Abstract
The liver plays a central role in the final elimination of cholesterol from the body either as bile acids or as free cholesterol (FC), and lipoprotein-derived cholesterol is the major source of total biliary cholesterol. HDL is the major lipoprotein responsible for removal and transport of cholesterol, mainly as cholesteryl esters (CEs), from the peripheral tissues to the liver. While HDL-FC is rapidly secreted into bile, the fate of HDL-CE remains unclear. We have earlier demonstrated the role of human CE hydrolase (CEH, CES1) in hepatic hydrolysis of HDL-CE and increasing bile acid synthesis, a process dependent on scavenger receptor BI expression. In the present study, we examined the hypothesis that by enhancing the elimination of HDL-CE into bile/feces, liver-specific transgenic expression of CEH will be anti-atherogenic. Increased CEH expression in the liver significantly increased the flux of HDL-CE to bile acids. In the LDLR−/− background, this enhanced elimination of cholesterol led to attenuation of diet-induced atherosclerosis with a consistent increase in fecal sterol secretion primarily as bile acids. Taken together with the observed reduction in atherosclerosis by increasing macrophage CEH-mediated cholesterol efflux, these studies establish CEH as an important regulator in enhancing cholesterol elimination and also as an anti-atherogenic target.
Keywords: cholesterol elimination, bile acid excretion, high density lipoprotein-cholesteryl ester hydrolysis, fecal bile acids, low density lipoprotein receptor
Homeostatic balance between dietary intake, endogenous synthesis, and fecal elimination of cholesterol is fessential to prevent pathological accumulation of cholesterol in macrophage foam cells that leads to the development of atherosclerosis. Unlike other macromolecules such as carbohydrates, proteins, or nucleic acids, once synthesized the steroid nucleus of cholesterol cannot be degraded within the human body and excess cholesterol can only be removed following biotransformation to more water soluble bile acids or as free cholesterol (FC) solubilized in bile acids. Because bile acid synthesis and secretion of cholesterol into the bile is largely restricted to the liver, the liver plays a central role in regulating the elimination of cholesterol from the body. Furthermore, as only a small portion (5–20%) of biliary cholesterol is derived from de novo synthesis (1, 2) and the bulk is supplied by the hepatic uptake of lipoproteins (3, 4), the liver also plays a key role in the flux of cholesterol returning to the liver from the peripheral tissues via lipoproteins. Chylomicrons carrying the dietary cholesterol return to the liver as remnants, and following delivery of associated TGs and cholesterol to the peripheral tissues, liver-derived VLDLs return to the liver as LDLs (5). This hepatic uptake of remnant or LDL-associated cholesterol is thought to regulate hepatic cholesterol synthesis or VLDL secretion (6), and recently Sniderman et al. (7) have demonstrated that while chylomicron remnant-associated cholesterol enters the regulatory pool and modulates hepatic de novo synthesis, LDL cholesterol is resecreted as VLDL. In contrast to LDL, HDL removes cholesterol from peripheral tissues, including artery wall-associated macrophage foam cells, delivers it to the liver and represents the major mechanism for the flux of cholesterol from the peripheral tissues to the liver by the process of reverse cholesterol transport (RCT). Because cholesterol is carried as cholesteryl esters (CEs) in all lipoproteins, intracellular hydrolysis of CEs is obligatory for the release of FC within the hepatocyte. While the remnants and LDLs are taken up by the endocytic pathway and the associated CEs are hydrolyzed within the acidic lysosomal compartment by acid CE hydrolase (CEH), CEs associated with HDLs enter the hepatocyte by selective uptake pathway via scavenger receptor BI (SR-BI) and hydrolysis of HDL-CEs is extra lysosomal and catalyzed by a neutral CEH (8).
We purified (9), characterized, and cloned rat liver neutral CEH (10), a member of the carboxylesterase family, and established its role in hepatic cholesterol homeostasis (11). Recently, we also cloned and characterized human liver CEH and demonstrated that transient over-expression of CEH increased bile acid synthesis and secretion from primary human hepatocytes (12). Furthermore, adenovirus-mediated over-expression of this enzyme enhanced cholesterol elimination by increasing the flux of cholesterol from macrophages to feces (in vivo RCT) (13). More importantly, SR-BI deficiency completely abolished CEH-mediated increase in in vivo RCT, demonstrating the requirement of functional SR-BI for CEH to channel HDL-derived CEs to bile and feces (13). Direct association of CEH with SR-BI delivered HDL-CEs, and conversion of HDL-CEs to bile acids further confirmed the role of hepatic CEH in metabolizing HDL-CEs and making the FC available for bile acid synthesis (14). Consistently, liver-specific deletion of Ces3, the murine homolog of human CEH (hCEH), led to a significant decrease in bile acid secretion and fecal elimination of bile acids resulting in an increase in diet-induced atherosclerosis in LDL receptor (LDLR)−/− mice (15).
In the present study we sought to further establish the anti-atherogenic role of hepatic CE hydrolysis by developing liver-specific hCEH transgenic mice. Hepatic over-expression of CEH did not affect hepatic cholesterol homeostasis but led to an increase in the flux of HDL-CEs to bile acids. The data presented here also demonstrates that by increasing fecal elimination of cholesterol as bile acids, liver-specific transgenic expression of CEH leads to attenuation of diet-induced atherosclerosis in LDLR−/− mice.
METHODS
Generation of transgenic mice
The plasmid pLIV.11, containing human apoE promoter as well as hepatic control region and all the necessary elements for chimeric transgene construction (poly linker region and heterologous intron), was used (16). Full-length hCEH cDNA (∼2 kb) with an inframe 3′-c-myc epitope was cloned into the MunI and MluI sites in the poly linker region (supplementary Fig. I), and the sequence of the chimeric transgene was confirmed by sequencing. The chimeric transgene was excised by digestion with SalI and SpeI, purified by agarose gel electrophoresis, and injected into the pronuclei of fertilized mouse eggs obtained from super-ovulated female mice (Balb-c/C57BL/6 hybrids). The injected eggs were surgically transferred to oviducts of surrogate females. Presence of hCEH transgene was confirmed either by PCR amplification of a 682 bp product using hCEH specific primers (133–155 bp and 814–792 bp) using mouse tail genomic DNA as a template or by Southern blot analysis using full-length hCEH as a probe to identify the ∼1,265 bp integrated DNA. The founder mice in the Balb-c/C57BL/6 hybrid background were backcrossed into the C57BL/6 background for 10 generations before experimentation and were labeled as liver-specific hCEH transgenic (LCEH2) mice. For evaluation of atherosclerosis, LCEH2 mice were crossed into the LDLR−/− background. To generate macrophage- and liver-specific double transgenics in the LDLR−/− background, LDLR−/−LCEH2 mice were crossed with LDLR−/−CEHTg mice generated in our laboratory earlier (17). Male and female littermates were included in the study at 10 weeks of age. The total number of animals in each group was, therefore, determined by the availability of the correct genotype and gender within a litter. For assessment of atherosclerosis, mice were fed a Western-type high-fat/high-cholesterol diet (TD88137, Harlan Teklad), which contained 21% fat, 0.15% cholesterol, and 19.5% casein by weight with no sodium cholate for 16 weeks. All procedures were approved by the Virginia Commonweath University Institutional Animal Care and Use Committee. Littermates were used for all experiments.
Tissue distribution of hCEH in LCEH2 mice
Tissues were harvested from WT (C57BL/6) and LCEH2 mice and total RNA was extracted using an RNeasy kit (Qiagen). hCEH mRNA expression was determined by real time PCR using optimized TaqMan gene expression assay (Hs00275607_m1), and mRNA copy number was determined using a standard curve as described earlier (12). To determine the hCEH protein expression, total protein extracts prepared from different tissues were analyzed by Western blot analysis using anti-c-myc antibody to specifically identify the c-myc tag on transgenic hCEH and species-specific fluorescently labeled secondary antibodies. Positive immune-reactivity was detected by scanning in the appropriate channels with an Odyssey infrared imaging system (LI-COR).
