Abstract
Due to the prevalence of oligo- and polysaccharides on the surfaces of pathogenic organisms, carbohydrates are primary targets for recognition by antibodies generated by the immune systems of higher organisms. Consequently, substantial effort has been expended in efforts to develop vaccines based on carbohydrate epitopes. Typical approaches involve multivalent presentation of carbohydrate targets on antigenic peptides or proteins, which often involve substantial synthetic commitments and/or vaccines that are heterogeneous and difficult to characterize. We have developed a simple, liposome-based approach to generate multivalent carbohydrate vaccines, and in place of an antigenic peptide or protein, we have used a potent antigen for natural killer T cells. This vaccine, based on the Streptococcus pneumoniae serotype 14 polysaccharide, gave a response superior to that from a clinically used vaccine (Prevnar). The dependence of this response on liposome formation was demonstrated by comparison to a simple mixture of the oligosaccharide and the natural killer T cell adjuvant. The importance of the strength of the adjuvant was observed by use of a potent synthetic adjuvant and a weaker, bacterial derived glycolipid adjuvant. These results demonstrate the effectiveness of this novel and relatively simple means of generating carbohydrate-based vaccines.
Introduction
Most cells are “sugar coated,” and the carbohydrates on cell surfaces provide information about the type of cell that lies beneath.1 Oligosaccharides presented by bacteria and parasites are generally distinct from those found on host cells.2 Consequently, recognition of cell-surface carbohydrates is a useful means of distinguishing and eliminating pathogens. In addition, tumor cells are also often coated with oligosaccharides that are different from non-transformed cells and consequently may be used to identify these cells.3 Tremendous effort has been and is currently expended in development of carbohydrate-based vaccines designed to turn adaptive immune responses against cells adorned with targeted oligosaccharides.1–7 Arguably, the most successful carbohydrate vaccines developed to date target multiple serotypes of pneumococcal bacteria.8 For example, the Prevnar vaccines elicit antibodies against the oligosaccharides found on pneumococcal bacteria and provide protection against infection in the majority of vaccinated populations. Current Prevnar vaccines consist of pneumococcal polysaccharides, isolated from bacteria, conjugated to a modified diphtheria toxin. While protective antibodies to the targeted polysaccharides are generated, concern remains that young children and the elderly do not respond to the vaccines as well as adults.9
Antibody responses originate in B cells through relatively weak interactions between carbohydrates and IgM on the cell surface. Class switching to IgG and affinity maturation are required for development of high affinity antibodies and long-term memory responses. For these processes to occur some degree of multivalency is required to promote aggregation of IgM on B cell surfaces and activated T cells must interact with B cells to provide the necessary “help” for class switching.1,6,7 Consequently, in carbohydrate vaccine design, multivalent presentation of the targeted oligosaccharides is often a design component. In addition, targeted oligosaccharides are typically conjugated to an antigenic peptide or protein. The peptide or protein is processed by antigen-presenting cells and presented to T cells, which in turn are activated and provide “help” to B cells.
The design approach of covalently attaching multivalent oligosaccharides to antigenic proteins or peptides has been widely used with varied levels of success.1,6,7 However, there are some drawbacks in preparing vaccines of this type. Polysaccharides isolated from biological sources are difficult to purify, and conjugation to a peptide or protein yields molecules of high molecular weight that are difficult to characterize. Use of synthetic oligosaccharides requires lengthy pathways to append multiple oligosaccharides on peptides or other scaffolds to achieve multivalency.
We have developed a novel approach to oligosaccharide-based vaccines that eliminates the need for covalent attachment to a peptide or protein for multivalent antigen presentation. In fact, this method requires no peptide or protein antigen and requires only a monomeric form of the targeted oligosaccharide. We reasoned that self-assembly of lipid-appended oligosaccharides in the outer leaflet of a liposome would provide the necessary multivalency for B cell stimulation. And rather than elicit responses through peptide recognition from conventional T cells, we targeted a subset of T cells, termed natural killer T cells (NKT cells), which respond to glycolipid presentation. An advantage of using glycolipid stimulation of NKT cells for B cell “help” is that the NKT cell antigen can be incorporated into the lipid bilayer of the same liposome presenting the oligosaccharide vaccine. Thus, both the vaccine and NKT cell adjuvant self assemble in a liposome allowing B cells to recognize the targeted oligosaccharide while presenting the NKT cell antigen.
