Abstract
We summarized the data related to foods high in saturated fat and risk of mortality. We searched Cochrane Library, MEDLINE, EMBASE, and ProQuest for studies from January 1952 to May 2012. We identified 26 publications with individual dietary data and all-cause, total cancer, or cardiovascular mortality as endpoints.
Pooled relative risk estimates demonstrated that high intakes of milk, cheese, yogurt, and butter were not associated with a significantly increased risk of mortality compared with low intakes. High intakes of meat and processed meat were significantly associated with an increased risk of mortality but were associated with a decreased risk in a subanalysis of Asian studies. The overall quality of studies was variable.
Associations varied by food group and population. This may be because of factors outside saturated fat content of individual foods. There is an ongoing need for improvement in assessment tools and methods that investigate food sources of saturated fat and mortality to inform dietary guidelines.
National dietary guidelines typically promote foods low in saturated fat.1–3 These guidelines have arisen from early epidemiological studies showing that increased serum cholesterol was associated with increased risk of cardiovascular disease (CVD) and feeding studies showing that some, but not all, saturated fats increased serum cholesterol in comparison with unsaturated fats.4,5 However, the effects of diet on CVD can be mediated through pathways other than total serum cholesterol or low-density lipoprotein cholesterol,6 and the use of intermediate measures such as cholesterol as outcomes could be misleading. Restriction of saturated fat is now being questioned,7 with a recent meta-analysis showing that intake of saturated fats was not significantly associated with an increased risk of CVD.8
As awareness of the relationship between diet, nutrition, and health increases in the general public, it is imperative that the dietary advice of health professionals be evidence based and reflect current scientific understanding. The recent debate regarding intake of saturated fats and risk of disease highlights 2 important questions for research.9 First, should dietary nutrients be considered in isolation? People consume foods, not individual nutrients. Thus, the effect of saturated fat needs to be considered in the context of its food sources. Individual saturated fatty acids may have different effects on mortality risk; for example, the type of saturated fat found in dairy products may be protective for chronic disease.10–13 Second, are individual biological markers sufficient measures of risk compared with clinical endpoints such as mortality, which give a more definitive outcome? We conducted a meta-analysis of cohort studies reporting the relationship between key food groups typically high in saturated fatty acids and mortality in initially healthy adults. We tested the null hypothesis that there would be no significant association of saturated fat sources with all-cause, CVD, or cancer mortality.
METHODS
We searched the bibliographical databases of the Cochrane Library, MEDLINE, Embase, ProQuest, and ProQuest Dissertations and Theses for studies published in journals from January 1952 to May 2012. We adapted the search queries for use in each database and included alternative spellings of search terms where appropriate. We conducted searches using the following search algorithm (as formatted for Ovid): (dairy OR milk OR cheese OR yogurt OR butter OR meat OR animal flesh OR animal organ OR palm oil OR soybean oil OR cocoa OR chocolate OR lard OR tallow OR coconut) AND (mortality OR cancer OR coronary heart disease OR cardiovascular), limited to (human AND [adult < 18–64 years > OR aged < 65+ years > ]). We sought to identify additional studies, conference presentations, and theses by searching lists of relevant trials, reference lists from review articles, and meta-analysis and key articles. We did not contact researchers and field experts to obtain additional references. We considered studies published in any language for inclusion.
Two authors (T. O. S. and K. H.) assessed 506 studies for relevance and independently read titles, abstracts, and key words of identified articles. We obtained full-text versions for studies reporting mortality in relation to intakes of food types considered to be clinically relevant sources of naturally occurring saturated fat. Inclusion criteria included initially healthy human adult participants and dietary data reported in sufficient detail on an individual rather than a population basis. We chose mortality as the outcome, as it is generally well quantified and represents a final health outcome. Mortality type included all-cause, CVD, or cancer. Exclusion criteria included animal models and populations defined by preexisting disease or participants younger than 16 years. We did not place any restrictions on follow-up time. We included 26 studies after exclusions, representing data from 1 800 418 participants.14–39 Figure 1 shows the flow of study selection for this meta-analysis.
FIGURE 1—
Flowchart of study inclusion: meta-analysis of the association of food sources of saturated fat with mortality: January 1952–May 2012.
Data Extraction
Two investigators (T. O. S. and K. H.) summarized studies, with any differences of opinion resolved after discussion and input from a third investigator as required. For studies meeting the inclusion criteria, we extracted relevant population and intervention characteristics with the use of standard extraction templates. Where results were reported for both high- and low-fat products for a food group (e.g., reduced fat milk and full cream milk or lean and fatty meats), we extracted the data for the high-fat product for inclusion in the analysis. For the meat category, we used results for red meat preferentially where specified. We extracted maximally adjusted results from each study. Principal summary measures were risk ratios (RRs) and hazard ratios (HRs).
Quality Assessment
We conducted the quality assessment to determine the likely risk of bias associated with each of the included studies and created a grading scheme to determine how appropriate the methods were in their ability to address our research hypothesis. Two authors (T. O. S. and K. H.) evaluated the included studies by using a modified quality assessment for cohort and case control studies derived from the Newcastle–Ottawa Quality Assessment Scale and checklists from Kmet et al.40,41 We derived quality scores from sample size, participant dropout, and adequacy of description of missing participants; length of follow-up; dietary assessment method; outcome ascertainment method; degree of control for potential biases; description of analysis; and results reporting. Investigated studies differed in 3 main areas: length of follow-up, dietary assessment method, and degree of control for potential biases, such as smoking, physical activity, socioeconomic factors, body mass index (defined as weight in kilograms divided by the square of height in meters), and adjustment for energy intake. We graded studies as comprehensive, adequate, or limited for each of these main criteria:
Sufficient length of follow-up in relation to age: youngest cohort members reach younger than 40 years = limited; 40–59 years = adequate; 60 years and older = comprehensive
Dietary assessment quality: food frequency only, not quantitative or semiquantitative or meat eaters versus non–meat eaters = limited; quantitative or semiquantitative food frequency questionnaire or single 24-hour recall = adequate; diet record or diet history or multiple 24-hour recalls = comprehensive (provides more complete information and is better able to assess portion sizes and capture longer term variation42)
Multivariate analysis controlling for potential bias: basic age, gender = limited; at least 1 additional factor besides age and gender variables = adequate; at least 4 additional factors besides age and gender variables = comprehensive
We assigned an overall grade of comprehensive, adequate, somewhat limited, or limited overall on the basis of the criteria. We graded on the basis of the ability of each study to address our research aim. Our aim was to report the relationship between key food groups typically high in saturated fatty acids and risk of mortality, and the included studies could have mortality as a primary or secondary measure. Therefore, we derived quality assessment and scores from the level of detail regarding individual intakes of food groups of interest, the likelihood that the study follow-up was sufficient to include mortality outcomes, and the degree to which studies controlled for potential bias in the analysis.
Synthesis of Results
We used Review Manager 5.143 to complete the analyses unless otherwise specified. The main outcome measures were all-cause, CVD, and cancer mortality. We included results from the most complex model reported that controlled for the greatest number of confounding factors. We performed subgroup analyses for main food categories. We tested fixed and random effects models. Because of the large heterogeneity in results, as determined by the I2 statistic, we have reported results for random effects models.
