Abstract
Vascular hyperpermeability, the excessive leakage of fluid and proteins from blood vessels to the interstitial space, commonly occurs in traumatic and ischemic injuries. This hyperpermeability causes tissue vasogenic edema, which often leads to multiple organ failure resulting in patient death. Vascular hyperpermeability occurs most readily in small blood vessels as their more delicate physical constitution makes them an easy target for barrier dysfunction. A single layer of endothelial cells, linked to one another by cell adhesion molecules, covers the interior surface of each blood vessel. The cell adhesion molecules play a key role in maintaining barrier functions like the regulation of permeability. Aging is a major risk factor for microvascular dysfunction and hyperpermeability. Apart from age-related remodeling of the vascular wall, endothelial barrier integrity and function declines with the advancement of age. Studies that address the physiological and molecular basis of vascular permeability regulation in aging are currently very limited. There have been many cellular and molecular mechanisms proposed to explain aging-related endothelial dysfunction but their true relationship to barrier dysfunction and hyperpermeability is not clearly known. Among the several mechanisms that promote vascular dysfunction and hyperpermeability, the following are considered major contributors: oxidative stress, inflammation, and the activation of apoptotic signaling pathways. In this review we highlighted (a) the physiological, cellular and molecular changes that occur in the vascular system as a product of aging; (b) the potential mechanisms by which aging leads to barrier dysfunction and vascular hyperpermeability in the peripheral and the blood-brain barrier; (c) the mechanisms by which the age-related increases in oxidative stress, inflammatory markers and apoptotic signaling etc. cause endothelial dysfunction and their relationship to hyperpermeability; and (d) the relationship between aging, vascular permeability and traumatic injuries.
Keywords: aging, vascular hyperpermeability, vascular endothelium, permeability regulation
In addition to transporting proteins, nutrients, cells and waste products throughout the body, the vasculature of humans and other vertebrates also allows the exchange of small compounds between the blood and tissues. This vascular permeability is essential for normal and healthy tissue function. However, vascular hyperpermeability—the excessive leakage of fluid and proteins from blood vessels to the interstitial space—is pathologic. It leads to tissue vasogenic edema, which is commonly seen in traumatic and ischemic injuries. Although vascular hyperpermeability is associated with these types of injuries in all age groups, its effect in the aging population is severe and warrants more attention and review. Studies that are focused on the physiological and molecular basis of vascular permeability regulation in aging are currently very limited. The main focus of this review article is to understand and correlate some significant findings in the field of endothelial dysfunction and hyperpermeability with human aging. Due to limitation of the available data on vascular permeability in aging, the potential relationship between vascular dysfunction and changes in permeability and its significance in aging has been discussed wherever possible.
Aging and vascular endothelium
In humans, the vascular endothelium is comprised of a single layer of endothelial cells located on the interior surface of blood vessels [1,2]. It forms a barrier between circulating blood and the vessel wall. This cellular layer has been identified as a dynamic structure, important in several key functions related to health and disease. In the peripheral microvasculature, neighboring endothelial cells in the layer maintain cell-to-cell contact predominantly via adherens junctions, formed by localized adherens junction proteins. The disruption of these proteins and their junctions leads to paracellular permeability [3–6]. One such adherens junction protein is the transmembrane adhesion protein VE-cadherin. It connects adjacent endothelial cells through calcium-dependent homophilic binding of its extracellular domain, while its intracellular domain interacts with the actin cytoskeleton via a family of catenins including α-catenin, β-catenin, γ-catenin, and p120. Dissociation of this complex has reportedly resulted in endothelial barrier dysfunction leading to vascular hyperpermeability [5–9]. Following cellular senescence, dysfunction of cell-cell junctions and an increase in microvascular permeability has been observed in human endothelial cells [10].
Microvascular hyperpermeability is tightly regulated in the brain by the blood-brain barrier (BBB). Age-related increase in BBB permeability has been reported in aged mice [11] and humans [12]. Although the scientific community’s understanding of the BBB and its dynamic functionality is constantly growing, for our purposes we discuss it primarily as a diffusion barrier. This extremely selective barrier is formed in part by the tight junctions (TJs) between the endothelial cells in its endothelial lining. These tight junctions, maintained by tight junction proteins (TJPs), help prevent most blood-borne substances from entering the brain parenchyma. Similar to epithelial TJs, endothelial TJs are composed of integral membrane proteins (occludin, claudins, junctional adhesion molecules (JAMs)), etc involved in intracellular interactions with cytoplasmic scaffolding proteins such as zonula occludens (ZO). These scaffolding proteins allow the indirect interaction of membrane proteins with the actin cytoskeleton [13]. ZO-1 is considered the predominant linker protein in the BBB that is involved in permeability regulation.
The key location of the endothelium at the interface between the blood and vessel wall endows it with an essential role in providing an anti-thrombotic vascular surface that promotes laminar blood flow, and maintains selective permeability to fluids, proteins, nutrients and hematopoietic cells. It is important to note that all of these activities occur mostly in the microcirculation, where the ratio of endothelial cell surface area to blood volume is at its highest, compared to the rest of the circulatory system. In addition to serving as a selective barrier, the vascular endothelium also participates in many physiological functions in the body. Vascular endothelial cells control vascular homeostasis, regulate blood pressure by vasoconstriction and vasodilation mechanisms, and promote angiogenesis. In the circulatory system, most changes in vascular permeability and instances of leukocyte transmigration occur in the postcapillary venules. These are the microvasculature located immediately following the capillaries.
During the process of aging, several structural and functional changes occur throughout the entire vascular system. This includes the heart, coronary arteries, peripheral arteries, and the microvasculature—including that of the BBB. A variety of physiological and molecular factors contribute to altered endothelial barrier integrity and hyperpermeability common in aging [14,15]. Importantly, vascular endothelial cells express several important molecules such as vascular endothelial growth factor (VEGF) and its receptors vascular endothelial growth factor receptor-1, 2 and 3 (VEGFR1, 2, 3) [16,17]. The VEGF/VEGFR2 signaling is critical for vasculogenesis as well as angiogenesis [17]. VEGF is a potent inducer of vascular permeability in the peripheral system and in the blood-brain barrier. Endothelial cells also express endothelial nitric oxide synthase (eNOS), (eNOS), which produces nitric oxide (NO). NO has many important physiological functions. For example, NO promotes vasodilation while it also inhibits leukocyte adhesion [18,19]. Under basal conditions eNOS is found inactive. However its activity is increased by many factors including bradykinin, thrombin and histamine. The release of these mediators leads to an increased production of NO, as well as induces vascular permeability.
It has been shown that endothelium-mediated vasodilatory function progressively declines with age [20]. This is associated with diminished eNOS expression and NO production in aging endothelial cells. A correlation between altered vasodilation and hyperpermeability has been made. Recently, Yoon et al. [21] demonstrated the decreased expression of eNOS in aged human umbilical vein endothelial cells, associated with dysfunction of cell-cell junctions and microvascular hyperpermeability [10]. It has been shown that eNOS induces vascular hyperpermeability in a VEGF-dependent fashion [22]. However, the precise age-associated mechanisms that cause a decrease in these molecules and their relationship to vascular hyperpermeability remain unknown. Interestingly, it has been observed that aging endothelial cells produce increased amounts of O2-anions [23], which scavenge NO and form peroxinitrite. Peroxintrite is a potent free radical that further decreases the activity of eNOS by inactivating it [21]. Peroxinitrite has also been shown to induce microvascular hyperpermeability by disrupting the adherens junction proteins [24]. These described mechanisms in part explain oxidative stress-mediated decrease of eNOS and NO in aging endothelial cells (refer to the section on oxidative stress in this review article for related information). On the other hand, it has been suggested that the age-associated changes occurring in eNOS regulatory proteins such as caveolin-1, pAkt, and heat shock protein 90 (Hsp90) contribute to the decreased activity of eNOS in aged endothelial cells [21]. In addition to these regulatory mechanisms, several other factors also regulate eNOS activity. For example, shear stress [25], estrogens [26], and growth factors [27] could also positively regulate eNOS expression. Aging is commonly associated with progressive deterioration in central nervous system function and blood-brain barrier (BBB) integrity [28]. Cerebrovascular aging can be viewed from several perspectives including: alterations in vascular density, vascular plasticity, and vascular reactivity (the adjustment of vessels to acute metabolic changes that takes place in tissues). Conditions which are commonly associated with aging, such as hypertension and cerebrovascular ischemia, aggravate these age-related alterations in the BBB integrity (refer to the section on BBB).
