Abstract
As the “baby boomers” age, the percentage of the population over sixty-five years of age is increasing rapidly. Chronic disease management is an important component in the care of the elderly. The effects of aging on different organ systems are also pertinent; such as the weakening homeostatic response to injury in the older individuals. Mucosal surfaces have the largest combined surface area in the body and are the site of important host microbe interactions, especially in the gut which is prone to injury, both from local and systemic insult. This susceptibility has been known to increase with age. Therefore it is important to understand the interplay between aging, injury and recovery at the mucosal surface. Sex hormones play an important role in the maintenance of the mucosal barrier function as well as the mucosa associated immune function in both genders. Menopause in women is a defined time period in which major hormonal changes occur such as a decline in systemic estradiol levels. The differential levels of sex hormones contribute to the sexual dimorphism seen in response to injury at the mucosal surface, prior to and following menopause. Thus the effect of sex hormone and aging on mucosal mechanisms in response to injury is an important area of investigation.
Keywords: aging, mucosal injury, gut mucosa, epithelial barrier function, microbial translocation, estradiol, hormones, menopause
By 2050, the total US population ages 65 and older is estimated to reach 89 million [1]. The prevalence of chronic diseases is very high in this age group, the likely consequence of which will include increased health care costs [2, 3]. Among the most prevalent chronic conditions are cardiovascular disorders, cancer, respiratory disease, arthritis and gastrointestinal disease [4–7]. All these diseases have an underlying immune dysregulation related etiology, which may cause persistent tissue damage. The increased propensity to tissue damage and reduced rate of tissue repair in the elderly contribute to disease progression [7, 8]. Thus, the study of injury and repair in the aging population is significant.
Mucosal surfaces, particularly those of the urogenital, repiratory and oro-gastro-intestinal (OGI) tracts, are often affected by chronic diseases and challenged by pathologies, due to factors such as infection and chemical insult. For example, sepsis associated with pneumonia and urinary tract infections (UTIs) is predominantly a disease of the aged, with increased incidence and mortality occurring in older individuals [9, 10]. Additionally, disorders like dysphagia and gastroesophageal reflux disease, present special management challenges and often led to complications such as impairment of nutritional status and a reduction in the quality of life [11, 12]. There is substantial evidence that the aging mucosal surfaces suffer both structural and functional defense defects, thus changing the homeostatic baseline and contributing to compounded pathology when challenged with disease [13, 14]. To be able to facilitate, via pharmaceutical intervention, enhancement of the body’s mucosal compartments and their repair following injury in an aging population, a better understanding of age-related changes is required. This review intends to address the current knowledge of the influence of age and gender on mucosal injury and repair; and specifically the contribution of sex hormones to these processes.
Role of sex hormones in immune modulation
In the aging female population increased susceptibility to mucosal injury comes on the heels of menopause-induced hormonal alterations. During the reproductive years, females exhibit a more robust humoral and cellular immune response as compared to age matched males or post-menopausal counterparts [15]. Immunosenescence in the aging population is thought, in part, to be a result of altered hormonal status and decreased production of estrogen (17β-estradiol; E2) [16]. The major effects of E2 are mediated through two receptors, ERα and ERβ, both of which are expressed on a variety of cell types including, but not limited to, immune cells, epithelial cells and muscle cells [17–19]. Some data also suggest a spatial difference in immune-regulation by the two receptors with respect to tissue type [20]. Estrogen has been shown to regulate many facets of the immune response such as immune cell differentiation, cytokine production and regulation of Ca2+ mobilization and release of inducible nitric oxide synthase within leukocytes [18, 21]. Thus, it is likely that decreased E2 production with aging substantially impacts mucosal health and recovery via loss of its immune-modulatory effects [22–24].
Like females, aging males also experience a profound reduction in the levels of sex hormones, specifically androgens. Though androgen decline in males is associated with similar defects in mucosal healing and repair, it should be stressed that “male menopause” follows a very different progression pattern as compared to female menopause. It has been documented that from the ages of 25–75 years a healthy male experiences approximately 30% loss of circulating testosterone, with over 50% of males over the age of 65 years meeting endocrine criteria for hypogonadism [25, 26]. In addition to testosterone’s direct immune-modulatory function, it also acts as a pro-hormone, converted to both 5α-hihydrotestosterone (DHT) and E2 [27]. Because E2 also contributes greatly to sex hormone regulation of immune response in males, the effects of androgen loss with age is compounded. Independently, androgens contribute to both pro- and anti-inflammatory states, modulating cytokines such as IL-1, IL-2, IL-6 and TNFα in a variety of cell types including macrophages, Kupffer cells, fibroblasts and splenocytes [28–30]. Thus, in males, it is proposed that the major actions of testosterone are mediated though both aromatization to E2 and E2-independent mechanisms; however, it is also the balance between testosterone and E2 which may be responsible for the immune regulation in mucosal healing following injury.
Gut mucosal function and aging
The oro-gastro-intestinal (OGI) tract carries out the functions of food processing and digestion, nutrient absorption, and expulsion of waste. Along with its role in digestion, the gut also harbors about 90% of the body’s lymphocytes within the gut associated lymphoid tissue (GALT) [31]. The intestinal structure is comprised of simple columnar epithelium, mucosa, submucosa, smooth muscle and serosa. The absorptive surface area of the intestine is increased by plicae circulares, villi, and microvilli. Glandular epithelium is present along the whole length of the gut in the form of goblet cells, which secrete mucous that lubricates the passage of food and protects the tissue from digestive enzymes (Figure 1). Changes in the microenvironment of the small intestine are associated with alterations in the composition, pH, and thickness of the mucous layer [32, 33]. Villi are in vaginations of the mucosa and increase the overall surface area of the intestine. The next layer is the muscularis mucosa, a layer of smooth muscle that aids in the action of continued peristalsis along the gut. The submucosa contains nerves, blood vessels, and elastic fiber with collagen that stretches with increased capacity but maintains the shape of the intestine. Surrounding this is the muscularis externa comprised of longitudinal and smooth muscle that helps with continued peristalsis and the movement of digested material out of and along the gut. Lastly there is the serosa, which is made up of loose connective tissue and is coated in mucus so as to prevent friction damage from the intestine rubbing against other tissue.
Figure 1.
