Abstract
Carnitine acetyltransferase (CrAT) is a mitochondrial matrix enzyme that catalyzes the interconversion of acetyl-CoA and acetylcarnitine. Emerging evidence suggests that this enzyme functions as a positive regulator of total body glucose tolerance and muscle activity of pyruvate dehydrogenase (PDH), a mitochondrial enzyme complex that promotes glucose oxidation and is feedback inhibited by acetyl-CoA. Here, we used tandem mass spectrometry-based metabolic profiling to identify a negative relationship between CrAT activity and muscle content of lipid intermediates. CrAT specific activity was diminished in muscles from obese and diabetic rodents despite increased protein abundance. This reduction in enzyme activity was accompanied by muscle accumulation of long-chain acylcarnitines (LCACs) and acyl-CoAs and a decline in the acetylcarnitine/acetyl-CoA ratio. In vitro assays demonstrated that palmitoyl-CoA acts as a direct mixed-model inhibitor of CrAT. Similarly, in primary human myocytes grown in culture, nutritional and genetic manipulations that promoted mitochondrial influx of fatty acids resulted in accumulation of LCACs but a pronounced decrease of CrAT-derived short-chain acylcarnitines. These results suggest that lipid-induced antagonism of CrAT might contribute to decreased PDH activity and glucose disposal in the context of obesity and diabetes.
Keywords: acetyl-coenzyme A, acylcarnitines, lipid metabolism, mitochondria, muscle, diabetes
l-Carnitine is a conditionally essential nutrient that serves as a substrate for a family of acyltransferase enzymes that catalyze the interconversion of acyl-CoAs and acylcarnitines. Unlike their acyl-CoA precursors, acylcarnitines can be transported across cellular membranes. Accordingly, carnitine is best known for its obligatory role in shuttling long-chain acyl-CoAs (LCACoAs) from the cytoplasm into the mitochondrial matrix for fatty acid oxidation, a function that is mediated by the outer mitochondrial membrane enzyme, carnitine palmitoyltransferase 1 (CPT1). The long-chain acylcarnitine (LCAC) products of CPT1 are transported across the inner mitochondrial membrane by carnitine acylcarnitine translocase and then converted back to LCACoAs by carnitine palimitoyltransferase 2 (CPT2), also localized to the inner membrane. By contrast, carnitine acetyltransferase (CrAT) resides in the mitochondrial matrix and has strong preference for short-chain acyl-CoA (SCACoA) intermediates of fatty acid, glucose, and amino acid catabolism. Thus, CrAT facilitates trafficking and efflux of carbon intermediates from the mitochondrial compartment to other cellular and extracellular sites.
Recent animal studies have established important roles for l-carnitine and CrAT in regulating glucose homeostasis and mitochondrial substrate switching (1). By converting acetyl-CoA to acetylcarnitine, CrAT not only buffers the mitochondrial acetyl-CoA pool but also regenerates free CoA, both of which influence the activities of several oxidative enzymes. Carnitine supplementation promotes acetylcarnitine efflux and encourages carbon flux through pyruvate dehydrogenase (PDH), the enzyme complex that connects glycolysis to glucose oxidation and that is feedback inhibited by its product, acetyl-CoA (2). Fitting with the notion that CrAT mitigates acetyl-CoA inhibition of PDH, mice with muscle-specific deletion of Crat show impaired switching from fatty acid to glucose-derived fuels during the fed-to-fasted transition (1). These perturbations in fuel metabolism were associated with intramuscular accumulation of SCACoAs, medium-chain acyl-CoAs (MCACoAs), and LCACoAs; decreased PDH activity; and development of whole-body insulin resistance (1).
Because PDH activity, substrate switching, and glucose tolerance are negatively impacted by obesity and high-fat (HF) feeding, the present study sought to determine whether these nutritional and pathophysiological conditions might likewise impinge upon CrAT activity. To this end, we examined changes in acylcarnitine/acyl-CoA balance, CrAT expression, and CrAT activity in a variety of rodent and cell culture models of nutrient-induced metabolic dysfunction. We found that CrAT activity was indeed decreased in response to genetic diabetes, HF feeding, and lipid exposure. Taken together with previous studies, these results suggest that diminished CrAT activity might contribute to low PDH activity and impaired glucose disposal in the context of obesity and diabetes.
MATERIALS AND METHODS
Animals
Animal studies were approved by the Duke University Institutional Animal Care and Use Committee. Male Wistar rats (150–175 g, Charles River) were single housed and allowed ad libitum access to food and water. Animals were randomly selected to receive 20 weeks of either a low-fat (LF) diet (D12450B) or a 45% HF diet (D12451; Research Diets) beginning at 3 months of age. Male Zucker diabetic fatty (ZDF) rats and lean controls (Charles River) were allowed ad libitum access to standard chow and water before harvest at 3 months of age. Rats were euthanized after intraperitoneal injection of Nembutal with the dose of 25 mg/kg body weight. Gastrocnemius samples were clamp frozen and stored at −80°C. Tissues were ground into powder and processed in CelLytic buffer (Sigma Chemicals, St. Louis, MO) by freeze fracturing three times and sonication at 5× 1 s pulses on setting five.
Mitochondrial isolation
Skeletal muscle mitochondria were prepared according to Kerner et al. (3) with modification. Mouse gastrocnemius muscles were removed under anesthesia and placed in ice-cold KMEM buffer (100 mM KCl, 50 mM MOPS, 1 mM EGTA, 5 mM MgSO4, pH 7.4). The tissue was cleaned, blotted, weighed, finely minced, and suspended at a 10-fold dilution in KMEM plus 1 mM ATP. The suspension was homogenized on ice using 10 passes with a Potter-Elvehjem homogenizer. KMEM/ATP buffer supplemented with 0.2% BSA was then added to achieve a 20-fold dilution. The homogenate was centrifuged at 500 g for 10 min at 4°C. The supernatant was then centrifuged at 10,000 g for 10 min at 4°C, and the pellet was resuspended in 500 µl of KMEM/ATP buffer and centrifuged at 7,000 g for 10 min at 4°C to wash. The pellet was resuspended in 500 µl KMEM and centrifuged at 7,000 g for 10 min at 4°C. The resulting mitochondrial pellet was resuspended in CelLytic lysis buffer (Sigma Chemicals) and processed by freeze fracturing three times and sonication at 5× 1 s pulses on setting five.