We recently reported that this approach to vaccine development generates high titers of high-affinity IgG antibodies and memory responses targeting an oligosaccharide from Streptococcus pneumoniae (serotype 14) and that NKT cell responses are essential for class switching and affinity maturation.10 We have further characterized this approach by comparing antibody responses from this vaccine and commercially available Prevnar in mice. We have demonstrated the importance of organization of the target oligosaccharide and the NKT cell adjuvant into a liposome, as compared to a simple mixture, and shown the necessity of using a strong NKT cell adjuvant in providing the T cell help required for class switching.
Results and Discussion
The structures of the capsular polysaccharides from multiple serotypes of pneumococcal bacteria are known,11 and these polysaccharides are produced by pneumococci in repeating oligosaccharide units (for example, see the repeating tetrasaccharide from S. pneumoniae in Figure 1). Prevnar is made from S. pneumoniae polysaccharides; however, it has been demonstrated that individual oligosaccharide subunits are sufficient epitopes for generation of opsonophagocytic antibodies.12 Thus, simpler, synthetically-derived oligosaccharides can be used for vaccination against pneumococci.
Figure 1.
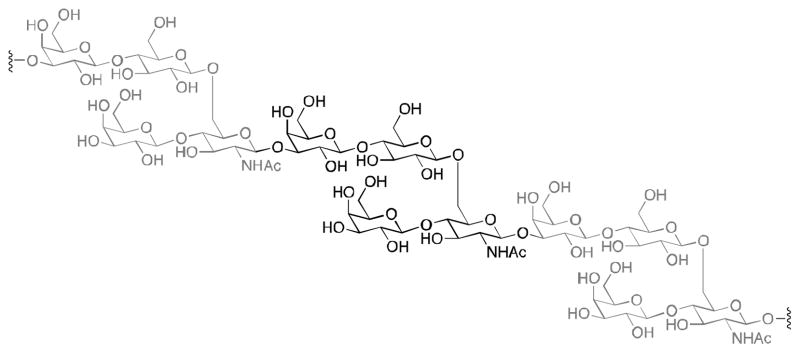
Structure of the repeating tetrasaccharide from S. pneumoniae serotype 14.
As a proof of concept of our vaccine design strategy, we elected to use the tetra-saccharide repeating unit from the polysaccharide from pneumococcal serotype 14. Covalent attachment of the oligosaccharide to diacylthioglycerol, to give PBS150 (Figure 2), allows the compound to stably assemble in liposomes. Furthermore, with multiple oligosaccharides adorning the surface of the liposome, the multivalency necessary for B cell activation is achieved.
Figure 2.
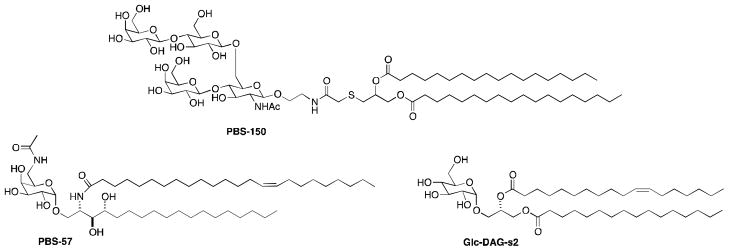
Structures of NKT cell agonists PBS57 and Glc-DAG-s2 and lipidated tetrasaccharide PBS150.
Glycosylceramide PBS57 (Figure 2) was developed as a highly potent stimulator of NKT cells.13 It readily loads into the antigen presentation protein CD1d and stimulates cytokine release from NKT cells in vivo at concentrations below 1 ng/mouse. We recently showed that NKT cells stimulated by PBS57 are effective in providing the T-cell help to B cells necessary for antibody class switching (from IgM to IgG).10 As described above, in the past, T cell help has been triggered through peptide recognition by T cells, and glycolipid-stimulated T cell help for class switching represents a new paradigm in vaccine development. Kinjo, et al.14 recently described glucosyldiacylglycerols produced by S. pneumoniae that stimulate NKT cells. These NKT cell antigens accompany capsular polysaccharides in S. pneumoniae, we investigated the ability of the most potent of these, glc-DAG-s2 (Figure 2), to stimulate NKT cells and provide B cell help.
