Abstract
Accumulated evidence shows that G protein-coupled receptor 119 (GPR119) plays a key role in glucose and lipid metabolism. Here, we explored the effect of GPR119 on cholesterol metabolism and inflammation in THP-1 macrophages and atherosclerotic plaque progression in apoE−/− mice. We found that oxidized LDL (Ox-LDL) significantly induced long intervening noncoding RNA (lincRNA)-DYNLRB2-2 expression, resulting in the upregulation of GPR119 and ABCA1 expression through the glucagon-like peptide 1 receptor signaling pathway. GPR119 significantly decreased cellular cholesterol content and increased apoA-I-mediated cholesterol efflux in THP-1 macrophage-derived foam cells. In vivo, apoE−/− mice were randomly divided into two groups and infected with lentivirus (LV)-Mock or LV-GPR119 for 8 weeks. GPR119-treated mice showed decreased liver lipid content and plasma TG, interleukin (IL)-1β, IL-6, and TNF-α levels, whereas plasma levels of apoA-I were significantly increased. Consistent with this, atherosclerotic lesion development was significantly inhibited by infection of apoE−/− mice with LV-GPR119. Our findings clearly indicate that, Ox-LDL significantly induced lincRNA-DYNLRB2-2 expression, which promoted ABCA1-mediated cholesterol efflux and inhibited inflammation through GPR119 in THP-1 macrophage-derived foam cells. Moreover, GPR119 decreased lipid and serum inflammatory cytokine levels, decreasing atherosclerosis in apoE−/− mice. These suggest that GPR119 may be a promising candidate as a therapeutic agent.
Keywords: long intervening noncoding ribonucleic acid-DYNLRB2-2, G protein-coupled receptor 119, glucagon-like peptide 1 receptor, ATP binding cassette transporter A1, atherosclerosis
Atherosclerosis is a complex disease, involving many cell types and circulating mediators and resulting in an inflammatory state. Atherosclerotic lesions form de novo from focal accumulation of lipoproteins, monocyte-derived macrophages, and lymphocytes within the arterial wall (1, 2). However, cholesterol and other lipids can be mobilized and excreted to prevent atherosclerosis in a process termed reverse cholesterol transport (RCT), in which ABCA1 plays a key role. ABCA1 is a member of a family of highly conserved transmembrane transport proteins. It plays a crucial role in the efflux of cellular cholesterol to HDL and its apolipoproteins (3). In humans, ABCA1 mutations can cause a severe HDL-deficiency syndrome, leading to the development of sterol deposits in tissue macrophages and premature atherosclerosis (4). Because of its role in macrophage lipid transport, ABCA1 is an important target for the prevention and treatment of atherosclerosis.
G protein-coupled receptor 119 (GPR119), which has been described as a deorphanized G protein-coupled receptor, plays a significant role in mediating systemic metabolic homeostasis. It has recently attracted attention because evidence from in vitro systems and animal models suggests that its modulation has a favorable effect on glucose homoeostasis (5, 6). Because GPR119 promotes glucose-stimulated insulin secretion, pancreatic β-cell function, and glucagon-like peptide 1 (GLP-1) release, its therapeutic potential for the treatment of type 2 diabetes mellitus is getting more attention (7, 8). Moreover, GPR119 coupling to Gα stimulatory proteins induces adenylate cyclase activity, resulting in an increase in intracellular cAMP, which regulates the GPR119-mediated release of GLP-1 (9). In addition, the GPR119-mediated release of GLP-1 from intestinal L-cells is protein kinase (PK)A dependent (10). Furthermore, cAMP, as a common second messenger that can activate MAPKs via PKA (11), can stimulate ABCA1 expression with species specific (12), alleviating the accumulation of cholesterol in macrophages by promoting ABCA1-mediated cellular cholesterol efflux (13, 14). Moreover, GPR119 can be activated by oleoylethanolamide and several other endogenous lipids containing oleic acid (15). These data suggest that GPR119 not only improves systemic glucose homeostasis but also affects lipid metabolism homeostasis.
Long noncoding RNAs (lncRNAs) are nonprotein coding transcribed RNA molecules consisting of more than 200 nucleotides and fall into one of three categories including: 1) long intervening noncoding RNAs (lincRNAs) are transcribed from regions far away from protein-coding genes; 2) natural antisense transcripts are transcribed from the opposite strand of a protein-coding gene; and 3) intronic lncRNAs are transcribed from within introns of protein-coding genes (16). lncRNAs have emerged as key components of the address code, allowing protein complexes, genes, and chromosomes to be trafficked to appropriate locations, and subjected to proper activation and deactivation (17). lncRNA-based mechanisms control cell fates during development, and their dysregulation underlies some human disorders caused by chromosomal deletions (18). An increasing number of studies have revealed that lncRNAs have a variety of important functions, including their role in transcription, splicing, translation, nuclear factor trafficking, imprinting, genome rearrangement, and chromatin modification (19–21). Aberrant lncRNA expression is associated with human diseases such as cancer, Alzheimer's disease, and cardiovascular disease (22–24). Thus, a better understanding of the roles of lncRNA will advance our understanding of cell regulatory and disease mechanisms.
In the present study, we demonstrated that oxidized LDL (Ox-LDL) significantly induced lincRNA-DYNLRB2-2 expression, which upregulated ABCA1 expression through GPR119. In addition, lincRNA-DYNLRB2-2 and GPR119 promoted ABCA1-mediated cholesterol efflux from THP-1 macrophage-derived foam cells, and inhibited inflammatory responses in these cells. Overexpression of GPR119 markedly decreased lipid levels and serum inflammatory cytokine levels to prevent atherosclerosis in apoE−/− mice.
MATERIALS AND METHODS
Materials
Human lipoproteins [acetylated (Ac)-LDL, Ox-LDL, and HDL] were obtained from Biomedical Technologies Inc. (Stoughton, MA). The PrimeScript RT reagent kit (Perfect Real Time) (DRR037A; TaKaRa Bio, Inc., Shiga, Japan) and SYBR® Premix Ex TaqTM II (Tli RNaseH Plus) (DRR820A; TaKaRa Bio, Inc.) were obtained as indicated. Chemicals were obtained from Sigma-Aldrich (St. Louis, MO) unless otherwise noted.
Animals and diets
The investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication number 85-23, revised 1996) and was approved by the Animal Experimental Committee at Nanfang Hospital. Male apoE−/− mice with a C57BL/6 background were purchased from the Laboratory Animal Center of Peking University (Beijing, China) and housed five per cage at 25°C on a 12 h light/dark cycle. To detect the effect of GPR119 on lipid metabolism, 6-week-old apoE−/− mice were randomized into two groups and injected via the tail vein with control lentivirus [lentivirus (LV)-Mock, n = 15] or LV encoding mouse GPR119 (LV-GPR119, n = 15), respectively. The mice were fed a high-fat diet (HFD) for a period of 8 weeks. The diet was a commercially prepared mouse food supplemented with 21% (w/w) butterfat, 0.15% (w/w) cholesterol, and 19.5% (w/w) casein (Beijing Keao Xieli Feed Co., LTD, Beijing, China). At week 8, mice were anesthetized and 1 ml of blood was collected by cardiac puncture before the mice were euthanized by cervical dislocation and tissues were collected for further analysis.
Cell culture
Human monocytic THP-1 cells were obtained from ATCC (Manassas, VA). THP-1 cells were maintained in Roswell Park Memorial Institute (RPMI) 1640 medium containing 10% FCS and differentiated for 72 h with 100 nM PMA. Macrophages were transformed into foam cells by incubation in the presence or absence of 50 μg/ml of Ox-LDL in serum-free RPMI 1640 medium containing 0.3% BSA for 48 h. Cells were incubated at 37°C in an atmosphere of 5% CO2. Cells were seeded in 6- or 12-well plates or 60 mm dishes and grown to 60–80% confluence before use.
lncRNA microarray analysis
Briefly, THP-1 macrophages (three samples) and THP-1 macrophage-derived foam cells (three samples) were used to isolate total RNA. Total RNA from each sample was quantified using the NanoDrop ND-1000 spectrophotometer and RNA integrity was assessed using standard denaturing agarose gel electrophoresis. For microarray analysis, the Agilent Array platform was employed. The sample preparation and microarray hybridization were performed using the manufacturer's standard protocols with minor modifications. Briefly, mRNA was purified from 1 μg of total RNA after removal of rRNA (mRNA-ONLY™ eukaryotic mRNA isolation kit; Epicentre Biotechnologies, Madison, WI). Then, each sample was amplified and transcribed into fluorescent cRNA along the entire length of the transcripts without 3′ bias utilizing a random priming method. The labeled cRNAs were hybridized onto the Human LncRNA Array v2.0 (8 × 60K; Arraystar, Inc., Rockville, MD). After the slides were washed, the arrays were scanned using the Agilent scanner G2505B. Agilent Feature Extraction software (version 10.7.3.1) was used to analyze acquired array images. Quantile normalization and subsequent data processing were performed using the GeneSpring GX v11.5.1 software package (Agilent Technologies, Santa Clara, CA). After quantile normalization of the raw data, lncRNAs and mRNAs, in which at least three out of six samples had flags in present or marginal (“All Targets Value”), were chosen for further data analysis. Differentially expressed lncRNAs and mRNAs with statistical significance were identified through Volcano Plot filtering. All of the microarray data were uploaded to the NCBI Gene Expression Omnibus (GEO) with a GEO accession number (GSE54039).
Cytokine assays and measurement of serum biochemical parameters
The levels of human TNF-α, interleukin (IL)-1β, and IL-6 present in the culture media (R&D Systems, Minneapolis, MN); the serum concentrations of IL-1β, IL-6, and TNF-α (R&D Systems); the serum serum amyloid A (SAA) amount (IBL, Hamburg, Germany); and the levels of serum apoA-I and apoB100 (Cusabio Biotech Co., Ltd., Wuhan, China) were measured by ELISA according to the manufacturer's instructions. The TG and total cholesterol (TC) concentrations were determined enzymatically using an automated analyzer.