To examine potential dose–response and nonlinear relationships, we used the method Greenland and Longnecker described.44 This method requires that the number of cases and total number of individuals be known for each category of intake. When these data were not provided, we approximated values from the number of person-years reported. Additionally, the mean or median level of intake is required for each category, and we estimated this value when it was not provided. For example, when density values such as grams of cheese per 1000 calories were reported, we converted values to grams per day using the average caloric intake of the relevant category. Or where intakes were reported as the number of servings consumed in a given time period, we converted these values to number of grams per day using the following serving sizes: meat 120 grams, processed meat 50 grams, milk 200 milliliters, yogurt 200 grams, cheese 40 grams, total dairy 200 grams, and butter 10 grams. For each food type, we determined the risk associated with each additional serving consumed per week. We used random effects models. To test for nonlinearity, we assessed the difference between the nonlinear and linear models. We completed all dose–response analyses in SAS 9.2.45
RESULTS
Key characteristics of the 26 included studies are displayed in Table 1. All were prospective observational studies. Populations were geographically diverse, with the majority of studies from the United States (n = 12) and Japan (n = 7). There were substantial differences in cohort size, with samples varying between 162 and 764 343. Study durations varied between 5 and 41 years, and participant age at enrollment ranged from 15 through 103 years. Most studies included both male and female cohorts.
TABLE 1—
Baseline Characteristics of Participants in 26 Prospective Cohort Studies of High–Saturated Fat Food Types and Mortality, Meta-Analysis of the Association of Food Sources of Saturated Fat With Mortality: January 1952–May 2012
| Study | Participants, No. | Age, Years | Follow-up, Years | Gender | Mortality Cause (No. Cases) | Food Type | Participant Group, Country of Residence |
| Bonthuis et al.14 | 1529 | 25–78 | 16 | Both | All-cause (n = 177), CVD (n = 61) | Dairy, milk, cheese, yogurt | Nambour Skin Cancer Prevention Trial, Australia |
| Bostick et al.15 | 34 486 | 55–69 | 8 | Female | CVD (n = 387) | Dairy, milk | Iowa Women’s Health Study, United States |
| Fortes et al.16 | 162 | ≥ 65 | 5 | Both | All-cause (n = 53) | Meat, dairy, milk, cheese, butter | Home for the Elderly, Rome, Italy |
| Fraser and Shavlik17 | 603 | ≥ 85 | 12 | Both | All-cause (n = 1387), CVD (n = 364) | Cheese | The Adventist Health Study |
| Goldbohm et al.18 | 20 782 | 55–69 | 10 | Both | All-cause (n = 16 136), CVD (n = 4288) | Milk, cheese, butter | Netherlands Cohort Study, Netherlands |
| Kinjo et al.19 | 223 170 | 40–69 | 15 | Both | CVD (n = 11 030) | Meat, milk | Japan |
| Kojima et al.20 | 107 824 | 40–79 | 10 | Both | Cancer (n = 284 colon, n = 173 rectal) | Meat, processed meat, milk, yogurt, cheese, butter | Japan Collaborative Cohort Study, Japan |
| Mann et al.21 | 10 802 | 16–79 | 13 | Both | All-cause (n = 392), IHD (n = 64) | Meat, eggs, milk, cheese | Vegetarian Society and Family/Friends, United Kingdom |
| Matsumoto et al.22 | 11 606 | 18–90 | 9 | Both | Cancer (n = 255) | Milk, yogurt, butter | Jichi Medical School Cohort Study, Japan |
| Mills et al.23 | 994 | 30–85 | 8 | Female | Cancer (n = 142; breast) | Meat, eggs, milk, cheese | Seventh-Day Adventists, United States |
| Mills et al.24 | 34 198 | 25–≥ 95 | 6 | Both | Cancer (n = 40; pancreatic) | Meat, eggs, milk, cheese | Seventh-Day Adventists, United States |
| Ngoan et al.25 | 13 250 | 15–96 | 13 | Both | Cancer (n = 116; stomach) | Meat, processed meat, milk | Fukuoka Prefecture, Japan |
| Ozasa et al.26 | 98 248 | 40–79 | 9 | Both | Cancer (n = 527; lung) | Processed meat, milk, yogurt, cheese, butter | Japan Collaborative Cohort Study, Japan |
| Pan et al.27 | 121 342 | 30–75 | 22–28 | Both | All-cause (n = 23 926), cancer (n = 9464), CVD (n = 5910) | Meat, processed meat | Health Professionals Follow-Up Study and Nurses’ Health Study, United States |
| Park et al.28 | 293 888 | 50–71 | 6 | Male | Cancer (n = 178; prostate) | Dairy, milk, yogurt, cheese | National Institutes of Health–AARP, United States |
| Phillips and Snowdon29 | 25 493 | ≥ 35 | 21 | Both | Cancer (n = 182; colorectal) | Meat, dairy, milk | Seventh-Day Adventists, United States |
| Qiu et al.30 | 50 069 | 40–≥ 80 | 6 | Both | CVD (n = 632; CHD) | Meat | Rural China |
| Sakauchi et al.31 | 114 517 | 40–≥ 80 | 10 | Both | Cancer (n = 85; urothelial) | Meat, processed meat, milk, yogurt, cheese, butter | Japan Collaborative Cohort Study, Japan |
| Sauvaget et al.32 | 37 130 | 34–103 | 16 | Both | CVD (n = 1462; stroke) | Meat, processed meat, dairy, milk | Hiroshima/Nagasaki Lifespan Study, Japan |
| Sinha et al.33 | 545 653 | 50–71 | 10 | Both | All-cause (n = 71 252), cancer (n = 25 362), CVD (n = 19 577) | Meat, processed meat | National Institutes of Health–AARP, United States |
| Smit et al.34 | 977 | 35–79 | 41 | Male | Cancer (n = 167; prostate) | Meat, dairy | Puerto Rico Heart Health Program, United States |
| Snowdon et al.35 | 6763 | ≥ 39 | 21 | Male | Cancer (n = 99; prostate) | Meat, milk, cheese | Seventh-Day Adventists, United States |
| Thorogood et al.36 | 11 130 | 39a | 12 | Both | All-cause (n = 200), cancer (n = 164), CVD (n = 94; IHD) | Meat | Vegetarian Society and Family/Friends, United Kingdom |
| Thun et al.37 | 764 343 | ≥ 30 | 6 | Both | Cancer (n = 1150; colon) | Meat | Cancer Prevention Study II, United States |
| Whiteman et al.38 | 10 522 | 35–64 | 9 | Both | All-cause (n = 514), cancer (n = 235), CVD (n = 107) | Meat, processed meat, milk, butter | OXCHECK, United Kingdom |
| Zheng et al.39 | 17 633 | ≥ 35 | 20 | Male | Cancer (n = 57; pancreatic) | Meat | Lutheran Brotherhood Insurance Policyholders, United States |
Note. CHD = coronary heart disease; CVD = cardiovascular disease; IHD = ischemic heart disease.
Mean age.
Total mortality was reported in 9 studies. Additionally, 5 reported total cancer, and 12 reported CVD-related mortality outcomes. Nine studies reported specific cancer mortality data. Some reported multiple outcomes of interest (Table 1).