Sub-cellular distribution of hCEH
Primary hepatocytes were prepared, and cytoplasm as well as endoplasmic reticulum (ER) were fractionated by differential centrifugation. The presence of hCEH was detected by Western blot analysis as described above. CE hydrolytic activity was determined using a micellar substrate as described earlier (18).
TG secretion from liver
Livers were harvested from WT and LCEH2 mice and precision cut liver slices were incubated with [3H]oleate for 3 h (19). Following three washes in PBS, total lipids were extracted and neutral lipids were separated by TLC using hexane:diethyl ether:acetic acid::90:10:1 (v/v). Spots corresponding to TG were marked, silica gel scraped, and associated radioactivity determined by liquid scintillation counting. To determine the rate of TG secretion in vivo, mice were fasted overnight and a baseline blood sample was collected via the tail vein. Mice were subsequently injected with tyloxapol (Sigma-Aldrich) at a concentration of 500 mg/kg body weight to inhibit lipoprotein lipase. Blood samples were subsequently collected at 1, 2, and 3 h postinjection and plasma TG levels were determined (L-Type TG-M kit, Wako Diagnostics). TG production rates were calculated as described (20).
Total plasma cholesterol and cholesterol distribution among plasma lipoproteins
A modified Column Lipoprotein Profile method was used. Whole plasma aliquots frozen and stored at −80°C were thawed at 4°C. Total plasma cholesterol (TPC) concentration was determined by a micro enzymatic method as described earlier (17). In brief, approximately 20 μg of cholesterol was injected onto a fast-protein liquid chromatography system (Superose 6 HR 10/30 column, Amersham Biosciences) with online mixing of the column effluent with enzymatic reagent (Cholesterol Liquid Stable, Thermo Electron) for acquiring lipoprotein cholesterol profiles. The data were acquired on a personal computer running ChromPerfect Spirit chromatography software (Justice Software). The system was optimized so that the area under the profiles was proportional to the cholesterol mass. Area percent in each lipoprotein fraction, VLDL, LDL, and HDL, was applied to TPC to calculate cholesterol concentration in the lipoprotein fractions.
Biochemical and histological analyses of liver tissue
About 100 mg of fresh liver tissue was homogenized in PBS and total lipids were extracted and amount of total cholesterol (TC), CEs, and TGs were determined, as described before, and normalized to wet weight. For histological analyses, liver tissue was fixed in 10% buffered formalin and paraffin embedded. Five micron sections were stained with hematoxylin and eosin and images were acquired using a Zeiss inverted microscope fitted with a digital camera as described earlier (19).
Intraperitoneal glucose tolerance tests
Ten-week-old LDLR−/− and LDLR−/−LCEH2 littermates were fed a Western diet for 16 weeks. After an overnight fast, a single bolus of glucose (2 mg/g body weight) was given intraperitoneally. Blood glucose levels were determined by commercially available glucometer using tail vein blood at 0, 15, 30, 60, 120, and 180 min.
Quantitative atherosclerosis analyses
The aorta was dissected from the heart to the iliac bifurcation, cleaned of any surrounding tissue, opened longitudinally, pinned on black wax, and fixed for 24 h in 10% buffered formalin. The fixed aortas were imaged on a black background using a Canon digital camera fitted with a 60 mm f/2.8 Macro lens. Total area and the area occupied by the lesions in the aortic arch and total aorta were determined using AxioVision™ image analysis software. The person quantifying the area occupied by lesions was blinded to the identity of the images.
Measurement of cholesterol movement from HDL to bile in vivo
Purified human HDL was purchased from Intracel and labeled with [3H]cholesteryl oleate using recombinant CETP as described earlier (15). Mice maintained on chow diet were injected (iv) with labeled HDL and transferred to metabolic cages. At 48 h, mice were euthanized and gall bladder bile was collected. Radioactivity associated with biliary cholesterol as well as bile acids was determined as described (17).
Fecal bile acid and cholesterol measurement
TC was extracted from the dried feces using chloroform-methanol (2:1, v/v). After evaporation under nitrogen, the extracts were solubilized in 2-propanol containing 10% Triton X-100 TC estimated by enzymatic assay using a Wako® Cholesterol E test kit. Bile acids extracted from dried feces were derivatized and the resulting phenacyl esters were separated using reverse phase HPLC (15). Total fecal bile acids and cholesterol were normalized to the dry weight of the feces and data presented as micromoles per gram.
Real time PCR
Total RNA was extracted using an RNeasy kit (Qiagen). cDNA was synthesized using a high capacity cDNA reverse transcription kit (Applied Biosystems). Real time PCR was performed on a Stratagene Mx3000P machine, using TaqMan Universal PCR Master Mix and optimized probe and primer sets from Applied Biosystems. The following optimized probes were used: Ces3, Mm00474816_m1; Ces1, Mm00491334_m1; Ces5, Mm00555211_m1; ES22, Mm00504914_m1; ES1, Mm00468347_m1; ES31, Mm00519905_m1; HSL, Mm00495359_m1; TG hydrolase (Tgh)2, Mm00523518_m1; Kiaa, Mm00626772_m1; BSEP, Mm00445168_m1; Abcg5, Mm00446249_m1; Abcg8, Mm00445970_m1; MDR2, Mm00435630_m1; HMGCR, Mm01282501_m1; ACAT-2, Mm00448823_m1; Ldlr, Mm00440169_m1; CYP7A1, Mm00484152_m1; and liver X receptor (LXR), Mm00437265_g1.
Statistical analyses
Data were analyzed by two-way ANOVA using GraphPad Prizm followed by Bonferroni post hoc tests to determine genotype and gender interactions, if any, as well as the significance of genotype and gender effects. Table 1 summarizes the results of these analyses including the actual P values. The differences were considered significant with P < 0.05 and are indicated in all figure legends.
TABLE 1.
Two-way ANOVA analyses
| Parameter | Genotype/Gender Interaction | Genotype Effects | Gender Effects |
| TPC | P = 0.002 | P = 0.01 | P = 0.0002 |
| VLDL | NS | P = 0.003 | NS |
| LDL | P = 0.002 | P = 0.09 | P = 0.0002 |
| HDL | NS | P = 0.02 | P = 0.05 |
| Arch lesion area (LDLR−/− vs. LDLR−/−LCEH2) | NS | P < 0.0001 | NS |
| Total lesion area (LDLR−/− vs. LDLR−/−LCEH2) | NS | P = 0.002 | NS |
| Arch lesion area (LDLR−/− vs. MLCL) | NS | P = 0.0004 | NS |
| Total lesion area (LDLR−/− vs. MLCL) | NS | P < 0.0001 | NS |
| Fecal BA (LDLR−/− vs. LDLR−/−LCEH2 | NS | P = 0.002 | P = 0.001 |
| Fecal cholesterol (LDLR−/− vs. LDLR−/−LCEH2) | NS | NS | P = 0.001 |
Two-way ANOVA analyses were performed for the indicated parameters and significant differences due to genotype or gender as well as genotype/gender interactions are shown. BA, bile acid.