RNA isolation and real-time quantitative PCR analysis
Total RNA from cultured cells or mouse tissues was extracted using the TRIzol reagent (Invitrogen, Carlsbad, CA) in accordance with the manufacturer's instructions. lincRNA-DYNLRB2-2 was detected with a commercial reagent (Promega, Madison, Wisconsin) according to the instruction manual in 20 μl reaction volumes. Real-time PCR was performed on a real-time PCR ABI 7500 Fast system (Applied Biosystems, Foster City, CA). The expression of U6 RNA was used as an endogenous control. Primers used for lincRNA-DYNLRB2-2 were as follows: lincRNA-DYNLRB2-2-F, 5′-TACAGGCATGAGGCATCGT-3′ lincRNA-DYNLRB2-2-R, 5′-AGAGGTTGGGATTCATGCTAGA-3′ and lincRNA-DYNLRB2-2-RT, 5′-GCAAGACTTGGAGTTGGTGAT-3′. Primers used for U6 were as follows: U6-F, 5′-CTCGCTTCG GCAGCACA-3′ U6-R, 5′-AACGCTTCACGAATTTGCGT-3′ and U6-RT, 5′-AACGCTTCACGAATTTGCGT-3′. The mRNA levels were evaluated by real-time quantitative PCR using an ABI 7500 Fast real-time PCR system with SYBR Green detection chemistry (TaKaRa Bio, Inc.). Glyceraldehyde 3-phosphate dehydrogenase expression was used as the internal control. Quantitative measurements were determined using the ΔΔCt method. All samples were measured in triplicate and the mean value was considered for comparative analysis.
Western blot analyses
Cells and tissues were harvested and protein extracts prepared according to established methods. Extracts were then separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and subjected to Western blot analysis using rabbit polyclonal anti-LXRα, -SRA1, -CD36, -SREBP1c, -SREBP2, and -GPR119 antibodies (Proteintech Group, Inc., Chicago, IL); rabbit polyclonal anti-NF-κB, -PPARγ, and -ABCA1 antibodies (Abcam Inc., Cambridge, MA); rabbit polyclonal anti-ABCG1, -SR-B1, -LDLR, -HMGCR, -HMGS, and -Niemann-Pick C1-Like 1 antibodies (Epitomics, Burlingame, CA); and rabbit polyclonal anti-ABCG5, -ABCG8, -MTP-PXR, and -β-actin antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). The proteins were visualized using a chemiluminescence method (ECL Plus Western blot detection system; Amersham Biosciences, Foster City, CA).
LV production and infection
Human monocytic THP-1 cells were cultured. Packed empty LVs with green fluorescent protein (LV-Mock) and LV-mediated lincRNA-DYNLRB2-2 overexpression vector (LV-DYNLRB2-2) were generated. The cells were infected with the LV stock at a multiplicity of infection of 100 (THP-1 cells) transducing units per cell in the presence of 8 μg/ml of polybrene. The cells were washed with fresh complete media after 24 h of incubation.
Plasmid construction and transfection
The PCR-XL-TOPO vector containing GPR119, vector PIRES2-EGFP, Platinum HIFI Taq polymerase, and Accuprime Pfx DNA polymerase were purchased from Invitrogen Biotechnology (Shanghai, China). The pCDNA3.1(+) vector, competent DH5α cells, XhoI and EcoRI restriction enzymes, and T4 DNA ligase were purchased from TaKaRa Biotechnology Co., Ltd. (Dalian, China). The fragment of EcoRI-GPR119-IRES-EGFP-XhoI was achieved from the PCR-XL-TOPO vector and PIRES2-EGFP vector by overlap extension PCR and was linked to pcDNA3.1 to create the recombinant plasmid pcDNA3.1-GPR119-IRES-EGFP. The inserted gene was identified by electrophoresis and sequencing. The recombinant plasmid was then transfected into the cultured cells by Lipofectamine 2000 (Invitrogen), and the over-expression of GPR119 was confirmed by RT-PCR and Western blotting.
Transfection with siRNA mimics
THP-1 macrophages were transfected with 50 nM siRNAs against GPR119, ABCA1, and an irrelevant 21-nucleotide control siRNA (negative control) purchased from Ribo Biotechnology. Cells (2 × 106 cells/well) were transfected using Lipofectamine 2000 transfection reagent for 48 h according to the manufacturer's instructions. All experimental control samples were treated with an equal concentration of a nontargeting control mimic sequence (negative controls). After 48 h of transfection, real-time RT-PCR and Western blotting were performed.
Dil-Ox-LDL uptake assays
PMA-differentiated THP-1 cells were treated with LV lincRNA-DYNLRB2-2 (LV-DYNLRB2-2) or plasmid pcDNA3.1-GPR119 and its own negative control as indicated, then fluorescent-tagged Dil-Ox-LDL was added, and the cells were incubated for 1 h. Adherent cells were harvested, and washed three times with PBS. Analysis was performed on a fluorescent activated cell sorting (FACScalibur) flow cytometer (Becton Dickinson, Franklin Lakes, NJ) with Cell Quest Pro software (BD Biosciences, San Jose, CA).
Cellular cholesterol efflux experiments
THP-1 macrophages were cultured and labeled with 0.2 μCi/ml [3H]cholesterol. After 72 h, cells were subsequently washed with PBS and incubated overnight in RPMI 1640 medium containing 0.1% (w/v) BSA to allow equilibration of [3H]cholesterol in all cellular pools. Equilibrated [3H]cholesterol-labeled cells were washed with PBS and incubated in 2 ml of efflux medium containing RPMI 1640 medium and 0.1% BSA with 25 μg/ml human plasma apoA-I for 12 h. A 150 μl sample of efflux medium was obtained at the times designated and passed through a 0.45 μm filter to remove any floating cells. Monolayers were washed twice in PBS, and cellular lipids were extracted with isopropanol. Medium and cell-associated [3H]cholesterol was then measured by liquid scintillation counting. Percent efflux was calculated by the following equation: [total media counts/(total cellular counts + total media counts)] × 100%.
Phospholipid efflux experiments
Cells were cultured as indicated above, and then incubated with 2 μCi/ml of [3H]choline chloride to label the phospholipids. After 72 h, cells were subsequently washed with PBS and incubated overnight in RPMI 1640 medium containing 0.1% (w/v) BSA. After 6 h of incubation in medium containing 10 mg/ml apoA-I, efflux medium was collected, centrifuged to remove cell debris as above, and aliquots were taken for extraction and separated by thin-layer chromatography with the use of silica G plates developed in chloroform/methanol/ammonia (25%, w/v)/water (50:65:5:4, v/v/v/v). Phospholipid spots were visualized by I2 vapors and identified by comigration with standards. Relative radioactivity was measured by Phosphoscreen and quantified by PhosphorImager (Molecular Dynamics Inc.). Phospholipid efflux was expressed as percent counts in the supernatant versus the total counts for each individual lipid.
En face plaque area
Immediately after the mice were euthanized, the aortas were excised and fixed in 10% buffered formalin for quantification of the en face plaque areas. Briefly, after the adventitial tissue was carefully removed, the aorta was opened longitudinally, stained with Oil Red O (Sigma), and pinned on a blue wax surface. En face images were obtained by a stereomicroscope (SZX12; Olympus, Tokyo, Japan) equipped with a digital camera (Dxm1200, Nikon, Tokyo, Japan) and analyzed using Adobe Photoshop version 7.0 and Scion Image software. The percentage of the luminal surface area stained by Oil Red O was determined.
Quantification of atherosclerosis in the aortic sinus
The upper portion of the heart and proximal aorta were obtained, embedded in OCT compound (Fisher, Tustin, CA), and stored at −70°C. Serial 10 μm thick cryosections of the aorta, beginning at the aortic root, were collected for a distance of 400 μm. Sections were stained with Oil Red O. The area of positive staining for Oil Red O was calculated as a percentage of the total section area and an average lipid droplet size was calculated utilizing ImagePro Plus software (Media Cybernetics) from five views per animal.
Liver histology and Oil Red O staining
To examine hepatic lipid deposition, lipid was assessed in samples collected in OCT compound by Oil Red O staining. Briefly, liver cryosections were fixed for 10 min in 60% isopropanol and stained with 0.3% Oil Red O in 60% isopropanol for 30 min and subsequently washed with 60% isopropanol. Sections were counterstained with Gill's hematoxylin, washed with acetic acid solution (4%), and mounted with aqueous solution. The area of positive staining for Oil Red O was calculated as a percentage of the total section area and an average lipid droplet size was calculated utilizing ImagePro Plus software (Media Cybernetics) from five views per animal.
Immunohistochemistry
For this procedure, each frozen liver tissue or aortic root sample was sectioned to 5 μM thickness and fixed in microscope slides. Sections of frozen tissue specimens were mounted on Poly-Lysine (Sigma)-coated slides, air dried, and fixed with acetone. Immunohistochemical staining was performed for CD68 using rabbit polyclonal antibody to CD68 at a dilution of 1:100 (Abcam, Cambridge, MA). Images were acquired and quantitated on an Olympus BX50 microscope using Optimis software (version 6.2) and digitized using a color video camera (three-charge coupled device; JVC, Wayne, NJ).
Statistical analysis
Data are expressed as mean ± SD. Results were analyzed by one-way ANOVA followed by the Student-Newman-Keuls test and the Student's t-test using SPSS v13.0 statistical software (SPSS, Inc., Chicago, IL). A two-tailed P value <0.05 was considered statistically significant.