Vascular dysfunction, permeability and aging
Peripheral Vascular Permeability
As previously mentioned disruption of adherens junctions and their associated proteins leads to increased paracellular permeability in the peripheral circulatory system. Such barrier dysfunction is a serious problem in hemorrhagic shock, burn, sepsis and cardiovascular disease. This pathological increase in permeability of this barrier permits extravasation of serum components into the surrounding tissue, leading to edema formation and multiple organ failure. While microvascular dysfunction occurs predominantly at endothelial cell-cell junctions, other intracellular components are also involved, such as linker proteins and the actin cytoskeleton [6,9]. Recent studies show that caspase-3 mediated breakdown of the adherens junctions is a major mechanism that leads to peripheral microvascular hyperpermeability [5,6,29]. Microvascular hyperpermeability is increased by actomyosin contractile activity in response to phosphorylation of the myosin light chain (MLC) via the activity of myosin light chain kinase (MLCK) [7,30]. MLCK is a serine/threonine-specific protein kinase that phosphorylates the regulatory light chain of myosin II. MLCK-dependent endothelial hyperpermeability occurs in response to inflammatory mediators such as activated neutrophils, thrombin, histamine and tumor necrosis factor alpha (TNF-α) [30,31]. It also involves multiple cell signaling pathways and signaling molecules that include Ca (++), protein kinase C, Src kinase and NO synthase [7,32]. Because pathological MLCK activity is so significantly involved in barrier dysfunction, MLCK-dependent signaling mechanisms provide multiple potential therapeutic targets for preventing vascular hyperpermeability, edema, and catastrophic organ failure. Another important molecule that protects against vascular endothelial hyperpermeability is Sphingosine-1-phosphate, a bioactive sphingolipid [33].
Blood-brain Barrier Permeability
Blood-brain barrier permeability increases with aging. Also, an age-related decrease in cerebral blood flow has been reported using arterial spin labeling. This method involves the measurement of mean transit time and capillary transmit time of magnetically labeled blood water was measured by MRI [34]. The perfusion and BBB permeability in aged rats was significantly altered compared with young rats, particularly in the hippocampus. One proposed consequence of these age-related perfusion and BBB permeability changes is a decreased ability in aged rats to sustain long-term potentiation [12]. Chronic hypoperfusion has been associated with a decrease in cognitive function and diminished neuronal plasticity [11]. Significant changes in age-related BBB permeability were observed in the hippocampus but not the cortex and correlated with the observation that the BBB in the hippocampus may be particularly vulnerable in senescence-accelerated mice, SAMP8 [11].
The normal barrier properties of the BBB plays a key role in regulating the entry of solutes and ions into the central nervous system (CNS) whereas the migration of cells appears to be significantly controlled by expression of chemokines and adhesion molecules. However, hyperpermeability in the BBB aids in the infiltration of circulating cells. Thus T cell infiltration has been found in CNS tissue of patients with Parkinson’s disease [35] and Alzheimer’s disease [36,37]. The infiltration of cells has also been reported following ischemic insult and is exacerbated by age. Popescu et al [38] demonstrated that an age-related decrease in tissue perfusion, together with the increase in BBB permeability, alter the microenvironment in the brain. These, in addition to the compromised homeostatic capability of microglia in response to aging, may be significant contributors to the neuroinflammatory changes described in the aged brain. Simpson et al [39] demonstrated that alterations in the BBB integrity occurs in cerebral white matter lesions in the ageing brain and are generally associated with dementia, a condition common in aging brain. Albumin extravasation studies of BBB permeability showed remarkable increase in permeability in the aging brain. This was enhanced in white matter lesions suggesting dysfunction of the BBB may contribute to the pathogenesis of white matter [39].
Age related alterations to the BBB of the cerebral cortex in relationship to Alzheimer-type pathology has been studied in humans. Viggars et al [40] investigated the BBB in the temporal cortex of humans. This longitudinal study was conducted in brain donations from a population-representative sample of participants. BBB leakage showed population-wide variation that increased with the progression of Alzheimer-type pathologies, though with considerable overlap between different levels of Alzheimer-type pathology. This was accompanied by increasing mean vascular density, but not by down-regulation of tight junction proteins. Mechanisms leading to BBB leakage in the aging brain remain to be defined, but the population-wide and BBB changes and its strong early correlation to Alzheimer-type pathology progression suggest that BBB dysfunction contributes to brain aging [40]. Lee et al [41] quantified the effects of age on BBB permeability following traumatic brain injury. They found evidence for a post-injury increase in BBB permeability in the aged brain, accompanied by a decrease in BBB repair responses [41].
Regulators of vascular dysfunction and permeability
Cell Adhesion Molecules
Vascular permeability is strictly regulated by interendothelial cell adhesion molecules. As discussed above, the endothelial cell adherens junction proteins, VE-cadherin and platelet endothelial cell adhesion molecule-1 (PECAM-1) play important roles in regulating peripheral microvascular permeability [5–7,42]. Cadherins are transmembrane proteins which form homophilic interactions in a calcium dependent fashion. They provide weak adhesive cell-cell forces, further stabilized by the catenins, which are intracellular proteins linking the cadherin cell surface molecule to the actin cytoskeleton. Inhibition of VE-cadherin proteasomal degradation enhanced barrier integrity and protected against microvascular hyperpermeability in lung microvascular endothelial cells in vitro and rat mesentery postcapillary venules in vivo [9]. β-Catenin is a key regulator of barrier integrity and following insult, translocate into the cytoplasm, resulting in microvascular hyperpermeability followed by a time-dependent recovery and relocation to the cell membrane [6]. This suggested a recycling pathway for β-catenin to the cell junction. In the BBB, tight junctions form the continuous intercellular barrier between endothelial cells, which is required to separate tissue spaces and regulate selective movement of solutes across the endothelium (refer section on BBB permeability). Cell adhesion molecules occludin, claudin family members, junctional adhesion molecules etc are important at the endothelial tight junctions of the BBB. A large number of cell adhesion molecules are substrates for proteolytic enzymes such as capase-3 and MMPs. Our studies have shown increased vascular permeability due to caspase-3 and MMP-9 [43,44] mediated breakdown of the adherens junction complex. An increase in the activities of caspase-3 and MMPs has been observed in aging and there is a possible link between enhanced vascular permeability and aging due to the changes in enzyme and activities.