RESULTS
Development of LCEH2 mice
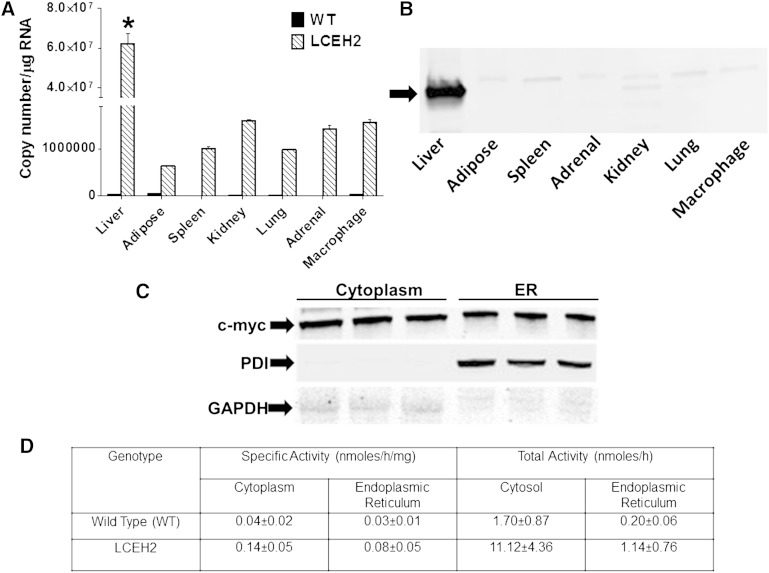
Liver-specific hCEH (gene symbol CES1) transgenic mice were developed and backcrossed into the C57BL/6 background. Total RNA from multiple tissues was used to determine the expression of hCEH. Consistent with the expression driven by the apoE promoter and the hepatocyte control region, liver-specific expression of hCEH mRNA was seen in LCEH2 mice (Fig. 1A). Furthermore, high expression of c-myc tagged hCEH protein was detected in total protein extracts from the liver, confirming liver-specific expression (Fig. 1B). The minor immune-reactive protein seen in kidney and lung extracts and the detection of a low copy number of hCEH in other tissues is likely due to the nonspecific proximal enhancer element in the apoE promoter as described by Simonet et al. (16).
Fig. 1.
Liver-specific expression of human CES1 in LCEH2 transgenic mice: Total RNA as well as protein from indicated tissues of WT C57BL/6 (WT) and transgenic (LCEH2) mice were isolated and hCEH expression determined. A: Human CES1 mRNA levels were determined by real time RT-PCR and CES1 copy number per microgram total RNA is shown (mean ± SD, n = 3). B: Expression of c-myc tagged human CES1 protein in different tissues was monitored by Western blot analysis using anti-c-myc antibody. The arrow points to the immunoreactive band. C: Sub-cellular localization of c-myc tagged human CES1 protein in primary hepatocytes from LCEH2 mice. D: CE hydrolytic activity associated with cytoplasm and ER fraction was determined as described in the Methods and expressed as specific as well as total activity. *P < 0.05.
Sub-cellular distribution of hCEH and CEH activity was determined in primary hepatocytes. The c-myc tagged CEH protein was associated with cytoplasm as well as ER; protein disulfide isomerase and GAPDH were used as markers for ER and cytoplasm, respectively (Fig. 1C). CEH-specific activity was increased by ∼3.5-fold in the cytoplasm and ∼2.6-fold in the ER of LCEH2 mice. However, transgenic CEH over-expression did not affect the relative distribution of total CEH activity; >85–90% of total activity was associated with cytoplasm, consistent with the cytoplasmic localization of CEH.
Two independent transgenic lines of mice (LCEH1 and LCEH2) were developed with similar liver-specific expression and the LCEH2 line was chosen for all further studies.
Human CES1 belongs to the carboxylesterase family and Ces3 is its murine homolog. To evaluate the effects, if any, on the expression of Ces3 and other members of the carboxylesterase family by transgenic expression of hCEH, expression of the indicated carboxylesterases was also monitored. No significant differences were noted in the expression of other genes (supplementary Fig. II) in mice on either a chow or a high-fat/high-cholesterol-containing Western diet.
To determine the potential effects of transgenic expression of hCEH in affecting genes involved in maintaining hepatic cholesterol homeostasis, expression of HMGCR, ACAT-2, Ldlr, and CYP7A1 was assessed. While a nonsignificant increase in CYP7A1 and significant increase in the expression of SR-BI (P = 0.02 for chow-fed mice, P = 0.04 for Western diet-fed mice) was observed, there was no change in the expression of other genes (supplementary Fig. III). Expression of LXR, a gene that plays an important role in hepatic lipogenesis, also remained unaltered (1.00 ± 0.02 in WT mice vs. 1.05 ± 0.11 in LCEH2 mice).
Because FC released as a result of CEH-mediated CE hydrolysis can either be directly secreted into bile via the FC transporter AbcG5/G8 or converted into bile acids before secretion via bile acid transporter (BSEP), expression of these transporters was evaluated. Ces3 deficiency did not affect the expression of these transporters (supplementary Fig. IV). There was also no change in the expression of phospholipid transporter MDR2.
Liver-specific transgenic expression of hCEH does not affect TG secretion from the liver
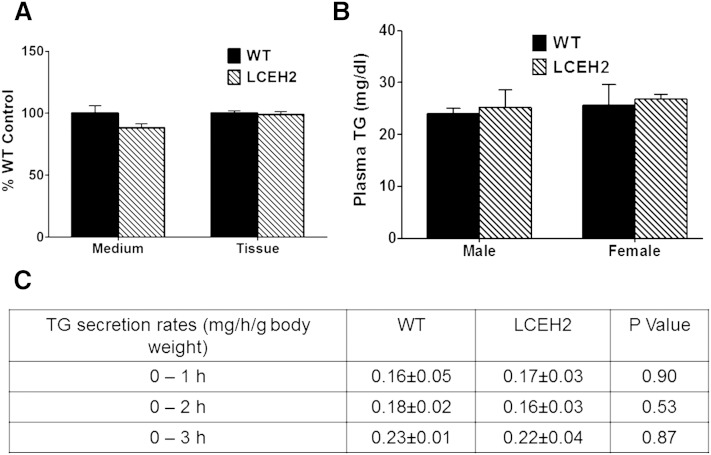
In addition to catalyzing the hydrolysis of CEs, hCEH also hydrolyzes TGs, and the murine homolog of hCEH, Ces3, has been characterized as Tgh and thought to play a role in VLDL secretion. To evaluate the effects of transgenic expression of hCEH, TG synthesis/secretion from precision cut liver slices was examined by monitoring incorporation of [3H]oleate in cellular as well as secreted TGs. There was no significant difference in [3H]oleate incorporation in cellular (tissue) as well as secreted (medium) TGs (Fig. 2A).
Fig. 2.
Transgenic expression of human CES1 does not increase hepatic TG secretion. A: Precision cut liver slices from WT C57BL/6 (WT) and transgenic (LCEH2) mice were incubated with [3H]oleic acid and incorporation of radiolabel into secreted (medium) and cellular (tissue) TGs was determined as described in the Methods. Data (mean ± SD, n = 6) are presented as percent WT control. B: Fasting plasma TG levels were determined and data are presented as mean ± SD, n = 6. C: TG secretion rates were also determined by measuring plasma TG levels at indicated times following toloxypol injection. Data are presented as mean ± SD, n = 6.
Plasma TG levels were also measured and are shown in Fig. 2B. There was no significant difference in plasma TG levels with CEH over-expression (P = 0.76 for males, P = 0.79 for females). Consistently, the rates of in vivo secretion of VLDLs were also not significantly different between WT and LCEH2 mice (Fig. 2C). These data demonstrate that transgenic expression of hCEH does not affect hepatic TG synthesis or secretion. Cholesterol content of plasma lipoproteins also remained unchanged in LCEH2 mice (data not shown).