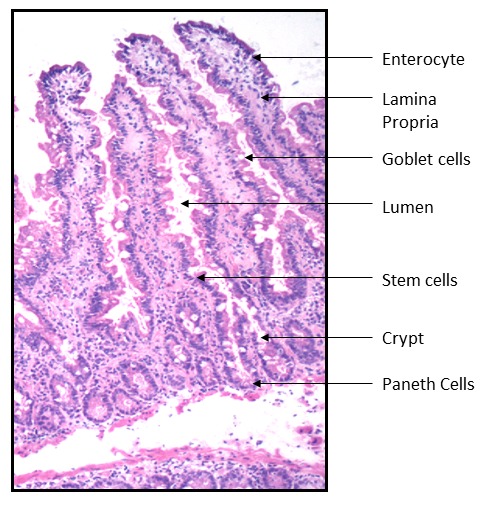
H&E staining of the jejunum. Goblet cells, Enterocytes and Paneth cells contribute to innate immunity. Lymphocytes present in the lamina propria and intra-epithelial areas provide acquired immunity. Image was obtained at 60X magnification.
One of the key characteristics of the GI tract epithelium is the rapid proliferation of cells that differentiate from immature stem cells, within the crypt, to terminally maturated cells, which move up the villus. In the murine model it has been observed that a state of hyperproliferation, not hypoproliferation, occurs in the gut mucosal epithelial cells of older (aged) rats compared to younger rats fed the same diet and with the same living conditions [34]. This increased turnover of epithelial cells was hypothesized to be due to increased loss of epithelial cells at the tips of the intestinal villi. Furthermore, abnormalities of the proliferative and differentiation responses became more evident when gastrointestinal tissues were stimulated by systemic injury.
Mucosal barrier function is essential to prevent potentially harmful pathogens within the gastrointestinal lumen, respiratory tract, and urogenital tract from gaining access to the body [35]. Increased microbial translocation into mucosal tissue and the blood stream results in increased systemic inflammatory cytokine production [36–39]. Cellular tight junctions (TJs) are dynamic structures located in the most apical region of cell-cell contact points and play a critical role in maintenance of epithelial barrier function, cell polarity, and intercellular adhesion [40, 41]. Tight junction proteins include zona occludens, occludin (OLCN), claudins (CLDNs) and others (Figure 2).
Figure 2.
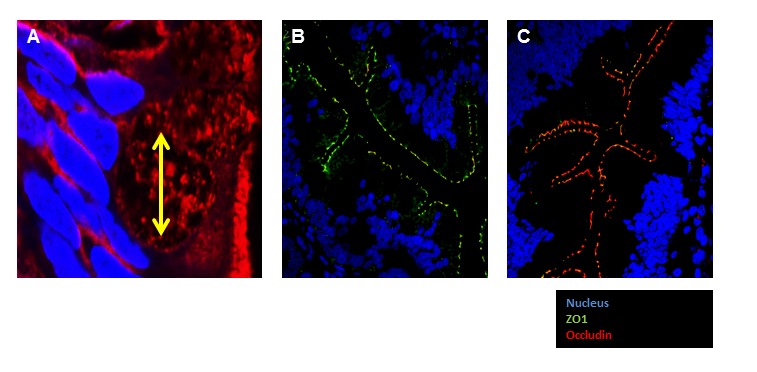
Fluorescent immunohistochemical staining of jejunum tight junction proteins. (A). Goblet cell diameter is marked in yellow. Nucleus: Blue Dapi, Cell Membrane: Orange mask. Image was obtained at 100X oil immersion confocal microscope. (B and C) IHC demonstrating the expression of Tight junction proteins, occluding (red) and ZO1 (Green) in small intestinal tissue (60X).
Disorders of the gastrointestinal tract, including increased incidence of diarrhea and constipation, are common in elderly people; however, the molecular mechanisms of aging that contribute to the vulnerability of the gastrointestinal tract have not been fully elucidated. Studies suggest that patients with gastrointestinal disorders have increased intestinal permeability. A study utilizing the baboon model has shown that gastrointestinal permeability was higher in colonic biopsies in aging monkeys [42]. Tight junction protein expression was decreased including zona occluden 1 (ZO-1), OCLN, and junctional adhesion molecule-A (JAM-A). Claudin 2 (CLDN2), a pore forming tight junction protein, expression was increased. Inflammatory cytokines interferon gamma (IFN-γ), interleukin 6 (IL-6), and interleukin 1 beta (IL-1β) were also found to be increased in colonic biopsies from old baboons compared to young baboons, and have previously been shown to directly hinder tight junction complex formation [14, 42, 43]. IL-1β also contributes to a disruption of tight junction integrity [44]. IL-1β levels are consistently higher in older individuals as well as during the course of systemic inflammation and injuries like severe burns. IL-1 receptor (IL1R) also plays an important role in the maintenance of epithelial integrity in aging [43]. These studies indicate that increased colonic permeability via age-associated remodeling of intestinal epithelial tight junction proteins may be an important component of gastrointestinal dysfunction.
Collagen provides the connective tissue backbone to the OGI mucosa (Figure 3). The effect of aging on connective tissue structure, as it pertains to mucosal tissue injury and repair, has been extensively investigated in the rodent model. Connective tissue and collagen deposition in the rat gastric mucosa increases with aging [46]. Studies have shown that age-induced changes in gastric connective tissue structure lead to a decreased capacity for tissue repair in response to gastric acid [45]. Furthermore, accumulation of oxidative products, observed in the stomach of older rats, is hypothesized to contribute to thickening of connective tissue deposition and replacement of mucosal tissue in the lower part of the gastric mucosa [46]. It is known that the intestinal milieu of inflammatory cytokines and systemic circulation of sex hormones play a significant role in both fibroblast and keratinocyte migration and proliferation. These cell types are critical in the deposition and restructuring of collagen throughout the body. Changes in collagen deposition, composition, and restructuring in aging may impact not only function of the OGI mucosa but also response to injury.
Figure 3.