Oxidative Stress
Aging is associated with increased oxidative stress and oxidative damage in the body [45,46]. Oxidative stress due to increased reactive oxygen species (ROS) is implicated in causing aging of vascular endothelium as well as promoting vascular hyperpermeability. In turn, aged endothelium produces increased free radicals, which might further accelerate aging. In the vascular wall, the vascular endothelium is an important source of free radicals, the formation of which increases during aging [47–49]. It has been demonstrated that biomarkers of oxidant damage, increased levels of nitrotyrosine were observed in human aged vascular endothelial cells [50], Moreover, oxidative stress markers were also observed in the arteries of aged animals [50,51], suggesting that aging is indeed associated with increased formation of ROS. Many different mechanisms are responsible for causing oxidative stress in endothelial cells that includes mitochondria-mediated production of ROS, decreases in free radical scavengers and increased susceptibility of macromolecules to free radical damage. The potential role of oxidative stress in vascular endothelium aging is also evident from the experiments carried out with antioxidants. The potent antioxidant, Vitamin C, as well as N-Acetylsyteine have been shown to increase the longevity of endothelial cells or decrease endothelial cell senescence [52,53]. Compounds that possess antioxidant properties—such as α-lipoic acid, curcumin and deprenyl—effectively prevented microvascular hyperpermeability in rat models of hemorrhagic shock [29,43,54]. Several other enzymes are thought to be involved in age-induced free radical formation. As mentioned above, NO synthases can be transformed into radical generating enzymes [55]. In rodents, a role for xanthine oxidase has been suggested [56]. Although direct evidence is lacking, NADPH oxidase might be a source of radical generation as activation of small GTPases [57] and potentially the NADPH oxidase subunit Nox4 [58] leads to O2 formation and senescence. That the NADPH oxidase may contribute to the aging phenomenon is also suggested by the observation that the stimulation of cultured cells with angiotensin II, a potent inducer and activator of NADPH oxidase in vascular cells, leads to DNA fragmentation [59] and treatment with angiotensin converting enzyme inhibitors prevents the aging-induced endothelial dysfunction in rats [60]. Super oxide anions are potent inducers of vascular hyperpermeability and the bioactivity of superoxide and other free radicals are increased with aging.
Inflammation
Inflammation is believed to play an essential role in the etiology of a variety of cardiovascular diseases [61,62] and there is increasing evidence for a similar involvement in vascular aging. Changes in vascular permeability are a significant problem in vascular inflammation associated with trauma, ischemia-reperfusion injury and sepsis. Also, inflammatory stimuli such as histamine, thrombin, VEGF, activated neutrophils and proinflammatory stimuli such as TNF-α and TRAIL induces disruption of endothelial cell-cell junctions as well as induces cytoskeleton disorganization [7,44,63]. This leads to a widened interendothelial space that facilitates extravasation of fluid and proteins to the extravascular space. It has been shown that such structural changes initiate with agonist-receptor binding followed by the activation of intracellular signaling molecules like calcium, protein kinase C, tyrosine kinases, myosin light chain kinase, and small Rho-GTPases. These kinases and GTPases then phosphorylate or alter the conformation of different subcellular components that control cell-cell adhesion, resulting in endothelial hyperpermeability [7]. Targeting key signaling molecules that mediate endothelial-junction-cytoskeleton dissociation demonstrates a therapeutic potential to improve vascular barrier function during inflammatory injury [6,7,63]. Also, plasma concentrations of inflammatory molecules can increase with age even in healthy adults [64–66] and is considered to correlate with changes in vascular permeability. In human vascular endothelial cells obtained from the brachial artery and/or antecubital veins of humans, expression of the pro-inflammatory nuclear transcription factor NF-κB and pro-inflammatory cytokine, interleukin-6, TNF-α and monocyte chemoattractant protein-1 are increased in older adults. It has been demonstrated that TNF-α and TRAIL induce microvascular endothelial cell permeability [44,63] via a mitochondrial apoptotic signaling pathway and an extrinsic signaling pathway respectively. The protective effects of recombinant Bcl-xL protein against TNF-α-induced endothelial cell adherens junction damage and microvascular endothelial cell hyperpermeability was observed [63]. TRAIL-induced microvascular hyperpermeability is phosphatidylinositol 3-kinase (PI3K)-dependent and may be mediated by caspase-3 cleavage of the endothelial adherens junctional complex [44]. Significant variations in the expression and levels of proinflammatory cytokines have been observed in aging [20,67–69].
The lymphatic system plays a major role in controlling acute and chronic inflammatory responses. Studies have indicated that the lymphatic system performs this function through increasing the drainage of extravasated fluid and inflammatory cells and by lymphatic vessel alteration of the immune response [70]. Although the effect of aging on lymphatic functions has not been extensively studied, recent investigations demonstrate that aging impairs the function of lymphatic vessels by pre-activating a higher degree of mast cells [71]. This would consequently hamper the ability of the lymphatic system to resolve inflammation.
Apoptotic Signaling
Apoptosis, or programmed cell death, involves a complex network of biochemical pathways that normally ensure a homeostatic balance between cellular proliferation and turnover in nearly all tissues [72,73]. A strong association between apoptosis, age-related diseases and aging has been observed, but the cellular and molecular mechanisms that control vascular permeability in aging are not clearly known. Vascular hyperpermeability has been considered as a phenomenon that occurs due to activation of the apoptotic signaling pathway which results in endothelial barrier dysfunction even without the instance of apoptotic cell death [6,8]. These apoptotic signaling cascades take place via either extrinsic or intrinsic (mitochondrial) pathways. The “intrinsic” pathway is mediated via activation of the mitochondria after hypoxia, ischemia and ROS generation [74–76]. Activated mitochondria release cytochrome c, AIF (apoptosis inducing factor), and smac (second mitochondrial-derived activator of caspases), all of which are regulated by members of the Bcl-2 family of proteins [77,78]. The Bcl-2 family consists of both anti-apoptotic (Bcl-2, Bcl-xL etc) and pro-apoptotic (Bak, Bax etc) members. The anti-apoptotic members of this family such as Bcl-xL are thought to prevent apoptosis by sequestering proforms of death-driving cysteine proteases or by preventing the release of mitochondrial apoptogenic factors. Thus, alterations in mitochondrial membrane integrity via pro-apoptotic factors and the subsequent release of cytochrome c are key components in the apoptotic-signaling cascade and may occur due to interplay between mitochondrial ROS levels and pro-and antiapoptotic molecules. Mitochondrial ROS formation and activation of the above pathway are major mechanisms regulating vascular hyperpermeability [5,29,43,54]. Increased ROS formation is one of the most significant regulators of endothelial aging and dysfunction (refer, section on ‘oxidative stress’, above). Our studies further showed evidence for the involvement of the pro-apoptotic factor Bak [42]. The caspase-mediated proteolytic cascade represents a central point in the apoptotic response and is influenced by both of the pathways. Recent evidence demonstrates several instances where caspase-3 activation doesn’t lead to cell death [8] and this explains why endothelial permeability is not correlated to endothelial cell death. Pro and antiapoptotic members of the Bcl-2 family proteins, TNF-α and p53 play pivotal roles in the regulation of caspase activation [72,73]. Non-human primates demonstrate increased apoptosis and reduced density of endothelial cells with aging, and this is associated with impaired endothelial function [79]. Thus an increased rate of endothelial apoptosis may decrease the number of healthy, normally functioning, vascular endothelial cells with aging and contribute to vascular endothelial dysfunction. Recent studies from our laboratory have demonstrated the involvement of apoptotic signaling as a key regulator of vascular hyperpermeability [5,6,42]. We have previously shown that the apoptotic signaling pathway is a key regulator of vascular permeability without inducing apoptotic cell death. The Bcl-2 family members are involved in controlling vascular hyperpermeability via this pathway. Proapoptotic Bcl-2 family protein Bak is upregulated following hemorrhagic shock induced vascular permeability and exogenously introduced Bak peptide effectively induced hyperpermeability [42]. Caspase-3 inhibition as well as protecting the mitochondrial integrity was effective against microvascular hyperpermeability in vitro and hemorrhagic shock induced vascular hyperpermeability in vivo [42,54]. As discussed above, paracellular permeability is regulated by the binding of the catenins to VE-cadherin. The catenin/cadherin complex is tethered to the endothelial cell cytoskeleton [4]. Dissociation of this complex has been reported to result in endothelial barrier dysfunction. Importantly, many of these molecules are substrates for caspase-3, the effector caspase in the apoptotic signaling cascade. A variety of compounds that inhibit apoptotic signaling pathways have been found to protect against hyperpermeability [29,43,54]. Their specific effect on vascular hyperpermeability in aging is not clearly known.