Liver-specific transgenic expression of hCEH increases the flux of CEs from HDLs to biliary bile acids
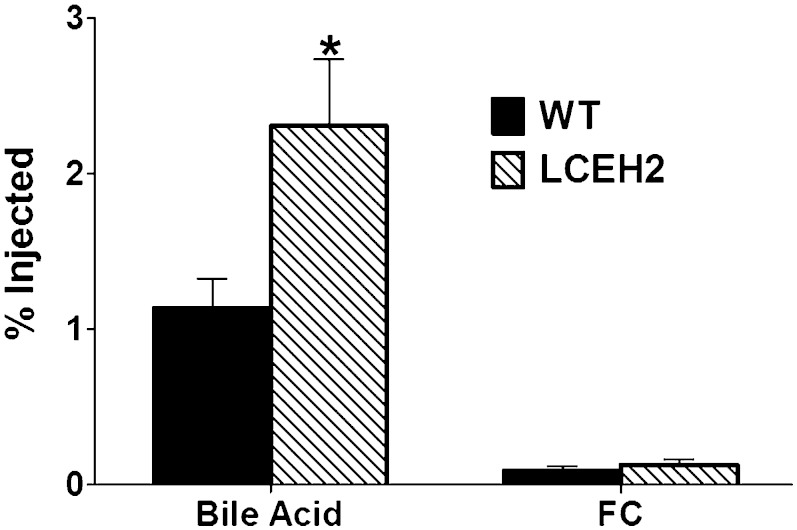
To examine the role of hepatic CE hydrolysis in regulating elimination of HDL-derived CEs into bile, flux of [3H]cholesterol from HDL-CEs into bile was monitored in vivo. As shown in Fig. 3, transgenic expression of hCEH significantly increased the elimination of [3H]cholesterol from HDL-CEs to biliary bile acids (2.31 ± 1.05 vs. 1.14 ± 0.45, P = 0.03). In contrast, there was no increase in the [3H]label associated with biliary FC. These data suggest that FC generated by hepatic CE hydrolysis of HDL-CEs is preferentially eliminated as bile acids and is consistent with the observed increase in bile acid secretion by adenovirus-mediated transient over-expression of hCEH in mice (13).
Fig. 3.
Transgenic expression of human CES1 increases the flux of [3H]cholesterol from HDL-CEs to bile. WT C57BL/6 (WT) and transgenic (LCEH2) mice were injected (iv) with [3H]CE-labeled HDL (HDL-[3H]CE) and gall bladder bile was collected after 48 h. Following phase separation of bile acids and biliary cholesterol, radioactivity associated with each fraction was determined. Data (mean ± SD, n = 6) are presented as percent of total radiolabel injected. *P < 0.05.
Effect of liver-specific hCEH expression in Western diet-fed LDLR−/− mice
LCEH2 mice were crossed into the LDLR−/− background and fed Western diet for 16 weeks and following parameters were examined.
Gene expression.
Hepatic expression of members of carboxylesterase family, as well as other known hydrolases, was examined and no significant differences were noted (supplementary Fig. V). Consistent with the data obtained in the C57BL/6 background, there was no significant change in the expression of genes involved in hepatic cholesterol homeostasis (HMG-CoAR, ACAT-2, Cyp7A1, and Cyp27A1) except that no significant increase in SR-BI expression was noted (supplementary Fig. VI). There was also no significant change in the expression of genes involved in cholesterol, phospholipid, and bile acid transport to the bile canaliculus, namely ABCCG5/G8, MDR2, and BSEP, respectively (supplementary Fig. VII).
Plasma lipoprotein profiles.
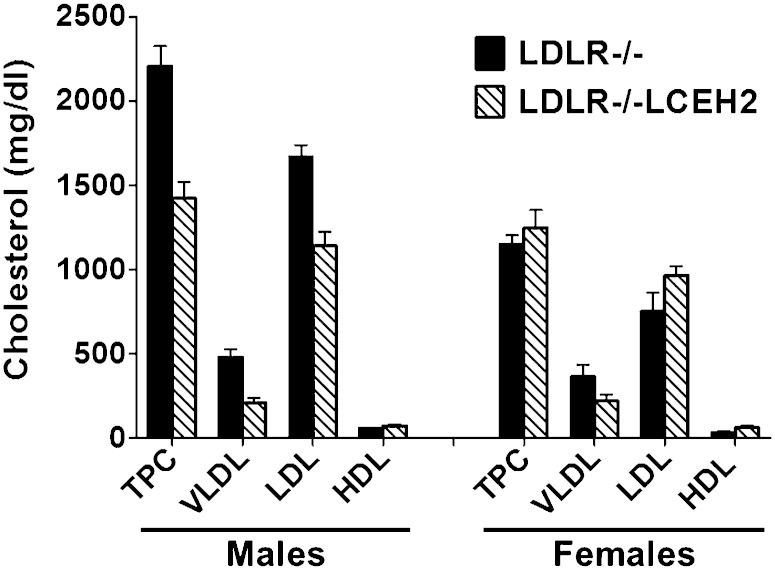
Fasting plasma TC and cholesterol associated with different lipoprotein fractions were measured and are shown in Fig. 4; representative fast-protein liquid chromatography profiles are shown in supplementary Fig. VIII. An overall significant effect of genotype (P = 0.008) and gender (P = 0.002) on TPC was detected by ANOVA (see Table 1). In addition, gender/genotype interaction was also considered very significant (P = 0.002). Analyses of the distribution of plasma cholesterol among the different lipoprotein fractions indicated significant effects of the genotype on VLDL (P = 0.003) and HDL (P = 0.025) cholesterol, but not LDL (P = 0.095) cholesterol. In contrast, gender/genotype interaction was considered very significant (P = 0.002) for LDL cholesterol only.
Fig. 4.
Plasma lipoprotein profiles. LDLR−/− and LDLR−/−LCEH2 mice were fed a high-fat/high-cholesterol Western diet for 16 weeks. Fasting plasma was collected at the time of euthanasia and TPC as well as cholesterol associated with different lipoprotein fractions (VLDL, LDL, and HDL) were determined as described in the Methods. Data (mean ± SD, n = 6) are shown for males and females. The effects of genotype (transgenic expression of CEH) and gender, as well as genotype/gender interactions on lipoprotein cholesterol content were determined to be significant by two-way ANOVA analyses and individual P values are included in Table 1.
Hepatic lipids.
Total hepatic lipids were analyzed for TC, FC, and CE as well as TG content. Liver-specific transgenic expression of hCEH did not affect the distribution of hepatic lipids (supplementary Fig. IXA). Histological analyses of livers from LDLR−/− and LDLR−/−LCEH2 mice showed lipid accumulation consistent with Western diet feeding but no difference was apparent between the two genotypes (supplementary Fig. IXB).
Glucose tolerance.
Loss of Ces3/Tgh is reported to improve glucose tolerance (21) and therefore, intra-peritoneal glucose tolerance tests were performed in Western diet-fed LDLR−/− and LDLR−/−LCEH2 mice. Over-expression of hCEH in the liver did not significantly affect glucose tolerance and the area under the curve (AUC) for males (LDLR−/− = 37,000 ± 2,700 and LDLR−/−LCEH2 = 39,000 ± 1,300; P = 0.54) and females (LDLR−/− = 29,000 ± 4,300 and LDLR−/−LCEH2 = 26,000 ± 8,000; P = 0.53) were not significantly different.
Atherosclerosis.
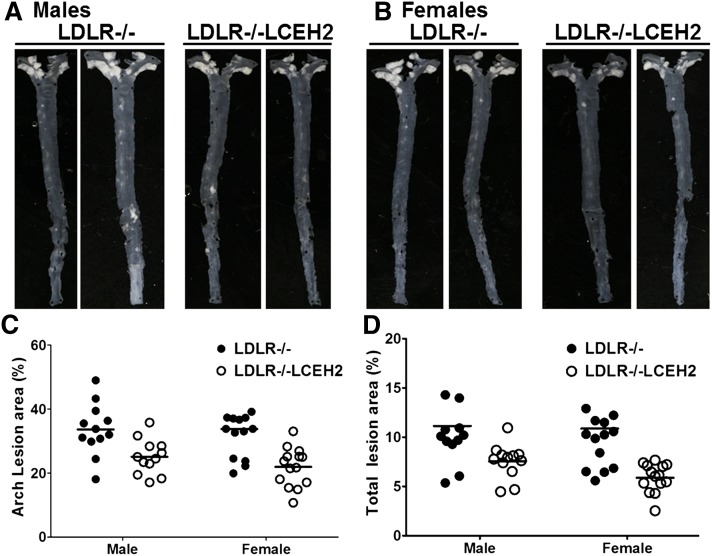
Western diet-induced atherosclerosis was monitored in LDLR−/− and LDLR−/−LCEH2 mice after 16 weeks of feeding. No mortality or morbidity was observed in mice with either genotype. Representative en face images are shown in Fig. 5A. The percent area of aorta covered with atherosclerotic lesions was determined and the quantification of the surface area occupied by atherosclerotic lesions is shown in Fig. 5B. Compared with LDLR−/− mice, liver-specific hCEH expression in LDLR−/−LCEH2 mice led to a significant decrease in the area occupied by the lesions in the aortic arch. Total area occupied by the lesions in the entire aorta was also determined and liver-specific hCEH expression significantly decreased the total area occupied by atherosclerotic lesions. An overall effect of genotype on total (P = 0.0002) and aortic arch (P < 0.0001) lesion area was detected by ANOVA. Effects of gender and gender/genotype interactions were not detected (see Table 1).