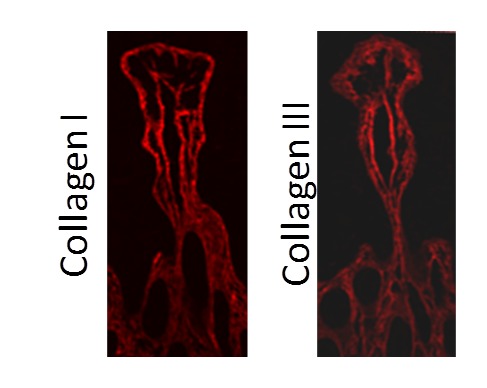
Collagen scaffold in small intestinal villus. Collagen is an important component of the connective tissue scaffold in the mucosa. Collagen I and III are demonstrated using histochemical staining in the jejunum (Collagen: red)
Sex hormones and mucosal barrier function
Mucosal surfaces are unique anatomical niches, as they are an interface between a sterile internal environment and a contaminated external environment [47, 48]. Mucosal sites require contact with the external environment to perform nutrient absorption in the small intestine, gas exchange in the lungs, water reabsorption in the colon etc. For this reason, one of the key functions of the epithelial cells which coat mucosal surfaces is to maintain barrier functions from the antigens of the external environment. The epithelial layer possesses polarity and requires close contact between the cells to function as an anatomical barrier. Maintenance of the barrier is critical for mucosal and systemic health, and is first to be damaged during external injury. Rapid healing of the epithelium and revival of barrier function is important in preventing ongoing immune activation and further infection at mucosal sites [49, 50]. With age, this process is slowed and results in some of the co-morbidities such as endometriosis, increased incidence of UTIs, diarrhea, as well as increased prevalence of pulmonary hypertension and protracted recovery from lung disease, associated with mucosal injuries in the aging population.
Disruption of TJ complexes is associated with a variety of human diseases including genital and gastric cancers, inflammatory bowel disease, and HIV infection [51–53]. Interestingly, sex hormones such as E2 play an important role in the maintenance of tight junctions. E2 levels decrease dramatically during the course of the menopausal transition [54–57]. E2 affects TJ formation, which can alter the level of bacterial translocation from any mucosal surface [58, 59]. A recent study showed that increased E2 in rats induced the expression of TJ protein OCLN via binding of ERβ [59]. This resulted in decreased intestinal epithelial permeability [59] and microbial translocation. Conversely, reduced levels of E2, as occurs with aging, particularly during female menopause, can potentially increase epithelial permeability and microbial translocation (Figure 4). No significant disparities are observed between the genders with regards to homeostatic epithelial barrier permeability [27]. Therefore, an E2-dependent mechanism for maintenance of the epithelial barrier may contribute equally in both males and females to mucosal integrity.
Figure 4.
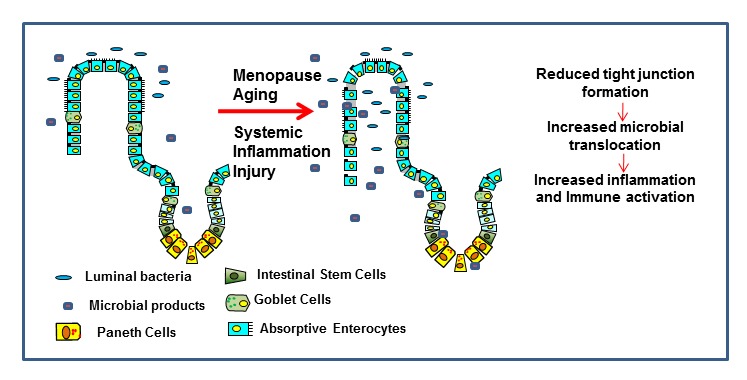
Schematic of the proposed effects of aging and hormonal changes. Proposed mechanism of the loss of mucosal epithelial integrity and microbial translocation and the effects of hormonal changes associated with aging.
Gender differences in OGI mucosal injury
Recently, several large-scale, cross-sectional epidemiological studies have been performed to determine whether there is gender-based skewing in populations of individuals with gastro-esophageal reflux diseases (GERD) [60–62]. The three conditions that comprise the majority of this spectrum include Barrett’s esophagus (BE), erosive reflux disease (ERD), and nonerosive reflux disease (non-ERD). Both Barrett’s esophagus and ERD are associated with erosive esophagitis and mucosal injury [61, 63]. Furthermore, it is generally accepted that there is a sequential progression from reflux erosive esophagitis, to Barrett’s esophagus, and finally to esophageal adenocarcinoma. Reflux erosive esophagitis results from exposure of the esophageal epithelium to the refluxed gastroduodenal contents. A male-predominant gender bias exists across the spectrum, although the ratios become higher with the progression towards esophageal adenocarcinoma. Meanwhile, nonerosive reflux diseases generally affect women more than men [60, 62]. These data allude to the gender differences in the vulnerability or resistance of the esophageal epithelium to caustic compounds of the gastroduodenal contents in males and females.
There have been reports that females are less affected than males by gastric or intestinal inflammation in response to chemical insult or bacterial infection [64–66].In these studies E2 was demonstrated to have anti-inflammatory activity and thus contributes to tissue resistance in females. A recent study by Masaka et al. explored the potential role of E2 in controlling esophageal tissue damage [63]. Employing a chronic rat reflux esophagitis model, a significant male-predominant, gender-related difference in esophageal tissue damage in the presence of exogenous nitric oxide (NO) as an exacerbating factor, was found [67]. While in the baseline model of reflux esophagitis macroscopic esophageal ulcers and microscopic inflammatory cell infiltrates were only mildly observed in both genders, in males, exogenous NO exacerbation induced deep esophageal ulcers and intense inflammation with polymophonuclear cell and lymphocyte infiltrates. In contrast, in female reflux esophasitis models, treatment with NO rarely exacerbated the mild tissue damage observed at baseline. Further, exogenous 17β-estradiol binding and signaling through E2 receptors attenuated esophageal tissue damage in males and ovariectomised rats via a reduction of mast cell-mediated cytotoxity and cytokine, specifically tumor necrosis factor alpha (TNFα), driven inflammation [63, 68]. Treatment with 17α-estradiol, which binds but does not induce downstream signaling, had no effect on tissue damage. This was the first study showing the prominent gender difference in the severity of esophageal tissue damage in a GERD-related animal model. Additional understanding of the causative luminal or genetic factors in yielding the gender-related difference would be clinically relevant to predict the etiological factors involved in the pathogenesis of reflux esophagitis in humans [69, 70].
Sex hormones in tissue repair
Gender variation in wound healing has been observed at various mucosal sites throughout the body. Specifically, variations in healing rates have been attributed to levels of circulating sex hormones and their effect on modulation of inflammation as well as fibroblast and keratinocytes cellular proliferation, differentiation and growth [71–73]. It has been established that both dermal and mucosal wound healing is significantly altered by the effects of E2 signaling [74–76]. Kumral et al. recently showed that gastric and colonic tissue damage is alleviated by E2, via both ERα and ERβ mediation as well as direct antioxidant effects [76]. In addition to its role in inflammatory regulation and re-epithelialization, an epidemiological study by Tuo et al. linked higher circulating E2 levels in women to the elevated production of duodenal mucosa bicarbonate secretion (DBS), a compound central in duodenal mucosal protection against acid-induced injury [77]. Duodenal ulcers are less prevalent in premenopausal women, compared to age matched men or post-menopausal women [78, 79]. In their findings, ex vivo stimulation of duodenal tissues with 17β-estradiol did not result in a difference in the levels of DBS secretion. The authors hypothesized that this stemmed from the result that males and females express similar levels of ERα and ERβ on duodenal epithelial cell surface [77]. This highlights that the observed sex differences of DBS were likely due to the gender differences in circulating E2 levels rather than a dimorphism in expression levels of E2 receptors between different sexes, and can possibly be extrapolated to the other effects of E2.