Non- or semiquantitative food frequency questionnaires were used to assess diet in 24 of the 26 studies. The number of items assessed by the questionnaires ranged from 8 to 166, with 9 studies assessing portion size in the questionnaires. One study compared vegetarian with nonvegetarian, and another used a single 24-hour recall. None of the included studies used multiple-day diet records or recalls. A description of the quality of included studies for the purpose of this analysis is displayed in Table 2. We were able to investigate relative risk of mortality in the meta-analysis for food groups of meat, processed meat, milk, butter, cheese, and dairy as a whole.
TABLE 2—
Quality Assessment of Studies Included: Meta-Analysis of the Association of Food Sources of Saturated Fat With Mortality: January 1952–May 2012
| Follow-Up/Youngest Participant Age, Yearsa | Dietary Assessmentb | Confounding Factors Consideredc (in Addition to Age and Gender) | |||||
| Study | Study Data | Study Grade | Study Data | Study Grade | Study Data | Study Grade | Overall Grade |
| Comprehensive | |||||||
| Bostick et al.15 | 8/55 | LL | 127-item SQ FFQ, validated weighed food records (dairy r = 0.75) | L | BMI, waist:hip, medical history, smoking, alcohol, education, physical activity, other dietary factors | LL | LL |
| Fortes et al.16 | 5/65 | LL | 114-item SQ FFQ, validated vs 7-d weighed record | L | BMI, smoking, cognitive status, medical history, education | LL | LL |
| Goldbohm et al.18 | 10/55 | LL | 150-item SQ FFQ, validated 9-d diet record (milk r = 0.60; cheese r = 0.61) | L | Education, smoking, BMI, physical activity, vitamin use, alcohol, other dietary factors | LL | LL |
| Sinha et al.33 | 10/50 | LL | 124-item SQ FFQ, validated 2 × 24-hr recalls (iron r = 0.59 male and 0.56 female, not validated on food group level) | L | Smoking, physical activity, education, marital status, medical history, race, BMI, alcohol, supplements, HRT, other dietary factors | LL | LL |
| Smit et al.34 | 41/35 | LL | 1 × 24-hr recall, quantitative | L | Smoking, BMI, physical activity, urban living, other dietary factors | LL | LL |
| Adequate | |||||||
| Bonthuis et al.14 | 16/25 | L | 129-item SQ FFQ, validated 5 × 24-hr recall (calcium r = 0.67; reliability ranged r = 0.57–0.82) | L | BMI, alcohol, education, physical activity, smoking, supplements, medical history, occupation, other dietary factors | LL | L |
| Fraser et al.17 | 12/84 | LL | 65-item FFQ | XXX | BMI, education, physical activity, medical history, smoking | LL | L |
| Kojima et al.20 | 10/40 | L | 33-item FFQ validated 4 × 3-d food record (all studied foods > r = 0.3; reproducibility ranged r = 0.4–0.8) | XXX | Physical activity, education, medical history, BMI, alcohol, smoking | LL | L |
| Pan et al.27 | 22–28/30 | L | 131–166-item SQ FFQ, validated 4 × 7-d diet record (r = 0.59 unprocessed red meat, 0.56 processed red meat); mean 12-month reliability (r = 0.57) | L | BMI, alcohol, physical activity, smoking, race, menopausal status and hormone use, family history, medical history, other dietary factors | LL | L |
| Park et al.28 | 6/50 | L | 124-item SQ FFQ, validated 2 × 24-hr recall (calcium r = 0.63, not validated on food group level) | L | Race, education, marital status, BMI, physical activity, smoking, alcohol, medical history, other dietary factors | LL | L |
| Qiu et al.30 | 6/40 | L | 10-item FFQ | XXX | Regional area, smoking, alcohol, medical history, BMI, marital status, sleep, other dietary factors | LL | L |
| Sauvaget et al.32 | 16/34 | L | 22-item FFQ, validated 1 × 24-hr diet record (range r = 0.17–0.32) | XXX | Smoking, BMI, education, medical history, radiation, regional area | LL | L |
| Snowdon et al.35 | 21/39 | LL | FFQ, 1 food group per Q | XXX | Education, % desirable wt, other dietary factors | L | L |
| Somewhat limited | |||||||
| Kinjo et al.19 | 15/40 | L | FFQ, 1 food group per Q (meat, milk, fish) | XXX | Occupation, smoking, alcohol | L | 1/2 |
| Mann et al.21 | 13/16 | XXX | SQ FFQ, 1 food group per Q, validity done for fiber only | L | Smoking, social class | L | 1/2 |
| Mills et al.23 | 8/30 | XXX | 21-item FFQ | XXX | Age at menarche, first pregnancy, and menopause, % desirable wt, education | LL | 1/2 |
| Ngoan et al.25 | 13/15 | XXX | 25-item FFQ | XXX | Smoking, alcohol, medical, occupation, coffee | LL | 1/2 |
| Ozasa et al.26 | 9/40 | L | 32-item FFQ, validated 4 × 3-d diet records (milk, ham, and sausage: r > 0.6) | XXX | Family history, smoking | L | 1/2 |
| Phillips et al.29 | 21/35 | L | 21-item FFQ | XXX | Coffee, % desirable wt, other dietary factors | L | 1/2 |
| Sakauchi et al.31 | 10/40 | L | 32-item FFQ, validated 4 × 3-d diet records (milk, ham, and sausage: r > 0.6) | XXX | Smoking | L | 1/2 |
| Thun et al.37 | 6/30 | XXX | 32-item FFQ | XXX | Family history, physical activity, BMI, aspirin use, other dietary factors | LL | 1/2 |
| Whiteman et al.38 | 9/35 | L | 8-item FFQ | XXX | Smoking, alcohol, SES | L | 1/2 |
| Zheng et al.39 | 20/35 | L | 35-item FFQ | XXX | Smoking, alcohol, energy intake | L | 1/2 |
| Limited | |||||||
| Mills et al.24 | 6/25 | XXX | FFQ past or current use by food group | XXX | Smoking | L | XXX |
| Thorogood et al.36 | 12/39e | XXX | Nonmeat eaters vs meat eaters | XXX | Smoking, BMI, SES | L | XXX |
| Matsumoto et al.22 | 9/18 | XXX | 30-item FFQ, validated 12-d diet records by other study that used same FFQ but “included more food items” (r = 0.65 milk) | XXX | … | XXX | XXX |
Note. BMI = body mass index; FFQ = food frequency questionnaire; HRT = hormone replacement therapy; SES = socioeconomic status; SQ = semiquantitative; Q = question, wt = weight. We derived grading from the ability of each study to address our research aims; it did not necessarily reflect the quality of research studies we investigated. We graded for each category of interest according to rankings of XXX = limited; L = adequate; LL = comprehensive. Total ranking represents average of the category grading and includes 1/2 = somewhat limited.
Sufficient length of follow-up in relation to age: youngest cohort members reach < 40 years = XXX; 40–59 years = L; ≥ 60 years = LL.
Dietary assessment quality: food frequency only, not quantitative or semiquantitative = XXX; quantitative or semiquantitative food frequency questionnaire or meat eaters versus nonmeat eaters or single 24-h recall = L; diet record or diet history or multiple 24-h recalls = LL.
dMultivariate analysis controlling for potential bias: basic age, gender = XXX; at least 1 additional factor besides age and gender variables = L; at least 4 additional factors besides age and gender variables = LL.