Fig. 5.
Liver-specific transgenic expression of human CES1 reduces diet-induced atherosclerosis. LDLR−/− and LDLR−/−LCEH2 mice were fed a high-fat/high-cholesterol Western diet for 16 weeks and development of atherosclerosis was assessed by en face analyses. A, B: Representative en face images from male and female mice, respectively. Quantitation of lesion area: data are presented as percent lesion area in aortic arch (C) or total aorta (D). The effects of genotype (transgenic expression of CEH) on lesion area and aortic cholesterol content were determined to be significant by two-way ANOVA analyses and individual P values are included in Table 1.
Fecal bile acid and cholesterol elimination.
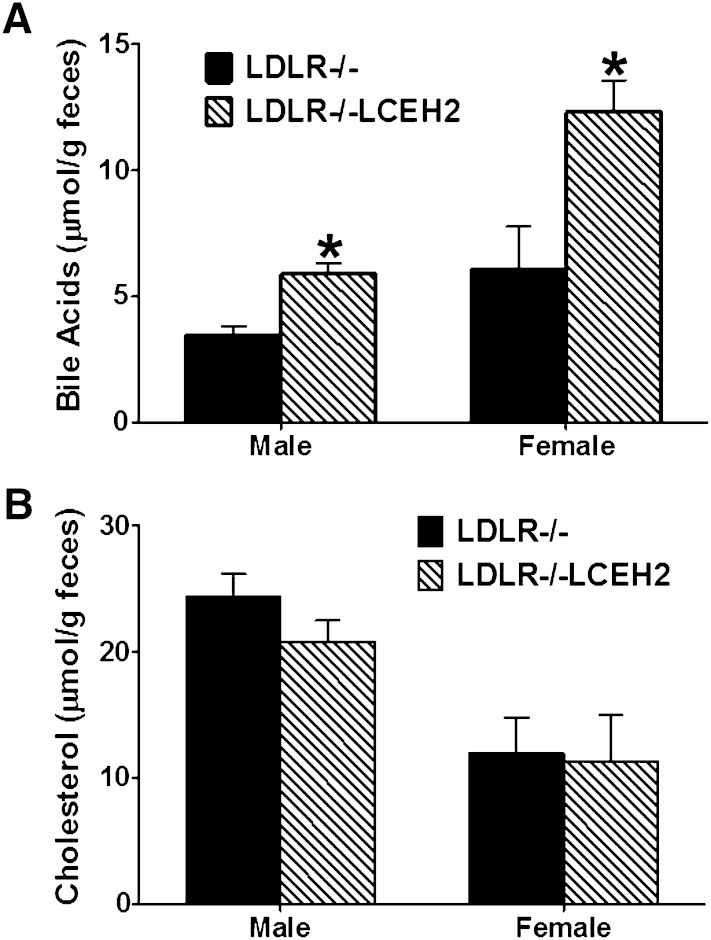
To examine whether transgenic over-expression of hCEH-mediated increase in removal of cholesterol in feces (either as FC or as bile acids) represents a potential mechanism for the observed decrease in atherosclerosis in LDLR−/−LCEH2 mice, total fecal bile acids as well as cholesterol levels were also monitored. As shown in Fig. 6, consistent with decreased flux of HDL-CE to bile in LCEH2 mice shown in Fig. 3, there was a significant increase in total fecal bile acids (P = 0.002) in LDLR−/−LCEH2 mice, and the magnitude of this increase was higher in female mice; these gender effects were highly significant (P = 0.001). In contrast, there was no significant change in fecal FC content in both genders suggesting a possible preferential flux of CEH-generated FC toward bile acid synthesis. These data demonstrate that hCEH mediates the increase in HDL-CE hydrolysis and subsequent conversion of released FC to bile acids for final removal from the body in the feces. It is noteworthy that while the fecal bile acid content was significantly higher in females, fecal FC content was higher in males (P = 0.001).
Fig. 6.
Liver-specific transgenic expression of human CES1 increases fecal bile acid elimination. LDLR−/− and LDLR−/−LCEH2 mice were fed a high-fat/high-cholesterol Western diet for 16 weeks. Total feces were collected over a period of 48 h and analyzed for cholesterol and bile acid content. Data (mean ± SD, n = 6) are presented as millimole per gram feces (dry weight). A: Total bile acids excreted in the feces. B: TC excreted in the feces. *P < 0.05.
Western diet-induced atherosclerosis in macrophage and liver hCEH double transgenics in LDLR−/− background
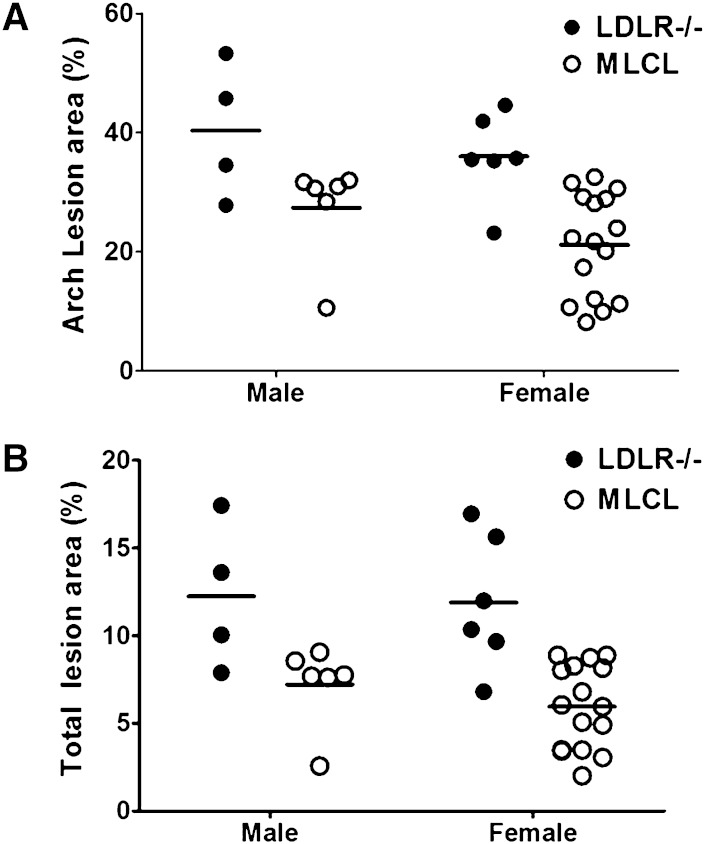
We earlier reported a significant reduction in diet-induced atherosclerosis in macrophage-specific CEH transgenic mice and the data presented above demonstrate the anti-atherogenic role of hepatic CEH (17). To examine whether transgenic over-expression of CEH in macrophage and liver would have additive effects, if any, double CEH transgenics in the LDLR−/− background (MLCL) were generated and Western diet-induced atherosclerosis was evaluated. While there was a significant reduction in aortic arch (P = 0.0004, Fig. 7A) and total (P < 0.0001, Fig. 7B) lesion area in double transgenic MLCL mice of both genders compared with LDLR−/− mice, this reduction was not significantly different from that seen in macrophage (P = 0.86 for arch, P = 0.53 for total lesion area) or liver single transgenic mice (P = 0.52 for arch, P = 0.29 for total lesion area). These data point to a potential maximum reduction that can possibly be achieved by enhancing CEH-mediated flux of cholesterol from macrophage foam cells to feces as bile acids by enhancing CEH-mediated elimination of cholesterol from the body either by increasing efflux from macrophage foam cells or by hepatic removal.