As discussed above, there are many studied mechanisms involved in apoptotic signaling, some of which cause hyperpermeability before reaching the point of causing cell death. In addition, increased apoptotic cell death in aging tissues can indirectly cause hyperpermeability.
Hormones
The significance of the age-associated decline in endogenous sex hormone levels, particularly levels of estradiol and testosterone, and their relation to disease and function is an area that require more research. Estrogens are known to regulate vascular functions and permeability [80,81]. 17β-estradiol shows protective benefits against vascular hyperpermeability following hemorrhagic shock in vivo [81] as well as endothelial cell monolayer permeability in vitro [80]. The effects of 17β-estradiol on vascular permeability is mediated via estrogen receptor-α and β (ERα and ERβ) [81]. The expression of ERα is lower in vascular endothelial cells obtained from estrogen-deficient postmenopausal women compared with premenopausal women [82,83]. In the overall group, ERα expression was positively related to brachial artery FMD, as well as to vascular endothelial cell expression of eNOS and eNOS phosphorylated at Ser [83]. These observations are consistent with the idea that circulating estrogen deficiency may lead to down-regulation of ERα and impaired endothelial function in postmenopausal women, in part as a result of reduced eNOS expression and activation. Recent studies also show that age and estradiol effects on BBB tight junctions and estrogen receptor proteins in ovariectomized rats. It has been found that 17β-estradiol treatment alters the expression of ERα and distinct TJ protein isoforms of the BBB without altering functional paracellular permeability [84].
Injuries and vascular permeability
Aging is a major risk factor in traumatic injuries including hemorrhagic shock, burn, and sepsis as well as in several cardiovascular diseases, stroke and cancer [85–87]. Microvascular barrier dysfunction is a significant complication in clinical conditions associated with traumatic injury [30]. Various epidemiological studies strongly suggest that these diseases are more often diagnosed in older people than they are in the younger population. It is important to give high priority to research that focuses on aging and age-associated disease in order to develop novel therapies that could treat age-associated diseases more effectively. The vascular system is a main target of the aging process, thus making it especially susceptible to disease and dysfunction in the elderly. This is, in part, due to the structural and functional changes that occur in the vascular system during aging. But aging also alters the ability of the brain to respond to injury. In response to all of the above, we discussed below how permeability regulation takes place in hemorrhagic shock, burn injury and septic shock.
Hemorrhagic Shock
Hemorrhagic shock (HS) is shock that occurs as a result of inadequate tissue perfusion. It is a life threatening medical emergency. This condition is produced by rapid and significant loss of intravascular volume, which may lead to hemodynamic instability, decreased tissue perfusion, cellular hypoxia, organ damage, and death [88]. The primary goals in a medical response are to stop the bleeding and restore circulating blood volume (resuscitation). However, this can lead to excessive fluid leakage, tissue edema, and multiple organ failure due to microvascular hyperpermeability [5,80]. The formation of ROS as a result of hemorrhagic shock and subsequent resuscitation plays an important role in vascular hyperpermeability, post-hemorrhage inflammation, and tissue injury. It has been found that the severity of tissue damage in mice caused by hemorrhagic shock is influenced by age [89]. HS also causes hypoperfusion of peripheral tissues which promotes endothelial dysfunction and may lead to further tissue injury. Aside from the aforementioned age-related decrease in the ability to mitigate tissue damage in mice, it is not clearly known how endothelial permeability regulation takes place following HS in aging. So, it is important to review the studies that generally deal with vascular permeability regulation following HS to understand the potential permeability mechanisms that are involved in aging endothelium.
As mentioned in the apoptotic signaling section, the apoptotic signaling pathway plays a significant role in vascular hyperpermeability. HS is a known trigger of the apoptotic signaling pathway, and this pathway is a major mechanism by which HS causes hyperpermeability. Studies have demonstrated that microvascular permeability regulation following HS is closely associated with mitochondrial ROS formation, mitochondrial release of cytochrome c and caspase-3 mediated breakdown of the adherens junction protein β-catenin [6,42].
A study of particular interest involving aged rats showed HS resulted in an age-correlated increased level of mitochondrial dysfunction, including an age-correlated increase in the mitochondrial release of cytochrome c [90]. Although a direct link has not been made, this increased release of cytochrome c in later-aged rats suggests a potential mechanism, involving apoptotic signaling, through which aged rats would have more severe tissue damage following HS induced hyperpermeability.
Mitigation of HS induced hyperpermeability, as discussed above, involves a variety of compounds with antioxidant properties in addition to their ability to block the apoptotic signaling pathway. These compounds effectively blocked HS-induced hyperpermeabiltiy in a regular rat model of HS injury [29,43,54] but have yet to be tested for their therapeutic potential against HS in aging. There is a clear lack of information on the relationship between HS, vascular permeability and aging. Thus studies that focus on HS-induced vascular hyperpermeability in aging are highly warranted.
Sepsis
Sepsis, a serious condition in all age groups, is especially problematic for the elderly population due to its association with high mortality rates in that population [91]. Aging alters the stress response, which compounds the increased susceptibility of the elderly to physiological stressors such as infection and sepsis [92]. The elderly population’s comparatively high sepsis-related mortality rate is due to their higher propensity for microvascular dysfunction and consequential multiorgan failure [93]. Treatment with septic sera collected from patients elicited greater increases in TNF-α expression in aged endothelial cells compared with younger cells. However, the induction of inducible NO synthase, intercellular adhesion molecule-1, and vascular cell adhesion molecule did not differ between the two groups. Collectively, aging increased sensitivity of microvascular endothelial cells to oxidative stress and increased cellular damage induced by inflammatory factors present in the circulation during septicemia [93]. Tucsek et al [93] demonstrated that the inflammatory response is exacerbated in a rodent endotoxemia model of sepsis in aged rats compared with young rats. To investigate the factors involving accelerated apoptosis in aged animals following sepsis, Zhou et al [94] analyzed the Fas/Fas ligand (Fas-L) pathway. Fas and Fas-L gene expression increased in the spleen in aged animals after the LPS treatment that was used to induce the symptoms of sepsis. Similarly, cleaved caspase-8 expression, a downstream element of Fas and Fas-L, was also significantly higher in the aged rats after LPS. This suggests that the Fas/Fas-L pathway may play an important role in sepsis-induced activation of apoptosis and hyperinflammation in aged animals [94]. Fas-Fas ligand interaction has been recently shown to regulate microvascular endothelial cell hyperpermeability [95]. More investigation is needed to determine whether this is a potential target for attenuation of sepsis-related hyperpermeability in aged subjects.