CrAT activity
Carnitine-dependent conversion of acetyl-CoA to acetylcarnitine and free CoA was measured as previously described with minor modifications (1). Cell lysates and isolated mitochondria were resuspended in CelLytic lysis buffer (Sigma Chemicals) and processed by freeze fracturing three times and sonication at 5× 1 s pulses on setting five. Acetyl-CoA and 0.1 mM DTNB were combined with purified enzyme, cell lysates, or isolated mitochondria. The assay buffer included 50 mM Tris and 1 mM EDTA in water at pH 7.8. CrAT activity was determined spectrophotometrically at 412 nm by evaluating the rate of reduction of DTNB by free CoA on a Spectramax M5 spectrophotometer (Molecular Devices). Samples were read for 2 min in the absence of carnitine to determine a baseline rate. Reactions were started with the addition of 5 mM l-carnitine (unless stated otherwise) and monitored every 20 s for 10 min. The 2 min baseline rate was then subtracted from the final linear rate to yield a corrected rate. Activity calculations were made using an instrument-specific extinction coefficient for TNB of 16,029 M−1 cm−1 determined using l-glutathione as a CoA donor and correcting for a path length of 0.641 cm (for a 0.2 ml reaction volume in Nunc 96-well plates). All substrates were purchased from Sigma and reconstituted in activity buffer.
Western blots
Protein was isolated using CelLytic lysis buffer (Sigma Chemicals). A BCA kit (Sigma Chemicals) was used to quantify protein. Protein (50 μg from cell/tissue lysates) was separated by SDS-PAGE, transferred to nitrocellulose, and incubated with antibodies prepared with 5% milk in TBS-Tween. Secondary antibodies were HRP-conjugated, and ECL detection reagent (Pierce, Rockford, IL) was used to visualize protein bands. Protein expression was normalized to total protein as determined by MemCode staining (Thermo Scientific). The primary CrAT antibody was a generous gift from the laboratory of Dr. Fausto Hegardt.
Metabolic profiling
Tissue and plasma samples were processed and analyzed by the Sarah W. Stedman Nutrition and Metabolism Metabolomics/Biomarker Core Laboratory. Acylcarnitine measurements were made using flow injection tandem mass spectrometry and sample preparation methods described by An et al. (4) and Haqq et al. (5). Acyl-CoA esters were analyzed using a method based on a previously published report (6) that relies on the extraction procedure described by Deutsch et al. (7). The CoAs were further purified by solid phase extraction as described by Minkler et al. (8).
Cell culture
Primary human skeletal myocytes (HSkMCs) were grown and differentiated as previously described (9), but with the addition of 100 μM l-carnitine in the differentiation medium. Cytomegalovirus promoter-driven recombinant adenoviruses (rAds) encoding either β-galactosidase (rAd-β-gal) or Myc-tagged rat carnitine palmitoyltransferase b (rAd-CPT1b) were constructed, amplified, and purified as previously described (10). On differentiation day 3, myotubes were treated overnight with 5.3 × 10−3 infectious units/cm2 rAd-β-gal or rAd-CPT1b. Medium was replaced on differentiation day 4. On day 6, cells were treated with differentiation medium containing 1% BSA, alone or complexed with 100 μM or 500 μM 1:1 oleate:palmitate, along with 1 mM l-carnitine. Conditioned culture medium and cell lysates were harvested on differentiation day 7. Cells were washed twice with PBS and flash frozen in liquid nitrogen, and lysates were harvested in water after scraping. Specimens were submitted to the Stedman Center Metabolomics Core Laboratory for profiling of acylcarnitines. Metabolite concentrations were normalized to total cellular protein. Similarly, rAd-CrAT treatment and processing was done according to Noland et al. (2).
Statistics
Statistical analyses were performed using SigmaStat (SysStat Software Inc., Point Richmond, CA) or the Microsoft Excel statistical package. Within-group responses to experimental manipulations were evaluated using a paired t-test, where appropriate. IC50 values were calculated using Prism GraphPad software. Data are presented as means ± SE, and the level of significance was established a priori at P ≤ 0.05.
RESULTS
Obesity and diabetes disrupt acyl-CoA buffering
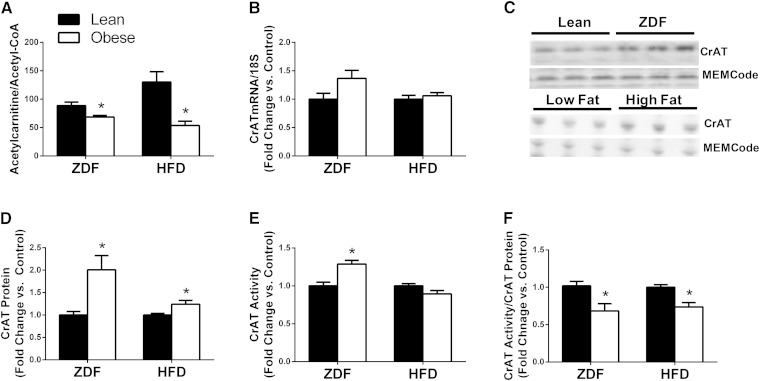
Obesity and diabetes are associated with muscle accumulation of medium-chain acylcarnitines (MCACs) and LCACs (2, 11). Because these intermediates are presumed to be in equilibrium with their cognate acyl-CoA precursors (12, 13), here we sought to examine tissue fluctuations in these two interconnected metabolite pools. As expected, LCACs and LCACoAs were increased (up to 4.6-fold) in both heart and gastrocnemius muscles from ZDF rats compared with lean controls, and to a lesser degree from those fed an HF diet versus an LF control diet (Table 1 and supplementary Fig. I). Despite pronounced accumulation of LCAC and MCAC species in heart and muscle of diabetic and/or obese rodents, acetylcarnitine (C2) levels were unchanged and tended to decrease in these same tissues (Table 1). Muscle levels of acetyl-CoA, the principal substrate of CrAT, were unchanged in the diabetic model but increased 1.8-fold in gastrocnemius muscles from rats fed an HF versus an LF diet.
TABLE 1.
Obesity and diabetes disrupt acyl-CoA buffering
| Heart |
Gastrocnemius Muscle |
Gastrocnemius Muscle |
||||
| Lean | ZDF | Lean | ZDF | LF | HF | |
| Total LCACs (pmol/mg tissue) | 7.7 ± 2.4 | 35.6 ± 7.1a | 8.3 ± 1.5 | 28.6 ± 3.5a | 2.3 ± 0.5 | 3.4 ± 0.6 |
| Total LCACoAs (pmol/mg tissue) | 12.6 ± 3.6 | 41.4 ± 5.8a | 5.0 ± 0.3 | 11.2 ± 0.9a | 7.2 ± 0.4 | 11.2 ± 1.0a |
| Total MCACs (pmol/mg tissue) | 0.6 ± 0.2 | 2.0 ± 0.4a | 1.1 ± 0.2 | 3.5 ± 0.4a | 0.6 ± 0.2 | 0.7 ± 0.1 |
| Total MCACoAs (pmol/mg tissue) | 5.3 ± 0.6 | 6.3 ± 0.5 | 1.6 ± 0.2 | 1.9 ± 0.1 | 2.0 ± 0.2 | 2.1 ± 0.2 |
| Acetylcarnitine (pmol/mg tissue) | 181.6 ± 16.3 | 135.6 ± 16.3 | 153.0 ± 10.7 | 153.6 ± 8.8 | 76.6 ± 12.2 | 54.4 ± 7.4 |
| Acetyl-CoA (pmol/mg tissue) | 15.4 ± 1.4 | 16.0 ± 0.5 | 1.8 ± 0.2 | 2.2 ± 0.07 | 0.58 ± 0.02 | 1.0 ± 0.07a |
Tandem mass spectrometry was used to assess total LCACs, LCACoAs, MCACs, MCACoAs, acetylcarnitine, and acetyl-CoA. Metabolites were measured in tissue homogenates from obese ZDF rats and lean control animals, and from adult Wistar rats fed a 10% LF or 45% HF diet for 20 weeks. Data are expressed as pmol/mg tissue and represent means ± SE from 5 to 8 animals per group.