Fig. 7.
Transgenic expression of human CES1 in liver and macrophages reduces diet-induced atherosclerosis. LDLR−/− and MLCL mice were fed a high-fat/high-cholesterol Western diet for 16 weeks and development of atherosclerosis was assessed by en face analyses. Data are presented as percent lesion area in aortic arch (A) or total aorta (B). The effects of genotype (transgenic expression of CEH) on lesion area and aortic cholesterol content were determined to be significant by two-way ANOVA analyses and individual P values are included in Table 1.
DISCUSSION
The liver plays a central role in the final elimination of cholesterol from the body either as FC or as bile acids, and lipoprotein-derived CEs represent the major source for biliary FC and bile acids. The data presented here clearly demonstrate that increasing hepatic CEH-mediated hydrolysis of lipoprotein-derived CEs leads to increased elimination of cholesterol as biliary and fecal bile acids. This enhanced elimination of cholesterol from the body is anti-atherogenic, resulting in significant attenuation of Western diet-induced atherosclerosis in LDLR−/−LCEH2 mice. These data confirm our previous results demonstrating a significant increase in atherogenesis by liver-specific deficiency of the murine homolog of hCEH, namely Ces3 (15). However, in contrast to liver-specific Ces deficiency, which did not alter plasma lipoprotein profiles, transgenic expression of hCEH significantly reduced total plasma and VLDL cholesterol levels and these effects were more pronounced in males. Although these changes in the plasma cholesterol levels can be viewed as anti-atherogenic, it needs to be emphasized that increasing evidence, however, points to an apparent lack of correlation between plasma lipid profiles and atherogenesis (22–25), and flux of cholesterol from the peripheral tissue, including the artery wall-associated macrophage foam cells, to feces is a more relevant parameter for predicting the risk for the development of atherosclerosis (26). In addition, Rowlan et al. (27) have recently demonstrated that most atherosclerosis susceptibility loci are distinct from those for plasma lipids.
In earlier studies, we have demonstrated a significant increase in bile acid secretion as well as flux of cholesterol from HDL to bile by adenovirus-mediated transient over-expression of hCEH, a process dependent on hepatic expression of SR-BI (13). Consistently, transgenic expression of hCEH significantly increased the flux of cholesterol from HDL to bile and this increase was associated with biliary bile acids and not biliary FC. FC associated with HDL is thought to be directly/rapidly secreted into bile (28) and intracellular sterol carrier protein-2 (SCP-2) plays an important role in the transport of FC from the basolateral to the cannilicular side of the hepatocyte (29). However, the fate of HDL-CEs taken up by selective uptake via SR-BI is not well-established. We have recently demonstrated cooperation between SR-BI and CEH in mediating the hydrolysis of HDL-CEs and the flux of FC generated toward bile acid synthesis (14). The data presented here also demonstrates that transgenic over-expression of hCEH increases the flux of HDL-CEs to biliary bile acids. It is noteworthy that hCEH over-expression led to a significant increase in the expression of SR-BI in LCEH2 mice and future studies will examine the coordinated regulation, if any, of hepatic CEH and SR-BI to further delineate the role of these two proteins in regulating the flux of HDL-CEs to bile acids. The lack of increase in flux of HDL-CEs to biliary FC in LCEH2 mice points to a preferential movement of CEH-generated FC toward bile acid synthesis and it remains to be seen whether intracellular cholesterol transport proteins such as SCP-2 or StAR proteins are involved in this process (30).
The murine homolog of hCEH, Ces3, is also known as Tgh, and as members of the carboxylesterase family of enzymes, both catalyze the hydrolysis of ester bonds in multiple substrates including CEs and TGs. Based on its role in TG hydrolysis, murine hepatic Ces3 is thought to play a role in VLDL secretion. Transgenic expression of hCEH did not affect hepatic TG synthesis or TG secretion in vivo; the observed secretion rates were comparable to those reported for C57BL/6 mice (21). Using a Zn-inducible metallothionein promoter-driven human TGH minigene in mice, Wei et al. (31) also reported unchanged hepatic TG secretion rates compared with WT mice.
Consistent with its role in regulating the flux of lipoprotein CEs to bile acids and biliary excretion, transgenic expression of hCEH significantly increased fecal sterol elimination. This increased fecal sterol elimination was predominantly associated with acidic sterol or bile acids. Furthermore, significant gender-related differences were observed; while total fecal FC was higher in males of both genotypes, total fecal bile acids were higher in females of both genotypes. Consistently, Turley et al. (32) have earlier reported a significantly higher bile acid synthesis and bile acid pool, as well as fecal bile acid excretion in female mice. Gender specificity of bile acid metabolic pathways has also been shown in Cyp7A1-deficient mice, where attenuated BSEP and changes in gall bladder bile composition is only observed in female mice (33). Charach et al. (34) described a strong correlation between bile acid secretion and coronary artery disease (CAD) in humans, where patients with established CAD excreted significantly lower amounts of bile acids, and concluded that impaired ability to excrete cholesterol may be an additional risk factor for CAD development. Additionally, while normal bile acid secretion in heterozygous familial hypercholesterolemic patients is not associated with CAD, suboptimal bile acid synthesis, especially in males, is strongly related to development of CAD (35). It remains to be seen whether these gender-related differences in bile acid secretion and elimination in feces is related to the reduced risk for CAD in premenopausal women. However, it is noteworthy that increased risk for CAD in postmenopausal women is associated with altered cholesterol metabolism and inefficient fecal elimination of cholesterol (36).
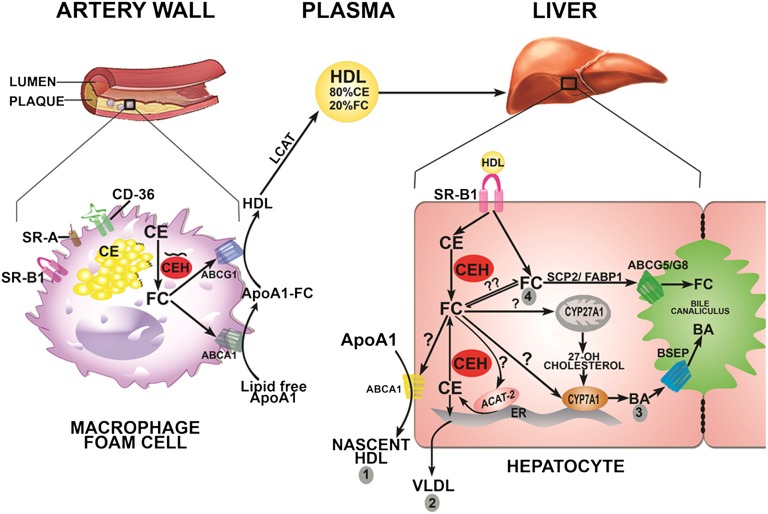
Mobilization of stored CE in lesion-associated macrophage foam cells is the obligatory first step toward regression of arterial plaque; and we have earlier demonstrated attenuation of diet-induced atherosclerosis in macrophage-specific CEH transgenic mice (17). Furthermore, reconstitution with CEH transgenic macrophages reduced the progression of existing plaques (37). The present study now establishes the anti-atherogenic role of hepatic CEH. Figure 8 summarizes the role of CEH in cholesterol elimination from the body. In artery wall-associated macrophage cells, CEH catalyzes the rate-limiting and obligatory first step in CE mobilization. The FC generated is effluxed to lipid-poor apoA1 or HDL and is carried to the liver via HDL predominantly as CEs. While HDL-FC is thought to be rapidly secreted into bile (29), HDL-CEs need to be hydrolyzed by hepatic CEH to generate FC; and currently it is not known whether CEH-generated FC equilibrates with other FC pools, including HDL-FC. Nonetheless, CEH-generated FC can theoretically have four fates: 1) resecretion via ABCA1 to apoA1 to generate nascent HDL; 2) translocation to the ER for esterification by ACAT-2 and secretion of the resulting CEs associated with VLDL; 3) transport to mitochondria or the ER to serve as a substrate for CYP27A1 or CYP7A1, respectively, for bile acid synthesis and secretion of bile acids; and 4) direct secretion into bile via ABCG5/G8, a process facilitated by intracellular sterol carrier proteins SCP-2 or FABP1 (30). While fates 1 and 2 recirculate the cholesterol returning from the periphery, fates 3 and 4 contribute to the final elimination of cholesterol from the body, and are hence anti-atherogenic. The data presented herein establishes the role of CEH in hydrolysis of HDL-CEs and elimination of the resulting FC primarily as bile acids, indicating a possible preferential translocation of CEH-generated FC to either mitochondria or ER for bile acid synthesis. Intracellular mechanisms involved in the translocation of FC to appropriate sub-cellular destinations (marked by “?” in Fig. 8) are currently undefined. Interactions between intracellular sterol binding proteins, such as SCP-2, FABP-1, or StAR proteins and CEH, could potentially regulate this process and facilitate the preferential flux of CEH-generated FC from HDL-CEs toward bile acid synthesis and final elimination from the body.