RESULTS
Ox-LDL induces GPR119 expression by upregulating lincRNA-DYNLRB2-2 in THP-1 macrophages
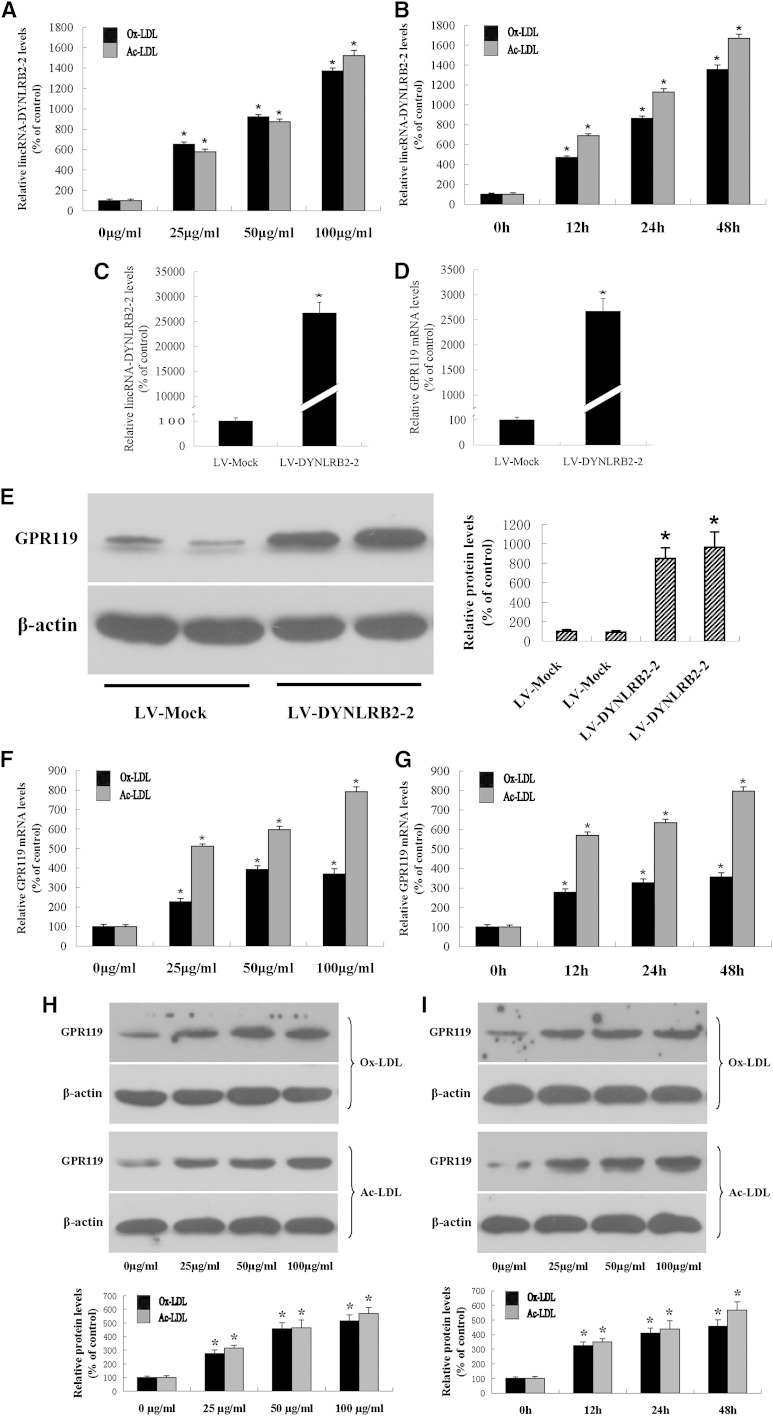
Monocyte recruitment to the vessel wall is a rate-limiting step in atherogenesis. Monocytes are recruited from the circulation into the subendothelial space, where they differentiate into mature macrophages and internalize modified lipoproteins to differentiate into lipid-laden foam cells (25). Studies have shown that lncRNAs play an essential role in cardiovascular diseases (24). To explore possible changes in RNA expression during macrophage formation, we performed microarray analysis of THP-1 macrophages and THP-1 macrophage-derived foam cells in the presence of Ox-LDL using the Arraystar probe dataset, which included 24,748 lncRNAs and 24,420 coding transcripts. lncRNA and mRNA expression profiles from three pairs of THP-1 macrophages and THP-1 macrophage-derived foam cells were produced using the Arraystar Human LncRNA Array v2.0 platform. Expression values were normalized based on the mean expression value for each probe set. Differently expressed probe sets were identified based on Student's t-test for paired samples’ normalized expression values using the following cutoff: absolute fold change value larger than 3 and P value less than 0.01. The results showed that lincRNA-DYNLRB2-2 (BM973870; 16q23.3, chr16:79,831,946-79,861,047) expression was upregulated in THP-1 macrophage-derived foam cells (4.688-fold, P < 0.001), suggesting that Ox-LDL and Ac-LDL affect the expression levels of lincRNA-DYNLRB2-2 in THP-1 macrophages. Ox-LDL/Ac-LDL increased lincRNA-DYNLRB2-2 expression in a dose- and time-dependent manner (Fig. 1A, B).
Fig. 1.
Dose-dependent and time-dependent effects of Ox-LDL/Ac-LDL on lincRNA-DYNLRB2-2 and GPR119 expression in THP-1 macrophages. A, B: THP-1 macrophages were treated with Ox-LDL/Ac-LDL at 0, 25, 50, and 100 μg/ml for 24 h, and THP-1 macrophages were treated with 50 μg/ml Ox-LDL/Ac-LDL for 0, 12, 24, and 48 h. lincRNA-DYNLRB2-2 levels were detected by real-time quantitative PCR. C–E: THP-1 macrophages were infected with LV-Mock or LV-DYNLRB2-2. lincRNA-DYNLRB2-2 levels and GPR119 gene levels were measured by real-time quantitative PCR and GPR119 protein expression was measured by Western blotting. F–I: THP-1 macrophages were treated with Ox-LDL/Ac-LDL at 0, 25, 50, and 100 μg/ml for 24 h, and THP-1 macrophages were treated with 50 μg/ml Ox-LDL/Ac-LDL for 0, 12, 24, and 48 h. F, G: GPR119 gene levels were measured by real-time quantitative PCR. H, I: GPR119 protein levels were measured by Western blotting. Similar results were obtained in three independent experiments. All the results are expressed as mean ± SD of three independent experiments, each performed in triplicate. *P < 0.05 versus vehicle.
To investigate the target candidates of lincRNA-DYNLRB2-2, we selected a series of genes showing altered expression according to the microarray analysis results. THP-1 macrophages were then transfected with LV-DYNRBP2-2 and the expression of candidate genes was measured by real-time quantitative PCR and Western blotting. LV-DYNLRB2-2 was created and transfected into THP-1 macrophages (Fig. 1C). The results showed that lincRNA-DYNLRB2-2 markedly increased GPR119 expression (Fig. 1D, E). We then investigated the effects of Ox-LDL/Ac-LDL on the expression levels of GPR119 in THP-1 macrophages, which showed that Ox-LDL/Ac-LDL treatment increased GPR119 expression in a dose- and time-dependent manner at both mRNA and protein levels (Fig. 1F–I). Treatment with 50 μg/ml Ox-LDL was the most effective at inducing GPR119 expression, and the strongest induction of GPR119 expression by Ox-LDL/Ac-LDL was observed after 24 h of culture.
The effect of lincRNA-DYNLRB2-2 and GPR119 on cholesterol metabolism and inflammation
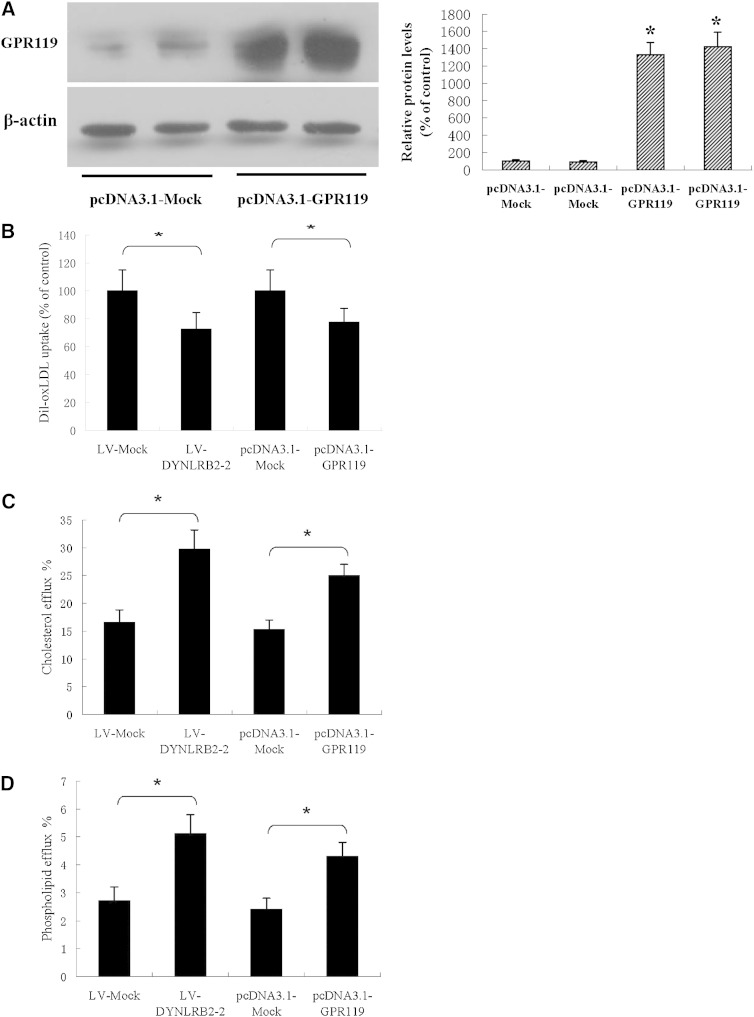
Atherosclerosis can be considered as both a lipid metabolism disorder and a chronic inflammatory disease. Innate immunity pathways have long been suspected to contribute to the initiation and progression of atherosclerosis. This suggests that crosstalk between lipid metabolism and innate immunity pathways play an important role in the development and/or prevention of atherosclerosis (26). To determine whether lincRNA-DYNLRB2-2 and GPR119 affect cholesterol metabolism and inflammation, we first examined the effect of lincRNA-DYNLRB2-2 and GPR119 on cholesterol metabolism in THP-1 macrophage-derived foam cells. LV-DYNLRB2-2 and plasmid vector pcDNA3.1-GPR119 were created and transfected into THP-1 macrophages (Fig. 1C, Fig. 2A). As shown in Fig. 2B, Dil-Ox-LDL uptake significantly decreased in GPR119 overexpressing or lincRNA-DYNLRB2-2 overexpressing THP-1 macrophages. Moreover, LV-DYNLRB2-2 and GPR119 significantly decreased TG, free cholesterol (FC), and cholesteryl ester (CE) levels (Tables 1, 2). The defect in lipidation of apoA-I has been suggested as a major cause of the impaired ability to stimulate cholesterol efflux from Tangier cells (27). Therefore, we examined whether apoA-I-mediated cholesterol efflux and phospholipid efflux were also regulated by lincRNA-DYNLRB2-2 and GPR119 in THP-1 macrophage-derived foam cells. As shown in Fig. 2C, D, the efflux of cholesterol and phospholipids was increased significantly when lincRNA-DYNLRB2-2 and GPR119 were overexpressed in THP-1 macrophage-derived foam cells. These results suggest that lincRNA-DYNLRB2-2 and GPR119 decrease cholesterol levels by impairing Ox-LDL uptake and promoting cholesterol efflux in THP-1 macrophage-derived foam cells.