Mean age.
Although all studies adjusted for age and gender when applicable, there was substantial variation in the degree of adjustment for potential confounding factors, such as level of physical activity, smoking, body mass index, socioeconomic factors, and alcohol consumption (Table 2).
Meta-Analysis
Individual study results and pooled estimates for all-cause mortality are provided in Figure 2. There was no significant relationship between intake of milk, cheese, butter, or all dairy and all-cause mortality. High intake of meat (RR = 1.17; 95% confidence interval [CI] = 1.08, 1.27) and processed meat (RR = 1.21; 95% CI = 1.16, 1.28) were significantly associated with an increased risk of all-cause mortality. Substantial heterogeneity was evident in studies reporting risk of mortality and consumption of meat (I2 = 85%), processed meat (I2 = 65%), and butter (I2 = 78%).
FIGURE 2—
High consumption of foods containing saturated fat and the associated risk of all-cause mortality: meta-analysis of the association of food sources of saturated fat with mortality: January 1952–May 2012.
Note. CI = confidence interval; F = female; M = male.
As displayed in Figure 3, there were no significant associations of high intake of meat, milk, cheese, or all dairy products with CVD mortality. However, high intake of processed meat was significantly associated with increased risk of CVD mortality (RR = 1.17; 95% CI = 1.02, 1.33). High heterogeneity existed between studies relating to consumption of meat (I2 = 93%), processed meat (I2 = 88%), and milk (I2 = 82%).
FIGURE 3—
High consumption of foods containing saturated fat and the associated risk of cardiovascular disease mortality: meta-analysis of the association of food sources of saturated fat with mortality: January 1952–May 2012.
Meat, processed meat, and milk were the only food groups with data from more than 1 study for all-cancer mortality. For individual food groups, high consumption of meat (RR = 1.14; 95% CI = 1.04, 1.24) and processed meat (RR = 1.13; 95% CI = 1.09, 1.17) were associated with an increased risk of cancer death; milk was not (Figure 4).
FIGURE 4—
High consumption of foods containing saturated fat and the associated risk of cancer mortality: meta-analysis of the association of food sources of saturated fat with mortality: January 1952–May 2012.
A significantly increased risk of specific cancer mortality was associated with high compared with low meat intake (RR = 1.25; 95% CI = 1.03, 1.52) but not processed meat (RR = 1.25; 95% CI = 0.91, 1.72) or milk (RR = 0.92; 95% CI = 0.79, 1.09). Moderate heterogeneity existed for studies relating to meat consumption (I2 = 60%; Figure A, available as a supplement to this article at http://www.ajph.org).
Subgroup Analysis
Subgroup analysis was limited because of the small number of studies in each food group and outcome. However, we examined the effect of eliminating studies classified as being of limited and somewhat limited quality. In addition, because of the large differences in the classification of meat intake in Asian and non-Asian populations, we conducted analyses separately for these 2 groups.
In terms of study quality, we graded 5 of 26 studies as being able to comprehensively address our research aim, whereas, at the other end of the spectrum, we graded 3 as limited (Table 2). We investigated changes in results when we excluded the limited studies from analysis. These studies were Matsumoto et al.,46 who investigated cancer mortality; Thorogood et al.,36 who examined all-cause mortality; and Mills et al.,24 who investigated pancreatic cancer mortality. Exclusion of the Matsumoto et al.46 study from the pooled analysis did not significantly change the results (RR = 1.14; 95% CI = 1.09, 1.19; I2 = 57%), nor did exclusion of Thorogood et al.36 (RR = 1.16; 95% CI = 1.13, 1.19; I2 = 3%), or the exclusion of both (RR = 1.16; 95% CI = 1.13, 1.19; I2 = 9%). For risk of specific cancer mortality, exclusion of the Mills et al. study24 resulted in a nonsignificant relationship between meat intake and mortality (RR = 1.20; 95% CI = 0.96, 1.50; I2 = 9%).
To determine whether differences in study quality were contributing to the significant heterogeneity evident in results, we removed those studies we considered of limited quality. In an additional step, we removed the studies we identified as of limited and somewhat limited quality to determine the impact on results. Although heterogeneity was not substantially reduced for most analyses, it was reduced for analysis of the relationship between meat and all-cause (I2 = 30.4%), all-cancer (I2 = 0%), and CVD (I2 = 73%) mortality as well as milk and CVD mortality (I2 = 0%).
The pooled analysis of non-Asian cohorts21,33,38 suggested a significantly increased risk of CVD mortality with high intake of meat (RR = 1.29; 95% CI = 1.17, 1.42; I2 = 70%). Conversely, studies of Asian populations19,30,32 demonstrated a significantly reduced risk of CVD mortality (RR = 0.82; 95% CI = 0.73, 0.91; I2 = 19%). Elimination of the 1 Asian cohort for processed meat did not change the direction of results with CVD mortality (RR = 1.24; 95% CI = 1.10, 1.40; I2 = 82%). There were no significant associations of the high intake of milk with CVD mortality in either Asian or non-Asian populations (RR = 0.84; 95% CI = 0.72, 1.00; I2 = 72% and RR = 1.07; 95% CI = 0.98, 1.17; I2 = 0%, respectively).
Nonlinear and Dose–Response Relationships
Where sufficient data were available, we investigated potential nonlinear and dose–response relationships between the number of weekly servings of meat, processed meat, milk, and cheese and risk of all-cause, cardiovascular, and cancer mortality (Table 3). We observed a significant positive linear relationship between risk of all-cause mortality and consumption of meat and processed meat (Figure B, available as a supplement to this article at http://www.ajph.org). We also observed a significant negative curvilinear relationship between number of servings of milk consumed per week and risk of all-cause mortality, but the decrease in risk was minimal (Figure C, available as a supplement to this article at http://www.ajph.org).
TABLE 3—
Mortality Risk Associated With Each Additional Serving of Food per Week, by Food Category and Mortality Outcome: Meta-Analysis of the Association of Food Sources of Saturated Fat With Mortality: January 1952–May 2012
| Food Type (Serving Size) | RR (95% CI) Linear Relationship | Studies, No. | Nonlinear Relationshipa | Heterogeneity (τ2) |
| All-cause mortality | ||||
| Milk (200 mL) | 1.00 (1.00, 1.00) | 4 | 0.036* | < 0.001 |
| Meat (120 g) | 1.02* (1.01, 1.04) | 7 | 0.007* | < 0.001 |
| Processed meat (50 g) | 1.04* (1.00, 1.07) | 2 | 0.139 | 0.001 |
| Cheeseb (40 g) | 1.00 (1.00, 1.00) | 5 | 0.106 | < 0.001 |
| CVD mortality | ||||
| Milk (200 mL) | 1.00 (0.99, 1.01) | 5 | 0.014* | 0.001 |
| Meat (120 g) | 1.01 (0.99, 1.04) | 9 | 0.323 | 0.001 |
| Processed meatb (50 g) | 1.02* (1.01, 1.03) | 2 | 0.005* | < 0.001 |
| Cheese (40 g) | 1.01 (0.99, 1.02) | 3 | 0.013* | 0.001 |
| Cancer mortality | ||||
| Meat (120 g) | 1.02* (1.02, 1.03) | 4 | 0.211 | < 0.001 |
| Processed meat (50 g) | 1.04 (1.00, 1.08) | 2 | < 0.001* | 0.001 |
Note. CI = confidence interval; CVD = cardiovascular disease; RR = risk ratio.