Fig. 8.
Anti-atherogenic roles of CEH. In arterial plaque-associated macrophage foam cells, CEH catalyzes the rate-limiting and obligatory first step in the process of CE mobilization required for plaque regression. Macrophage CEH (depicted as a black squiggle) is shown to be associated with lipid droplets and hydrolyzes associated CEs. FC is effluxed via ABCA1 or ABCG1, esterified by plasma LCAT, and carried predominantly as CEs in HDL to the liver. SR-BI-delivered HDL-CEs are hydrolyzed by hepatic CEH and the resulting FC can have four potential fates: 1) resecretion via ABCA1 to apoA1 to generate nascent HDL; 2) translocation to the ER for esterification by ACAT-2 and secretion of resulting CEs associated with VLDL; 3) transport to mitochondria or ER to serve as a substrate for CYP27A1 or CYP7A1 for bile acid (BA) synthesis and secretion of bile acids; and 4) direct secretion into bile via ABCG5/G8, a process facilitated by intracellular sterol carrier proteins SCP-2 or FABP1. The intracellular processes involved in translocation of FC to appropriate sub-cellular organelles are likely to regulate the fate of FC and are currently undefined (indicated by ?).
An increase in hepatic CEH in addition to an increase in macrophage CEH in MLCL mice did not result in additive reduction in atherosclerosis. It is likely that the maximum possible effect is obtained by either increasing macrophage or hepatic CEH. Nonetheless, these data confirm that a simultaneous increase in CEH-mediated CE hydrolysis in both macrophages and liver does not have any deleterious effects, opening the way for the development of pharmacological agents for increasing CEH activity. This is specifically relevant because LXR ligands that enhance FC efflux from macrophages and reduce atherosclerosis in mice (38) have undesirable lipogenic effects in the liver (39); increased expression of hCEH in LCEH2 mice examined here did not affect hepatic TG synthesis/secretion or accumulation.
In conclusion, by mediating the hydrolysis of HDL-CEs in the liver and increasing elimination of cholesterol as bile acids in the feces, hepatic CEH is anti-atherogenic.
Supplementary Material
Acknowledgments
The authors thank Dr. Liqing Yu of Wake Forest University School of Medicine for providing the pLIV.11 vector.
Footnotes
Abbreviations:
- CAD
- coronary artery disease
- CE
- cholesteryl ester
- CEH
- cholesteryl ester hydrolase
- ER
- endoplasmic reticulum
- FC
- free cholesterol
- hCEH
- human cholesteryl ester hydrolase
- LCEH2
- liver-specific human CEH transgenic
- LDLR
- LDL receptor
- LXR
- liver X receptor
- RCT
- reverse cholesterol transport
- SCP-2
- sterol carrier protein-2
- SR-BI
- scavenger receptor BI
- TC
- total cholesterol
- Tgh
- TG hydrolase
- TPC
- total plasma cholesterol
This work was supported by research grant HL097346 from the National Heart, Lung, and Blood Institute (NHLBI) to S.G. The transgenic mice were developed in the Institutional Massey Cancer Center Transgenic/Knock-out Mouse Core facility supported in part by grant P30 CA016059 from the National Cancer Institute.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of nine figures.
REFERENCES
- 1.Robins S. J., Fasulo J. M., Lessard D., Patton G. M. 1993. Hepatic cholesterol synthesis and the secretion of newly synthesized cholesterol in bile. Biochem. J. 289: 41–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Empen K., Lange K., Stange E. F. 1997. Newly synthesized cholesterol in human bile and plasma: quantification by mass isotopomer distribution analysis. Am. J. Physiol. Gastrointest. Liver Physiol. 272: G367–G373. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz C. C., Berman M., Vlahcevic Z. R., Halloran L. G., Gregory D. H., Swell L. 1978. Multicompartmental analysis of cholesterol metabolism in man. Characterization of the hepatic bile acid and biliary cholesterol precursor sites. J. Clin. Invest. 61: 408–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartz C. C., Halloran L. G., Vlahcevic Z. R., Gregory D. H., Swell L. 1978. Preferential utilization of free cholesterol from high density lipoproteins for biliary cholesterol secretion in man. Science. 200: 62–64. [DOI] [PubMed] [Google Scholar]
- 5.Gotto A. M., Jr 1990. Interrelationship of triglycerides with lipoproteins and high-density lipoproteins. Am. J. Cardiol. 66: 20A–23A. [DOI] [PubMed] [Google Scholar]
- 6.Brown M. S. J. L. G. 1986. A receptor-mediated pathway for cholesterol homeostasis. Science. 232: 34–47. [DOI] [PubMed] [Google Scholar]
- 7.Sniderman A. D., Qi Y., Ma C. L., Wang R. H., Naples M., Baker C., Zhang J., Adeli K., Kiss R. S. 2013. Hepatic cholesterol homeostasis: is the low-density lipoprotein pathway a regulatory or a shunt pathway? Arterioscler. Thromb. Vasc. Biol. 33: 2481–2490. [DOI] [PubMed] [Google Scholar]
- 8.Shimada A., Tamai T., Oida K., Takahashi S., Suzuki J., Nakai T., Miyabo S. 1994. Increase in neutral cholesteryl ester hydrolase activity produced by extralysosomal hydrolysis of high-density lipoprotein cholesteryl esters in rat hepatoma cells (H-35). Biochim. Biophys. Acta. 1215: 126–132. [DOI] [PubMed] [Google Scholar]
- 9.Ghosh S., Grogan W. M. 1991. Rapid three-step purification of a hepatic neutral cholesteryl ester hydrolase which is not the pancreatic enzyme. Lipids. 26: 793–798. [DOI] [PubMed] [Google Scholar]
- 10.Ghosh S., Mallonee D. H., Hylemon P. B., Grogan W. M. 1995. Molecular cloning and expression of rat hepatic neutral cholesteryl ester hydrolase. Biochim. Biophys. Acta. 1259: 305–312. [DOI] [PubMed] [Google Scholar]
- 11.Ghosh S., Natarajan R., Pandak W. M., Hylemon P. B., Grogan W. M. 1998. Regulation of hepatic neutral cholesteryl ester hydrolase by hormones and changes in cholesterol flux. Am. J. Physiol. 274: G662–G668. [DOI] [PubMed] [Google Scholar]
- 12.Zhao B., Natarajan R., Ghosh S. 2005. Human liver cholesteryl ester hydrolase: cloning, molecular characterization, and role in cellular cholesterol homeostasis. Physiol. Genomics. 23: 304–310. [DOI] [PubMed] [Google Scholar]
- 13.Zhao B., Song J., Ghosh S. 2008. Hepatic overexpression of cholesteryl ester hydrolase enhances cholesterol elimination and in vivo reverse cholesterol transport. J. Lipid Res. 49: 2212–2217. [DOI] [PubMed] [Google Scholar]
- 14.Yuan Q., Bie J., Wang J., Ghosh S. S., Ghosh S. 2013. Cooperation between hepatic cholesteryl ester hydrolase and scavenger receptor BI for hydrolysis of HDL-CE. J. Lipid Res. 54: 3078–3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bie J., Wang J., Marqueen K. E., Osborne R., Kakiyama G., Korzun W., Ghosh S. S., Ghosh S. 2013. Liver-specific cholesteryl ester hydrolase deficiency attenuates sterol elimination in the feces and increases atherosclerosis in ldlr-/- mice. Arterioscler. Thromb. Vasc. Biol. 33: 1795–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simonet W. S., Bucay N., Lauer S. J., Taylor J. M. 1993. A far-downstream hepatocyte-specific control region directs expression of the linked human apolipoprotein E and C–I genes in transgenic mice. J. Biol. Chem. 268: 8221–8229. [PubMed] [Google Scholar]