Fig. 2.
Effect of lincRNA-DYNLRB2-2 and GPR119 on lipid absorption, lipid content and lipid efflux. A–D: THP-1 macrophages were treated as indicated. A: GPR119 protein levels were measured by Western blotting. B: Cells were incubated with 5 μg/ml of DiI-labeled Ox-LDL for 1 h and uptake of DiI-labeled Ox-LDL was analyzed by flow cytometry. C, D: The cells were labeled with 0.2 μCi/ml [3H]cholesterol or 2 μCi/ml of [3H]choline chloride for 72 h and then apoA-I-mediated cellular cholesterol was analyzed using liquid scintillation counting assays. apoA-I-mediated phospholipid efflux was calculated by subtracting the efflux to the medium and expressed as the percentage of total cellular and medium phospholipid amounts. All the results are expressed as mean ± SD from three independent experiments, each performed in triplicate. *P < 0.05 versus control group.
TABLE 1.
Effect of lincRNA-DYNLRB2-2 and GPR119 on cholesterol content in THP-1 macrophage
| TC (μg/mg cell protein) | FC (μg/mg cell protein) | CE (μg/mg cell protein) | CE/TC (%) | |
| Control | 115 ± 11 | 109 ± 9 | 6 ± 3 | 5.2 |
| LV-Mock | 120 ± 9 | 113 ± 8 | 7 ± 3 | 5.8 |
| LV-DYNLRB2-2 | 95 ± 6a | 89 ± 5a | 6 ± 2 | 6.3 |
| pcDNA3.1-Mock | 116 ± 10 | 110 ± 7 | 6 ± 3 | 5.2 |
| pcDNA3.1-GPR119 | 87 ± 6b | 82 ± 5b | 5 ± 2 | 5.7 |
THP-1 cells were differentiated for 72 h with 100 nM PMA and then THP-1 macrophage cells were divided into five groups as indicated. Cellular TC, FC, and CE were determined by HPLC. The results are expressed as mean ± SD from three independent experiments, each performed in triplicate.
P < 0.05 versus LV-Mock group.
P < 0.05 versus pcDNA3.1-Mock group.
TABLE 2.
Effect of lincRNA-DYNLRB2-2 and GPR119 on cholesterol content in THP-1 macrophage-derived foam cells
| TC (μg/mg cell protein) | FC (μg/mg cell protein) | CE (μg/mg cell protein) | CE/TC (%) | |
| Control | 489 ± 15 | 190 ± 12 | 299 ± 15 | 61.1 |
| LV-Mock | 495 ± 16 | 195 ± 13 | 300 ± 13 | 60.7 |
| LV-DYNLRB2-2 | 300 ± 13a | 128 ± 11a | 172 ± 14a | 57.5 |
| pcDNA3.1-Mock | 482 ± 13 | 186 ± 11 | 296 ± 14 | 61.5 |
| pcDNA3.1-GPR119 | 306 ± 8b | 131 ± 10b | 175 ± 16b | 57.1 |
THP-1 cells were differentiated for 72 h with 100 nM PMA and then macrophages were transformed into foam cells by incubation in the presence of 50 μg/ml of Ox-LDL for 48 h. THP-1 macrophage-derived foam cells were divided into five groups as indicated. Cellular TC, FC, and CE were determined by HPLC. The results are expressed as mean ± SD from three independent experiments, each performed in triplicate.
P < 0.05 versus LV-Mock group.
P < 0.05 versus pcDNA3.1-Mock group.
Atherosclerosis is a complex inflammatory disease and macrophage foam cells are the major cell type involved in this disease (28). Therefore, we next investigated the effect of lincRNA-DYNLRB2-2 and GPR119 on inflammation in THP-1 macrophages. As shown in Table 3, lincRNA-DYNLRB2-2 and GPR119 inhibited the LPS-induced release of inflammatory cytokines including TNF-α, IL-1β, and IL-6 in THP-1 macrophages, suggesting that lincRNA-DYNLRB2-2 and GPR119 play an important role in anti-inflammatory processes.
TABLE 3.
Effect of lincRNA-DYNLRB2-2 and GPR119 on LPS-induced inflammatory cytokines in THP-1 macrophage cells
| TNF-α (pg/ml) | IL-1β (pg/ml) | IL-6 (pg/ml) | |
| Control | 136 ± 29 | 42 ± 11 | 186 ± 31 |
| LPS | 861 ± 45a | 521 ± 35a | 506 ± 43a |
| LV-DYNLRB2-2 | 121 ± 37 | 53 ± 15 | 178 ± 39 |
| LV-Mock + LPS | 879 ± 56 | 551 ± 41 | 525 ± 39 |
| LV-DYNLRB2-2 + LPS | 392 ± 55b | 262 ± 28b | 312 ± 43b |
| pcDNA3.1-GPR119 | 115 ± 32 | 48 ± 9 | 173 ± 31 |
| pcDNA3.1-Mock + LPS | 839 ± 46 | 542 ± 47 | 531 ± 42 |
| pcDNA3.1-GPR119 + LPS | 352 ± 46c | 281 ± 33c | 295 ± 35c |
THP-1 macrophage cells were divided into eight groups as indicated and cultured in medium at 37°C for 24 h. Secreted inflammatory cytokines were quantified by ELISA. LPS (lipopolysaccharide) indicates treatment with 10 ng/ml LPS. The results are expressed as the mean ± SD of three independent experiments, each performed in triplicate.
P < 0.05 versus control group.
P < 0.05 versus LV-Mock + LPS group.
P < 0.05 versus pcDNA3.1-Mock + LPS group.
GPR119 is involved in lincRNA-DYNLRB2-2-induced upregulation of ABCA1 expression and function
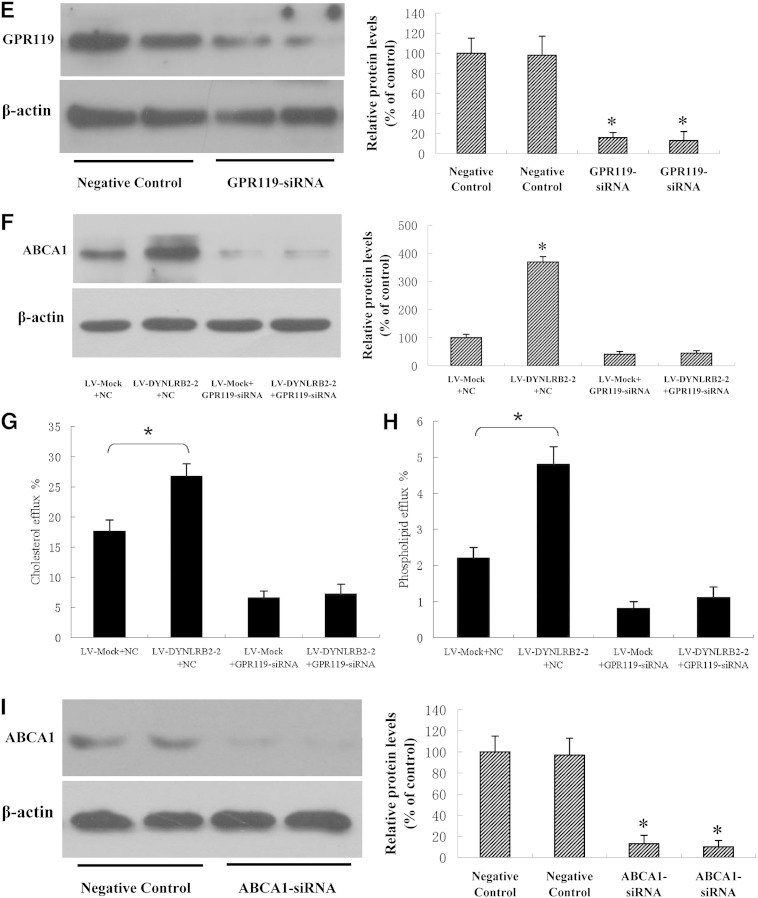
ABCA1 plays an essential role in cholesterol metabolism and affects cellular inflammatory cytokine secretion by modulating cholesterol content both in the plasma membrane and in the intracellular compartment (1). Here, we showed that lincRNA-DYNLRB2-2 and GPR119 could affect Ox-LDL uptake and efflux, and the release of inflammatory factors. Therefore, we hypothesized that GPR119 might induce the expression and function of ABCA1. We then examined the effect of lincRNA-DYNLRB2-2 and GPR119 on ABCA1 expression in THP-1 macrophages. As shown in Fig. 3A–D, overexpression of both lincRNA-DYNLRB2-2 and GPR119 significantly increased ABCA1 expression levels. Because lincRNA-DYNLRB2-2 was shown to upregulate GPR119 expression, we further investigated to determine whether the positive effect of lincRNA-DYNLRB2-2 on ABCA1 expression was mediated by the modulation of GPR119. Treatment with siRNA against GPR119 downregulated GPR119 protein expression by 89.5% (Fig. 3E). Treatment with LV-DYNLRB2-2 increased ABCA1 expression and this effect was completely abolished by siRNA-mediated silencing of GPR119 in THP-1 macrophages (Fig. 3F). Furthermore, LV-DYNLRB2-2 increased the ABCA1-induced cholesterol and phospholipid efflux and this effect was completely abolished by siRNA-mediated silencing of GPR119 in THP-1 macrophages (Fig. 3G, H). These results suggested that GPR119 was involved in the lincRNA-DYNLRB2-2 induced upregulation of ABCA1 expression and function.