Oligosaccharides from pneumococcal serotype 14 have been synthesized,15,16 but published procedures are lengthy and were designed to generate the tetrasaccharide and higher order oligomers. Consequently, we developed our own synthesis of the tetrasaccharide appropriately derivatized for attachment to thiodiacylglycerol (Scheme 1). Beginning with protected glucosamine 1,17 a glycosidic bond was formed with 2-bromoethanol, and the bromide was then displaced with azide. Protecting group manipulation gave 3 and then 4, which was suitable for coupling with 5.18 Reaction of disaccharides 6 and 7 gave tetrasaccharide 8 in good yield. A sequence of deprotection, acylation and deprotection steps yielded 9. Reduction of the azide and coupling with 10 gave PBS150.
Scheme 1.
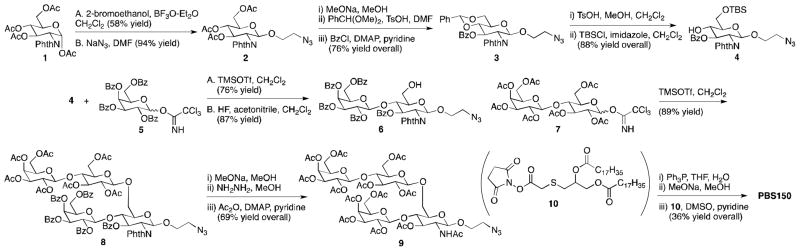
Synthesis of PBS150.
The lipid tether (10) for the tetrasaccharide was prepared by reaction of para-methoxybenzyl alcohol (PMBOH) with chloroacetic anhydride, followed by reaction with 1-thioglycerol yielding 11 (Scheme 2). Ester formation with stearoyl chloride was followed by selective cleavage of the PMB ester and ester formation with N-hydroxysuccinimide, giving 10.
Scheme 2.
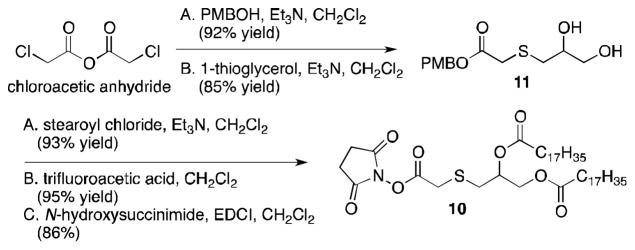
Synthesis of 10.
To provide multivalency of the tetrasaccharide in PBS150, the compound was incorporated into liposomes prepared with dioleoylphosphatidylcholine (DOPC) and cholesterol using well-established methods.19 To provide NKT cell stimulation, PBS57 was also incorporated into the liposomes constituting the vaccine. The molar ratio of DOPC, cholesterol, PBS150 and PBS57 were 35, 40, 20 and 5, respectively.
As described above, Prevnar vaccines have proven effective in generating specific antibodies to pneumococcal oligosaccharides. As a comparator for the liposomal vaccine containing PBS150 and PBS57, the vaccine Prevnar13 was used. And to ensure that comparable amounts of antigen were administered the amount of oligosaccharide antigen (serotype 14) in the liposomal vaccine was matched to that found in the Prevnar vaccine.
The liposomal vaccine was designed to provide multivalency with PBS150 and the NKT cell agonist, PBS57, in the same particles. To establish the importance of liposome formulation and PBS150 multivalency, we also prepared a vaccine comprised of PBS150 and PBS57 as a simple mixture. As with the liposomal vaccine, the amount of oligosaccharide was matched with that found in the Prevnar vaccine. The same amount of PBS57 was used in the two glycolipid vaccines.
