Abstract
Chloroplasts were isolated from wild type (DG) and heterozygous mutant (LG) soybean (Glycine max) leaves, and various biochemical functions were compared. Noncyclic electron transport, and its coupled phosphorylation, cyclic phosphorylation and H+ ion transport in both systems, were 3 to 5 times faster in rate (on a chlorophyll basis) in the mutant plastids. On a chloroplast lamellar protein basis, the mutant plastid rates were 1.5 to 2.5 times the wild type rates.
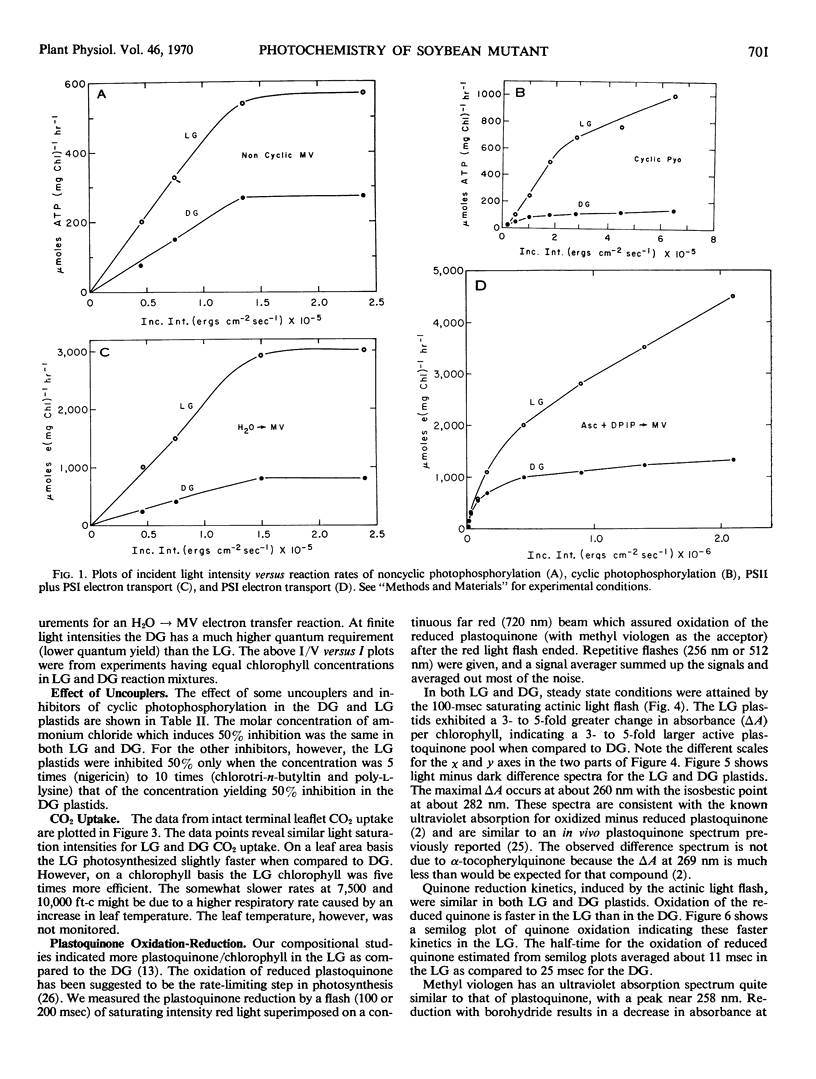
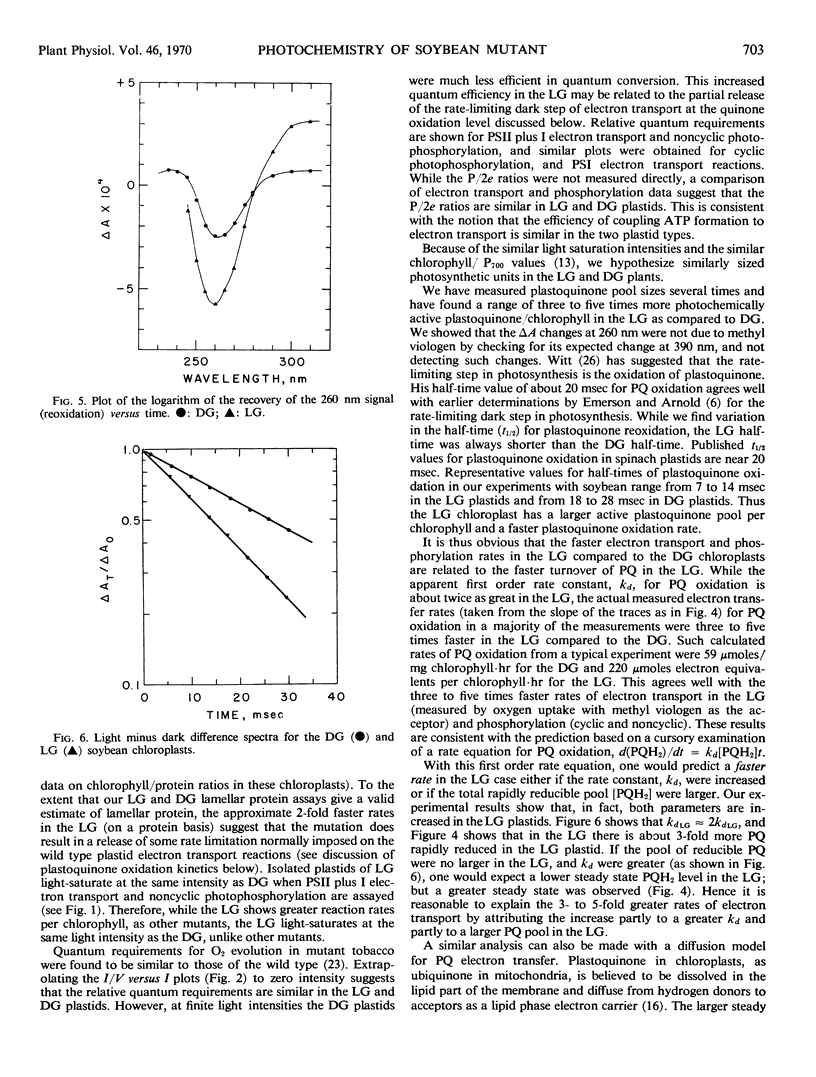
Plastoquinone (PQ) reduction and oxidation (rates and extent) were measured by following absorbance changes at 260 nanometers with the repetitive flash technique. Mutant plastids have about a 2-fold greater apparent first order rate constant for PQ oxidation and a 3- to 5-fold larger pool of rapidly reducible PQ. Plastoquinone oxidation has been identified by other workers as the rate-limiting step in electron transport. Assuming the PQ oxidation is a first order process (d(PQH2)/dt = kD[PQH2]t), the observed increase in kd for the LG (kdLG ≈ 2kdDG) and the greater steady state amount of rapidly turning over PQ, [PQH2]LG>[PQH2]DG, could account for the 3- to 5-fold greater rates of electron transport and phosphorylation found in the mutant chloroplasts.
Light saturation for noncyclic photophosphorylation and photosystem 2 plus 1 electron transport occurred at similar intensities for both LG and DG plastids. Relative quantum requirements extrapolated to zero intensity were similar in the LG and DG, although at finite light intensities the LG had a better relative quantum efficiency.