Likewise, metabolites of testosterone, as well as other androgens [5α-dihydrotestosterone (DHT) and Dehydroepiandrosterone (DHEA)], have been shown to affect dermal wound closure by impairing reepithelialization and inducing immunosuppressive effects [80]. As in dermal wounds, a study by Engeland et al. reported that in oral mucosal damage testosterone levels were inversely correlated with wound healing rates in premenopausal women and age matched males. Conversely, in post-menopausal women a positive correlation of testosterone levels and wound healing rates was observed [81]. It was hypothesized that the immunomodulatory role of testosterone in reducing IL-6, which is mitogenic to keratinocytes, contributes to the effect observed in premenopausal women and men. The effect observed in post-menopausal women was not linked to age specifically, but hormonal status. Authors put forth the idea that with the increased immune activation observed in post-menopausal women at baseline, higher levels of anti-inflammatory testosterone decreased this activation thus being beneficial to tissue healing [82, 83].
Estrogen and Urinary Tract Infections
While UTIs are most prevalent in females aged 18–24, a significant number of women over 50 still contract UTIs [84]. Recurrent infections in healthy, aging women ages 50–70 have been linked to decreased levels of estradiol [85]. After menopause, decreased levels of E2 cause vulvovaginal atrophy in 25–50% of women [13]. Symptoms such as vaginal dryness, itching, increase in vaginal pH, urinary frequency and incontinence, contribute to the impairment of defenses against incoming pathogens at the urogenital mucosa [86]. Thus estradiol supplementation has been considered as a way to decrease the risk of recurrent infections in the postmenopausal population and has demonstrated moderate success [87].
One of the mechanisms by which estradiol therapy in post-menopausal women has proven successful may be related to tight junction formation enhancement by E2. Numerous studies have shown that estrogen treatment, in vitro, increases tight junction protein expression including ZO1 and CLDN in the vaginal epithelium [88]. In both, a urothelial cell line and exfoliated bladder cells from postmenopausal women, estradiol treatment increased transcripts of ZO1 and OCLN as well as e-cadherin protein [86]. This demonstrates that estrogen’s beneficial effects on tight junction proteins may occur on mucosal surfaces outside of the vagina [86]. Estrogen-mediated restoration of a diminished antimicrobial response in post-menopausal women could also contribute to the decreases seen in UTIs following treatment. When post-menopausal women were given estradiol supplementation for two weeks, 75% showed increases in at least three antimicrobial peptides in urinary tract cells. The most highly increased peptides were beta-defensin 3 (hBD3), beta-defensin 1 (hBD1), and RNase 7 [86].
An alternate mechanism by which estradiol supplementation can contribute to urogential mucosal health in post-menopausal women is via its role in vaginal pH regulation. A number of theories have been proposed for the contribution of E2 toward vaginal pH control, including direct effects on the epithelium and altering vaginal microflora populations [89, 90]. In healthy, young, menstruating women vaginal microflora is dominated by Lactobacilli which produce lactic acid, hydrogen peroxide, and various bacterial proteins which together inhibit overgrowth of any pathogenic bacterial species [91]. Thus, the vaginal microflora confers protection against pathogens which are associated with UTIs. Estrogen has been shown to promote lactobacilli colonization and growth by increasing storage of glycogen, the substrate for acid production by bacteria, in vaginal epithelial cells [90]. With a decline in E2 levels during menopause, along with decreased glycogen stores and quantity of lactobacilli, acid production decreases leading to an elevation in vaginal pH, which facilitates growth of potential UTI-causing pathogens.
Sex Hormones in Lung Disease
While the prevalence of chronic obstructive pulmonary disease (COPD) is greater in men, women appear to be more sensitive to the effects of cigarette smoke in developing COPD, often developing COPD after smoking less than men [92]. This may be related to an E2-mediated difference in metabolism of cigarette smoke toxins at the lung mucosa. Estradiol increases the activity of cytochrome P450 (CPY) enzymes, which break down toxins but create harmful metabolites which can be more toxic than their precursors [93]. Because complete metabolism of these toxins requires a rate limiting process which is not increased by E2, harmful intermediate metabolites may remain in the lungs of women smokers longer than their male counterparts, increasing lung damage and resulting in poorer clinical outcomes.
A gender discrepancy in outcomes of cystic fibrosis (CF) patients has been seen clinically, with females having much higher early mortality rates than males [94]. This may be due to an E2-mediated increase in mucus production, a principal determinant of mortality in CF patients. Estradiol has been shown to increase mucin gene expression in lung epithelium, which may result in increased mucus overall [95]. Additionally, female CF patients show lowest lung function just before ovulation, suggesting that this surge of E2 may be enhancing mucus production and thereby decreasing lung function [96].
Taken together, these studies demonstrate the wide variety of ways E2 modulates lung function and affects disease morbidity. In some instances, modulation of sex hormones may be a viable clinical intervention worthy of further investigation.
Summary
Aging and sex differences play an important role in the development of mucosal injury as well as its repair. The role of sex hormones is controversial at best. The part that is clear is that aging mucosal surfaces are extremely susceptible to injury. New studies indicate that the microbiome may also play an important role in mucosal health. This too is a rapidly developing area of research that is poorly is understood and likely plays a role in healthy aging. The molecular mechanisms that regulate aging are poorly understood. Taken together comprehensive studies using relevant animal models are needed to better understand the interplay between mucosal injury, gender, sex hormones and aging.
Acknowledgments
We would like to thank the Department of Medical Microbiology and Immunology, Professor and Chair, Dr. Satya Dandekar for her support. Dr. Sankaran is supported by a Building Interdisciplinary Research Careers in Women’s Health award (K12 HD051958) funded by the NICHD, ORWH, and the NIA. Irinia Grishina is supported by California HIV Research Program: D10-D-303.