P < 0.05, lean versus obese.
CrAT specific activity is diminished in rodent models of obesity and diabetes
Collectively, the results in Table 1 revealed discordant fluctuations in the myocellular acyl-CoA and acylcarnitine pools in response to obesity and diabetes. Fig. 1A highlights the obesity-associated shift in the acetylcarnitine/acetyl-CoA ratio, which decreased 23% and 58% in muscles from the ZDF and HF fed rats, respectively, as compared with controls. Because a similar imbalance resulted from muscle-specific deletion of Crat (1), we questioned whether these observations might be indicative of reduced enzyme activity. To test this possibility, we first measured changes in CrAT mRNA and protein expression in the same models. Interestingly, CrAT mRNA levels tended to increase (Fig. 1B), whereas CrAT protein abundance (Fig. 1C, D) was doubled in gastrocnemius muscles from ZDF compared with lean control rats. Likewise, the obesogenic HF diet did not affect CrAT mRNA but increased protein abundance by 24% compared with the LF diet controls (Fig. 1B–D). Despite the 2-fold rise in CrAT protein levels measured in tissue lysates from ZDF compared with control rats, CrAT activity (measured in homogenates prepared from the same tissues) was elevated by only 20% (Fig. 1E). Similarly, CrAT activity was unchanged in homogenates prepared using muscles from the HF-fed rats, despite a 24% increase in CrAT protein levels (Fig. 1E). Thus, when CrAT activity was corrected for protein abundance, specific activity of the enzyme was reduced 30% and 26%, in the ZDF and HF diet models, respectively (Fig. 1F). Protein linearity of the CrAT activity assay was confirmed using muscle homogenates from lean rats (supplementary Fig. II). Similar results were observed in heart homogenates from ZDF compared with lean control rats (supplementary Fig. III) and skeletal muscle homogenates of C57BL6/J mice fed an HF compared with an LF diet (supplementary Fig. IV). Thus, this response is consistent across tissues and species.
Fig. 1.
CrAT specific activity is diminished by obesity and diabetes. Gastrocnemius muscles were harvested from obese ZDF rats and lean control animals, and from adult Wistar rats fed a 10% LF or 45% HF diet for 20 weeks. (A) Tandem mass spectrometry-based measurement of the acetylcarnitine:acetyl-CoA ratio. (B) CrAT mRNA expression normalized to 18S. (C) Representative western blots and (D) protein abundance of CrAT normalized to the MEMCode stain. (E) Total CrAT enzyme activity and (F) CrAT specific activity corrected for CrAT protein abundance. Data are expressed as fold change relative to the lean control group and represent means ± SE of 5–8 animals per group. * P < 0.05 lean versus obese.
LCACoAs inhibit CrAT activity
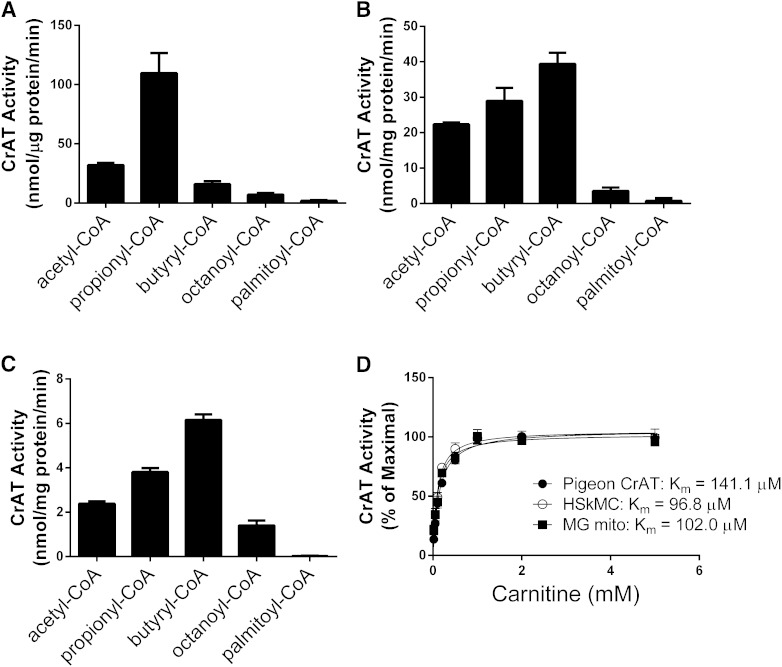
Results in Fig. 1 revealed a negative relationship between intramuscular lipid accumulation and CrAT activity. Relevant to this observation, earlier studies reported that LCACoAs inhibited the activity of purified pigeon CrAT (14–16). Here, we sought to examine the inhibitory actions of palmitoyl-CoA on the activity of rat and mouse CrAT when assayed in a more native environment. To this end, we first compared CrAT substrate preference and enzyme kinetics using purified pigeon protein (Fig. 2A), lysates of HSkMC treated with an rAd encoding rCrAT (Fig. 2B), and isolated mitochondria from mouse gastrocnemius muscle (Fig. 2C). Consistent with earlier reports, both pigeon and rodent CrAT strongly preferred SCACoAs and had essentially no activity with palmityol-CoA. Preference for specific short-chain substrates differed by species. Whereas purified pigeon CrAT preferred propionyl-CoA>acetyl-CoA>butyryl-CoA, mouse and rat CrAT had greatest preference for butyryl-CoA, followed by propionyl-CoA>acetyl-CoA (Fig. 2B, C). Affinity for l-carnitine was similar in the three systems assayed, each showing a Km of ∼0.10 mM (Fig. 2D).
Fig. 2.
CrAT prefers SCACoAs. CrAT enzyme activity was measured with 0.45 mM acyl-CoA substrates of various chain lengths and 5.0 mM l-carnitine using (A) purified CrAT from pigeon breast muscle, (B) lysates from primary HSkMCs expressing recombinant rat CrAT, and (C) isolated mitochondria from mouse gastrocnemius muscle (MG). (D) CrAT activity as a function of increasing carnitine concentration measured in the presence of 0.45 mM acetyl-CoA. Results are expressed as percent of maximal CrAT activity. Data represent means ± SE from 3 to 5 separate experiments.