Test of significant departure from linear.
Fixed effects and random effects results are the same trend.
P < .05.
There was no significant relationship between number of servings of cheese consumed per week and mortality. Significant curvilinear relationships existed between CVD mortality and intake of milk (negative association; Figure D, available as a supplement to this article at http://www.ajph.org), cheese (Figure E, available as a supplement to this article at http://www.ajph.org), and processed meat (Figure F, available as a supplement to this article at http://www.ajph.org). We observed the greatest reduction in risk for milk around 12 servings per week. Cheese displayed an incline in risk from around 8 servings per week, and processed meat from around 3 servings per week. There was a significant positive relationship between intake of meat and cancer mortality (Figure G, available as a supplement to this article at http://www.ajph.org).
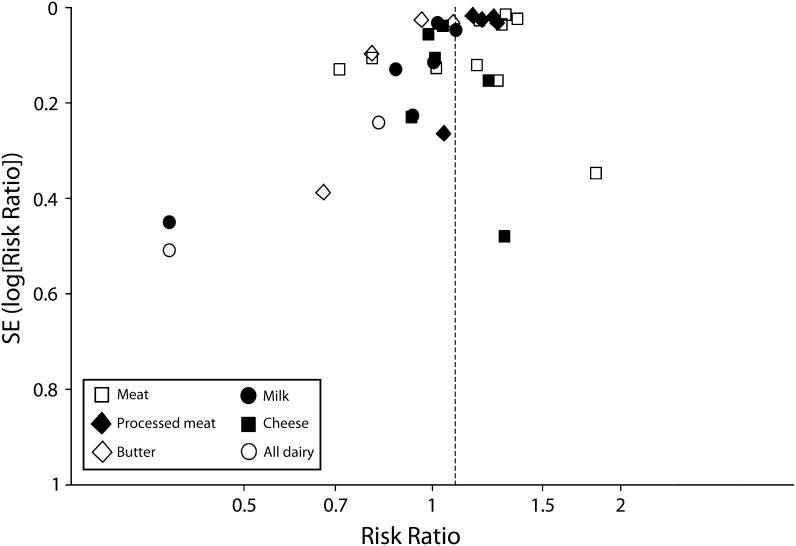
Because of the small number of studies in each analysis, there was limited statistical power to detect publication bias; therefore, we assessed bias with the use of funnel plots. We observed little evidence of bias (Figure 5).
FIGURE 5—
Funnel plot of publication bias for all-cause mortality, by food group: meta-analysis of the association of food sources of saturated fat with mortality: January 1952–May 2012.
DISCUSSION
Early guidelines regarding intake of saturated fat, which remain largely unchanged today, lacked a strong evidence base. These guidelines were derived from assumptions about the causal pathway of disease development, specifically that replacing saturated fat in the diet with polyunsaturated fat lowered total serum cholesterol, which in turn reduced risk of CVD.47,48 This is now thought to be an oversimplification of the multiple processes that influence the impact of dietary composition on human health. Our review, which summarizes the currently available evidence, is unable to support a strong recommendation regarding restricting intake of foods high in saturated fat for the prevention of mortality.
The results of our review suggest milk, cheese, butter, and total dairy intake were not significantly associated with increased risk of mortality. By contrast, higher intakes of meat and processed meat were significantly associated with modest increased risk of all-cause and cancer mortality, and higher intakes of processed meat were also significantly associated with a modest increase in CVD mortality. We observed dose–response relationships for meat and processed meat across mortality categories, with the majority displaying a curvilinear relationship with increasing risk at higher intakes. The total relative risk estimate for all-cause mortality for all included food groups suggested a small increase in risk with higher intakes (RR = 1.09; 95% CI = 1.03, 1.14). However, considering the low quality of most of the included studies and the large variation in the results between individual food groups, we were unable to provide support for, or refute, the existing guidelines regarding consumption of saturated fat. These results highlight the need for further high-quality research regarding intake of foods high in saturated fats and disease outcomes before dietary guidelines or public health recommendations are proposed or implemented.
The positive association observed between meat intake and mortality risk agrees with those previously reported in relation to cancer risk. A meta-analysis of almost 8000 cases from 19 prospective studies found consumption of red meat and processed meat to be linked with risk of developing both colon and rectal cancer.49 However, research investigating mechanisms behind this result suggests that these associations are likely to be because of factors outside saturated fat content. Unlike dairy products, meat contains high amounts of heme iron, which has been shown to damage the colonic mucosa in rat models, resulting in a hyperproliferation of the epithelium, which may have carcinogenic effects.50 A prospective cohort study supports this concept, showing intake of heme iron from red meat was positively associated with risk of CVD, whereas non–heme iron intake was not significant.51 Nitrosation may also increase the toxicity of heme in cured products such as processed meats.52 Cooking meat at high temperatures is also thought to result in other potential mutagens and carcinogens such as heterocyclic amines and polycyclic aromatic hydrocarbons.52 Notably, our results showed a significantly reduced risk of CVD mortality associated with high compared with low consumption of meat in Asian populations, who are reported to have lower intakes of saturated fat than do Western populations.53 Meat intake may modify the effects of saturated fat on atherogenic lipoproteins, as suggested by results of a diet trial that showed that replacing carbohydrate with beef resulted in improvements when combined with diets that were overall low in saturated fat but not high in saturated fat.54 Therefore, the dietary context of foods may also be important along with the foods themselves. Another potential explanation is that the population subgroup differences may be a result of disease etiology rather than meat intake. Studies that reported outcomes for stroke and cerebrovascular disease predominately examined Asian populations, whereas those that reported cardiovascular outcomes examined non-Asian cohorts.
The meta-analysis by Siri-Tarino et al.8 found no significant associations with total saturated fat intake and risk of CVD. This research relates directly to recommendations to limit saturated fat as a whole in the diet. De Oliveira Otto et al.55 used a food-based approach and reported that a higher intake of dairy saturated fat was associated with lower CVD risk, whereas a higher intake of meat saturated fat was associated with higher risk. The authors state that associations between saturated fat and health may depend on food-specific fatty acids or other nutrient constituents in addition to saturated fat. Taken together with our findings, it appears that the role of saturated fat in health may differ on the basis of the source and type of saturated fat consumed rather than on the total amount.