References
- [1].Vincent GK, Velkoff VA.(May 2010THE NEXT FOUR DECADESThe Older Population in the United States: 2010 to 2050 Population Estimates and Projections 14 US Census Bureau [Google Scholar]
- [2].Hussain A, Rivers PA. Confronting the challenges of long-term health care crisis in the United States. J Health Care Finance. 2009;36:71–82. [PubMed] [Google Scholar]
- [3].Waite LJ. The demographic face of America’s elderly. Inquiry. 1996;33:220–224. [PubMed] [Google Scholar]
- [4].Ultori C, Cimetti L, Stefanoni P, Pellegrini R, Rapazzini P, Capella C. Merkel cell carcinoma in elderly: case report and review of the literature. Aging Clin Exp Res. 2013;25:211–214. doi: 10.1007/s40520-013-0020-2. [DOI] [PubMed] [Google Scholar]
- [5].Taffet GE, Donohue JF, Altman PR. Considerations for managing chronic obstructive pulmonary disease in the elderly. Clin Interv Aging. 2014;9:23–30. doi: 10.2147/CIA.S52999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kwak HB. Effects of aging and exercise training on apoptosis in the heart. J Exerc Rehabil. 2013;9:212–219. doi: 10.12965/jer.130002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Britton E, McLaughlin JT. Ageing and the gut. Proc Nutr Soc. 2013;72:173–177. doi: 10.1017/S0029665112002807. [DOI] [PubMed] [Google Scholar]
- [8].Nair S, Ren J. Autophagy and cardiovascular aging: lesson learned from rapamycin. Cell Cycle. 2012;11:2092–2099. doi: 10.4161/cc.20317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Taniguchi T, Tsuha S, Takayama Y, Shiiki S. Shaking chills and high body temperature predict bacteremia especially among elderly patients. Springerplus. 2013;2:624. doi: 10.1186/2193-1801-2-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Heppner HJ, Cornel S, Peter W, Philipp B, Katrin S. Infections in the elderly. Crit Care Clin. 2013;29:757–774. doi: 10.1016/j.ccc.2013.03.016. [DOI] [PubMed] [Google Scholar]
- [11].Becher A, Dent J. Systematic review: ageing and gastro-oesophageal reflux disease symptoms, oesophageal function and reflux oesophagitis. Aliment Pharmacol Ther. 2011;33:442–454. doi: 10.1111/j.1365-2036.2010.04542.x. [DOI] [PubMed] [Google Scholar]
- [12].Poh CH, Navarro-Rodriguez T, Fass R. Review: treatment of gastroesophageal reflux disease in the elderly. Am J Med. 2010;123:496–501. doi: 10.1016/j.amjmed.2009.07.036. [DOI] [PubMed] [Google Scholar]
- [13].Sturdee DW, Panay N. Recommendations for the management of postmenopausal vaginal atrophy. Climacteric. 2010;13:509–522. doi: 10.3109/13697137.2010.522875. [DOI] [PubMed] [Google Scholar]
- [14].Tran L, Greenwood-Van Meerveld B. Age-associated remodeling of the intestinal epithelial barrier. J Gerontol A Biol Sci Med Sci. 2013;68:1045–1056. doi: 10.1093/gerona/glt106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Grossman C. Possible underlying mechanisms of sexual dimorphism in the immune response, fact and hypothesis. J Steroid Biochem. 1989;34:241–251. doi: 10.1016/0022-4731(89)90088-5. [DOI] [PubMed] [Google Scholar]
- [16].Olsen NJ, Kovacs WJ. Gonadal steroids and immunity. Endocr Rev. 1996;17:369–384. doi: 10.1210/edrv-17-4-369. [DOI] [PubMed] [Google Scholar]
- [17].Velders M, Schleipen B, Fritzemeier KH, Zierau O, Diel P. Selective estrogen receptor-beta activation stimulates skeletal muscle growth and regeneration. FASEB J. 2012;26:1909–1920. doi: 10.1096/fj.11-194779. [DOI] [PubMed] [Google Scholar]
- [18].Bird MD, Karavitis J, Kovacs EJ. Sex differences and estrogen modulation of the cellular immune response after injury. Cell Immunol. 2008;252:57–67. doi: 10.1016/j.cellimm.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Buchanan DL, Kurita T, Taylor JA, Lubahn DB, Cunha GR, Cooke PS. Role of stromal and epithelial estrogen receptors in vaginal epithelial proliferation, stratification, and cornification. Endocrinology. 1998;139:4345–4352. doi: 10.1210/endo.139.10.6241. [DOI] [PubMed] [Google Scholar]
- [20].Hildebrand F, Hubbard WJ, Choudhry MA, Thobe BM, Pape HC, Chaudry IH. Are the protective effects of 17beta-estradiol on splenic macrophages and splenocytes after trauma-hemorrhage mediated via estrogen-receptor (ER)-alpha or ER-beta? J Leukoc Biol. 2006;79:1173–1180. doi: 10.1189/jlb.0106029. [DOI] [PubMed] [Google Scholar]
- [21].Kovats S, Carreras E. Regulation of dendritic cell differentiation and function by estrogen receptor ligands. Cell Immunol. 2008;252:81–90. doi: 10.1016/j.cellimm.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Seko K, Kagami H, Senga K, Ozeki K, Mizutani H, Ueda M. Effects of ovariectomy and estrogen replacement on rat oral mucosa. Maturitas. 2005;50:44–51. doi: 10.1016/j.maturitas.2004.03.015. [DOI] [PubMed] [Google Scholar]
- [23].Degano B. [The effect of estrogens on the permeability of the bronchial mucosa] Annales de dermatologie et de venereologie. 1998;125(Suppl 2):S21–22. [PubMed] [Google Scholar]
- [24].Diebel ME, Diebel LN, Liberati DM. Gender dimorphism in the gut: mucosal protection by estrogen stimulation of IgA transcytosis. J Trauma. 2011;71:474–479. doi: 10.1097/TA.0b013e318228239d. [DOI] [PubMed] [Google Scholar]
- [25].Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- [26].Morley JE, Kaiser FE, Perry HM, 3rd, Patrick P, Morley PM, Stauber PM, et al. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metabolism. 1997;46:410–413. doi: 10.1016/s0026-0495(97)90057-3. [DOI] [PubMed] [Google Scholar]
- [27].Fimmel S, Zouboulis CC. Influence of physiological androgen levels on wound healing and immune status in men. Aging Male. 2005;8:166–174. doi: 10.1080/13685530500233847. [DOI] [PubMed] [Google Scholar]