It was expected that IgM levels specific for the targeted oligosaccharide would increase substantially after vaccination with all three vaccines. However, the more important gauge of success would be generation of IgG as a consequence of class switching. An additional gauge would be if substantial IgG generation could be achieved through a single vaccination rather than multiple injections.
C57BL/6 mice were vaccinated intraperitoneally with each of the three vaccines (200 μL volume) on day 0 and a second vaccination was administered on day 57 (Figure 3). Due to solubility and viscosity constraints with the Prevnar vaccine (containing alum), it was necessary to inject the vaccines in relatively large volumes. Consequently, intraperitoneal rather than intramuscular administration was used. Antibody counts and isotypes were measured on a bi-weekly basis. As was expected, all three vaccines caused comparable and substantial production of IgM, observed at week 2, and this level of production was consistent through the end of the experiment (25 weeks). However, the amounts of IgG produced in response to the three vaccine classes were distinct. Vaccination with Prevnar13 triggered class switching from IgM to IgG at a significant level (p-value < 0.05) after nine weeks and administration of the second dose of vaccine at week eight. Vaccination with the PBS57 + PBS150 mix achieved significance at week eight, before administration of the second dose of the vaccine. In contrast, PBS57/PBS150-containing liposomes caused substantial class switching with a single dose of vaccine. At two weeks, a statistically significant amount of IgG was produced, and by six weeks substantial antibody levels were observed in all test animals (p-value of 0.00024).
Figure 3.
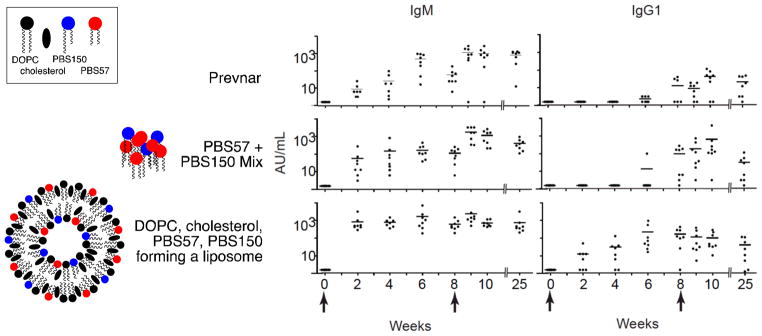
Antibody (IgM and IgG1) formation in response to vaccination (intraperitoneal injection on days 0 and 57) with Prevnar1320 (2.2 μg of each polysaccharide) or with PBS57+PBS150 as a simple mixture (4 μg of PBS150 and 1 μg of PBS57) or in liposome formulation, as indicated. Each injection contained an equivalent amount of pneumococcal polysaccharide serotype 14. Sera were collected at indicated intervals to measure IgM and IgG1 antibody titers against S. pneumoniae serotype 14. Each dot represents the serum titer of an individual mouse at the indicated time. Statistical analysis: * p-value < 0.05; *** p-value < 0.001. The first time periods at which p-values below 0.05 or 0.001 are indicated.
Pneumococcal bacteria display antigenic oligosaccharides in multivalent form and produce NKT cell antigens, such as Glc-DAG-s2. Thus, these bacteria are similar to the PBS57/PBS150 liposomal vaccine (i.e., they present the oligosaccharide epitope in multivalent form and provide an NKT cell antigen). NKT cell antigens produced by pneumococcal bacteria (e.g., Glc-DAG-s2) are much weaker than alpha-galactosylceramides (e.g., PBS57);14 nevertheless, we reasoned that even a weak NKT cell antigen might be a sufficient adjuvant to contribute to an effective vaccine.