Burn
Burn injury to individuals older than 60 years occurs at a frequency disproportionate to all other age groups except the very young [96]. Burns are associated with a significant leak of intravascular fluid into the interstitial space and require large volumes of resuscitation fluids [97]. Major cutaneous burns result not only in localized tissue damage, but also in broad systemic inflammation that causes organ system damage distal to the burn site. The pulmonary microvessels are particularly susceptible to functional abnormalities after exposure to burn-induced inflammatory mediators [31]. Tinsely et al [31] found that the phosphorylation of MLC is a required element of burn-induced hyperpermeability in rat lung microvascular endothelial cell monolayers. It was also discovered that MLCK and Rho kinase inhibition blocked actin stress fiber formation and MLC phosphorylation after exposure to burn plasma. Another study showed that age-associated pulmonary congestion observed following burn injury may be due to differences in lung endothelial adhesion responses and are compounded by elevated numbers of hyperchemokinetic circulating neutrophils in aged mice [98]. In rat lung microvascular endothelial cells, activation of intrinsic apoptotic pathways has been associated with microvascular hyperpermeability resulting from burn injury [97]. (−)-Deprenyl, an antioxidant and antiapoptotic drug, has been shown to modulate intrinsic apoptotic signaling and attenuate burn-induced hyperpermeability [97]. Damage to the adherens junction complexes between endothelial cells plays an integral role in the pathophysiology of microvascular hyperpermeability following burn trauma [31,97,99]. The changes that take place in the adherens junction complex during aging as well as its significance in vascular hyperpermeability following burn trauma needs to be studied.
Conclusion
Although vascular hyperpermeability is associated with injuries and diseases in all age groups, its effect in the aging population is severe and warrants urgent attention in basic research. Aging elicits several changes in the vascular endothelium that effect permeability regulation. Age-related oxidative stress, via ROS, is one of the major contributing factors in the loss of endothelial cell function in advanced age. This oxidative stress, in addition to inflammatory molecules, may be involved in endothelial aging by affecting vascular function, endothelial gene expression, the activation of the apoptotic signaling pathways, as well as breaking down barrier integrity and deregulating permeability. Future studies are needed to understand the changes that take place in the adherens and tight junction complex during aging and to elucidate whether aging, as well as alterations in the circulating level of endothelial progenitor cells, are relevant to age-associated vascular permeability. Advances in the understanding of endothelial function/physiology have been the basis for many therapeutic strategies. The discovery of novel and potent antioxidants and antiapoptotic molecules may control aging-induced vascular dysfunction and thereby improve the function of aging endothelial cells. This would include the attenuation of vascular hyperpermeability. It is conceivable that expanding the understanding of endothelial cell function in advanced age would lead to targeted therapies against vascular dysfunction and hyperpermeability associated with traumatic and ischemic injuries and other diseases in aging.
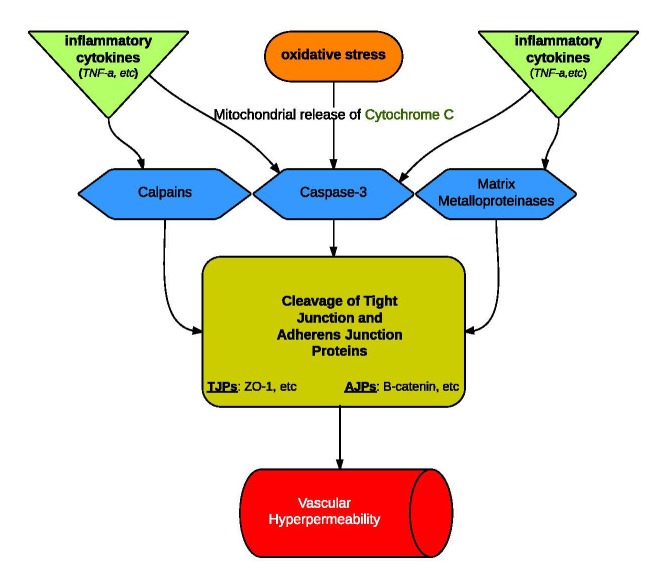
Figure 1.
Regulation of Vascular Permeability. Hyperpermeability seems to be caused by similar pathways in both young and aged blood vessels. The increase in permeability that comes with age is attributed to the scale of regulator release and activity at each level of the pathway. In aged endothelial cells: (a) oxidative stress intensifies with an increased release of ROS, (b) more cytochrome C, an inducer of proteolytic Caspase-3, is released from mitochondria (c) there is an increase in inflammatory cytokine release.
References
- [1].Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- [2].Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- [3].Michel CC. Fluid exchange in the microcirculation. J Physiol. 2004 Jun 15;557(pt 3):701–2. doi: 10.1113/jphysiol.2004.063511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dejana E, Bazzone G, Lampugnani MG. Vascular endothelial (VE)-cadherin: only an intercellular glue? Exp Cell Res. 1999;252:13–19. doi: 10.1006/excr.1999.4601. [DOI] [PubMed] [Google Scholar]
- [5].Childs EW, Tharakan B, Hunter FA, Tinsley JH, Cao X. Apoptotic signaling induces hyperpermeability following hemorrhagic shock. Am J Physiol Heart Circ Physiol. 2007;292:H3179–89. doi: 10.1152/ajpheart.01337.2006. [DOI] [PubMed] [Google Scholar]
- [6].Tharakan B, Hellman J, Sawant DA, Tinsley JH, Parrish AR, Hunter FA, Smythe WR, Childs EW. β-Catenin dynamics in the regulation of microvascular endothelial cell hyperpermeability. Shock. 2012;37:306–11. doi: 10.1097/SHK.0b013e318240b564. [DOI] [PubMed] [Google Scholar]
- [7].Kumar P, Shen Q, Pivetti CD, Lee ES, Wu MH, Yuan SY. Molecular mechanisms of endothelial hyperpermeability: implications in inflammation. Expert Rev Mol Med. 2009;11:e19. doi: 10.1017/S1462399409001112. 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tharakan B, Chowdhury I, Bhat GK. Fundamental mechanisms and implications of neuronal cell apoptosis. In: Schmid CJ, Wolfe JL, editors. Neuronal Cell Apoptosis. New York: Nova Scientific Publishers; 2011. pp. 143–166. [Google Scholar]
- [9].Sawant DA, Tharakan B, Adekanbi A, Hunter FA, Smythe WR, Childs EW. Inhibition of VE-cadherin proteasomal degradation attenuates microvascular hyperpermeability. Microcirculation. 2011;18:46–55. doi: 10.1111/j.1549-8719.2010.00067.x. [DOI] [PubMed] [Google Scholar]
- [10].Krouwer VJ, Hekking LH, Langelaar-Makkinje M, Regan-Klapisz E, Post JA. Endothelial cell senescence is associated with disrupted cell-cell junctions and increased monolayer permeability. Vasc Cell. 2012;4:12. doi: 10.1186/2045-824X-4-12. 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Pelegrí C, Canudas AM, del Valle J, et al. Increased permeability of blood-brain barrier on the hippocampus of a murine model of senescence. Mech Ageing Dev. 2007;128:522–528. doi: 10.1016/j.mad.2007.07.002. [DOI] [PubMed] [Google Scholar]
- [12].Blau CW, Cowley TR, O’Sullivan J, et al. The age-related deficit in LTP is associated with changes in perfusion and blood-brain barrier permeability. Neurobiol Aging. 2012;33:1005. doi: 10.1016/j.neurobiolaging.2011.09.035. [DOI] [PubMed] [Google Scholar]
