Abstract
The timing of a child's first acute lower respiratory infection (ALRI) is important, because the younger a child is when he or she experiences ALRI, the greater the risk of death. Indoor exposure to particulate matter less than or equal to 2.5 µm in diameter (PM2.5) has been associated with increased frequency of ALRI, but little is known about how it may affect the timing of a child's first ALRI. In this study, we aimed to estimate the association between a child's age at first ALRI and indoor exposure to PM2.5 in a low-income community in Dhaka, Bangladesh. We followed 257 children from birth through age 2 years to record their age at first ALRI. Between May 2009 and April 2010, we also measured indoor concentrations of PM2.5 in children's homes. We used generalized gamma distribution models to estimate the relative age at first ALRI associated with the mean number of hours in which PM2.5 concentrations exceeded 100 µg/m3. Each hour in which PM2.5 levels exceeded 100 µg/m3 was independently associated with a 12% decrease (95% confidence interval: 2, 21; P = 0.021) in age at first ALRI. Interventions to reduce indoor exposure to PM2.5 could increase the ages at which children experience their first ALRI in this urban community.
Keywords: acute lower respiratory infection, Bangladesh, child health, particulate matter, survival analysis
Acute lower respiratory infection (ALRI) is the leading cause of death in Bangladeshi children (1), and 30%–50% of all children under 2 years of age suffer from ALRI each year (2, 3). The greatest burden of child death in Bangladesh is among infants, who are at increased risk of death from ALRI compared with older children. From 2002 to 2006, the estimated mortality rate among children under age 5 years in Bangladesh was 65 per 1,000 livebirths, but 90% of the burden (61 per 1,000) occurred among children under 12 months of age and 37% (37 per 1,000) occurred in neonates (4). Forty-eight percent of all deaths among children aged more than 29 days but less than 1 year were caused by acute respiratory infection in 2004, but only 17% of deaths were precipitated by acute respiratory infection among children aged 1–4 years (5).
The age at which a child develops ALRI has been shown to be an important determinant of severity of ALRI and death from ALRI. In a California study, Izurieta et al. (6) reported that rates of hospitalization for pneumonia, which is a marker of severity, were 2–10 times higher for infants than for children aged 1–4 years, depending on the season. Another study from rural Indonesia suggested that children under 4 months of age with severe pneumonia were 5.6 times more likely to die from their illness than older children (7). One review of pneumonia mortality rates showed that the case fatality ratio among infants was 15–19 times higher than that among 1- to 4-year-olds (8). Most epidemiologic studies on ALRI have investigated exposures associated with increased frequency of ALRI but not the timing of the first ALRI. Efforts to identify modifiable risk factors associated with younger age at first ALRI could lead to interventions to reduce severity of and risk of death from ALRI.
Children in both urban and rural Bangladeshi households are exposed to concentrations of particulate matter inside their homes many times higher than the World Health Organization recommended guideline of <25 μg/m3 (9–12). Indoor exposure to particulate matter has been consistently associated with increased risk of lower respiratory infection in children (13–17). One study from urban Bangladesh showed that infants, in particular, were at increased risk of ALRI from indoor exposure to particulate matter less than or equal to 2.5 µm in diameter (PM2.5) (14), but there are no data available on how exposure to PM2.5 may affect the timing of a child's first ALRI. Our objective in this analysis was to estimate the association between indoor PM2.5 concentration and age at first ALRI among children under 2 years of age in a low-income, urban cohort in Dhaka, Bangladesh.
METHODS
Enrollment in the birth cohort
Between January 2008 and March 2009, investigators at the International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,B) enrolled a cohort of newborns in Mirpur, a densely populated, low-income community in Dhaka (18), to study the etiology of childhood infections and cognitive development (19). The study area comprised approximately 3 km2 with a population of approximately 19,000 persons. All pregnant women in the study area were identified through house-to-house visits, and all women who were planning to reside there for the next 2 years were invited to participate in the study. In exchange for participation, mothers were offered free primary medical care for their children from the study clinic. Mothers of all children enrolled in this birth cohort who were still participating in the study during April 2009 were invited to participate in our study examining the relationship between indoor air pollution and acute respiratory infection (10, 14).
Child follow-up and surveillance for ALRI
A detailed description of the methods used for children's enrollment and follow-up has been published elsewhere (14). Trained research assistants used standard protocols to measure children's weight and length within 72 hours of birth and at approximately 3 and 6 months of age. All enrolled children were visited every 3–4 days by trained community health workers for the duration of the study to identify signs and symptoms of illness. Children were referred to the study clinic if they experienced 1 major sign of illness (subjective fever; rapid, labored, or noisy breathing; inability to eat or drink; convulsion; cyanosis) or 2 minor signs (cough; rhinorrhea; sore throat; muscle or joint pain; chills; headache; irritability; repeated vomiting) on the day of the visit (2). Study children lived within approximately 2 km of the study clinic. Pediatricians at the study clinic recorded the symptoms reported by the mother or caretaker and physically examined the children.
An incident of ALRI was defined as an acute respiratory illness observed by the study physician that included either cough or difficult breathing and either age-specific tachypnea or physician-observed chest in-drawing, per criteria proposed by the World Health Organization (20). Tachypnea was defined as a measurement of ≥60 breaths/minute for children aged <2 months, ≥50 breaths/minute for children aged 2–11 months, and ≥40 breaths/minute for children aged 12–23 months (21).