It has been suggested that it is not feasible to separate different types of saturated fat with respect to food choices because the foods contain a combination of several different saturated fatty acids.9 The types of saturated fat in meat, processed meat, milk, yogurt, cheese, and butter are predominantly palmitic acid (16:0), followed by stearic acid (18:0), and then myristic acid (14:0; Table 4). Both stearic acid and palmitic acid, the predominant saturated fatty acids in the food types studied, have been shown to have a neutral to favorable impact on serum lipid profiles compared with lauric acid and myristic acid.57 Stearic acid seems to be more beneficial than is palmitic acid when the 2 are compared,58 and stearic acid but not trans fatty acid has been shown to significantly reduce concentrations of low-density lipoprotein cholesterol.59 Although there is not yet enough evidence to give dietary recommendations for individual saturated fatty acids, our research raises questions regarding the traditional belief that all high–saturated fat foods lead to increased mortality. As people consume foods as a whole rather than individual nutrients, food-based dietary advice (i.e., consuming more or less of particular foods) rather than nutrient-based dietary advice (i.e., consuming more or less of individual nutrients) may be more practical for health professionals to communicate to the public.
TABLE 4—
Saturated Fatty Acid Profile of Food Types High in Naturally Occurring Saturated Fat: Meta-Analysis of the Association of Food Sources of Saturated Fat With Mortality: January 1952–May 2012
| Food Type | Saturated Fat Content, g/100 g | Predominant Saturated Fatty Acid, Type (g/100 g) | Other Major Contributing Saturated Fatty Acids, Type (g/100 g) |
| Meata | 7.2 | 16:0 (4.1) | 18:0 (2.0), 14:0 (0.6) |
| Processed meatb | 11.6 | 16:0 (7.1) | 18:0 (3.9), 14:0 (0.5) |
| Milkc | 1.9 | 16:0 (0.8) | 18:0 (0.4), 14:0 (0.3) |
| Yogurtd | 2.1 | 16:0 (0.9) | 14:0 (0.3), 18:0 (0.3) |
| Cheesee | 21.1 | 16:0 (9.8) | 18:0 (4.0), 14:0 (3.3) |
| Butterf | 51.4 | 16:0 (21.7) | 18:0 (10.0), 14:0 (7.4) |
Source. US Department of Agrictulture.56
Derived from beef, ground, 70% lean meat or 30% fat, cooked.
Derived from luncheon meat, pork, beef.
Derived from milk, whole, 3.25% milk fat.
Derived from yogurt, plain, whole milk.
Derived from cheese, cheddar.
Derived from butter, without salt.
Limitations
Strengths of our study include the use of the relatively well-measured and decisive measure of mortality as an outcome, the inclusion of many large-scale and diverse studies, and the evaluation of food groups rather than nutrients. However, the small number of studies in some food categories was a limitation for evaluating associations of mortality with certain foods. A further limitation was the wide variation in the quality of dietary assessment tools. Food frequency questionnaires, which almost all studies included in our analysis used, are useful for assessing large cohorts, but some did not attempt to directly quantify serving size, were not validated with food group–specific intake, or reported correlation coefficients as low as 0.17. The degree of accounting for potential confounding factors studies used also varied.
Appropriate adjustment for confounding factors is important considering that a healthy lifestyle effect may have existed in some studies, whereby saturated fat would generally be limited with healthier lifestyles in accordance with dietary guidelines or those with existing disease or a family history of heart disease. This is also true for studies of populations who have a relatively strict dietary and lifestyle approach, such as Seventh-Day Adventists. Although our focus was on foods providing saturated fat in the diet, it was not possible to extract the effect of saturated fat from other nutrients in a food, and some nutrients present in food we investigated may obscure the relationship between saturated fat and mortality. On the other hand, some studies used models including numerous factors, thereby increasing the possibility of overadjustment, resulting in loss of power to see an association where a true association exists. In the absence of any randomized experiments, observational studies provide the best evidence we have available. However, these weaknesses are inherent to observational epidemiology as a field and should be considered in the interpretation of our results.
A potential criticism of our study is that deriving a quantitative estimate of risk though meta-analysis is not appropriate because of the quality deficiencies and substantial differences between the studies included in this analysis (Table 1). Although results should be interpreted in the context of this heterogeneity, we feel that there is value in combining study results to provide the best quantitative assessment of the current evidence because of the existence of longstanding and specific dietary guidelines related to saturated fat consumption. On the basis of our meta-analysis we are unable to provide support for or propose changes to current guidelines and recommendations. Instead, this review highlights the need for high-quality research regarding intakes of saturated fats, in particular regarding food sources of saturated fats and disease outcomes.
Most dietary guidelines recommend limiting saturated fat to 10% or less of total energy intake; however, these guidelines generally do not specify the replacement nutrient.9 Replacing saturated fats with polyunsaturated fats is considered to be beneficial to health7: Jakobsen et al.60 demonstrated a significant inverse association of polyunsaturated fats with risk of coronary events in a pooled analysis of cohort studies; Mozafarrian et al.61 showed that consumption of polyunsaturated fat in place of saturated fat reduces coronary heart disease events in randomized controlled trials. Furthermore, Jakobsen et al. suggested that replacing high–saturated fat foods in the diet with refined carbohydrate foods may be detrimental to health.60 Therefore, it may be more in keeping with available evidence if guidelines say to either replace saturated fats with polyunsaturated fats or focus more on food-based recommendations.
Conclusions
Our study reveals the ongoing need for improvement in assessment tools and methods that investigate food sources of saturated fat and mortality. We believe the dietary assessment tools for such studies would be improved through the use of more comprehensive estimation of diet in the form of multiple 24-hour recalls, diet histories, or diet records rather than the heavy reliance on food frequency questionnaires, which often do not assess portion size and may not be well validated on a specific food group level. Despite the central role that dietary advice plays in public health promotion and prevention, our quality assessment identified a lack of comprehensive studies in the area of mortality and foods high in saturated fat. Studies that use quantitative dietary assessments, with a sufficient length of follow-up and sufficient personal and lifestyle data to adequately adjust for potential confounders, would be a welcome addition to the literature and bring needed clarity to the scientific evidence of the association of saturated fat with health.
Acknowledgments
This study was supported by the National Health and Medical Research Council (grant 572742).
We would like to thank Eve Blair, PhD, and Sonya Girdler, PhD, for sharing their knowledge on systematically reviewing literature and for providing inspiration to start this research and Stephen R. Zubrick, PhD, for his support and valuable feedback on the article.
Note. T. O. S. was previously awarded a Dairy Innovation Australia grant (DHNC-MetX06-2011) separate to this project.