Fig. 3.
GPR119 is involved in lincRNA-DYNLRB2-2-induced upregulation of ABCA1 expression and function. A–I: THP-1 macrophages were treated as indicated. A, C: The expression of ABCA1 was detected by real-time quantitative PCR. B, D–F, I: Protein expression was detected by Western blotting. E, F: THP-1 macrophages were treated as indicated and protein expression was detected by Western blotting. G, H: The cells were labeled with 0.2 μCi/ml [3H]cholesterol or 2 μCi/ml of [3H]choline chloride for 72 h and then apoA-I-mediated cellular cholesterol efflux and phospholipid efflux were analyzed using liquid scintillation counting assays. All the results are expressed as mean ± SD from three independent experiments, each performed in triplicate. *P < 0.05 versus control group.
Next, we investigated the effects of siRNA silencing of ABCA1 on the expression of pro-inflammatory cytokines modulated by GPR119 in LPS-stimulated THP-1 macrophages. As shown in Fig. 3I and Table 4, treatment with siRNA against ABCA1 downregulated ABCA1 protein expression by 91.2%, and the suppression of pro-inflammatory cytokine expression by GPR119 was markedly compensated by treatment with ABCA1 siRNA in LPS-stimulated THP-1 macrophages.
TABLE 4.
ABCA1 is involved in the regulation of LPS-induced inflammatory cytokines by GPR119 in THP-1 macrophage cells
| TNF-α (pg/ml) | IL-1β (pg/ml) | IL-6 (pg/ml) | |
| Control | 131 ± 27 | 39 ± 12 | 197 ± 35 |
| pcDNA3.1-GPR119 + NC | 121 ± 22 | 30 ± 13 | 181 ± 43 |
| pcDNA3.1-GPR119 + ABCA1-siRNA | 145 ± 39 | 46 ± 15 | 191 ± 47 |
| pcDNA3.1-Mock + NC + LPS | 796 ± 53 | 529 ± 42 | 506 ± 35 |
| pcDNA3.1-GPR119 + NC + LPS | 387 ± 46a | 296 ± 42a | 268 ± 52a |
| pcDNA3.1-GPR119 + ABCA1-siRNA + LPS | 731 ± 55 | 563 ± 57 | 491 ± 42 |
THP-1 macrophage cells were divided into six groups as indicated and cultured in medium at 37°C for 24 h. Secreted inflammatory cytokines were quantified by ELISA. LPS indicates treatment with 10 ng/ml LPS. The results are expressed as the mean ± SD of three independent experiments, each performed in triplicate.
P < 0.05 versus pcDNA3.1-Mock + NC + LPS group.
GLP-1 receptor is involved in the GPR119-induced upregulation of ABCA1
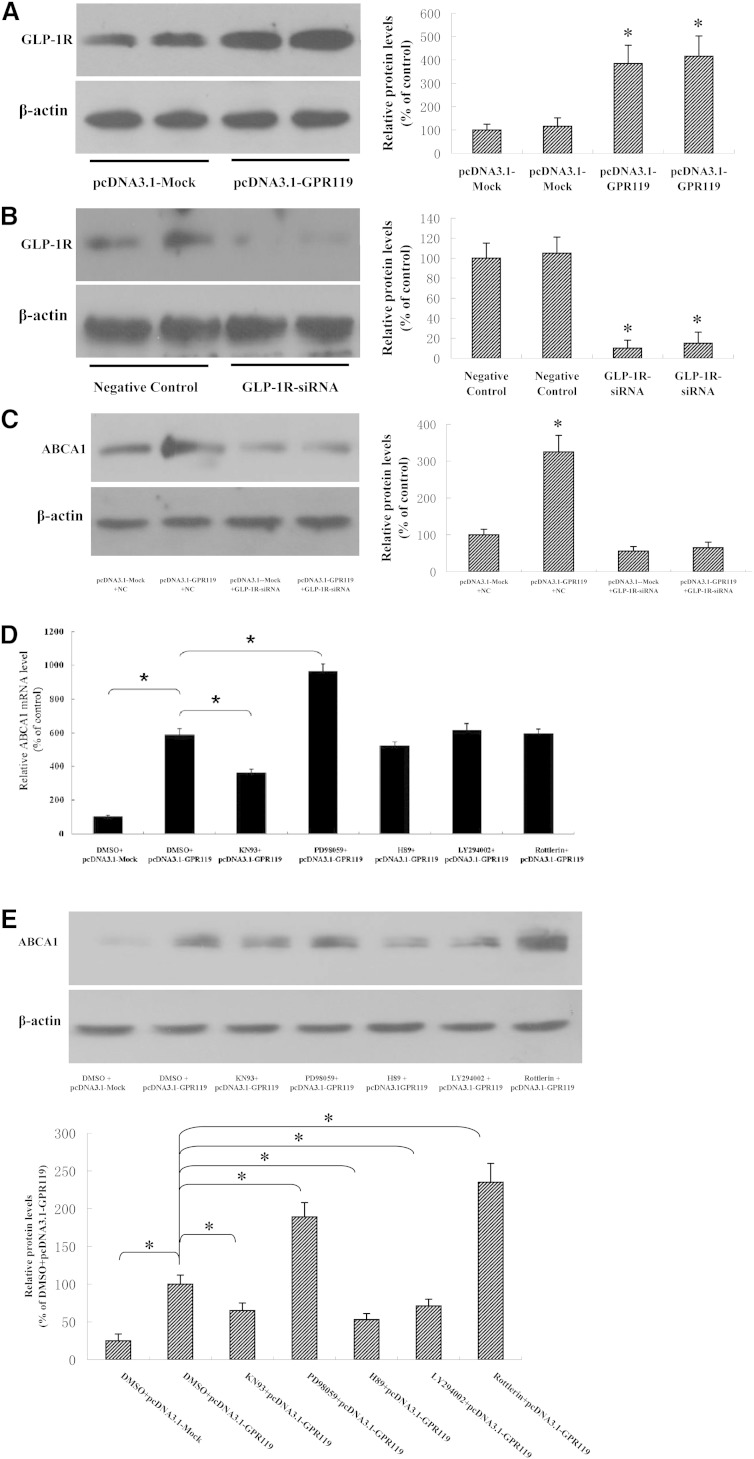
GPR119 can activate GLP-1 receptor (GLP-1R) secretion in insulin resistance (7). Therefore, we explored whether the GPR119-mediated regulation of ABCA1 expression is mediated by GLP-1R in THP-1 macrophages. As shown in Fig. 4A, B, the expression of the GLP-1R protein was markedly increased by treatment with pcDNA3.1-GPR119, whereas it was decreased in response to GLP-1R siRNA silencing. Next, we examined the effects of GLP-1R siRNA on the upregulation of ABCA1 induced by GPR119. Treatment with pcDNA3.1-GPR119 significantly increased ABCA1 expression, whereas this effect was completely abolished by siRNA-mediated silencing of GLP-1R in THP-1 macrophages (Fig. 4C). These results suggest that the GPR119-induced upregulation of ABCA1 expression is mediated by GLP-1R.
Fig. 4.
GLP-1R is involved in GPR119-induced upregulation of ABCA1. A–C: THP-1 macrophages were treated as indicated and Western blot assays were performed to detect protein expression. D, E: THP-1 macrophages were treated with or without inhibitors of CaMK (KN93, 5 μM), ERK1/2 (PD98059, 50 μM), PKA (H89, 10 μM), PKB (LY294002, 10 μM), and inhibitor of PKC-ζ (rottlerin, 10 μM) for 30 min, then transfected with pc-DNA3.1-GPR119 or pc-DNA3.1-Mock for 48 h. ABCA1 mRNA and protein levels were measured by real-time quantitative PCR and Western blotting, respectively. All results are expressed as mean ± SD of three independent experiments, each performed in triplicate. *P < 0.05 versus control group.
GLP-1R signaling involves five pathways including Ca2+/calmodulin-dependent protein kinase (CaMK), MAPKs/ERK1/2, cAMP/PKA, phosphoinositide 3-kinase (PI-3K)/AKT/PKB, and atypical PKC-ζ pathway (5, 29). To test whether GPR119 promotes ABCA1 expression by stimulating GLP-1R and its downstream pathways, we examined the effect of inhibitors of each signaling pathway on ABCA1 expression in THP-1 macrophages. Thus, the CaMK inhibitor KN93, the ERK1/2 inhibitor PD98059, the PKA inhibitor H89, the PKB inhibitor LY294002, and the PKC-ζ inhibitor rottlerin were used to determine which pathway is involved in the induction of ABCA1 expression by treatment with pcDNA3.1-GPR119. As shown in Fig. 4D, E, KN93 significantly suppressed GPR119-induced expression of ABCA1, whereas PD98059 significantly increased GPR119-induced ABCA1 expression at the mRNA and protein levels. In addition, H89 and LY294002 downregulated ABCA1 protein expression, whereas rottlerin increased ABCA1 protein levels. These results demonstrated that GPR119 upregulated ABCA1expression through the activation of GLP-1R and its downstream signaling pathways.