Ammonium chloride concentrations needed to inhibit cyclic photophosphorylation 50% were similar in both LG and DG plastids. Nigericin, poly-l-lysine, and chlorotri-n-butyltin, were needed in concentrations 5 to 10 times greater in the LG to yield 50% inhibition at comparable chlorophyll concentrations.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boardman N. K., Highkin H. R. Studies on a barley mutant lacking chlorophyll b. I. Photochemical activity of isolated chloroplasts. Biochim Biophys Acta. 1966 Oct 10;126(2):189–199. doi: 10.1016/0926-6585(66)90054-9. [DOI] [PubMed] [Google Scholar]
- CRANE F. L., DILLEY R. A. DETERMINATION OF COENZYME Q (UBIQUINONE). Methods Biochem Anal. 1963;11:279–306. doi: 10.1002/9780470110294.ch6. [DOI] [PubMed] [Google Scholar]
- Dilley R. A. Effect of poly-L-lysine on energy-linked chloroplast reactions. Biochemistry. 1968 Jan;7(1):338–346. doi: 10.1021/bi00841a043. [DOI] [PubMed] [Google Scholar]
- Dilley R. A., Vernon L. P. Quantum requirement of the light-induced proton uptake by spinach chloroplasts. Proc Natl Acad Sci U S A. 1967 Feb;57(2):395–400. doi: 10.1073/pnas.57.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fork D. C., Heber U. W. Studies on electron-transport reactions of photosynthesis in plastome mutants of oenothera. Plant Physiol. 1968 Apr;43(4):606–612. doi: 10.1104/pp.43.4.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highkin H. R., Boardman N. K., Goodchild D. J. Photosynthetic Studies on a Pea-mutant Deficient in Chlorophyll. Plant Physiol. 1969 Sep;44(9):1310–1320. doi: 10.1104/pp.44.9.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn J. S. Chlorotri-n-butylin. An inhibitor of photophosphorylation in isolated chloroplasts. Biochim Biophys Acta. 1968 Jan 15;153(1):203–210. doi: 10.1016/0005-2728(68)90161-8. [DOI] [PubMed] [Google Scholar]
- Keck R. W., Dilley R. A. Chloroplast composition and structure differences in a soybean mutant. Plant Physiol. 1970 Nov;46(5):692–698. doi: 10.1104/pp.46.5.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok B., Rurainski H. J., Owens O. V. The reducing power generated in photoact I of photosynthesis. Biochim Biophys Acta. 1965 Nov 29;109(2):347–356. doi: 10.1016/0926-6585(65)90162-7. [DOI] [PubMed] [Google Scholar]
- LEVINE R. P., SMILLIE R. M. THE PHOTOSYNTHETIC ELECTRON TRANSPORT CHAIN OF CHLAMYDOMONAS REINHARDI. I. TRIPHOSPHOPYRIDINE NUCLEOTIDE PHOTOREDUCTION IN WILD-TYPE AND MUTANT STRAINS. J Biol Chem. 1963 Dec;238:4052–4057. [PubMed] [Google Scholar]
- Lavorel J. Fluorescence Properties of Wild-Type Chlamydomonas reinhardi and Three Mutant Strains Having Impaired Photosynthesis. Plant Physiol. 1968 Jul;43(7):1049–1055. doi: 10.1104/pp.43.7.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISHIMURA M., ITO T., CHANCE B. Studies on bacterial photophosphorylation. III. A sensitive and rapid method of determination of photophosphorylation. Biochim Biophys Acta. 1962 May 7;59:177–182. [PubMed] [Google Scholar]
- Rieske J. S., Lumry R., Spikes J. D. The Mechanism of the Photochemical Activity of Isolated Chloroplasts. III. Dependence of Velocity on Light Intensity. Plant Physiol. 1959 May;34(3):293–300. doi: 10.1104/pp.34.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell G. K., Lyman H., Heath R. L. Absence of fluorescence quenching in a photosynthetic mutant of Euglena gracilis. Plant Physiol. 1969 Jun;44(6):929–931. doi: 10.1104/pp.44.6.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMILLIE R. M., LEVINE R. P. THE PHOTOSYNTHETIC ELECTRON TRANSPORT CHAIN OF CHLAMYDOMONAS REINHARDI. II. COMPONENTS OF THE TRIPHOSPHOPYRIDINE NUCLEOTIDE-REDUCTIVE PATHWAY IN WILD-TYPE AND MUTANT STRAINS. J Biol Chem. 1963 Dec;238:4058–4062. [PubMed] [Google Scholar]
- Schmid G., Gaffron H. Chloroplast structure and the photosynthetic unit. Brookhaven Symp Biol. 1966;19:380–392. [PubMed] [Google Scholar]
- Shavit N., Dilley R. A., San Pietro A. Ion translocation in isolated chloroplasts. Uncoupling of photophosphorylation and translocation of K+ and H+ ions induced by Nigericin. Biochemistry. 1968 Jun;7(6):2356–2363. doi: 10.1021/bi00846a043. [DOI] [PubMed] [Google Scholar]