References
- 1.National Health and Medical Research Council. Dietary Guidelines for Australian Adults. Canberra, Australia: Department of Health and Ageing, Commonwealth of Australia; 2003. [Google Scholar]
- 2.Health Canada. Canada’s Food Guide. Ottawa, Ontario: Minister of Health Canada; 2011. [Google Scholar]
- 3.US Department of Health and Human Services. Dietary Guidelines for Americans. Washington, DC: US Government Printing Office; 2010. [Google Scholar]
- 4.Keys A, Anderson JT, Grande F. Serum cholesterol response to changes in the diet: IV. Particular saturated fatty acids in the diet. Metabolism. 1965;14(7):776–787. doi: 10.1016/0026-0495(65)90004-1. [DOI] [PubMed] [Google Scholar]
- 5.Hegsted DM, McGandy RB, Myers ML, Stare FJ. Quantitative effects of dietary fat on serum cholesterol in man. Am J Clin Nutr. 1965;17(5):281–295. doi: 10.1093/ajcn/17.5.281. [DOI] [PubMed] [Google Scholar]
- 6.Hu FB, Willett WC. Optimal diets for prevention of coronary heart disease. JAMA. 2002;288(20):2569–2578. doi: 10.1001/jama.288.20.2569. [DOI] [PubMed] [Google Scholar]
- 7.Zelman K. The great fat debate: a closer look at the controversy—questioning the validity of age-old dietary guidance. J Am Diet Assoc. 2011;111(5):655–658. doi: 10.1016/j.jada.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 8.Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am J Clin Nutr. 2010;91(3):535–546. doi: 10.3945/ajcn.2009.27725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Astrup A, Dyerberg J, Elwood P et al. The role of reducing intakes of saturated fat in the prevention of cardiovascular disease: where does the evidence stand in 2010? Am J Clin Nutr. 2011;93(4):684–688. doi: 10.3945/ajcn.110.004622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warensjö E, Jansson J-H, Cederholm T et al. Biomarkers of milk fat and the risk of myocardial infarction in men and women: a prospective, matched case–control study. Am J Clin Nutr. 2010;92(1):194–202. doi: 10.3945/ajcn.2009.29054. [DOI] [PubMed] [Google Scholar]
- 11.Soedamah-Muthu SS, Ding EL, Al-Delaimy WK et al. Milk and dairy consumption and incidence of cardiovascular diseases and all-cause mortality: dose–response meta-analysis of prospective cohort studies. Am J Clin Nutr. 2011;93(1):158–171. doi: 10.3945/ajcn.2010.29866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azadbakht L, Mirmiran P, Esmaillzadeh A, Azizi F. Dairy consumption is inversely associated with the prevalence of the metabolic syndrome in Tehranian adults. Am J Clin Nutr. 2005;82(3):523–530. doi: 10.1093/ajcn.82.3.523. [DOI] [PubMed] [Google Scholar]
- 13.Gibson RA, Makrides M, Smithers LG, Voevodin M, Sinclair AJ. The effect of dairy foods on CHD: a systematic review of prospective cohort studies. Br J Nutr. 2009;102(9):1267–1275. doi: 10.1017/S0007114509371664. [DOI] [PubMed] [Google Scholar]
- 14.Bonthuis M, Hughes MCB, Ibiebele TI, Green AC, Van Der Pols JC. Dairy consumption and patterns of mortality of Australian adults. Eur J Clin Nutr. 2010;64(6):569–577. doi: 10.1038/ejcn.2010.45. [DOI] [PubMed] [Google Scholar]
- 15.Bostick RM, Kushi LH, Wu Y, Meyer KA, Sellers TA, Folsom AR. Relation of calcium, vitamin D, and dairy food intake to ischemic heart disease mortality among postmenopausal women. Am J Epidemiol. 1999;149(2):151–161. doi: 10.1093/oxfordjournals.aje.a009781. [DOI] [PubMed] [Google Scholar]
- 16.Fortes C, Forastiere F, Farchi S, Rapiti E, Pastori G, Perucci CA. Diet and overall survival in a cohort of very elderly people. Epidemiology. 2000;11(4):440–445. doi: 10.1097/00001648-200007000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Fraser GE, Shavlik DJ. Risk factors for all-cause and coronary heart disease mortality in the oldest-old: the Adventist Health Study. Arch Intern Med. 1997;157(19):2249–2258. [PubMed] [Google Scholar]
- 18.Goldbohm RA, Chorus AMJ, Garre FG, Schouten LJ, Van Den Brandt PA. Dairy consumption and 10-y total and cardiovascular mortality: a prospective cohort study in the Netherlands. Am J Clin Nutr. 2011;93(3):615–627. doi: 10.3945/ajcn.110.000430. [DOI] [PubMed] [Google Scholar]
- 19.Kinjo Y, Beral V, Akiba S et al. Possible protective effect of milk, meat and fish for cerebrovascular disease mortality in Japan. J Epidemiol. 1999;9(4):268–274. doi: 10.2188/jea.9.268. [DOI] [PubMed] [Google Scholar]
- 20.Kojima M, Wakai K, Tamakoshi K et al. Diet and colorectal cancer mortality: results from the Japan collaborative cohort study. Nutr Cancer. 2004;50(1):23–32. doi: 10.1207/s15327914nc5001_4. [DOI] [PubMed] [Google Scholar]
- 21.Mann JI, Appleby PN, Key TJ, Thorogood M. Dietary determinants of ischaemic heart disease in health conscious individuals. Heart. 1997;78(5):450–455. doi: 10.1136/hrt.78.5.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsumoto M, Ishikawa S, Nakamura Y, Kayaba K, Kajii E. Consumption of dairy products and cancer risks. J Epidemiol. 2007;17(2):38–44. doi: 10.2188/jea.17.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mills PK, Annegers JF, Phillips RL. Animal product consumption and subsequent fatal breast cancer risk among Seventh-Day Adventists. Am J Epidemiol. 1988;127(3):440–453. doi: 10.1093/oxfordjournals.aje.a114821. [DOI] [PubMed] [Google Scholar]
- 24.Mills PK, Beeson WL, Abbey DE, Fraser GE, Phillips RL. Dietary habits and past medical history as related to fatal pancreas cancer risk among Adventists. Cancer. 1988;61(12):2578–2585. doi: 10.1002/1097-0142(19880615)61:12<2578::aid-cncr2820611232>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 25.Ngoan LT, Mizoue T, Fujino Y, Tokui N, Yoshimura T. Dietary factors and stomach cancer mortality. Br J Cancer. 2002;87(1):37–42. doi: 10.1038/sj.bjc.6600415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozasa K, Watanabe Y, Ito Y et al. Dietary habits and risk of lung cancer death in a large-scale cohort study (JACC Study) in Japan by sex and smoking habit. Jpn J Cancer Res. 2001;92(12):1259–1269. doi: 10.1111/j.1349-7006.2001.tb02148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan A, Sun Q, Bernstein AM et al. Red meat consumption and mortality: results from 2 prospective cohort studies. Arch Intern Med. 2012;172(7):555–563. doi: 10.1001/archinternmed.2011.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park Y, Mitrou PN, Kipnis V, Hollenbeck A, Schatzkin A, Leitzmann MF. Calcium, dairy foods, and risk of incident and fatal prostate cancer. Am J Epidemiol. 2007;166(11):1270–1279. doi: 10.1093/aje/kwm268. [DOI] [PubMed] [Google Scholar]
- 29.Phillips RL, Snowdon DA. Dietary relationships with fatal colorectal cancer among Seventh-Day Adventists. J Natl Cancer Inst. 1985;74(2):307–317. [PubMed] [Google Scholar]