- [28].Schroder J, Kahlke V, Staubach KH, Zabel P, Stuber F. Gender differences in human sepsis. Arch Surg. 1998;133:1200–1205. doi: 10.1001/archsurg.133.11.1200. [DOI] [PubMed] [Google Scholar]
- [29].Gornstein RA, Lapp CA, Bustos-Valdes SM, Zamorano P. Androgens modulate interleukin-6 production by gingival fibroblasts in vitro. J Periodontol. 1999;70:604–609. doi: 10.1902/jop.1999.70.6.604. [DOI] [PubMed] [Google Scholar]
- [30].Messingham KA, Shirazi M, Duffner LA, Emanuele MA, Kovacs EJ. Testosterone receptor blockade restores cellular immunity in male mice after burn injury. J Endocrinol. 2001;169:299–308. doi: 10.1677/joe.0.1690299. [DOI] [PubMed] [Google Scholar]
- [31].Macal M, Sankaran S, Chun TW, Reay E, Flamm J, Prindiville TJ, et al. Effective CD4+ T-cell restoration in gut-associated lymphoid tissue of HIV-infected patients is associated with enhanced Th17 cells and polyfunctional HIV-specific T-cell responses. Mucosal Immunol. 2008;1:475–488. doi: 10.1038/mi.2008.35. [DOI] [PubMed] [Google Scholar]
- [32].Ikuma M, Hanai H, Kaneko E, Hayashi H, Hoshi T. Effects of aging on the microclimate pH of the rat jejunum. Biochimica et biophysica acta. 1996;1280:19–26. doi: 10.1016/0005-2736(95)00261-8. [DOI] [PubMed] [Google Scholar]
- [33].Choi SH, Kornegay ET, Eigel WN. Characterization of small intestinal mucus glycoproteins from pigs of various ages. Comparative biochemistry and physiology. A, Comparative physiology. 1991;99:677–680. doi: 10.1016/0300-9629(91)90149-7. [DOI] [PubMed] [Google Scholar]
- [34].Holt PR, Yeh KY. Small intestinal crypt cell proliferation rates are increased in senescent rats. J Gerontol. 1989;44:B9–14. doi: 10.1093/geronj/44.1.b9. [DOI] [PubMed] [Google Scholar]
- [35].Viswanathan VK, Hecht G. Innate immunity and the gut. Curr Opin Gastroenterol. 2000;16:546–551. doi: 10.1097/00001574-200011000-00015. [DOI] [PubMed] [Google Scholar]
- [36].Balzan S, de Almeida Quadros C, de Cleva R, Zilberstein B, Cecconello I. Bacterial translocation: overview of mechanisms and clinical impact. J Gastroenterol Hepatol. 2007;22:464–471. doi: 10.1111/j.1440-1746.2007.04933.x. [DOI] [PubMed] [Google Scholar]
- [37].Aloi M, Cucchiara S. Extradigestive manifestations of IBD in pediatrics. Eur Rev Med Pharmacol Sci. 2009;13(Suppl 1):23–32. [PubMed] [Google Scholar]
- [38].Inagaki-Ohara K, Sasaki A, Matsuzaki G, Ikeda T, Hotokezaka M, Chijiiwa K, et al. Suppressor of cytokine signalling 1 in lymphocytes regulates the development of intestinal inflammation in mice. Gut. 2006;55:212–219. doi: 10.1136/gut.2004.062653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].McGuckin MA, Eri R, Simms LA, Florin TH, Radford-Smith G. Intestinal barrier dysfunction in inflammatory bowel diseases. Inflamm Bowel Dis. 2009;15:100–113. doi: 10.1002/ibd.20539. [DOI] [PubMed] [Google Scholar]
- [40].Marchiando AM, Graham WV, Turner JR. Epithelial barriers in homeostasis and disease. Annu Rev Pathol. 2010;5:119–144. doi: 10.1146/annurev.pathol.4.110807.092135. [DOI] [PubMed] [Google Scholar]
- [41].Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- [42].Tran L, Greenwood-Van Meerveld B. Age-associated remodeling of the intestinal epithelial barrier. J Gerontol A Biol Sci Med Sci. 2013;68:1045–1056. doi: 10.1093/gerona/glt106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Song J, Wolf SE, Wu XW, Finnerty CC, Herndon DN, Jeschke MG. Proximal gut mucosal epithelial homeostasis in aged IL-1 type I receptor knockout mice after starvation. The Journal of surgical research. 2011;169:209–213. doi: 10.1016/j.jss.2010.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Al-Sadi R, Ye D, Said HM, Ma TY. Cellular and molecular mechanism of interleukin-1beta modulation of Caco-2 intestinal epithelial tight junction barrier. J Cell Mol Med. 2011;15:970–982. doi: 10.1111/j.1582-4934.2010.01065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Newton JL. Changes in upper gastrointestinal physiology with age. Mech Ageing Dev. 2004;125:867–870. doi: 10.1016/j.mad.2004.05.007. [DOI] [PubMed] [Google Scholar]
- [46].Kang JM, Kim N, Kim JH, Oh E, Lee BY, Lee BH, et al. Effect of aging on gastric mucosal defense mechanisms: ROS, apoptosis, angiogenesis, and sensory neurons. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1147–1153. doi: 10.1152/ajpgi.00218.2010. [DOI] [PubMed] [Google Scholar]
- [47].Bengmark S. Gut microenvironment and immune function. Curr Opin Clin Nutr Metab Care. 1999;2:83–85. doi: 10.1097/00075197-199901000-00014. [DOI] [PubMed] [Google Scholar]
- [48].Westermann J, Engelhardt B, Hoffmann JC. Migration of T cells in vivo: molecular mechanisms and clinical implications. Ann Intern Med. 2001;135:279–295. doi: 10.7326/0003-4819-135-4-200108210-00013. [DOI] [PubMed] [Google Scholar]
- [49].Frank JA. Claudins and alveolar epithelial barrier function in the lung. Ann N Y Acad Sci. 2012;1257:175–183. doi: 10.1111/j.1749-6632.2012.06533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Iizuka M, Konno S. Wound healing of intestinal epithelial cells. World J Gastroenterol. 17:2161–2171. doi: 10.3748/wjg.v17.i17.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Zhang JB, Du XG, Zhang H, Li ML, Xiao G, Wu J, et al. Breakdown of the gut barrier in patients with multiple organ dysfunction syndrome is attenuated by continuous blood purification: effects on tight junction structural proteins. Int J Artif Organs. 2010;33:5–14. [PubMed] [Google Scholar]
