factor (sTNF) receptors: A possible prognostic marker for bone marrow transplantation-related complications. Cytokines Mol Ther. 1996;2:243–250. [PubMed] [Google Scholar]
- 27.Grimm J, Zeller W, Zander AR. Soluble interleukin-2 receptor serum levels after allogeneic bone marrow transplantations as a marker for GVHD. Bone Marrow Transplant. 1998;21:29–32. doi: 10.1038/sj.bmt.1701041. [DOI] [PubMed] [Google Scholar]
- 28.Paczesny S, Braun TM, Levine JE, et al. Elafin is a biomarker of graft-versus-host disease of the skin. Sci Transl Med. 2010;2:13ra2. doi: 10.1126/scitranslmed.3000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrara JL, Harris AC, Greenson JK, et al. Regenerating islet-derived 3-alpha is a biomarker of gastrointestinal graft-versus-host disease. Blood. 2011;118:6702–6708. doi: 10.1182/blood-2011-08-375006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vander Lugt MT, Braun T, Ferrara JLM, et al. Plasma concentration of suppressor of tumorigenicity 2 (ST2), the IL33 receptor, at initiation of graft versus host disease therapy predicts day 28 response and day 180 survival post-treatment. Biol Blood Marrow Transplant. 2012;18:S201–S202. [abstr.]. [Google Scholar]
- 31.Ayabe T, Satchell DP, Wilson CL, et al. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol. 2000;1:113–118. doi: 10.1038/77783. [DOI] [PubMed] [Google Scholar]
- 32.Yilmaz OH, Katajisto P, Lamming DW, et al. mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature. 2012;486:490–495. doi: 10.1038/nature11163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luft T, Dietrich S, Falk C, et al. Steroid-refractory GVHD: T-cell attack within a vulnerable endothelial system. Blood. 2011;118:1685–1692. doi: 10.1182/blood-2011-02-334821. [DOI] [PubMed] [Google Scholar]
- 34.Harris AC, Ferrara JL, Braun TM, et al. Plasma biomarkers of lower gastrointestinal and liver acute GVHD. Blood. 2012;119:2960–2963. doi: 10.1182/blood-2011-10-387357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruutu T, Eriksson B, Remes K, et al. Ursodeoxycholic acid for the prevention of hepatic complications in allogeneic stem cell transplantation. Blood. 2002;100:1977–1983. doi: 10.1182/blood-2001-12-0159. [DOI] [PubMed] [Google Scholar]
- 36.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 37.Ponec RJ, Hackman RC, McDonald GB. Endoscopic and histologic diagnosis of intestinal graft-versus-host disease after marrow transplantation. Gastrointest Endosc. 1999;49:612–621. doi: 10.1016/s0016-5107(99)70390-1. [DOI] [PubMed] [Google Scholar]
- 38.Faca V, Coram M, Phanstiel D, et al. Quantitative analysis of acrylamide labeled serum proteins by LC-MS/MS. J Proteome Res. 2006;5:2009–2018. doi: 10.1021/pr060102+. [DOI] [PubMed] [Google Scholar]
- 39.Faca V, Pitteri SJ, Newcomb L, et al. Contribution of protein fractionation to depth of analysis of the serum and plasma proteomes. J Proteome Res. 2007;6:3558–3565. doi: 10.1021/pr070233q. [DOI] [PubMed] [Google Scholar]
- 40.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3 doi: 10.2202/1544-6115.1027. Article3. [DOI] [PubMed] [Google Scholar]
- 41.Meyers JH, Sabatos CA, Chakravarti S, Kuchroo VK. The TIM gene family regulates autoimmune and allergic diseases. Trends Mol Med. 2005;11:362–369. doi: 10.1016/j.molmed.2005.06.008. (Review). [DOI] [PubMed] [Google Scholar]
- 42.Anderson AC, Anderson DE, Bregoli L, et al. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science. 2007;318:1141–1143. doi: 10.1126/science.1148536. [DOI] [PubMed] [Google Scholar]
- 43.Geng H, Zhang GM, Li D, et al. Soluble form of T cell Ig mucin 3 is an inhibitory molecule in T cell-mediated immune response. J Immunol. 2006;176:1411–1420. doi: 10.4049/jimmunol.176.3.1411. [DOI] [PubMed] [Google Scholar]
- 44.Khademi M, Illes Z, Gielen AW, et al. T Cell Ig- and mucin-domain-containing molecule-3 (TIM-3) and TIM-1 molecules are differentially expressed on human Th1 and Th2 cells and in cerebrospinal fluid-derived mononuclear cells in multiple sclerosis. J Immunol. 2004;172:7169–7176. doi: 10.4049/jimmunol.172.11.7169. [DOI] [PubMed] [Google Scholar]
- 45.Frisancho-Kiss S, Nyland JF, Davis SE, et al. Cutting edge: T cell Ig mucin-3 reduces inflammatory heart disease by increasing CTLA-4 during innate immunity. J Immunol. 2006;176:6411–6415. doi: 10.4049/jimmunol.176.11.6411. [DOI] [PubMed] [Google Scholar]
- 46.Monney L, Sabatos CA, Gaglia JL, et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415:536–541. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- 47.Nakae S, Iikura M, Suto H, et al. TIM-1 and TIM-3 enhancement of Th2 cytokine production by mast cells. Blood. 2007;110:2565–2568. doi: 10.1182/blood-2006-11-058800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanchez-Fueyo A, Tian J, Picarella D, et al. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat Immunol. 2003;4:1093–1101. doi: 10.1038/ni987. [DOI] [PubMed] [Google Scholar]
- 49.Sabatos CA, Chakravarti S, Cha E, et al. Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nat Immunol. 2003;4:1102–1110. doi: 10.1038/ni988. [DOI] [PubMed] [Google Scholar]
- 50.Koguchi K, Anderson DE, Yang L, et al. Dysregulated T cell expression of TIM3 in multiple sclerosis. J Exp Med. 2006;203:1413–1418. doi: 10.1084/jem.20060210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang L, Anderson DE, Kuchroo J, Hafler DA. Lack of TIM--3 immunoregulation in multiple sclerosis. J Immunol. 2008;180:4409–4414. doi: 10.4049/jimmunol.180.7.4409. [DOI] [PubMed] [Google Scholar]
- 52.Ndhlovu LC, Lopez-Verges S, Barbour JD, et al. Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood. 2012;119:3734–3743. doi: 10.1182/blood-2011-11-392951. [DOI] [PMC free article] [PubMed] [Google Scholar]