- 30.Qiu D, Mei J, Tanihata T, Kawaminami K, Minowa M. A cohort study on cerebrovascular disease in middle-aged and elderly population in rural areas in Jiangxi Province, China. J Epidemiol. 2003;13(3):149–156. doi: 10.2188/jea.13.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakauchi F, Mori M, Washio M et al. Dietary habits and risk of urothelial cancer death in a large-scale cohort study (JACC Study) in Japan. Nutr Cancer. 2004;50(1):33–39. doi: 10.1207/s15327914nc5001_5. [DOI] [PubMed] [Google Scholar]
- 32.Sauvaget C, Nagano J, Allen N, Grant EJ, Beral V. Intake of animal products and stroke mortality in the Hiroshima/Nagasaki Life Span Study. Int J Epidemiol. 2003;32(4):536–543. doi: 10.1093/ije/dyg151. [DOI] [PubMed] [Google Scholar]
- 33.Sinha R, Cross AJ, Graubard BI, Leitzmann MF, Schatzkin A. Meat intake and mortality: a prospective study of over half a million people. Arch Intern Med. 2009;169(6):562–571. doi: 10.1001/archinternmed.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smit E, Garcia-Palmieri MR, Figueroa NR et al. Protein and legume intake and prostate cancer mortality in Puerto Rican men. Nutr Cancer. 2007;58(2):146–152. doi: 10.1080/01635580701328206. [DOI] [PubMed] [Google Scholar]
- 35.Snowdon DA, Phillips RL, Choi W. Diet, obesity, and risk of fatal prostate cancer. Am J Epidemiol. 1984;120(2):244–250. doi: 10.1093/oxfordjournals.aje.a113886. [DOI] [PubMed] [Google Scholar]
- 36.Thorogood M, Mann J, Appleby P, McPherson K. Risk of death from cancer and ischaemic heart disease in meat and non-meat eaters. BMJ. 1994;308(6945):1667–1670. doi: 10.1136/bmj.308.6945.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thun MJ, Calle EE, Namboodiri MM et al. Risk factors for fatal colon cancer in a large prospective study. J Natl Cancer Inst. 1992;84(19):1491–1500. doi: 10.1093/jnci/84.19.1491. [DOI] [PubMed] [Google Scholar]
- 38.Whiteman D, Muir J, Jones L, Murphy M, Key T. Dietary questions as determinants of mortality: the OXCHECK experience. Public Health Nutr. 1999;2(4):477–487. doi: 10.1017/s136898009900066x. [DOI] [PubMed] [Google Scholar]
- 39.Zheng W, McLaughlin JK, Gridley G et al. A cohort study of smoking, alcohol consumption, and dietary factors for pancreatic cancer (United States) Cancer Causes Control. 1993;4(5):477–482. doi: 10.1007/BF00050867. [DOI] [PubMed] [Google Scholar]
- 40.Wells GA, Shea B, O’Connell D The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed May 1, 2011.
- 41.Kmet LM, Lee RC, Cook LS. Standard Quality Assessment Criteria for Evaluating Primary Research Papers From a Variety of Fields. Edmonton, Alberta: Alberta Heritage Foundation for Medical Research; 2004. [Google Scholar]
- 42.Cameron M, Van Staveren W, editors. Manual on Methodology for Food Consumption Studies. New York, NY: Oxford University Press; 1988. [Google Scholar]
- 43.Review Manager, Version 5.1 [computer program] Copenhagen: The Nordic Cochrane Centre; 2011. [Google Scholar]
- 44.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose–response data, with applications to meta-analysis. Am J Epidemiol. 1992;135(11):1301–1309. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 45.SAS, Version 9.2 [computer program] Cary, NC: SAS Institute; 2008. [Google Scholar]
- 46.Matsumoto M, Ishikawa S, Nakamura Y, Kayaba K, Kajii E. Consumption of dairy products and cancer risks. J Epidemiol. 2007;17(2):38–44. doi: 10.2188/jea.17.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Katan MB, Zock PL, Mensink RP. Effects of fats and fatty acids on blood lipids in humans: an overview. Am J Clin Nutr. 1994;60(6):1017S–1022S. doi: 10.1093/ajcn/60.6.1017S. [DOI] [PubMed] [Google Scholar]
- 48.Rivellese AA, Auletta P, Marotta G et al. Long term metabolic effects of two dietary methods of treating hyperlipidaemia. BMJ. 1994;308(6923):227–231. doi: 10.1136/bmj.308.6923.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Larsson SC, Wolk A. Meat consumption and risk of colorectal cancer: a meta-analysis of prospective studies. Int J Cancer. 2006;119(11):2657–2664. doi: 10.1002/ijc.22170. [DOI] [PubMed] [Google Scholar]
- 50.Sesink AL, Termont DS, Kleibeuker JH, Van der Meer R. Red meat and colon cancer: the cytotoxic and hyperproliferative effects of dietary heme. Cancer Res. 1999;59(22):5704–5709. [PubMed] [Google Scholar]
- 51.de Oliveira Otto MC, Alonso A, Lee D-H et al. Dietary intakes of zinc and heme iron from red meat, but not from other sources, are associated with greater risk of metabolic syndrome and cardiovascular disease. J Nutr. 2012;142(3):526–533. doi: 10.3945/jn.111.149781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Santarelli RL, Pierre F, Corpet DE. Processed meat and colorectal cancer: a review of epidemiologic and experimental evidence. Nutr Cancer. 2008;60(2):131–144. doi: 10.1080/01635580701684872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou BF, Stamler J, Dennis B et al. Nutrient intakes of middle-aged men and women in China, Japan, United Kingdom, and United States in the late 1990s: the INTERMAP study. J Hum Hypertens. 2003;17(9):623–630. doi: 10.1038/sj.jhh.1001605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mangravite LM, Chiu S, Wojnoonski K, Rawlings RS, Bergeron N, Krauss RM. Changes in atherogenic dyslipidemia induced by carbohydrate restriction in men are dependent on dietary protein source. J Nutr. 2011;141(12):2180–2185. doi: 10.3945/jn.111.139477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Oliveira Otto MC, Mozaffarian D, Kromhout D et al. Dietary intake of saturated fat by food source and incident cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr. 2012;96(2):397–404. doi: 10.3945/ajcn.112.037770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. US Department of Agriculture. Nutrient database. Available at: http://www.nal.usda.gov/fnic/foodcomp/search. Accessed October 15, 2011.
- 57.Hayes KC, Pronczuk A, Lindsey S, Diersen-Schade D. Dietary saturated fatty acids (12:0, 14:0, 16:0) differ in their impact on plasma cholesterol and lipoproteins in nonhuman primates. Am J Clin Nutr. 1991;53(2):491–498. doi: 10.1093/ajcn/53.2.491. [DOI] [PubMed] [Google Scholar]
- 58.Bonanome A, Grundy SM. Effect of dietary stearic acid on plasma cholesterol and lipoprotein levels. N Engl J Med. 1988;318(19):1244–1248. doi: 10.1056/NEJM198805123181905. [DOI] [PubMed] [Google Scholar]
- 59.Aro A, Jauhiainen M, Partanen R, Salminen I, Mutanen M. Stearic acid, trans fatty acids, and dairy fat: effects on serum and lipoprotein lipids, apolipoproteins, lipoprotein(a), and lipid transfer proteins in healthy subjects. Am J Clin Nutr. 1997;65(5):1419–1426. doi: 10.1093/ajcn/65.5.1419. [DOI] [PubMed] [Google Scholar]
- 60.Jakobsen MU, O’Reilly EJ, Heitmann BL et al. Major types of dietary fat and risk of coronary heart disease: a pooled analysis of 11 cohort studies. Am J Clin Nutr. 2009;89(5):1425–1432. doi: 10.3945/ajcn.2008.27124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mozaffarian D, Micha R, Wallace S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2010;7(3):e1000252. doi: 10.1371/journal.pmed.1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]