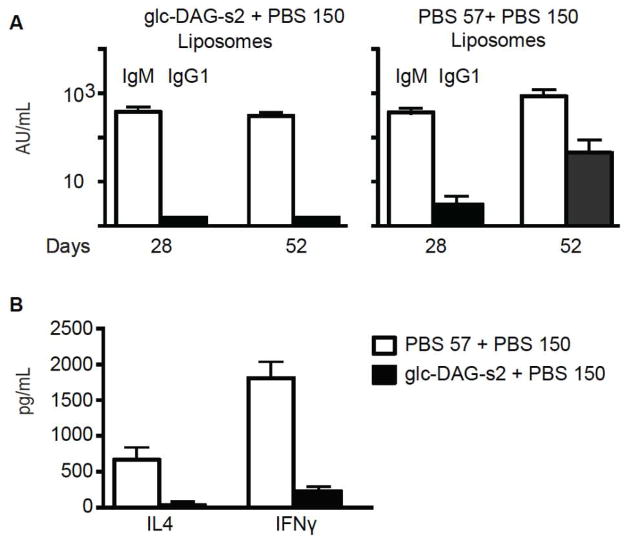
To test this hypothesis, we prepared liposomes containing PBS150 and Glc-DAG-s2 in the same proportions described for the PBS57/PBS150 liposomes. Mice were vaccinated with both types of liposomes, and antibody responses were quantified. Both types of liposome gave strong IgM responses (Figure 4A). However, only vaccination with the liposomes containing PBS57 caused class switching to IgG. To better understand why Glc-DAG-s2/PBS150 liposomes did not lead to class switching, we measured cytokine release in response to the two liposomal vaccines. Though dosed at the same level, PBS57-containing liposomes caused substantially higher levels of cytokine release (both IL-4 and IFN-γ) than Glc-DAG-s2-containing liposomes (Figure 4B).
Figure 4.
Comparative adjuvant effects of PBS57 and Glc-DAG-s2. Mice were injected intraperitoneally with liposomes carrying PBS57 + PBS150 or Glc-DAG-s2 + PBS150, as indicated, at days 0 and 38. A: anti-S. pneumoniae serotype 14 antibody IgM and IgG1 titers measured 4 weeks after the first injection (day 28) and 2 weeks after the boost (day 52), as indicated. Data compiled from a total of 8 mice immunized with PBS57 and 8 mice immunized with Glc-DAG-s2. B: IL4 and IFNγ cytokines (pg/mL) released in the serum of a total of 7 mice injected with PBS57 and 11 mice injected with Glc-DAG-s2, as indicated.
Conclusions
Vaccination with a liposomal formulation of the oligosaccharide epitope in PBS150 and the NKT cell antigen PBS57 results in generation of high titers of IgG. The importance of the liposomal formulation is evidenced by weaker responses to a vaccine comprised of the same amounts of PBS150 and PBS57 in a non-liposomal form. Comparison of IgG production in response to vaccination with liposomes containing either PBS57 or Glc-DAG-s2, both with PBS150, demonstrates the importance of a strong NKT cell antigen in the vaccine.
Notably, substantial IgG is produced after a single vaccination with PBS57/PBS150 liposomes. After a second vaccination with either Prevnar13 or with the simple PBS57/PBS150 mixture, comparable titers of IgG for the targeted epitope were generated. The recommended dosing of Prevnar requires four vaccinations,21 which may cause challenges for those in rural areas or in the developing world. A shorter dosing regimen would be a substantial improvement in vaccines designed to prevent pneumococcal diseases. The liposomal vaccine described herein is prepared using well-characterized target epitopes, and an adjuvant that activates a well-defined subset of T cells. It provides a proof of concept for a simple means of generating protective antibody responses to carbohydrate epitopes, and it is anticipated that it can be expanded to include epitopes for other pneumococcal serotypes as well as those for other pathogens.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant P01AI053725 (to A.B., L.T., and P.B.S.), Major State Basic Research Development Program of China (973 Program 2012CB825806), National Natural Sciences Foundation of China 31271430, Fundamental Research Funds for Central Universities and NNCAS-2011-5 (to L.B.), and the University of Chicago Digestive Diseases Research Core Center (P30 DK42086). A.B. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Electronic Supplementary Information (ESI) available: Experimental details for synthesis of PBS150, vaccination and antibody and cytokine quantification. See DOI: 10.1039/b000000x/