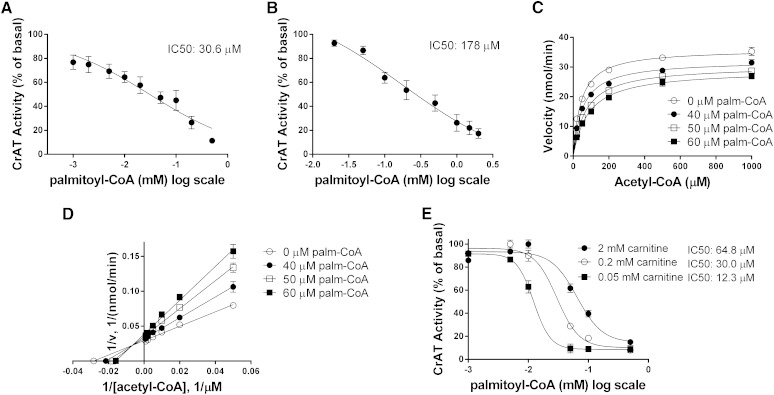
We next examined palmitoyl-CoA inhibition of CrAT activity using the same three systems. Palmitoyl-CoA inhibited purified pigeon CrAT with an IC50 of 30.6 µM (Fig. 3A), whereas the IC50 values for recombinant rat CrAT expressed in HSkMC and mouse CrAT measured in isolated muscle mitochondria were 178 µM and 65 µM, respectively (Fig. 3B, E). Oleoyl-CoA inhibited CrAT activity in a similar manner (data not shown). In isolated mitochondria, 40–60 μM palmitoyl-CoA reduced the Vmax of CrAT by 11% to 23% (Fig. 3C). Intersection of the double reciprocal (Lineweaver-Burk) plots occurred to the left of the vertical (1/v) axis (Fig. 3D), reflecting a mixed-model form of inhibition. This result implies a combination of uncompetitive inhibition (inhibitor binds only to the enzyme-substrate complex) and competitive inhibition (inhibitor binds only in the absence of substrate). Thus, palmitoyl-CoA appears to bind CrAT regardless of whether SCACoA substrate is already bound. Interestingly, however, the concentration of palmitoyl-CoA necessary for half-maximal inhibition of CrAT increased from 12 µM to 65 µM in the presence of low (0.05 mM) as compared with high (2 mM) carnitine concentrations (Fig. 3E). This implies that binding of carnitine to CrAT reduces its affinity for palmitoyl-CoA, suggesting that LCACoAs function as more potent inhibitors of the enzyme when mitochondrial concentrations of free carnitine are low.
Fig. 3.
LCACoAs inhibit CrAT activity. CrAT activity as a function of increasing palmitoyl-CoA concentration was measured using (A) purified protein from pigeon breast, (B) isolated mitochondria from primary HSkMCs expressing recombinant rat CrAT, and (C, D) isolated mitochondria from mouse gastrocnemius muscle. Assays in A, B, and E were performed with 0.45 mM acetyl-CoA and 5 mM l-carnitine. (E) Palmitoyl-CoA inhibition of CrAT activity as a function of l-carnitine concentration using isolated mitochondria from mouse gastrocnemius muscle. Data represent means ± SE from 3 to 5 separate experiments (A, B) or 5–8 animals per group (C–E).
Lipid exposure and increased CPT1 activity reduced acetylcarnitine production in primary HSkMCs
We next sought to determine whether chronic exposure of muscle mitochondria to elevated fatty acids is sufficient to inhibit CrAT activity in an intact cellular system. To this end, primary HSkMCs were incubated with increasing doses of a 1:1 mixture of oleate:palmitate, and short-chain acylcarnitine (SCAC) profiles were used as a surrogate index of CrAT activity. Additionally, to promote fatty acid influx into the mitochondrial matrix where CrAT resides, HSkMCs were also treated with rAd encoding rat CPT1b. This experimental paradigm was designed to mimic both the lipid conditions and increased expression of CPT1b that are characteristic of obesity and diabetes (17).
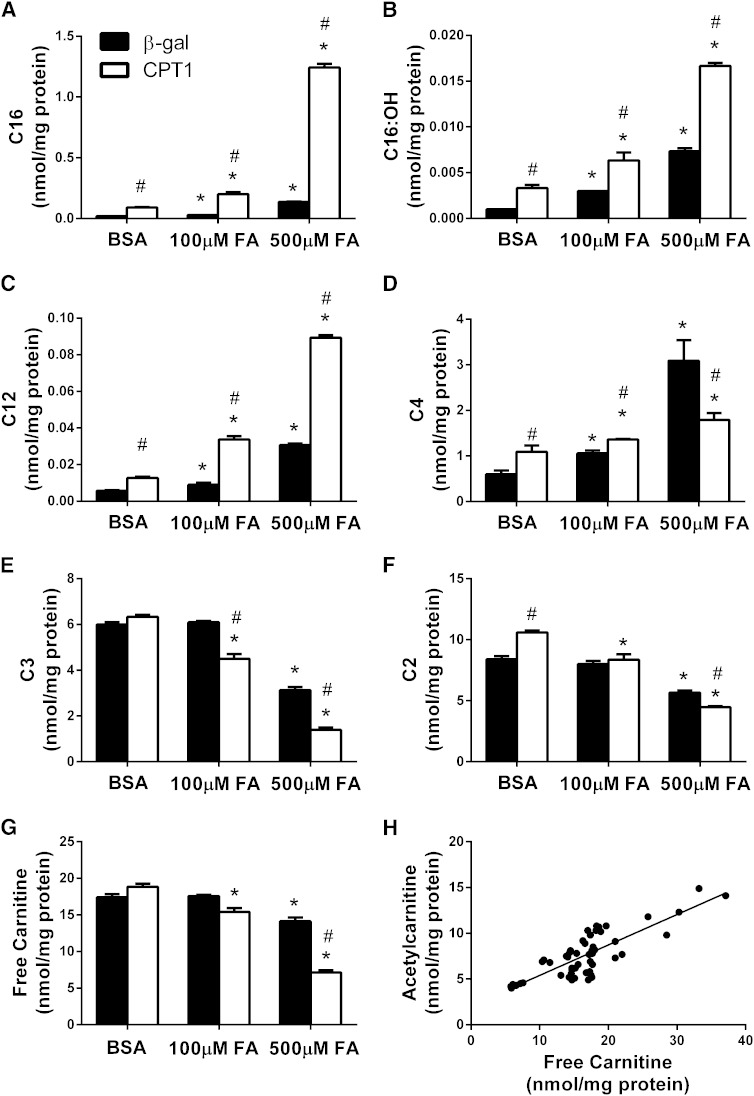
After 24 h exposure to fatty acids, control cells treated with rAd encoding β-gal increased production of palmitoylcarnitine (C16), 3-hydroxypalmitoylcarnitine (C16:OH), and lauroylcarnitine (C12) in a dose-dependent fashion (Fig. 4A–C). The C12 and C16:OH carnitine species are generated as by-products of β-oxidation. Thus, cellular accumulation of these intermediates is likely to reflect increased production of the respective acyl-CoA precursors within the mitochondrial compartment. Compared with the rAd-β-gal control group, treatment of cells with rAd-CPT1b caused a 4- to 9-fold increase in cellular levels of palmitoylcarnitine (C16), the direct product of the enzyme. Overexpression of rAd-CPT1b also elevated most LCACs and MCACs, suggesting increased mitochondrial transport and catabolism of fatty acids (Fig. 4A–C). Because MCACs and LCACs are thought to be in equilibrium with their acyl-CoA counterparts, these results support the notion that lipid exposure, especially in combination with CPT1b overexpression, leads to a rise in the mitochondrial pool of LCACoAs.