Effect of GPR119 on plasma lipid parameters and circulating cytokine levels
Because of the key role of GPR119 in cholesterol and lipid metabolism, we further examined the effect of GPR119 on the terminal plasma lipid levels in apoE−/− mice fed a HFD. As shown in Table 5, overexpression of LV-GPR119 led to a moderate decrease in plasma TG levels (28.1%). Concomitantly, plasma apoA-I levels (40.0%) showed a moderate increase in the GPR119 group compared with the control group. Moreover, to investigate whether GPR119-mediated changes in cellular pro-inflammatory gene expression could result in the corresponding changes in plasma inflammatory cytokines, we conducted a series of ELISA tests (Table 6). Consistent with the inflammatory gene expression data in our in vitro experiment, infection with LV-GPR119 reduced the plasma concentrations of IL-1β, IL-6, TNF-α, and SAA by 32.0, 28.0, 37.0, and 39.8%, respectively.
TABLE 5.
Effect of GPR119 on serum lipids and lipoprotein values in apoE−/− mice (n = 10)
| TG (mmol/L) | TC (mmol/l) | apoA-I (g/l) | apoB (g/l) | |
| LV-Mock | 1.35 ± 0.17 | 32.04 ± 2.65 | 0.05 ± 0.01 | 0.16 ± 0.02 |
| LV-GPR119 | 0.97 ± 0.15a | 32.32 ± 3.13 | 0.07 ± 0.02a | 0.15 ± 0.03 |
Data are expressed as mean ± SD.
P < 0.05 versus LV-Mock group.
TABLE 6.
Effect of GPR119 on serum cytokine levels in apoE−/− mice (n = 10)
| IL-1β (pg/ml) | IL-6 (pg/ml) | TNF-α (pg/ml) | SAA (μg/ml) | |
| LV-Mock | 12.37 ± 1.18 | 78.67 ± 5.82 | 11.35 ± 1.56 | 30.35 ± 3.23 |
| LV-GPR119 | 8.41 ± 1.25a | 56.65 ± 4.27a | 7.15 ± 1.28a | 18.26 ± 2.50a |
Data are expressed as mean ± SD.
P < 0.05 versus LV-Mock group.
Effect of GPR119 on hepatic and intestinal lipid metabolism
The liver is the major organ responsible for the production and degradation of apoB-100-containing lipoproteins (30). Because of the central role of the liver in determining plasma lipoprotein levels, several therapeutic strategies designed to modulate hepatic lipid metabolism have been developed to reduce the susceptibility to atherosclerosis. Furthermore, the definitive identification of intestinal cholesterol transporters and an understanding of their roles in the cholesterol absorption process may improve the effectiveness of treatments aimed at suppressing cholesterol absorption, thereby reducing hypercholesterolemia and lowering the risk of cardiovascular disease (31). Therefore, we next analyzed the effect of GPR119 on the lipid content of the liver of apoE−/− mice by Oil Red O staining. The liver GPR119 protein levels were markedly increased by LV-GPR119 treatment in apoE−/− mice (Fig. 5A). Representative images of randomly selected sections of the liver stained for Oil Red O in the control group and the GPR119 group are shown in Fig. 5B. Oil Red O staining revealed that the liver lipid content was reduced in the LV-GPR119-treated animals compared with the controls. Assessment of the effect of GPR119 on TG and TC content by enzymatic analysis showed a moderate decrease of TG (26.6%, P < 0.05) and TC (28.2%, P < 0.05) in the LV-GPR119 group (Table 7).
Fig. 5.
Effect of GPR119 on hepatic and intestinal lipid metabolism. A–D: ApoE−/− mice were randomized into the control group and the GPR119 group and fed a HFD. A: GPR119 protein levels in liver tissues were measured by Western blotting. All results are presented as mean ± SD (n = 5). All experiments were performed in triplicate. *P < 0.05 versus control group. B: Liver cryosections were stained with Oil Red O. Original magnification, 100×. C, D: Protein expression was measured by Western blotting. All results are presented as mean ± SD (n = 5). All experiments were performed in triplicate. *P < 0.05 versus control group.
TABLE 7.
Effect of GPR119 on hepatic lipid deposition in apoE−/− mice (n = 10)
Data are expressed as mean ± SD.
P < 0.05 versus LV-Mock group.
The protein expression of a series of genes involved in lipid metabolism in the mouse liver was investigated by Western blot analysis. As shown in Fig. 5C, the GPR119 group had significantly higher expression of ABCA1 and SR-B1 than the control group, but lower expression of SREBP1c and SREBP2. However, no statistically significant differences in the expression of HMGS, HMGCR, LDLR, and ABCG1 were detected between the GPR119 group and the control group. Furthermore, we investigated the effects of GPR119 on intestinal cholesterol absorption in apoE−/− mice. Assessment of protein expression levels in intestinal tissues of apoE−/− mice by Western blotting (Fig. 5D) showed that GPR119 upregulated the expression of ABCA1, downregulated ABCG5 expression, and had no effect on ABCG8, Niemann-Pick C1-Like 1, and MTP expression (P > 0.05).
Effect of GPR119 on plaque formation
To investigate the impact of GPR119 on atherogenesis in apoE−/− mice, atherosclerotic lesions were evaluated by aortic valve section and en face analyses. In mice infected with LV-GPR119, the average lesion area was decreased compared with that in controls by both en face and aortic valve section analyses (Fig. 6A). Quantification of Oil Red O-stained lesions in en face preparations of aortas revealed that treatment with LV-GPR119 resulted in a significant 30% decrease in lesion area in apoE−/− mice compared with the controls. To confirm the positive effects of GPR119 on atherosclerosis, Oil Red O-stained aortic valve sections were quantified, which showed a significant 27% reduction in lesion area in apoE−/− mice treated with LV-GPR119 compared with controls.
Fig. 6.
Effect of GPR119 on atherosclerosis initiation and development in apoE−/− mice. A–C: apoE−/− mice were randomized into a control group and a GPR119 group and fed a HFD. A: Representative staining of en face aorta (a) with Oil Red O and aortic valves (b) with Oil Red O. En face lesions (c) and total lesions in the aortic valves (d) were analyzed in apoE−/− mice. B: Cryosections of aortic valves from apoE−/− mice were immunohistochemically stained for the macrophage marker CD68 (a). The integral optical density of CD68 in the aortic valve cryosections from apoE−/− mice was analyzed (b). C: Protein expression levels in tissues of apoE−/− mice were analyzed by Western blotting (n = 5 mice/group). All data are presented as mean ± SD (n = 10). All experiments were performed in triplicate, except as indicated. *P < 0.05 versus control group.
Immunohistochemical staining was performed to visualize macrophages in atherosclerotic lesions (using an antibody against CD68). As shown in Fig. 6B, LV-GPR119-treated mice showed a considerably lower number of CD68+ cells than control mice, indicating that macrophage infiltration was significantly decreased in lesions of apoE−/− mice (P < 0.05). These observations indicated that the positive effect of GPR119 is ongoing in atherosclerotic lesions of apoE−/− mice.
Western blot assessment of the levels of proteins related to cholesterol metabolism and inflammation in aortic tissue of apoE−/− mice showed that the levels of SR-A1, CD36, and NF-κB were decreased, whereas protein levels of ABCA1 and SR-B1 were increased in the GPR119 group (Fig. 6C). However, no effect was observed on ABCG1 protein expression in the GPR119 group compared with the control group (P > 0.05). In addition, assessment of the levels of nuclear receptor proteins related to cholesterol metabolism and inflammation, including LXRα, PPARα, PPARγ, PXR, HNF-1α, and LRH1, showed no significant differences between the GPR119 and control groups (P > 0.05, data not shown).
DISCUSSION
Atherosclerosis is responsible for cardiovascular disease, which is the leading cause of death in developed countries. Hypercholesterolemia is a well-established risk factor for the development of atherosclerosis and its pathologic complications. Evidence from clinical trials indicates that reduction of plasma cholesterol levels by dietary and/or pharmacological means leads to a reduction in the risk of death from cardiovascular disease (32, 33). In the present study, we showed for the first time that GPR119 could decrease lipid content and enhance cholesterol efflux in THP-1 macrophage-derived foam cells. Moreover, GPR119 markedly decreased plasma levels of TG and increased plasma levels of apoA-I, leading to a significant inhibition of atherosclerotic plaque formation in apoE−/− mice fed a high-fat/high-cholesterol diet.