- [13].Luissint AC, Artus C, Glacial F, Ganeshamoorthy K, Couraud PO. Tight junctions at the blood brain barrier: physiological architecture and disease-associated dysregulation. Fluids Barriers CNS. 2012;9:23. doi: 10.1186/2045-8118-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a ‘set up’ for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- [15].Brandes RP, Fleming I, Busse R. Endothelial aging. Cardiovasc Res. 2005;66:286–294. doi: 10.1016/j.cardiores.2004.12.027. [DOI] [PubMed] [Google Scholar]
- [16].Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, et al. Failure of bloodisland formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- [17].Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9–22. [PubMed] [Google Scholar]
- [18].Geary GG, Buchholz JN. Selected contribution: effects of aging on cerebrovascular tone and [Ca2+]i. J Appl Physiol. 2003;95:1746–54. doi: 10.1152/japplphysiol.00275.2003. [DOI] [PubMed] [Google Scholar]
- [19].Muller-Delp JM, Spier SA, Ramsey MW, Delp MD. Aging impairs endothelium-dependent vasodilation in rat skeletal muscle arterioles. Am J Physiol, Heart Circ Physiol. 2002;283:H1662–72. doi: 10.1152/ajpheart.00004.2002. [DOI] [PubMed] [Google Scholar]
- [20].Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Aging-induced proinflammatory shift in cytokine expression profile in coronary arteries. FASEB J. 2003;17:1183–1185. doi: 10.1096/fj.02-1049fje. [DOI] [PubMed] [Google Scholar]
- [21].Yoon HJ, Cho SW, Ahn BW, Yang SY. Alterations in the activity and expression of endothelial NO synthase in aged human endothelial cells. Mech Ageing Dev. 2010;131:119–123. doi: 10.1016/j.mad.2009.12.010. [DOI] [PubMed] [Google Scholar]
- [22].Durán WN, Breslin JW, Sánchez FA. The NO cascade, eNOS location, and microvascular permeability. Cardiovasc Res. 2010;87:254–61. doi: 10.1093/cvr/cvq139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tanabe T, Maeda S, Miyauchi T, Iemitsu M, Takanashi M, et al. Exercise training improves ageing-induced decrease in eNOS expression of the aorta. Acta Physiol Scand. 2003;178:3–10. doi: 10.1046/j.1365-201X.2003.01100.x. [DOI] [PubMed] [Google Scholar]
- [24].Zhang Y, Zhao S, Gu Y, Lewis DF, Alexander JS, Wang Y. Effects of peroxynitrite and superoxide radicals on endothelial monolayer permeability: potential role of peroxynitrite in preeclampsia. J Soc Gynecol Investig. 2005;12:586–92. doi: 10.1016/j.jsgi.2005.09.003. [DOI] [PubMed] [Google Scholar]
- [25].Davis ME, Cai H, Drummond GR, Harrison DG. Shear stress regulates endothelial nitric oxide synthase expression through c-Src by divergent signaling pathways. Circ Res. 2001;89:1073–1080. doi: 10.1161/hh2301.100806. [DOI] [PubMed] [Google Scholar]
- [26].Kleinert H, Wallerath T, Euchenhofer C, Ihrig-Biedert I, Li H, et al. Estrogens increase transcription of the human endothelial NO synthase gene: analysis of the transcription factors involved. Hypertension. 1998;31:582–588. doi: 10.1161/01.hyp.31.2.582. [DOI] [PubMed] [Google Scholar]
- [27].Bouloumie A, Schini-Kerth VB, Busse R. Vascular endothelial growth factor up-regulates nitric oxide synthase expression in endothelial cells. Cardiovasc Res. 1999;41:773–780. doi: 10.1016/s0008-6363(98)00228-4. [DOI] [PubMed] [Google Scholar]
- [28].Shah GN, Mooradian AD. Age-related changes in the blood-brain barrier. Exp Gerontol. 1997;32:501–19. doi: 10.1016/s0531-5565(96)00158-1. [DOI] [PubMed] [Google Scholar]
- [29].Tharakan B, Hunter FA, Smythe WR, Childs EW. Alpha-lipoic acid attenuates hemorrhagic shock-induced apoptotic signaling and vascular hyperpermeability. Shock. 2008;30:571–7. doi: 10.1097/SHK.0b013e31816a7308. [DOI] [PubMed] [Google Scholar]
- [30].Shen Q, Wu MH, Yuan SY. Endothelial contractile cytoskeleton and microvascular permeability. Cell Health Cytoskelet. 2009;2009:43–50. doi: 10.2147/chc.s5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Tinsley JH, Teasdale NR, Yuan SY. Myosin light chain phosphorylation and pulmonary endothelial cell hyperpermeability in burns. Am J Physiol Lung Cell Mol Physiol. 2004;286:L841–7. doi: 10.1152/ajplung.00341.2003. [DOI] [PubMed] [Google Scholar]
- [32].Rigor RR, Shen Q, Pivetti CD, Wu MH, Yuan SY. Myosin light chain kinase signaling in endothelial barrier dysfunction. Med Res Rev. 2013;33:911–33. doi: 10.1002/med.21270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Curry FE, Clark JF, Adamson RH. Erythrocyte-derived sphingosine-1-phosphate stabilizes basal hydraulic conductivity and solute permeability in rat microvessels. Am J Physiol Heart Circ Physiol. 2012;303:H825–34. doi: 10.1152/ajpheart.00181.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mitschelen M, Garteiser P, Carnes BA, Farley JA, Doblas S, Demoe JH, Warrington JP, Yan H, Nicolle MM, Towner R, Sonntag WE. Basal and hypercapnia-altered cerebrovascular perfusion predict mild cognitive impairment in aging rodents. 2009;164:918–28. doi: 10.1016/j.neuroscience.2009.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Stone DK, Reynolds AD, Mosley RL, Gendelman HE. Innate and adaptive immunity for the pathobiology of Parkinson’s disease. Antioxid. Redox Signal. 2009;11:2151–2166. doi: 10.1089/ars.2009.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Schindowski K, Eckert A, Peters J, et al. Increased T-cell reactivity and elevated levels of CD8_ memory T-cells in Alzheimer’s disease-patients and T-cell hyporeactivity in an Alzheimer’s diseasemouse model: implications for immunotherapy. Neuromol Med. 2007;9:340–354. doi: 10.1007/s12017-007-8015-9. [DOI] [PubMed] [Google Scholar]
- [37].Togo T, Akiyama H, Iseki E, et al. Occurrence of T cells in the brain of Alzheimer’s disease and other neurological diseases. J Neuroimmunol. 2002;124:83–92. doi: 10.1016/s0165-5728(01)00496-9. [DOI] [PubMed] [Google Scholar]
- [38].Popescu BO, Toescu EC, Popescu LM, Bajenaru O, Muresanu DF, Schultzberg M, Bogdanovic N. Blood-brain barrier alterations in ageing and dementia. J Neurol Sci. 2009;283:99–106. doi: 10.1016/j.jns.2009.02.321. [DOI] [PubMed] [Google Scholar]
- [39].Simpson JE, Wharton SB, Cooper J, Gelsthorpe C, Baxter L, Forster G, Shaw PJ, Savva G, Matthews FE, Brayne C, Ince PG. Alterations of the blood-brain barrier in cerebral white matter lesions in the ageing brain. Neurosci Lett. 2010;486:246–51. doi: 10.1016/j.neulet.2010.09.063. [DOI] [PubMed] [Google Scholar]
- [40].Viggars AP, Wharton SB, Simpson JE, Matthews FE, Brayne C, Savva GM, Garwood C, Drew D, Shaw PJ, Ince PG. Alterations in the blood brain barrier in ageing cerebral cortex in relationship to Alzheimer-type pathology: a study in the MRC-CFAS population neuropathology cohort. 2011;505:25–30. doi: 10.1016/j.neulet.2011.09.049. [DOI] [PubMed] [Google Scholar]
- [41].Lee P, Kim J, Williams R, Sandhir R, Gregory E, Brooks WM, Berman NE. Effects of aging on blood brain barrier and matrix metalloproteases following controlled cortical impact in mice. Exp Neurol. 2012;234:50–61. doi: 10.1016/j.expneurol.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Childs EW, Tharakan B, Byrge N, Tinsley JH, Hunter FA, Smythe WR. Angiopoietin-1 inhibits intrinsic apoptotic signaling and vascular hyperpermeability following hemorrhagic shock. Am J Physiol Heart Circ Physiol. 2008;294:H2285–95. doi: 10.1152/ajpheart.01361.2007. [DOI] [PubMed] [Google Scholar]