- 17.Zhao B., Song J., Chow W. N., St Clair R. W., Rudel L. L., Ghosh S. 2007. Macrophage-specific transgenic expression of cholesteryl ester hydrolase significantly reduces atherosclerosis and lesion necrosis in Ldlr mice. J. Clin. Invest. 117: 2983–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao B., Bie J., Wang J., Marqueen S. A., Ghosh S. 2012. Identification of a novel intracellular cholesteryl ester hydrolase (carboxylesterase 3) in human macrophages: compensatory increase in its expression after carboxylesterase 1 silencing. Am. J. Physiol. Cell Physiol. 303: C427–C435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bie J., Zhao B., Marqueen K. E., Wang J., Szomju B., Ghosh S. 2012. Macrophage-specific transgenic expression of cholesteryl ester hydrolase attenuates hepatic lipid accumulation and also improves glucose tolerance in ob/ob mice. Am. J. Physiol. Endocrinol. Metab. 302: E1283–E1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shachter N. S., Hayek T., Leff T., Smith J. D., Rosenberg D. W., Walsh A., Ramakrishnan R., Goldberg I. J., Ginsberg H. N., Breslow J. L. 1994. Overexpression of apolipoprotein CII causes hypertriglyceridemia in transgenic mice. J. Clin. Invest. 93: 1683–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei E., Ben Ali Y., Lyon J., Wang H., Nelson R., Dolinsky V. W., Dyck J. R., Mitchell G., Korbutt G. S., Lehner R. 2010. Loss of TGH/Ces3 in mice decreases blood lipids, improves glucose tolerance, and increases energy expenditure. Cell Metab. 11: 183–193. [DOI] [PubMed] [Google Scholar]
- 22.Lipinski M. J., Campbell K. A., Duong S. Q., Welch T. J., Garmey J. C., Doran A. C., Skaflen M. D., Oldham S. N., Kelly K. A., McNamara C. A. 2012. Loss of id3 increases VCAM-1 expression, macrophage accumulation, and atherogenesis in Ldlr-/- mice. Arterioscler. Thromb. Vasc. Biol. 32: 2855–2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cardilo-Reis L., Gruber S., Schreier S. M., Drechsler M., Papac-Milicevic N., Weber C., Wagner O., Stangl H., Soehnlein O., Binder C. J. 2012. Interleukin-13 protects from atherosclerosis and modulates plaque composition by skewing the macrophage phenotype. EMBO Mol. Med. 4: 1072–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park S. H., Sui Y., Gizard F., Xu J., Rios-Pilier J., Helsley R. N., Han S. S., Zhou C. 2012. Myeloid-specific IκB kinase β deficiency decreases atherosclerosis in low-density lipoprotein receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 32: 2869–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.So J. S., Hur K. Y., Tarrio M., Ruda V., Frank-Kamenetsky M., Fitzgerald K., Koteliansky V., Lichtman A. H., Iwawaki T., Glimcher L. H., et al. 2012. Silencing of lipid metabolism genes through IRE1α-mediated mRNA decay lowers plasma lipids in mice. Cell Metab. 16: 487–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larach D. B., deGoma E. M., Rader D. J. 2012. Targeting high density lipoproteins in the prevention of cardiovascular disease? Curr. Cardiol. Rep. 14: 684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rowlan J. S., Li Q., Manichaikul A., Wang Q., Matsumoto A. H., Shi W. 2013. Atherosclerosis susceptibility loci identified in an extremely atherosclerosis-resistant mouse strain. J. Am. Heart Assoc. 2: e000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kozarsky K. F., Donahee M. H., Rigotti A., Iqbal S. N., Edelman E. R., Krieger M. 1997. Overexpression of the HDL receptor SR-BI alters plasma HDL and bile cholesterol levels. Nature. 387: 414–417. [DOI] [PubMed] [Google Scholar]
- 29.Fuchs M., Lammert F., Wang D. Q., Paigen B., Carey M. C., Cohen D. E. 1998. Sterol carrier protein 2 participates in hypersecretion of biliary cholesterol during gallstone formation in genetically gallstone-susceptible mice. Biochem. J. 336: 33–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ren S., Hylemon P. B., Marques D., Gurley E., Bodhan P., Hall E., Redford K., Gil G., Pandak W. M. 2004. Overexpression of cholesterol transporter StAR increases in vivo rates of bile acid synthesis in the rat and mouse. Hepatology. 40: 910–917. [DOI] [PubMed] [Google Scholar]
- 31.Wei E., Alam M., Sun F., Agellon L. B., Vance D. E., Lehner R. 2007. Apolipoprotein B and triacylglycerol secretion in human triacylglycerol hydrolase transgenic mice. J. Lipid Res. 48: 2597–2606. [DOI] [PubMed] [Google Scholar]
- 32.Turley S. D., Schwarz M., Spady D. K., Dietschy J. M. 1998. Gender-related differences in bile acid and sterol metabolism in outbred CD-1 mice fed low- and high-cholesterol diets. Hepatology. 28: 1088–1094. [DOI] [PubMed] [Google Scholar]
- 33.Erickson S. K., Lear S. R., Deane S., Dubrac S., Huling S. L., Nguyen L., Bollineni J. S., Shefer S., Hyogo H., Cohen D. E., et al. 2003. Hypercholesterolemia and changes in lipid and bile acid metabolism in male and female cyp7A1-deficient mice. J. Lipid Res. 44: 1001–1009. [DOI] [PubMed] [Google Scholar]
- 34.Charach G., Grosskopf I., Rabinovich A., Shochat M., Weintraub M., Rabinovich P. 2011. The association of bile acid excretion and atherosclerotic coronary artery disease. Therap. Adv. Gastroenterol. 4: 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simonen H., Miettinen T. A. 1987. Coronary artery disease and bile acid synthesis in familial hypercholesterolemia. Atherosclerosis. 63: 159–166. [DOI] [PubMed] [Google Scholar]
- 36.Rajaratnam R. A., Gylling H., Miettinen T. A. 2001. Cholesterol absorption, synthesis, and fecal output in postmenopausal women with and without coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 21: 1650–1655. [DOI] [PubMed] [Google Scholar]
- 37.Bie J., Zhao B., Ghosh S. 2011. Atherosclerotic lesion progression is attenuated by reconstitution with bone marrow from macrophage-specific cholesteryl ester hydrolase transgenic mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 301: R967–R974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joseph S. B., McKilligin E., Pei L., Watson M. A., Collins A. R., Laffitte B. A., Chen M., Noh G., Goodman J., Hagger G. N., et al. 2002. Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proc. Natl. Acad. Sci. USA. 99: 7604–7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schultz J. R., Tu H., Luk A., Repa J. J., Medina J. C., Li L., Schwendner S., Wang S., Thoolen M., Mangelsdorf D. J., et al. 2000. Role of LXRs in control of lipogenesis. Genes Dev. 14: 2831–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.