RCT is a multi-step process by which cholesterol is transported from peripheral tissues back to the liver for excretion via the plasma, thus preventing atherosclerosis. Cholesterol from nonhepatic peripheral tissues is transferred to HDLs by ABCA1. apoA-I acts as an acceptor, and the phospholipid component of HDLs acts as a sink for the mobilized cholesterol (34). The control of macrophage cholesterol homeostasis is of critical importance in the pathogenesis of atherosclerosis, as alterations in the balance between cholesterol influx, intracellular transport and efflux can lead to excessive accumulation of cholesterol in macrophages and their transformation into foam cells (35). In the present study, GPR119 exerted an anti-atherogenic effect by decreasing cellular cholesterol content and upregulating the expression of ABCA1, which plays a crucial role in mediating the transport of cholesterol across cellular membranes (36–38). Its regulation is cAMP- and sterol-dependent, and cAMP is responsible for the functional activation of ABCA1 or the upregulation of ABCA1 expression (39, 40). In the present study, we showed that GPR119 activates GLP-1R and its downstream pathways (CaMK and cAMP/PKA) and PI-3K/AKT/PKB pathways, and it inhibits the MAPK-ERK1/2 and PKC-ζ pathways, finally resulting in the upregulation of ABCA1 expression and apoA-I mediated cholesterol efflux. These results provide strong evidence supporting the notion that GPR119 exerts its anti-atherogenic effects by increasing the rate of RCT. Furthermore, GPR119 enhanced the overall rate of RCT by activating pathways that mediate the delivery of HDL-cholesterol to the liver. Mature HDL can transfer its cholesterol to the liver directly via SR-B1 or indirectly via CETP-mediated transfer to apoB-containing lipoproteins, with subsequent uptake by the liver via the LDLR (41). We showed that GPR119 upregulated the expression of SR-B1 in apoE−/− mice and induced the expression of LDL receptors in the liver, increasing the catabolism of plasma LDL and decreasing the plasma concentration of cholesterol. In addition, apolipoproteins are important constituents of plasma lipoproteins and essential for RCT. We performed in vivo experiments and showed that GPR119 can significantly increase apoA-I levels in apoE−/− mice. apoA-I is secreted predominantly by the liver and is present in the majority of HDL particles, and its concentration is closely correlated with plasma HDL (42)·
Atherosclerosis is a chronic disease that can remain asymptomatic for decades. Inflammatory processes take part in all stages of the atherosclerotic process, from lesion initiation to plaque rupture (43). Macrophages, whether engorged with lipids or not, play a key role in the mediation and modulation of inflammation, and much atherosclerosis research has targeted the role of macrophages in the inflammatory pathways that underlie atherogenesis (44, 45). Evidence from several recent studies indicate that inflammation, along with other atherogenic-related mediators, plays a distinct regulating role in ABCA1 expression. Proatherogenic cytokines such as IFN-γ and IL-1β inhibit the expression of ABCA1, while anti-atherogenic cytokines, including IL-10 and transforming growth factor TGF-β1, promote the expression of ABCA1. Moreover, some cytokines such as TNF-α regulate ABCA1 expression in a species-specific and dose-dependent manner (46). Chronic activation of inflammatory pathways mediates the pathogenesis of insulin resistance, and the macrophage/adipocyte nexus provides a key mechanism underlying decreased insulin sensitivity. Recently, a family of G protein-coupled receptors has been identified that exhibits high affinity for inflammation, glucose metabolism, and insulin sensitivity (47). In the present study, we showed that GPR119 significantly downregulated IL-1β, IL-6, and TNF-α levels through the regulation of ABCA1 expression in LPS-induced THP-1 macrophage-derived foam cells. To further investigate the mechanisms whereby GPR119 treatment inhibited plaque progression and stabilization, changes in gene expression of inflammatory molecules were explored in apoE−/− mice fed a high-fat/high-cholesterol diet. We found that IL-1β, IL-6, TNF-α, and SAA were markedly repressed in GPR119-overexpressing apoE−/− mice. Our results suggest that GPR119-induced suppression of inflammatory molecule expression could block or retard the development of atherosclerotic lesions and thus have a positive influence on disease outcomes.
The diverse roles of regulatory RNAs extend well beyond the epigenome, as they regulate transcription in different ways (48). lncRNAs are nonprotein coding transcribed RNAs that target the minor dihydrofolate reductase gene promoter, which binds to the general transcription factor IIB and to the DNA of the dihydrofolate reductase major promoter forming a specific and stable tripartite complex, resulting in the dissociation of the preinitiation complex and transcriptional repression (49). The lncRNAs also function as transcriptional coactivators, modulating protein localization and function. Ox-LDL contributes to atherosclerotic plaque formation and progression by several mechanisms, including the induction of endothelial cell activation and dysfunction, macrophage foam cell formation, and smooth muscle cell migration and proliferation. Ox-LDL upregulates ABCA1 expression at both protein and mRNA levels (31). In the present study, we showed that Ox-LDL induces GPR119 expression through lincRNA-DYNLRB2-2 in THP-1 macrophages. In response to extracellular signals, lincRNA-DYNLRB2-2 upregulated GPR119 expression, which increased ABCA1 expression resulting in promotion of ABCA1-mediated cholesterol efflux leading to the protection from atherosclerosis. However, the detailed mechanism of lincRNA-DYNLRB2-2-induced upregulation of GPR119 expression needs further clarification. Moreover, in the present study, we showed that Ox-LDL and Ac-LDL had similar effects on lincRNA-DYNLRB2-2 levels. Remarkably, little is known about the direct downstream target lncRNAs of Ox-LDL and Ac-LDL. Thus, whether lincRNA-DYNLRB2-2 can be directly regulated by Ox-LDL or Ac-LDL and whether another mechanism is involved needs to be further explored.
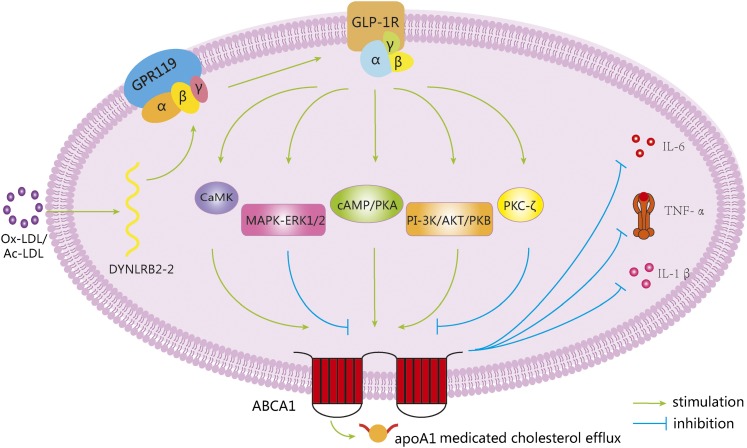
In conclusion, we have demonstrated that Ox-LDL significantly induced lincRNA-DYNLRB2-2 expression (Fig. 7), which promoted ABCA1-mediated cholesterol efflux and inhibited inflammation through GPR119 in THP-1 macrophage-derived foam cells. In addition, GPR119 decreased lipid and inflammatory cytokine levels in serum to further prevent atherosclerosis in apoE−/− mice, indicating that GPR119 may represent a promising candidate as a therapeutic agent.
Fig. 7.
ABCA1 expression and function can be induced by GPR119 to protect from atherosclerosis. Ox-LDL upregulates lincRNA-DYNLRB2-2 expression, resulting in increased ABCA1 expression through GPR119. GLP-1R-mediated signaling pathways including CaMK, cAMP/PKA, PI-3K/AKT/PKB, MAPK-ERK1/2, and PKC-ζ are involved in GPR119-induced ABCA1 expression. Moreover, GPR119 inhibits inflammation and promotes apoA-I-mediated cellular cholesterol efflux through inducing ABCA1 expression. These beneficial effects are accompanied by a reduction of macrophage-derived foam cell formation, inhibition of inflammatory gene expression, adhesion molecule expression in the aorta, and increase of cholesterol efflux from peripheral tissues, thus preventing of atherosclerotic plaque formation.
Footnotes
Abbreviations:
- Ac-LDL
- acetylated LDL
- CaMK
- Ca2+/calmodulin-dependent protein kinase
- CE
- cholesteryl ester
- FC
- free cholesterol
- GLP-1R
- glucagon-like peptide 1 receptor
- GPR119
- G protein-coupled receptor 119
- HFD
- high-fat diet
- IL
- interleukin
- lincRNA
- long intervening noncoding RNA
- lncRNA
- long noncoding RNA
- LPS
- lipopolysaccharide
- LV
- lentivirus
- Ox-LDL
- oxidized LDL
- PI-3K
- phosphoinositide 3-kinase
- PK
- protein kinase
- RCT
- reverse cholesterol transport
- SAA
- serum amyloid A
- TC
- total cholesterol
This study was supported by the National Natural Sciences Foundation of China (81271905 and 81301489), Guangdong Provincial Natural Sciences Foundation of China (S2012020010920), and the President Foundation of Nanfang Hospital, Southern Medical University (2013B004). The authors declare no conflicts of interest.