- [52].Shen L, Su L, Turner JR. Mechanisms and functional implications of intestinal barrier defects. Dig Dis. 2009;27:443–449. doi: 10.1159/000233282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Edelblum KL, Turner JR. The tight junction in inflammatory disease: communication breakdown. Curr Opin Pharmacol. 2009;9:715–720. doi: 10.1016/j.coph.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Burger HG, Hale GE, Robertson DM, Dennerstein L. A review of hormonal changes during the menopausal transition: focus on findings from the Melbourne Women’s Midlife Health Project. Hum Reprod Update. 2007;13:559–565. doi: 10.1093/humupd/dmm020. [DOI] [PubMed] [Google Scholar]
- [55].Arnal JF, Laurell H, Fontaine C, Billon A, Calippe B, Lenfant F, et al. Estrogen receptor actions on vascular biology and inflammation: implications in vascular pathophysiology. Climacteric. 2009;12(Suppl 1):12–17. doi: 10.1080/13697130902820006. [DOI] [PubMed] [Google Scholar]
- [56].Al-Azzawi F, Palacios S. Hormonal changes during menopause. Maturitas. 2009;63:135–137. doi: 10.1016/j.maturitas.2009.03.009. [DOI] [PubMed] [Google Scholar]
- [57].Wierman ME, Kohrt WM. Vascular and metabolic effects of sex steroids: new insights into clinical trials. Reprod Sci. 2007;14:300–314. doi: 10.1177/1933719107303673. [DOI] [PubMed] [Google Scholar]
- [58].Braniste V, Jouault A, Gaultier E, Polizzi A, Buisson-Brenac C, Leveque M, et al. Impact of oral bisphenol A at reference doses on intestinal barrier function and sex differences after perinatal exposure in rats. Proc Natl Acad Sci U S A. 2010;107:448–453. doi: 10.1073/pnas.0907697107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Braniste V, Leveque M, Buisson-Brenac C, Bueno L, Fioramonti J, Houdeau E. Oestradiol decreases colonic permeability through oestrogen receptor beta-mediated up-regulation of occludin and junctional adhesion molecule-A in epithelial cells. The Journal of physiology. 2009;587:3317–3328. doi: 10.1113/jphysiol.2009.169300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Cook MB, Wild CP, Forman D. A systematic review and meta-analysis of the sex ratio for Barrett’s esophagus, erosive reflux disease, and nonerosive reflux disease. Am J Epidemiol. 2005;162:1050–1061. doi: 10.1093/aje/kwi325. [DOI] [PubMed] [Google Scholar]
- [61].Ford AC, Forman D, Reynolds PD, Cooper BT, Moayyedi P. Ethnicity, gender, and socioeconomic status as risk factors for esophagitis and Barrett’s esophagus. Am J Epidemiol. 2005;162:454–460. doi: 10.1093/aje/kwi218. [DOI] [PubMed] [Google Scholar]
- [62].Lin M, Gerson LB, Lascar R, Davila M, Triadafilopoulos G. Features of gastroesophageal reflux disease in women. Am J Gastroenterol. 2004;99:1442–1447. doi: 10.1111/j.1572-0241.2004.04147.x. [DOI] [PubMed] [Google Scholar]
- [63].Masaka T, Iijima K, Endo H, Asanuma K, Ara N, Ishiyama F, et al. Gender differences in oesophageal mucosal injury in a reflux oesophagitis model of rats. Gut. 2013;62:6–14. doi: 10.1136/gutjnl-2011-301389. [DOI] [PubMed] [Google Scholar]
- [64].Fox JG, Rogers AB, Ihrig M, Taylor NS, Whary MT, Dockray G, et al. Helicobacter pylori-associated gastric cancer in INS-GAS mice is gender specific. Cancer research. 2003;63:942–950. [PubMed] [Google Scholar]
- [65].Houdeau E, Moriez R, Leveque M, Salvador-Cartier C, Waget A, Leng L, et al. Sex steroid regulation of macrophage migration inhibitory factor in normal and inflamed colon in the female rat. Gastroenterology. 2007;132:982–993. doi: 10.1053/j.gastro.2006.12.028. [DOI] [PubMed] [Google Scholar]
- [66].Kruidenier L, van Meeteren ME, Kuiper I, Jaarsma D, Lamers CB, Zijlstra FJ, et al. Attenuated mild colonic inflammation and improved survival from severe DSS-colitis of transgenic Cu/Zn-SOD mice. Free Radic Biol Med. 2003;34:753–765. doi: 10.1016/s0891-5849(02)01426-0. [DOI] [PubMed] [Google Scholar]
- [67].Ishiyama F, Iijima K, Asanuma K, Ara N, Yoshitake J, Abe Y, et al. Exogenous luminal nitric oxide exacerbates esophagus tissue damage in a reflux esophagitis model of rats. Scand J Gastroenterol. 2009;44:527–537. doi: 10.1080/00365520802699260. [DOI] [PubMed] [Google Scholar]
- [68].Verdu EF, Deng Y, Bercik P, Collins SM. Modulatory effects of estrogen in two murine models of experimental colitis. Am J Physiol Gastrointest Liver Physiol. 2002;283:G27–36. doi: 10.1152/ajpgi.00460.2001. [DOI] [PubMed] [Google Scholar]
- [69].Shenderov BA. Gut indigenous microbiota and epigenetics. Microb Ecol Health Dis. 2012:23. doi: 10.3402/mehd.v23i0.17195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kang GH, Lee HJ, Hwang KS, Lee S, Kim JH, Kim JS. Aberrant CpG island hypermethylation of chronic gastritis, in relation to aging, gender, intestinal metaplasia, and chronic inflammation. Am J Pathol. 2003;163:1551–1556. doi: 10.1016/S0002-9440(10)63511-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Mealey BL, Moritz AJ. Hormonal influences: effects of diabetes mellitus and endogenous female sex steroid hormones on the periodontium. Periodontol. 2003;2000;32:59–81. doi: 10.1046/j.0906-6713.2002.03206.x. [DOI] [PubMed] [Google Scholar]
- [72].Bhardwaj A, Bhardwaj SV. Effect of menopause on women’s periodontium. J Midlife Health. 2012;3:5–9. doi: 10.4103/0976-7800.98810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Gilliver SC, Ruckshanthi JP, Hardman MJ, Nakayama T, Ashcroft GS. Sex dimorphism in wound healing: the roles of sex steroids and macrophage migration inhibitory factor. Endocrinology. 2008;149:5747–5757. doi: 10.1210/en.2008-0355. [DOI] [PubMed] [Google Scholar]