Notes and references
- 1.Morelli L, Poletti L, Lay L. Eur J Org Chem. 2011:5723–5777. [Google Scholar]
- 2.Pozsgay V. Curr Topic Med Chem. 2008;8:126–140. doi: 10.2174/156802608783378864. [DOI] [PubMed] [Google Scholar]
- 3.Heimburg-Molinaro J, Lum M, Vijay G, Jain M, Almogren A, Rittenhouse-Olson K. Vaccine. 2011;29:8802–8826. doi: 10.1016/j.vaccine.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daum RS, Spellberg B. Clin Infect Dis. 2012;54:560–567. doi: 10.1093/cid/cir828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joshi VS, Bajaj IB, Survase SA, Singhal RS, Kennedy JF. Carbo Poly. 2009;75:553–565. [Google Scholar]
- 6.Peri F. Chem Soc Rev. 2013;42:4543–4556. doi: 10.1039/c2cs35422e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galan MC, Dumy P, Renaudet O. Chem Soc Rev. 2013;42:4599–4612. doi: 10.1039/c2cs35413f. [DOI] [PubMed] [Google Scholar]
- 8.Hausdorff WP, Dagan R, Bechers F, Schuerman L. Vaccine. 2009;27:7257–7269. doi: 10.1016/j.vaccine.2009.09.111. [DOI] [PubMed] [Google Scholar]
- 9.González-Fernández A, Faro J, Fernández C. Vaccine. 2008;26:292–300. doi: 10.1016/j.vaccine.2007.11.042. [DOI] [PubMed] [Google Scholar]
- 10.Bai L, Deng S, Reboulet R, Matthew R, Teyton L, Savage PB, Bendelac A. Proc Nat Acad Sci USA. 2013;110:16097–16102. doi: 10.1073/pnas.1303218110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ovodov YS. Biochem (Moscow) 2006;71:937–954. doi: 10.1134/s000629790609001x. [DOI] [PubMed] [Google Scholar]
- 12.Safari D, Dekker HAT, Joosten JA, Michalik D, Carvalho de Souza A, Adamo R, Lahmann M, Sundgren A, Oscarson S, Kamerling JP, Snippe H. Infect Immun. 2008;76:4615–4623. doi: 10.1128/IAI.00472-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y, Goff RD, Zhou D, Mattner J, Sullivan BA, Khurana A, Cantu C, Altman JD, Teyton L, Bendelac A, Savage PB. J Immun Methods. 2006;312:34–39. doi: 10.1016/j.jim.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 14.Kinjo Y, Illarionov P, Vela JL, Pei B, Gerardi E, Li X, Li Y, Imamura M, Kaneko Y, Okawara A, Miyazaki Y, Gómez-Velasco A, Rogers P, Dahesh S, Uchiyama S, Khurana A, Kawahara K, Yesilkaya H, Andrew PW, Wong CW, Kawakami K, Nizet V, Besra GS, Tsuji M, Zajonic DM, Kronenberg M. Nat Immunol. 2011;12:966–974. doi: 10.1038/ni.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joosten JAF, Kamerling JP, Vliegenthart JFG. Carb Res. 2003;338:2611–2627. doi: 10.1016/s0008-6215(03)00313-6. [DOI] [PubMed] [Google Scholar]
- 16.Sundgren A, Lahmann M. J Carb Chem. 2005;24:379–391. [Google Scholar]
- 17.Kochetkov NK, Byramova NE, Tsvetkov YE, Backinovskii LV. Tetrahedron. 1985;41:3363–3375. [Google Scholar]
- 18.Zhu X, Schmidt RR. In: Handbook of Chemical Glycosylation. Demchenko AV, editor. Wiley-VCH; Weinheim, Germany: 2008. pp. 143–185. [Google Scholar]
- 19.Szoka F, Jr, Papahadjopoulos D. Ann Rev Biophys Bioeng. 1980;9:467–508. doi: 10.1146/annurev.bb.09.060180.002343. [DOI] [PubMed] [Google Scholar]
- 20.Bryan KA, Block SL, Baker SA, Gruber WC, Scott DA. Pediatrics. 2010;125:866–875. doi: 10.1542/peds.2009-1405. [DOI] [PubMed] [Google Scholar]
- 21.Sucher AJ, Chahine EB, Nelson M, Sucher BJ. Ann Pharmacother. 2011;45:1516–1524. doi: 10.1345/aph.1Q347. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.