Fig. 4.
Fatty acid exposure and CPT1 overexpression decrease SCAC production in HSkMCs. Acylcarnitine levels were measured in lysates prepared from primary HSkMCs treated with rAd encoding β-gal (filled bars) or rat CPT1b (open bars). Cells were treated with adenoviruses on differentiation day 3 and harvested on day 7 following 24 h exposure to 1% BSA alone or complexed with 100 or 500 µM 1:1 oleate:palmitate, along with 1 mM l-carnitine. Specific acylcarnitine species shown include (A) palmitoylcarnitine (C16), (B) 3-hydroxypalmitoylcarnitine (C16:OH), (C) lauroylcarnitine (C12), (D) butyrylcarnitine (C4), (E) propionylcarnitine (C3), (F) acetylcarnitine (C2), and (G) free carnitine. (H) Cellular levels of free carnitine and acetylcarnitine were strongly correlated. * P < 0.05 fatty acid treatment versus BSA control; # P < 0.05 rAd-CPT1b versus β-gal control virus.
Interestingly, despite marked lipid-induced increases in LCACs and MCACs, a disparate pattern emerged for the short-chain products of the CrAT reaction. For example, cellular accumulation of butyrylcarnitine (C4) increased dose dependently in the control cells but not the rAd-CPT1b-treated group. On the contrary, conditions of high lipid exposure (500 μM O:P) caused C4 levels to decrease 50% in the cells overexpressing CPT1b as compared with controls (Fig. 4D). Moreover, fatty acid exposure resulted in a dose-dependent decrease in both propionylcarnitine (C3) and acetylcarnitine, and this effect was exacerbated by CPT1b overexpression (Fig. 4E–G). A nearly identical pattern emerged when we measured acylcarnitine efflux into the culture medium during the same 24 h period of lipid exposure (supplementary Fig. V). Despite ample provision of extracellular carnitine (1 mM), intracellular content of free carnitine declined in response to lipid exposure, particularly in the rAd-CPT1b group. Also noteworthy, cellular concentrations of free carnitine correlated strongly with acetylcarnitine (Fig. 4H) but not LCAC (not shown). These observations are consistent with the notion that lipid exposure antagonizes CrAT activity, not only by increasing mitochondrial concentrations of LCACoAs but also by reducing availability of free carnitine within the mitochondrial matrix (Fig. 5).
Fig. 5.
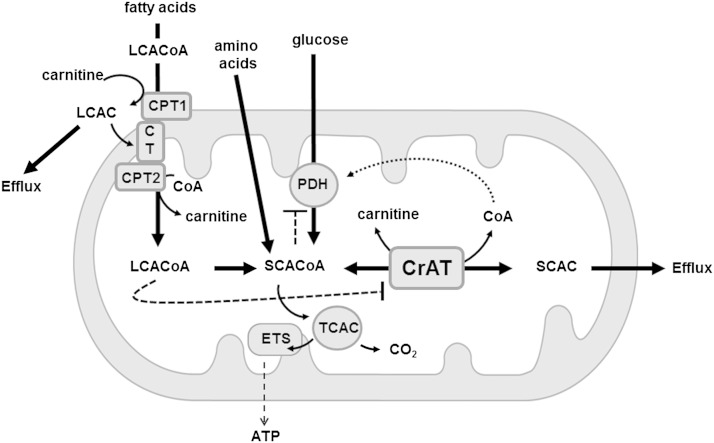
Proposed model of lipid-induced inhibition of CrAT activity. Fatty acid-derived LCACoAs cross the mitochondrial double membrane via the carnitine/acylcarnitine transport system. After traversing the inner membrane through the action of carnitine acylcarnitine translocase (CT), the acylcarnitine products of CPT1 are converted back to LCACoAs by CPT2, thereby replenishing the matrix pool of free carnitine. CrAT coverts SCACoA intermediates of fat, glucose, and amino acid metabolism to SCAC metabolites that can efflux from the mitochondria when nutrient supply and catabolism exceed flux through the tricarboxylic acid cycle (TCAC) and the electron transport system (ETS). CrAT lowers acetyl-CoA and regenerates free CoA, which together disinhibits PDH and promotes glucose oxidation. Excessive formation and cellular efflux of LCAC depletes the matrix pool of free carnitine, thereby limiting CrAT activity. Additionally, LCACoAs act as direct allosteric inhibitors of CrAT. These mechanisms might serve to counterregulate the activities of CrAT and PDH to spare glucose and promote β-oxidation when lipids are plentiful.
DISCUSSION
CrAT regulates the intramitochondrial pool of SCACoAs. Because emerging evidence points to a critical role for this enzyme in regulating whole-body glucose homeostasis, the present investigation sought to determine whether nutritional stresses that perturb metabolic control alter CrAT expression and/or function. Our study produced three major findings. First, in skeletal muscle and heart from obese rodents, tissue balance between acetylcarnitine and acetyl-CoA was disrupted in accordance with reduced CrAT specific activity and elevated concentrations of LCACoAs. Second, we confirmed earlier reports that CrAT activity is potently inhibited by LCACoAs. Finally, experiments in primary human myocytes showed that nutritional and genetic manipulations that promote fatty acid delivery to mitochondria caused a marked decline in cellular production and efflux of CrAT-derived acylcarnitines. In aggregate, these results suggest that CrAT function is compromised by chronic overnutrition, due at least in part to lipid-induced inhibition of enzyme activity.
Chronic overfeeding leads to increased adiposity, hyperlipidemia, and ectopic accumulation of triacylglycerol and other lipid-derived metabolites in tissues such as liver, pancreas, skeletal muscle, and heart (18, 19). Consistent with previous reports (2, 5), the current study found that muscle levels of MCACoAs and LCACoAs, along with several even chain acylcarnitine intermediates of fat oxidation, were elevated in rodent models of obesity and diabetes. β-Oxidation is a rich source of SCACoAs, and CrAT uses acetyl-CoA substrate derived from both glucose and fat catabolism (1, 20). We therefore anticipated that muscle concentrations of acetyl-CoA and acetyl-carnitine might rise in concert during periods of nutrient surplus. Instead, tissue levels of acetylcarnitine were either diminished or remained unchanged at times when acetyl-CoA levels trended upward (Table 1). The corresponding decline in the acetylcarnitine/acetyl-CoA ratio was accompanied by diminished CrAT specific activity, which was evident in each of three rodent obesity models examined in this study (Fig. 1 and supplementary Figs. III and IV).