- [43].Tharakan B, Whaley JG, Hunter FA, Smythe WR, Childs EW. (-)-Deprenyl inhibits vascular hyperpermeability after hemorrhagic shock. Shock. 2010;33:56–63. doi: 10.1097/SHK.0b013e3181a7fb7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Stagg HW, Bowen KA, Sawant DA, Rodriguez M, Tharakan B, Childs EW. Tumor necrosis factor-related apoptosis-inducing ligand promotes microvascular endothelial cell hyperpermeability through phosphatidylinositol 3-kinase pathway. Am J Surg. 2013;205:419–25. doi: 10.1016/j.amjsurg.2012.10.027. [DOI] [PubMed] [Google Scholar]
- [45].Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–47. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- [46].Harman D. The free radical theory of aging. Antioxid Redox Signal. 2003;5:557–61. doi: 10.1089/152308603770310202. [DOI] [PubMed] [Google Scholar]
- [47].Hamilton CA, Brosnan MJ, Mcintyre M, Graham D, Dominiczak AF. Superoxide excess in hypertension and aging: a common cause of endothelial dysfunction. Hypertension. 2011;37:529–34. doi: 10.1161/01.hyp.37.2.529. [DOI] [PubMed] [Google Scholar]
- [48].Gorlach A, Brandes RP, Nguyen K, Amidi M, Dehghani F, Busse R. A gp91phox containing NADPH oxidase selectively expressed in endothelial cells is a major source of oxygen radical generation in the arterial wall. Circ Res. 2000;87:26–32. doi: 10.1161/01.res.87.1.26. [DOI] [PubMed] [Google Scholar]
- [49].Jung O, Schreiber JG, Geiger H, Pedrazzini T, Busse R, Brandes RP. gp91phox-containing NADPH oxidase mediates endothelial dysfunction in renovascular hypertension. Circulation. 2004;109:1795–1801. doi: 10.1161/01.CIR.0000124223.00113.A4. [DOI] [PubMed] [Google Scholar]
- [50].Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, et al. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–1166. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- [51].Francia P, delli Gatti C, Bachschmid M, Martin-Padura I, Savoia C, Migliaccio E, Pelicci PG, Schiavoni M, Luscher TF, Volpe M, Cosentino F. Deletion of p66shc gene protects against age-related endothelial dysfunction. Circulation. 2004;110:2889–2895. doi: 10.1161/01.CIR.0000147731.24444.4D. [DOI] [PubMed] [Google Scholar]
- [52].Furumoto K, Inoue E, Nagao N, Hiyama E, Miwa N. Age-dependent telomere shortening is slowed down by enrichment of intracellular vitamin C via suppression of oxidative stress. Life Sci. 1998;63:935–948. doi: 10.1016/s0024-3205(98)00351-8. [DOI] [PubMed] [Google Scholar]
- [53].Haendeler J, Hoffmann J, Diehl JF, Vasa M, Spyridopoulos I, et al. Antioxidants inhibit nuclear export of telomerase reverse transcriptase and delay replicative senescence of endothelial cells. Circ Res. 2004;94:768–775. doi: 10.1161/01.RES.0000121104.05977.F3. [DOI] [PubMed] [Google Scholar]
- [54].Tharakan B, Hunter FA, Smythe WR, Childs EW. Curcumin inhibits reactive oxygen species formation and vascular hyperpermeability following haemorrhagic shock. Clin Exp Pharmacol Physiol. 2010;37:939–44. doi: 10.1111/j.1440-1681.2010.05414.x. [DOI] [PubMed] [Google Scholar]
- [55].Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, et al. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–9. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Chung HY, Song SH, Kim HJ, Ikeno Y, Yu BP. Modulation of renalxanthine oxidoreductase in aging: gene expression and reactive oxygen species generation. J Nutr Health Aging. 1999;3:19–23. [PubMed] [Google Scholar]
- [57].Deshpande SS, Qi B, Park YC, Irani K. Constitutive activation of rac1 results in mitochondrial oxidative stress and induces premature endothelial cell senescence. Arterioscler Thromb Vasc Biol. 2003;23:e1–6. doi: 10.1161/01.atv.0000047869.13737.53. [DOI] [PubMed] [Google Scholar]
- [58].Geiszt M, Kopp JB, Varnai P, Leto TL. Identification of renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci USA. 2000;97:8010–4. doi: 10.1073/pnas.130135897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Mazza F, Goodman A, Lombardo G, Vanella A, Abraham NG. Heme oxygenase-1 gene expression attenuates angiotensin II-mediatedDNA damage in endothelial cells. Exp Biol Med (Maywood) 2003;228:576–83. doi: 10.1177/15353702-0322805-31. [DOI] [PubMed] [Google Scholar]
- [60].Atkinson J. Effect of aging and chronic angiotensin I converting enzyme inhibition on the endothelial function of the mesenteric arterial bed of the rat. Am J Cardiol. 1995;76:19E–23E. doi: 10.1016/s0002-9149(99)80498-6. [DOI] [PubMed] [Google Scholar]
- [61].Tousoulis D, Charakida M, Stefanadis C. Endothelial function and inflammation in coronary artery disease. Heart. 2006;92:441–444. doi: 10.1136/hrt.2005.066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, III, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- [63].Sawant DA, Tharakan B, Wilson RL, Stagg HW, Hunter FA, Childs EW. Regulation of tumor necrosis factor-α-induced microvascular endothelial cell hyperpermeability by recombinant B-cell lymphoma-extra large. J Surg Res. 2013;184:628–37. doi: 10.1016/j.jss.2013.04.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res. 2007;100:1659–66. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- [65].Donato AJ, Black AD, Jablonski KL, Gano LB, Seals DR. Aging is associated with greater nuclear NF kappa B, reduced I kappa B alpha, and increased expression of proinflammatory cytokines in vascular endothelial cells of healthy humans. Aging Cell. 2008;7:805–12. doi: 10.1111/j.1474-9726.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Bruunsgaard H, Pedersen M, Pedersen BK. Review Aging and proinflammatory cytokines. Curr Opin Hematol. 2001;8:131–6. doi: 10.1097/00062752-200105000-00001. [DOI] [PubMed] [Google Scholar]
- [67].Arenas IA, Armstrong SJ, Xu Y, Davidge ST. Tumor necrosis factor-α and vascular angiotensin II in estrogen-deficient rats. Hypertension. 2006;48:497–503. doi: 10.1161/01.HYP.0000235865.03528.f1. [DOI] [PubMed] [Google Scholar]
- [68].Csiszar A, Labinskyy N, Smith K, Rivera A, Orosz Z, Ungvari Z. Vasculoprotective effects of anti-tumor necrosis factor-α treatment in aging. Am J Pathol. 2007;170:388–398. doi: 10.2353/ajpath.2007.060708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Csiszar A, Wang M, Lakatta EG, Ungvari Z. Inflammation and endothelial dysfunction during aging: role of NF-κB. J. Appl Physiol. 2008;105:1333–1341. doi: 10.1152/japplphysiol.90470.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Dieterich LC, Seidel CD, Detmar M. Lymphatic vessels: new targets for the treatment of inflammatory diseases. Angiogenesis. 2013 Nov 9; doi: 10.1007/s10456-013-9406-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [71].Chatterjee V, Gashev AA. Aging-associated shifts in functional status of mast cells located by adult and aged mesenteric lymphatic vessels. Am J Physiol Heart Circ Physiol. 2012 Sep 15;303(6):H693–702. doi: 10.1152/ajpheart.00378.2012. Epub 2012 Jul 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Chowdhury I, Tharakan B, Bhat GK. Current concepts in apoptosis: the physiological suicide program revisited. 2006;11:506–25. doi: 10.2478/s11658-006-0041-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Chowdhury I, Tharakan B, Bhat GK. Caspases - an update. Comp Biochem Physiol B Biochem Mol Biol. 2008;151:10–27. doi: 10.1016/j.cbpb.2008.05.010. [DOI] [PubMed] [Google Scholar]