- [74].Campbell L, Emmerson E, Davies F, Gilliver SC, Krust A, Chambon P, et al. Estrogen promotes cutaneous wound healing via estrogen receptor beta independent of its antiinflammatory activities. J Exp Med. 2010;207:1825–1833. doi: 10.1084/jem.20100500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Gilliver SC, Emmerson E, Campbell L, Chambon P, Hardman MJ, Ashcroft GS. 17beta-estradiol inhibits wound healing in male mice via estrogen receptor-alpha. Am J Pathol. 2010;176:2707–2721. doi: 10.2353/ajpath.2010.090432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Kumral ZN, Memi G, Ercan F, Yegen BC. Estrogen Alleviates Acetic Acid-Induced Gastric or Colonic Damage via Both ERalpha- and ERbeta-Mediated and Direct Antioxidant Mechanisms in Rats. Inflammation, in press. 2013 doi: 10.1007/s10753-013-9786-9. [DOI] [PubMed] [Google Scholar]
- [77].Tuo B, Wen G, Wei J, Liu X, Wang X, Zhang Y, et al. Estrogen regulation of duodenal bicarbonate secretion and sex-specific protection of human duodenum. Gastroenterology. 2011;141:854–863. doi: 10.1053/j.gastro.2011.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Kurata JH, Honda GD, Frankl H. The incidence of duodenal and gastric ulcers in a large health maintenance organization. Am J Public Health. 1985;75:625–629. doi: 10.2105/ajph.75.6.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Rosenstock SJ, Jorgensen T. Prevalence and incidence of peptic ulcer disease in a Danish County--a prospective cohort study. Gut. 1995;36:819–824. doi: 10.1136/gut.36.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Gilliver SC, Ruckshanthi JP, Hardman MJ, Zeef LA, Ashcroft GS. 5alpha-dihydrotestosterone (DHT) retards wound closure by inhibiting re-epithelialization. J Pathol. 2009;217:73–82. doi: 10.1002/path.2444. [DOI] [PubMed] [Google Scholar]
- [81].Engeland CG, Sabzehei B, Marucha PT. Sex hormones and mucosal wound healing. Brain Behav Immun. 2009;23:629–635. doi: 10.1016/j.bbi.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Hum Reprod Update. 2005;11:411–423. doi: 10.1093/humupd/dmi008. [DOI] [PubMed] [Google Scholar]
- [83].Kovacs EJ. Aging, traumatic injury, and estrogen treatment. Exp Gerontol. 2005;40:549–555. doi: 10.1016/j.exger.2005.04.009. [DOI] [PubMed] [Google Scholar]
- [84].Foxman B, Somsel P, Tallman P, Gillespie B, Raz R, Colodner R, et al. Urinary tract infection among women aged 40 to 65: behavioral and sexual risk factors. J Clin Epidemiol. 2001;54:710–718. doi: 10.1016/s0895-4356(00)00352-8. [DOI] [PubMed] [Google Scholar]
- [85].Stamm WE, Raz R. Factors contributing to susceptibility of postmenopausal women to recurrent urinary tract infections. Clin Infect Dis. 1999;28:723–725. doi: 10.1086/515209. [DOI] [PubMed] [Google Scholar]
- [86].Luthje P, Linden Hirschberg A, Brauner A. Estrogenic action on innate defense mechanisms in the urinary tract. Maturitas. 2014;77:32–36. doi: 10.1016/j.maturitas.2013.10.018. [DOI] [PubMed] [Google Scholar]
- [87].Perrotta C, Aznar M, Mejia R, Albert X, Ng CW. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Obstet Gynecol. 2008;112:689–690. doi: 10.1097/AOG.0b013e318185f7a5. [DOI] [PubMed] [Google Scholar]
- [88].Gorodeski GI. Aging and estrogen effects on transcervical-transvaginal epithelial permeability. J Clin Endocrinol Metab. 2005;90:345–351. doi: 10.1210/jc.2004-1223. [DOI] [PubMed] [Google Scholar]
- [89].Aroutcheva A, Gariti D, Simon M, Shott S, Faro J, Simoes JA, et al. Defense factors of vaginal lactobacilli. Am J Obstet Gynecol. 2001;185:375–379. doi: 10.1067/mob.2001.115867. [DOI] [PubMed] [Google Scholar]
- [90].Baldassarre M, Giannone FA, Foschini MP, Battaglia C, Busacchi P, Venturoli S, et al. Effects of long-term high dose testosterone administration on vaginal epithelium structure and estrogen receptor-alpha and - beta expression of young women. Int J Impot Res. 2013;25:172–177. doi: 10.1038/ijir.2013.9. [DOI] [PubMed] [Google Scholar]
- [91].Gupta K, Stapleton AE, Hooton TM, Roberts PL, Fennell CL, Stamm WE. Inverse association of H2O2-producing lactobacilli and vaginal Escherichia coli colonization in women with recurrent urinary tract infections. J Infect Dis. 1998;178:446–450. doi: 10.1086/515635. [DOI] [PubMed] [Google Scholar]
- [92].Gillum RF. Frequency of attendance at religious services and cigarette smoking in American women and men: the Third National Health and Nutrition Examination Survey. Prev Med. 2005;41:607–613. doi: 10.1016/j.ypmed.2004.12.006. [DOI] [PubMed] [Google Scholar]
- [93].Tam TW, Akhtar H, Arnason JT, Cvijovic K, Boon H, Cameron DW, et al. Inhibition of human cytochrome p450 metabolism by blended herbal products and vitamins. J Pharm Pharm Sci. 2011;14:1–16. doi: 10.18433/j3n30w. [DOI] [PubMed] [Google Scholar]
- [94].Kerem E, Reisman J, Corey M, Canny GJ, Levison H. Prediction of mortality in patients with cystic fibrosis. N Engl J Med. 1992;326:1187–1191. doi: 10.1056/NEJM199204303261804. [DOI] [PubMed] [Google Scholar]
- [95].Choi SM, Seo MJ, Lee YG, Lee MJ, Jeon HJ, Kang KK, et al. Effects of DA-6034, a flavonoid derivative, on mucin-like glycoprotein and ocular surface integrity in a rabbit model. Arzneimittelforschung. 2009;59:498–503. doi: 10.1055/s-0031-1296433. [DOI] [PubMed] [Google Scholar]
- [96].Johannesson M, Ludviksdottir D, Janson C. Lung function changes in relation to menstrual cycle in females with cystic fibrosis. Respir Med. 2000;94:1043–1046. doi: 10.1053/rmed.2000.0891. [DOI] [PubMed] [Google Scholar]