CrAT interconverts acetyl-CoA and acetyl-carnitine with an equilibrium constant of 1.5–1.8 (21, 22). Accordingly, carbon flux through this enzyme is thought to depend simply on local concentrations of its substrates and products. However, we found that obesity and diabetes lowered CrAT specific activity at a posttranslational level. Our search to explain this observation uncovered earlier reports showing that LCACoAs inhibited purified preparations of pigeon, rat, and bovine CrAT (14–16). Importantly, we were able to reproduce these results in the context of a more native enzyme environment. Using isolated mitochondria and muscle cell lysates, we found that palmitoyl-CoA potently inhibited CrAT enzyme activity with an IC50 in the range of 12–65 μM. Likewise, 24 h exposure of primary HSkMCs to high concentrations of fatty acids resulted in a marked decrease in cellular production of SCAC species, especially when CPT1b was overexpressed. The suppressive effects of fatty acid exposure and CPT1b overexpression were specific for CrAT-derived metabolites, whereas the medium- and long-chain species changed in the opposite direction (Fig. 3). These findings support the notion that intramitochondrial accumulation of LCACoAs leads to a reduction in CrAT activity. Although the foregoing in vitro assays clearly point to allosteric enzyme inhibition as a potential mode of regulation, we cannot rule out the possibility that CrAT activity is also modulated by covalent modification. For example, protein palmitoylation of critical cysteine residues has been shown to inhibit the activities of glycolytic enzymes such as glycerol aldehyde phosphate dehydrogenase (23) and phosphofructokinase-1 (24), thereby contributing to lipid-induced lowering of glucose oxidation (25). The mechanism by which palmitoylation occurs is not entirely clear. Although acylation can occur enzymatically via the action of palmitoyltransferase (26), concentration-dependent autoacylation has been observed in vitro using various purified proteins (reviewed in Ref. 27). Another factor that might contribute to changes in CrAT activity in vivo is a shift in enzyme localization between the mitochondrial matrix and inner membrane (28, 29). Additional studies are necessary to explore these possibilities.
Fitting with earlier reports, palmitoyl-CoA inhibition of CrAT conformed to a mixed-model interaction with more potent inhibition occurring at low carnitine conditions. This finding is reminiscent of the interaction between CPT1 and its natural inhibitor, malonyl-CoA. Thus, carnitine competes with malonyl-CoA for binding to CPT1 and thereby defends enzyme activity (30). Because CPT1 and CrAT share similar carnitine binding motifs (31), these data support the notion that carnitine acyltransferases are susceptible to inhibition when local carnitine concentrations fall. This observation is particularly relevant to obesity, diabetes, and other disease conditions associated with carnitine insufficiency (2, 32–40). Moreover, results from the current study suggest that the mitochondrial pool of free carnitine is more prone to depletion than other compartments. For instance, lipid-induced accumulation of LCACs in primary HSkMCs was accompanied by diminished intracellular levels of free carnitine and a marked reduction in SCAC production. This finding has two important functional implications. First, owing to its position on the outer mitochondrial membrane, CPT1 gains preferred access to carnitine. Second, persistent production and efflux of LCACs appears to lower carnitine delivery to the mitochondrial matrix. These conditions set the stage for a scenario wherein chronic overexposure to lipids limits carnitine availability for the CrAT reaction without inhibiting CPT1 activity and/or fat oxidation.
The possibility that reduced CrAT activity might impact energy metabolism at a physiological level is supported by two lines of evidence. First, muscle-specific ablation of Crat disrupts whole-body glucose homeostasis in mice (1). The glucose intolerant phenotype of CratM−/− mice was linked to impaired substrate switching from fatty acid to glucose oxidation during the fasted-to-fed transition. In the loss-of-function model, preference for fat oxidation was evident systemically by indirect calorimetry as well as in isolated mitochondria. At a molecular level, CrAT deficiency resulted in elevated tissue levels of acetyl-CoA and complete loss of carnitine-stimulated PDH activity (1). Second, dietary l-carnitine supplementation has been shown to improve insulin sensitivity and increase whole-body glucose oxidation. In both animal and human studies, the salutary effects of carnitine supplementation were associated with marked increases in circulating and urinary concentrations of acetylcarnitine, along with elevated PDH activity measured in tissue homogenates. Likewise, overexpression of CrAT in primary human myocytes increases acetylcarnitine efflux and lowers fat oxidation while promoting glucose uptake (1, 2). The major regulatory effect of acetyl-CoA on PDH occurs via its activation of the four known PDH kinases (PDK1–4), which phosphorylate and inactivate the E1a subunit of the complex (41). Acetyl-CoA and free CoA compete for binding to the complex, and the half-maximal ratio for PDH phosphorylation is estimated at 0.4 (42). The acetyl-CoA/CoA ratio in muscle fluctuates between 0.1 and 0.8, depending on nutritional and physiological circumstances. Thus, small shifts in this ratio can contribute to substantive changes in PDH activity. Considering that CrAT lowers acetyl-CoA and regenerates free CoA, chronic inhibition of CrAT by palmitoyl-CoA has the potential to elevate the mitochondrial acetyl-CoA/CoA ratio to a level that promotes PDH phosphorylation and inhibits glucose oxidation, both of which are commonly observed in obese rodents and humans (43–46). In aggregate, these findings align with the notion that insufficient CrAT activity might exacerbate obesity-related metabolic derangements.
Finally, we consider the biological advantage of diminished CrAT activity at times when LCACoA concentrations rise. Given that CrAT promotes carbon flux through the PDH complex, perhaps this mechanism contributes to the glucose-fatty acid cycle described by Randle et al. (25). In this model, increased supply of lipid substrate promotes high rates of β-oxidation and expansion of the mitochondrial acetyl-CoA pool, which in turn inhibits PDH activity and glucose disposal. CrAT opposes the Randle mechanism by lowering acetyl-CoA and disinhibiting PDH, thereby facilitating glucose oxidation (25). The aforementioned reports strongly imply that CrAT-mediated production and efflux of acetylcarnitine promotes muscle glucose disposal during the fasted-to-fed transition (1). Conversely, lipid-induced antagonism of CrAT might serve to counterregulate glucose oxidation during circumstances of lipid surplus (Fig. 5). Whereas this mode of regulation would be predicted to confer a survival advantage by sparing glucose during starvation, we suggest that chronic inhibition of CrAT in the context of overnutrition contributes to low PDH activity and glucose intolerance. Studies to better understand the pathophysiological implications of reduced CrAT activity now await further investigation.