REFERENCES
- 1.Yin K., Liao D. F., Tang C. K. 2010. ATP-binding membrane cassette transporter A1 (ABCA1): a possible link between inflammation and reverse cholesterol transport. Mol. Med. 16: 438–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burnett J. R. 2004. Lipids, lipoproteins, atherosclerosis and cardiovascular disease. Clin. Biochem. Rev. 25: 2. [PMC free article] [PubMed] [Google Scholar]
- 3.Ye D., Lammers B., Zhao Y., Meurs I., Van Berkel T. J., Van Eck M. 2011. ATP-binding cassette transporters A1 and G1, HDL metabolism, cholesterol efflux, and inflammation: important targets for the treatment of atherosclerosis. Curr. Drug Targets. 12: 647–660. [DOI] [PubMed] [Google Scholar]
- 4.Oram J. F., Lawn R. M. 2001. ABCA1. The gatekeeper for eliminating excess tissue cholesterol. J. Lipid Res. 42: 1173–1179. [PubMed] [Google Scholar]
- 5.Overton H. A., Babbs A. J., Doel S. M., Fyfe M. C., Gardner L. S., Griffin G., Jackson H. C., Procter M. J., Rasamison C. M., Tang-Christensen M., et al. 2006. Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab. 3: 167–175. [DOI] [PubMed] [Google Scholar]
- 6.Swaminath G. 2008. Fatty acid binding receptors and their physiological role in type 2 diabetes. Arch. Pharm. (Weinheim). 341: 753–761. [DOI] [PubMed] [Google Scholar]
- 7.Ohishi T., Yoshida S. 2012. The therapeutic potential of GPR119 agonists for type 2 diabetes. Expert Opin. Investig. Drugs. 21: 321–328. [DOI] [PubMed] [Google Scholar]
- 8.Leech C. A., Dzhura I., Chepurny O. G., Kang G., Schwede F., Genieser H. G., Holz G. G. 2011. Molecular physiology of glucagon-like peptide-1 insulin secretagogue action in pancreatic β cells. Prog. Biophys. Mol. Biol. 107: 236–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ning Y., O'Neill K., Lan H., Pang L., Shan L. X., Hawes B. E., Hedrick J. A. 2008. Endogenous and synthetic agonists of GPR119 differ in signalling pathways and their effects on insulin secretion in MIN6c4 insulinoma cells. Br. J. Pharmacol. 155: 1056–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lauffer L. M., Iakoubov R., Brubaker P. L. 2009. GPR119 is essential for oleoylethanolamide-induced glucagon-like peptide-1 secretion from the intestinal enteroendocrine L-cell. Diabetes. 58: 1058–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Semple G., Fioravanti B., Pereira G., Calderon I., Uy J., Choi K., Xiong Y., Ren A., Morgan M., Dave V., et al. 2008. Discovery of the first potent and orally efficacious agonist of the orphan G-protein coupled receptor 119. J. Med. Chem. 51: 5172–5175. [DOI] [PubMed] [Google Scholar]
- 12.Le Goff W., Zheng P., Brubaker G., Smith J. D. 2006. Identification of the cAMP-responsive enhancer of the murine ABCA1 gene: requirement for CREB1 and STAT3/4 elements. Arterioscler. Thromb. Vasc. Biol. 26: 527–533. [DOI] [PubMed] [Google Scholar]
- 13.Nishiuchi Y., Murao K., Imachi H., Nishiuchi T., Iwama H., Ishida T. 2010. Transcriptional factor prolactin regulatory element-binding protein-mediated gene transcription of ABCA1 via 3′,5′-cyclic adenosine-5′-monophosphate. Atherosclerosis. 212: 418–425. [DOI] [PubMed] [Google Scholar]
- 14.Hu Y. W., Ma X., Li X. X., Liu X. H., Xiao J., Mo Z. C., Xiang J., Liao D. F., Tang C. K. 2009. Eicosapentaenoic acid reduces ABCA1 serine phosphorylation and impairs ABCA1-dependent cholesterol efflux through cyclic AMP/protein kinase A signaling pathway in THP-1 macrophage-derived foam cells. Atherosclerosis. 204: e35–e43. [DOI] [PubMed] [Google Scholar]
- 15.Hansen H. S., Rosenkilde M. M., Holst J. J., Schwartz T. W. 2012. GPR119 as a fat sensor. Trends Pharmacol. Sci. 33: 374–381. [DOI] [PubMed] [Google Scholar]
- 16.Rinn J. L., Chang H. Y. 2012. Genome regulation by long noncoding RNAs. Annu. Rev. Biochem. 81: 145–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Batista P. J., Chang H. Y. 2013. Long noncoding RNAs: cellular address codes in development and disease. Cell. 152: 1298–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ng S. Y., Lin L., Soh B. S., Stanton L. W. 2013. Long noncoding RNAs in development and disease of the central nervous system. Trends Genet. 29: 461–468. [DOI] [PubMed] [Google Scholar]
- 19.Birney E., Stamatoyannopoulos J. A., Dutta A., Guigó R., Gingeras T. R., Margulies E. H., Weng Z., Snyder M., Dermitzakis E. T., Thurman R. E., et al. ; ENCODE Project Consortium. 2007. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature. 447: 799–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amaral P. P., Dinger M. E., Mercer T. R., Mattick J. S. 2008. The eukaryotic genome as an RNA machine. Science. 319: 1787–1789. [DOI] [PubMed] [Google Scholar]
- 21.Tripathi V., Ellis J. D., Shen Z., Song D. Y., Pan Q., Watt A. T., Freier S. M., Bennett C. F., Sharma A., Bubulya P. A., et al. 2010. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol. Cell. 39: 925–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H., Chen Z., Wang X., Huang Z., He Z., Chen Y. 2013. Long non-coding RNA: a new player in cancer. J. Hematol. Oncol. 6: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qureshi I. A., Mehler M. F. 2013. Long non-coding RNAs: novel targets for nervous system disease diagnosis and therapy. Neurotherapeutics. 10: 632–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scheuermann J. C., Boyer L. A. 2013. Getting to the heart of the matter: long non-coding RNAs in cardiac development and disease. EMBO J. 32: 1805–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han K. H., Han K. O., Green S. R., Quehenberger O. 1999. Expression of the monocyte chemoattractant protein-1 receptor CCR2 is increased in hypercholesterolemia. Differential effects of plasma lipoproteins on monocyte function. J. Lipid Res. 40: 1053–1063. [PubMed] [Google Scholar]
- 26.Shibata N., Glass C. K. 2009. Regulation of macrophage function in inflammation and atherosclerosis. J. Lipid Res. 50(Suppl): S277–S281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawn R. M., Wade D. P., Garvin M. R., Wang X., Schwartz K., Porter J. G., Seilhamer J. J., Vaughan A. M., Oram J. F. 1999. The Tangier disease gene product ABC1 controls the cellular apolipoprotein-mediated lipid removal pathway. J. Clin. Invest. 104: R25–R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osterud B., Bjorklid E. 2003. Role of monocytes in atherogenesis. Physiol. Rev. 83: 1069–1112. [DOI] [PubMed] [Google Scholar]
- 29.Holz G. G. 2004. Epac: A new cAMP-binding protein in support of glucagon-like peptide-1 receptor-mediated signal transduction in the pancreatic beta-cell. Diabetes. 53: 5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pittman R. C., Carew T. E., Attie A. D., Witztum J. L., Watanabe Y., Steinberg D. 1982. Receptor-dependent and receptor-independent degradation of low density lipoprotein in normal rabbits and in receptor-deficient mutant rabbits. J. Biol. Chem. 257: 7994–8000. [PubMed] [Google Scholar]
- 31.Hui D. Y., Labonté E. D., Howles P. N. 2008. Development and physiological regulation of intestinal lipid absorption. III. Intestinal transporters and cholesterol absorption. Am. J. Physiol. Gastrointest. Liver Physiol. 294: G839–G843. [DOI] [PubMed] [Google Scholar]
- 32.Shepherd J., Cobbe S. M., Ford I., Isles C. G., Lorimer A. R., MacFarlane P. W., McKillop J. H., Packard C. J.; West of Scotland Coronary Prevention Study Group. 2004. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. 1995. Atheroscler. Suppl. 5: 91–97. [DOI] [PubMed] [Google Scholar]
- 33.Panel A. P. 2006. Reducing residual cardiovascular risk: the relevance of raising high-density lipoprotein cholesterol in patients on cholesterol-lowering treatment. Diab. Vasc. Dis. Res. 3: S1–S12. [DOI] [PubMed] [Google Scholar]
- 34.Tall AR. An overview of reverse cholesterol transport. Eur Heart J. 1998; 19 Suppl A:A31–5. [PubMed] [Google Scholar]
- 35.Tabas I. 2000. Cholesterol and phospholipid metabolism in macrophages. Biochim. Biophys. Acta. 1529: 164–174. [DOI] [PubMed] [Google Scholar]
- 36.Rader D. J., Alexander E. T., Weibel G. L., Billheimer J., Rothblat G. H. 2009. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J. Lipid Res. 50(Suppl): S189–S194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohashi R., Mu H., Wang X., Yao Q., Chen C. 2005. Reverse cholesterol transport and cholesterol efflux in atherosclerosis. QJM. 98: 845–856. [DOI] [PubMed] [Google Scholar]
- 38.Hu Y. W., Zheng L., Wang Q. 2010. Regulation of cholesterol homeostasis by liver X receptors. Clin. Chim. Acta. 411: 617–625. [DOI] [PubMed] [Google Scholar]
- 39.Haidar B., Denis M., Krimbou L., Marcil M., Genest J., Jr 2002. cAMP induces ABCA1 phosphorylation activity and promotes cholesterol efflux from fibroblasts. J. Lipid Res. 43: 2087–2094. [DOI] [PubMed] [Google Scholar]
- 40.Lin G., Bornfeldt K. E. 2002. Cyclic AMP-specific phosphodiesterase 4 inhibitors promote ABCA1 expression and cholesterol efflux. Biochem. Biophys. Res. Commun. 290: 663–669. [DOI] [PubMed] [Google Scholar]
- 41.Rigotti A., Miettinen H. E., Krieger M. 2003. The role of the high-density lipoprotein receptor SR-BI in the lipid metabolism of endocrine and other tissues. Endocr. Rev. 24: 357–387. [DOI] [PubMed] [Google Scholar]
- 42.Rubin E. M., Krauss R. M., Spangler E. A., Verstuyft J. G., Clift S. M. 1991. Inhibition of early atherogenesis in transgenic mice by human apolipoprotein AI. Nature. 353: 265–267. [DOI] [PubMed] [Google Scholar]
- 43.Navab K. D., Elboudwarej O., Gharif M., Yu J., Hama S. Y., Safarpour S., Hough G. P., Vakili L., Reddy S. T., Navab M., et al. 2011. Chronic inflammatory disorders and accelerated atherosclerosis: chronic kidney disease. Curr. Pharm. Des. 17: 17–20. [DOI] [PubMed] [Google Scholar]
- 44.Libby P. 2012. Inflammation in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 32: 2045–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mantovani A., Garlanda C., Locati M. 2009. Macrophage diversity and polarization in atherosclerosis: a question of balance. Arterioscler. Thromb. Vasc. Biol. 29: 1419–1423. [DOI] [PubMed] [Google Scholar]
- 46.Hu Y. W., Ma X., Huang J. L., Mao X. R., Yang J. Y., Zhao J. Y., Li S. F., Qiu Y. R., Yang J., Zheng L., et al. 2013. Dihydrocapsaicin attenuates plaque formation through a PPARγ/LXRα pathway in apoE-/- mice fed a high-fat/high-cholesterol diet. PLoS ONE. 8: e66876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oh D. Y., Lagakos W. S. 2011. The role of G-protein-coupled receptors in mediating the effect of fatty acids on inflammation and insulin sensitivity. Curr. Opin. Clin. Nutr. Metab. Care. 14: 322–327. [DOI] [PubMed] [Google Scholar]
- 48.Goodrich J. A., Kugel J. F. 2006. Non-coding-RNA regulators of RNA polymerase II transcription. Nat. Rev. Mol. Cell Biol. 7: 612–616. [DOI] [PubMed] [Google Scholar]
- 49.Schonrock N., Harvey R. P., Mattick J. S. 2012. Long noncoding RNAs in cardiac development and pathophysiology. Circ. Res. 111: 1349–1362. [DOI] [PubMed] [Google Scholar]