- [74].Green DR, Evan GI. A matter of life and death. Cancer Cell. 2002;1:19–30. doi: 10.1016/s1535-6108(02)00024-7. [DOI] [PubMed] [Google Scholar]
- [75].Kim R, Emi M, Tanabe K. Role of mitochondria as the gardens of cell death. Cancer Chemother Pharmacol. 2005;57:1–9. doi: 10.1007/s00280-005-0111-7. [DOI] [PubMed] [Google Scholar]
- [76].Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, Newmeyer DD. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17:525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- [77].Newmeyer DD, Ferguson-Miller S. Mitochondria: releasing power for life and unleashing the machineries of death. Cell. 2003;112:481–490. doi: 10.1016/s0092-8674(03)00116-8. [DOI] [PubMed] [Google Scholar]
- [78].Desagher S, Osen-Sand A, Nichols A, Eskes R, Montessuit S, Lauper S, Maundrell K, Antonsson B, Martinou JC. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J Cell Biol. 1999;144:891–901. doi: 10.1083/jcb.144.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Asai K, Kudej RK, Shen YT, Yang GP, Takagi G, Kudej AB, Geng YJ, Sato N, Nazareno JB, Vatner DE, et al. Peripheral vascular endothelial dysfunction and apoptosis in old monkeys. Arterioscler Thromb Vasc Biol. 2000;20:1493–1499. doi: 10.1161/01.atv.20.6.1493. [DOI] [PubMed] [Google Scholar]
- [80].Tharakan B, Corprew R, Hunter FA, Whaley JG, Smythe WR, Childs EW. 17beta-estradiol mediates protection against microvascular endothelial cell hyperpermeability. Am J Surg. 2009;197:147–54. doi: 10.1016/j.amjsurg.2008.10.003. [DOI] [PubMed] [Google Scholar]
- [81].Childs EW, Tharakan B, Hunter FA, Smythe WR. 17beta-estradiol mediated protection against vascular leak after hemorrhagic shock: role of estrogen receptors and apoptotic signaling. Shock. 34:229–35. doi: 10.1097/SHK.0b013e3181d75b50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Mendelsohn ME. Mechanisms of estrogen action in the cardiovascular system. J Steroid Biochem Mol Biol. 2000;74:337–343. doi: 10.1016/s0960-0760(00)00110-2. [DOI] [PubMed] [Google Scholar]
- [83].Gavin KM, Seals DR, Silver AE, Moreau KL. Vascular endothelial estrogen receptor α is modulated by estrogen status and related to endothelial function and endothelial nitric oxide synthase in healthy women. J Clin Endocrinol Metab. 2009;94:3513–3520. doi: 10.1210/jc.2009-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Sandoval KE, Witt KA. Age and 17β-estradiol effects on blood-brain barrier tight junction and estrogen receptor proteins in ovariectomized rats. Microvasc Res. 2011;81:198–205. doi: 10.1016/j.mvr.2010.12.007. [DOI] [PubMed] [Google Scholar]
- [85].Driver JA, Djousse L, Logroscino G, Gaziano JM, Kurth T. Incidence of cardiovascular disease and cancer in advanced age: prospective cohort study. BMJ. 2008;337:a2467. doi: 10.1136/bmj.a2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Kelly-Hayes M. Influence of age and health behaviors on stroke risk: lessons from longitudinal studies. J Am Geriatr Soc. 2010;2:S325–328. doi: 10.1111/j.1532-5415.2010.02915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Lakatta EG. Age-associated cardiovascular changes in health: impact on cardiovascular disease in older persons. Heart Fail Rev. 2002;7:29–49. doi: 10.1023/a:1013797722156. [DOI] [PubMed] [Google Scholar]
- [88].Gutierrez G, Reines HD, Wulf-Gutierrez ME Z. Clinical review: hemorrhagic shock. Crit Care. 2004;8:373–81. doi: 10.1186/cc2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Mees ST, Gwinner M, Marx K, Faendrich F, Schroeder J, Haier J, Kahlke V. Influence of sex and age on morphological organ damage after hemorrhagic shock. 2008;29:670–4. doi: 10.1097/shk.0b013e31815c3ea0. [DOI] [PubMed] [Google Scholar]
- [90].Jian B, Yang S, Chen D, Chaudry I, Raju R. Influence of aging and hemorrhage injury on Sirt1 expression: possible role of myc-Sirt1 regulation in mitochondrial function. Biochim Biophys Acta. 2011;1812:1446–51. doi: 10.1016/j.bbadis.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Lufrano M, Jacob A, Zhou M, Wang P. Sphingosine kinase-1 mediates endotoxemia-induced hyperinflammation in aged animals. Mol Med Rep. 2013;8:645–9. doi: 10.3892/mmr.2013.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Saito H, Sherwood ER, Varma TK, Evers BM. Effects of aging on mortality, hypothermia, and cytokine induction in mice with endotoxemia or sepsis. Mech Ageing Dev. 2003;124:1047–58. doi: 10.1016/j.mad.2003.08.002. [DOI] [PubMed] [Google Scholar]
- [93].Tucsek Z, Gautam T, Sonntag WE, et al. Aging exacerbates microvascular endothelial damage induced by circulating factors present in the serum of septic patients. J Gerontol A Biol Sci Med Sci. 2013;68:652–60. doi: 10.1093/gerona/gls232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Zhou M, Wu R, Dong W, Leong J, Wang P. Accelerated apoptosis contributes to aging-related hyperinflammation in endotoxemia. Int J Mol Med. 2010;25:929–35. doi: 10.3892/ijmm_00000424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Sawant DA, Tharakan B, Tobin RP, et al. Inhibition of Fas-Fas ligand interaction attenuates microvascular hyperpermeability following hemorrhagic shock. Shock. 2013;39:161–7. doi: 10.1097/SHK.0b013e31827bba73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Grant EJ. Preventing burns in the elderly: a guide for home healthcare professionals. Home Healthc Nurse. 2013;31:561–73. doi: 10.1097/01.NHH.0000436217.56972.58. [DOI] [PubMed] [Google Scholar]
- [97].Whaley JG, Tharakan B, Smith B, Hunter FA, Childs EW. (−)-Deprenyl inhibits thermal injury-induced apoptotic signaling and hyperpermeability in microvascular endothelial cells. J Burn Care Res. 2009;30:1018–27. doi: 10.1097/BCR.0b013e3181bfb825. [DOI] [PubMed] [Google Scholar]
- [98].Nomellini V, Brubaker AL, Mahbub S, et al. Dysregulation of neutrophil CXCR2 and pulmonary endothelial icam-1 promotes age-related pulmonary inflammation. Aging Dis. 2012;3:234–47. [PMC free article] [PubMed] [Google Scholar]
- [99].Stagg HW, Whaley JG, Tharakan B, et al. Doxycycline attenuates burn-induced microvascular hyperpermeability. J Trauma Acute Care Surg. 2013;75:1040–6. doi: 10.1097/TA.0b013e3182aa9c79. [DOI] [PubMed] [Google Scholar]