Supplementary Material
Footnotes
Abbreviations:
- β-gal
- β-galactosidase
- CPT1
- carnitine palmitoyltransferase 1
- CrAT
- carnitine acetyltransferase
- HF
- high fat
- HSkMC
- human skeletal myocyte
- LCAC
- long-chain acylcarnitine
- LCACoA
- long-chain acyl-CoA
- LF
- low fat
- MCAC
- medium-chain acylcarnitine
- MCACoA
- medium-chain acyl-CoA
- PDH
- pyruvate dehydrogenase
- rAd
- recombinant adenovirus
- SCAC
- short-chain acylcarnitine
- SCACoA
- short-chain acyl-CoA
- ZDF
- Zucker diabetic fatty
The authors are supported by grants from the United States Public Health Service: R01 DK089312 (D.M.M.) and 2P01-DK058398 (D.M.M. and C.B.N.), as well as funding from a Duke/National University of Singapore Collaborative Research Grant (D.M.M. and C.B.N.). D.M. Muoio and C.B. Newgard receive funding from Pfizer Incorporated. The remaining co-authors declare no conflict of interest.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of five figures.
REFERENCES
- 1.Muoio D. M., Noland R. C., Kovalik J. P., Seiler S. E., Davies M. N., DeBalsi K. L., Ilkayeva O. R., Stevens R. D., Kheterpal I., Zhang J., et al. 2012. Muscle-specific deletion of carnitine acetyltransferase compromises glucose tolerance and metabolic flexibility. Cell Metab. 15: 764–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noland R. C., Koves T. R., Seiler S. E., Lum H., Lust R. M., Ilkayeva O., Stevens R. D., Hegardt F. G., Muoio D. M. 2009. Carnitine insufficiency caused by aging and overnutrition compromises mitochondrial performance and metabolic control. J. Biol. Chem. 284: 22840–22852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kerner J., Turkaly P. J., Minkler P. E., Hoppel C. L. 2001. Aging skeletal muscle mitochondria in the rat: decreased uncoupling protein-3 content. Am. J. Physiol. Endocrinol. Metab. 281: E1054–E1062. [DOI] [PubMed] [Google Scholar]
- 4.An J., Muoio D. M., Shiota M., Fujimoto Y., Cline G. W., Shulman G. I., Koves T. R., Stevens R., Millington D., Newgard C. B. 2004. Hepatic expression of malonyl-CoA decarboxylase reverses muscle, liver and whole-animal insulin resistance. Nat. Med. 10: 268–274. [DOI] [PubMed] [Google Scholar]
- 5.Haqq A. M., Lien L. F., Boan J., Arlotto M., Slentz C. A., Muehlbauer M. J., Rochon J., Gallup D., McMahon R. L., Bain J. R., et al. 2005. The Study of the Effects of Diet on Metabolism and Nutrition (STEDMAN) weight loss project: rationale and design. Contemp. Clin. Trials. 26: 616–625. [DOI] [PubMed] [Google Scholar]
- 6.Magnes C., Sinner F. M., Regittnig W., Pieber T. R. 2005. LC/MS/MS method for quantitative determination of long-chain fatty acyl-CoAs. Anal. Chem. 77: 2889–2894. [DOI] [PubMed] [Google Scholar]
- 7.Deutsch J., Grange E., Rapoport S. I., Purdon A. D. 1994. Isolation and quantitation of long-chain acyl-coenzyme A esters in brain tissue by solid-phase extraction. Anal. Biochem. 220: 321–323. [DOI] [PubMed] [Google Scholar]
- 8.Minkler P. E., Kerner J., Ingalls S. T., Hoppel C. L. 2008. Novel isolation procedure for short-, medium-, and long-chain acyl-coenzyme A esters from tissue. Anal. Biochem. 376: 275–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muoio D. M., Way J. M., Tanner C. J., Winegar D. A., Kliewer S. A., Houmard J. A., Kraus W. E., Dohm G. L. 2002. Peroxisome proliferator-activated receptor-alpha regulates fatty acid utilization in primary human skeletal muscle cells. Diabetes. 51: 901–909. [DOI] [PubMed] [Google Scholar]
- 10.Becker T. C., BeltrandelRio H., Noel R. J., Johnson J. H., Newgard C. B. 1994. Overexpression of hexokinase I in isolated islets of Langerhans via recombinant adenovirus. Enhancement of glucose metabolism and insulin secretion at basal but not stimulatory glucose levels. J. Biol. Chem. 269: 21234–21238. [PubMed] [Google Scholar]
- 11.Koves T. R., Ussher J. R., Noland R. C., Slentz D., Mosedale M., Ilkayeva O., Bain J., Stevens R., Dyck J. R., Newgard C. B., et al. 2008. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 7: 45–56. [DOI] [PubMed] [Google Scholar]
- 12.Brass E. P., Hoppel C. L. 1980. Relationship between acid-soluble carnitine and coenzyme A pools in vivo. Biochem. J. 190: 495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carter A. L., Lennon D. L., Stratman F. W. 1981. Increased acetyl carnitine in rat skeletal muscle as a result of high-intensity short-duration exercise. Implications in the control of pyruvate dehydrogenase activity. FEBS Lett. 126: 21–24. [DOI] [PubMed] [Google Scholar]
- 14.Chase J. F. 1967. The substrate specificity of carnitine acetyltransferase. Biochem. J. 104: 510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huckle W. R., Tamblyn T. M. 1983. Purification and properties of carnitine acetyltransferases from bovine spermatozoa and heart. Arch. Biochem. Biophys. 226: 94–110. [DOI] [PubMed] [Google Scholar]
- 16.Mittal B., Kurup C. K. 1980. Purification of clofibrate-induced carnitine acetyltransferase from rat liver mitochondria. Biochim. Biophys. Acta. 619: 90–97. [DOI] [PubMed] [Google Scholar]
- 17.Zou Y., Du H., Yin M., Zhang L., Mao L., Xiao N., Ren G., Zhang C., Pan J. 2009. Effects of high dietary fat and cholesterol on expression of PPAR alpha, LXR alpha, and their responsive genes in the liver of apoE and LDLR double deficient mice. Mol. Cell. Biochem. 323: 195–205. [DOI] [PubMed] [Google Scholar]
- 18.Goodpaster B. H., Kelley D. E. 2002. Skeletal muscle triglyceride: marker or mediator of obesity-induced insulin resistance in type 2 diabetes mellitus? Curr. Diab. Rep. 2: 216–222. [DOI] [PubMed] [Google Scholar]
- 19.Muoio D. M., Newgard C. B. 2008. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat. Rev. Mol. Cell Biol. 9: 193–205. [DOI] [PubMed] [Google Scholar]
- 20.Ramsay R. R. 2000. The carnitine acyltransferases: modulators of acyl-CoA-dependent reactions. Biochem. Soc. Trans. 28: 182–186. [DOI] [PubMed] [Google Scholar]
- 21.Farrell S. O., Fiol C. J., Reddy J. K., Bieber L. L. 1984. Properties of purified carnitine acyltransferases of mouse liver peroxisomes. J. Biol. Chem. 259: 13089–13095. [PubMed] [Google Scholar]
- 22.Pieklik J. R., Guynn R. W. 1975. Equilibrium constants of the reactions of choline acetyltransferase, carnitine acetyltransferase, and acetylcholinesterase under physiological conditions. J. Biol. Chem. 250: 4445–4450. [PubMed] [Google Scholar]
- 23.Yang J., Gibson B., Snider J., Jenkins C. M., Han X., Gross R. W. 2005. Submicromolar concentrations of palmitoyl-CoA specifically thioesterify cysteine 244 in glyceraldehyde-3-phosphate dehydrogenase inhibiting enzyme activity: a novel mechanism potentially underlying fatty acid induced insulin resistance. Biochemistry. 44: 11903–11912. [DOI] [PubMed] [Google Scholar]
- 24.Jenkins C. M., Yang J., Sims H. F., Gross R. W. 2011. Reversible high affinity inhibition of phosphofructokinase-1 by acyl-CoA: a mechanism integrating glycolytic flux with lipid metabolism. J. Biol. Chem. 286: 11937–11950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Randle P. J., Garland P. B., Hales C. N., Newsholme E. A. 1963. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1: 785–789. [DOI] [PubMed] [Google Scholar]
- 26.Dunphy J. T., Greentree W. K., Manahan C. L., Linder M. E. 1996. G-protein palmitoyltransferase activity is enriched in plasma membranes. J. Biol. Chem. 271: 7154–7159. [DOI] [PubMed] [Google Scholar]
- 27.Dietrich L. E., Ungermann C. 2004. On the mechanism of protein palmitoylation. EMBO Rep. 5: 1053–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brdiczka D., Gerbitz K., Pette D. 1969. Localization and function of external and internal carnitine acetyltransferases in mitochondria of rat liver and pig kidney. Eur. J. Biochem. 11: 234–240. [DOI] [PubMed] [Google Scholar]
- 29.Bakker A., Biermans W., Van Belle H., De Bie M., Bernaert I., Jacob W. 1994. Ultrastructural localisation of carnitine acetyltransferase activity in mitochondria of rat myocardium. Biochim. Biophys. Acta. 1185: 97–102. [DOI] [PubMed] [Google Scholar]
- 30.López-Viñas E., Bentebibel A., Gurunathan C., Morillas M., de Arriaga D., Serra D., Asins G., Hegardt F. G., Gómez-Puertas P. 2007. Definition by functional and structural analysis of two malonyl-CoA sites in carnitine palmitoyltransferase 1A. J. Biol. Chem. 282: 18212–18224. [DOI] [PubMed] [Google Scholar]
- 31.Jogl G., Hsiao Y. S., Tong L. 2004. Structure and function of carnitine acyltransferases. Ann. N. Y. Acad. Sci. 1033: 17–29. [DOI] [PubMed] [Google Scholar]
- 32.De Palo E., Gatti R., Sicolo N., Padovan D., Vettor R., Federspil G. 1981. Plasma and urine free L-carnitine in human diabetes mellitus. Acta Diabetol. Lat. 18: 91–95. [DOI] [PubMed] [Google Scholar]
- 33.Soltész G., Melegh B., Sándor A. 1983. The relationship between carnitine and ketone body levels in diabetic children. Acta Paediatr. Scand. 72: 511–515. [DOI] [PubMed] [Google Scholar]
- 34.Winter S. C., Simon M., Zorn E. M., Szabo-Aczel S., Vance W. H., O'Hara T., Higashi L. 1989. Relative carnitine insufficiency in children with type I diabetes mellitus. Am. J. Dis. Child. 143: 1337–1339. [DOI] [PubMed] [Google Scholar]
- 35.Pregant P., Schernthaner G., Legenstein E., Lienhart L., Bruck S., Schnack C., Kaiser E. 1991. [Decreased plasma carnitine in type I diabetes mellitus]. Klin. Wochenschr. 69: 511–516. German. [DOI] [PubMed] [Google Scholar]
- 36.Tamamoğullari N., Siliğ Y., Içağasioğlu S., Atalay A. 1999. Carnitine deficiency in diabetes mellitus complications. J. Diabetes Complications. 13: 251–253. [DOI] [PubMed] [Google Scholar]
- 37.Poorabbas A., Fallah F., Bagdadchi J., Mahdavi R., Aliasgarzadeh A., Asadi Y., Koushavar H., Vahed Jabbari M. 2007. Determination of free L-carnitine levels in type II diabetic women with and without complications. Eur. J. Clin. Nutr. 61: 892–895. [DOI] [PubMed] [Google Scholar]
- 38.Okudaira N., Fujigaki M., Nakayoshi T., Komiya I., Sugiyama Y. 2001. Up-regulation of carnitine transporters helps maintain tissue carnitine levels in carnitine deficiency induced by pivalic acid. Pharm. Res. 18: 439–445. [DOI] [PubMed] [Google Scholar]
- 39.Longo N., Amat di San Filippo C., Pasquali M. 2006. Disorders of carnitine transport and the carnitine cycle. Am. J. Med. Genet. C. Semin. Med. Genet. 142C: 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tein I. 2003. Carnitine transport: pathophysiology and metabolism of known molecular defects. J. Inherit. Metab. Dis. 26: 147–169. [DOI] [PubMed] [Google Scholar]
- 41.Strumiło S. 2005. Short-term regulation of the mammalian pyruvate dehydrogenase complex. Acta Biochim. Pol. 52: 759–764. [PubMed] [Google Scholar]
- 42.Kerbey A. L., Radcliffe P. M., Randle P. J., Sugden P. H. 1979. Regulation of kinase reactions in pig heart pyruvate dehydrogenase complex. Biochem. J. 181: 427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Randle P. J., Priestman D. A., Mistry S. C., Halsall A. 1994. Glucose fatty acid interactions and the regulation of glucose disposal. J. Cell. Biochem. 55(Suppl.): 1–11. [DOI] [PubMed] [Google Scholar]
- 44.Sugden M. C., Orfali K. A., Holness M. J. 1995. The pyruvate dehydrogenase complex: nutrient control and the pathogenesis of insulin resistance. J. Nutr. 125: 1746S–1752S. [DOI] [PubMed] [Google Scholar]
- 45.Putman C. T., Spriet L. L., Hultman E., Lindinger M. I., Lands L. C., McKelvie R. S., Cederblad G., Jones N. L., Heigenhauser G. J. 1993. Pyruvate dehydrogenase activity and acetyl group accumulation during exercise after different diets. Am. J. Physiol. 265: E752–E760. [DOI] [PubMed] [Google Scholar]
- 46.Mondon C. E., Jones I. R., Azhar S., Hollenbeck C. B., Reaven G. M. 1992. Lactate production and pyruvate dehydrogenase activity in fat and skeletal muscle from diabetic rats. Diabetes. 41: 1547–1554. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.