Measurement of household risk factors and indoor particulate matter levels
All children still participating in the birth cohort during April 2009 were eligible to participate in our study of particulate matter exposure and incidence of ALRI. One child was enrolled per household. Every child was visited during May 2009, and characteristics of the household were recorded on a structured questionnaire, as previously described (14). We recorded the type of stove and fuel used in the home, indoor cigarette smoking, the number of people residing in the house, and measures of education and household wealth.
The yearly average concentrations of fine particulate matter in the child's sleeping space were measured. Typically, the place where the child slept and spent the vast majority of his or her time was also the primary living space for the family. Details on the methods used for measurement of indoor particulate matter concentrations in these households have been published elsewhere (10, 14). In brief, once per month from May 2009 through April 2010, a particulate matter air monitor manufactured by the Berkeley Air Monitoring Group (University of California, Berkeley, California) (10, 22, 23) was placed on the wall approximately 2 feet (0.6 m) above the child's sleeping space. The monitor logged the average PM2.5 concentration in the preceding 60 seconds once per minute for a 24-hour period, following the manufacturer's instructions. Children who had at least 11 monthly measurements, with at least 1,300 minute readings each, were included in the analysis.
Statistical analyses
We used descriptive statistics to characterize study children and their households. The particulate matter monitors we used had a minimum limit of detection of 50 μg/m3 (24). Therefore, we used threshold metrics to summarize PM2.5 concentration measurements from each child's sleeping space. Specifically, we calculated the mean number of hours for which the PM2.5 concentration exceeded 100 μg/m3 (daily hours >100 μg/m3) over the year in which PM2.5 concentrations were measured. Although daily hours of a PM2.5 level greater than 100 μg/m3 is not a threshold known to be associated with illness, we chose this threshold because it represented twice the limit of detection of the monitors and 4 times the World Health Organization guidelines for mean daily indoor-air particulate matter concentrations (25 μg/m3) (9). We also calculated the mean number of hours in which PM2.5 exceeded thresholds of 50 μg/m3 and 250 μg/m3 (5 times the limit of detection) and the time-weighted mean of PM2.5 for sensitivity analyses.
The time origin for the survival analysis was birth, the time axis was the age (in months) of the child, and events were defined as the first ALRI. Children were censored from the analysis after their first ALRI or when they reached their second birthday. First, we fitted Kaplan-Meier curves (25) to graph the proportion of children who had not yet experienced their first ALRI over time. To explore whether children's ages at first development of ALRI differed by particulate matter exposure, we classified children as being exposed either above the median PM2.5 concentration (>100 µg/m3 for ≥5.3 hours/day) or below the median PM2.5 concentration (>100 µg/m3 for <5.3 hours/day) and fitted Kaplan-Meier curves (25) for each group. We recorded the signs and symptoms children experienced during their first ALRI.
Our primary interest was to quantify the relative time to first ALRI associated with PM2.5 exposure rather than the relative hazard. We therefore used the generalized gamma distribution, which is a parametric approach that yields relative times as the measure of association (26). The time axis represented the age of the child, so the relative time measure of association in this model was equivalent to the relative age of children at the first ALRI. An added benefit of using the generalized gamma distribution is that the method does not assume that the ratio of hazards remains constant over time (26). This model was implemented using the streg command in Stata 10 (StataCorp LP, College Station, Texas) (26).
We first estimated the association between exposures and children's ages at first ALRI in bivariate analyses. We primarily investigated the relationship between the number of hours in which PM2.5 levels exceeded 100 μg/m3 and first ALRI. Then, we constructed a multivariate model using hours of PM2.5 >100 μg/m3 as the exposure variable and adjusted for potential confounders between the risk of ALRI and particulate matter exposure as described in the meta-analysis by Dherani et al. (27). The potential confounders we considered included socioeconomic status, mother's education, household crowding, malnutrition, and duration of breastfeeding. Ownership of both a television and a cell phone was used as an indicator of household wealth. We created a dichotomous variable for mother's education to indicate whether or not the mother had more than an elementary school education. We used the indicator of household wealth and mother's education as proxies for socioeconomic status. Crowding was represented in the model by the number of persons per square meter of household floor space. Children's mean weight-for-age z scores observed at birth, 3 months of age, and 6 months of age and the duration of breastfeeding (in months) were included in the model as linear variables. Children who weighed less than 2,500 g when they were enrolled in the study within 72 hours of birth were classified as being low birth weight (28).
Exposure to indoor tobacco smoke and vaccination status were also proposed as possible confounders of the relationship between indoor particulate matter exposure and ALRI (27). Exposure to indoor cigarette smoking was not included in our multivariate model because we considered indoor tobacco smoke to be a contributor to our measurement of PM2.5, not a confounder. We did not include vaccination status in the model because coverage for measles, diphtheria, and pertussis vaccines was greater than or equal to 95% among cohort children (Rashidul Haque, ICDDR,B, personal communication, 2011). At the time this study was conducted, neither influenza virus nor pneumococcal vaccine was included in the immunization schedule or available commercially in the local market. Haemophilus influenzae type b vaccines were not included in the immunization schedule but were commercially available in the study community for approximately US$7 per dose (Nadira Sultana Kakoly, ICDDR,B, personal communication, 2011). However, we did not collect information about H. influenzae type b vaccination status from study children.
Sensitivity analyses
To explore the sensitivity of our results to the threshold used in the analysis (mean hours of PM2.5 levels >100 μg/m3), we conducted the same multivariate analyses using alternative thresholds (50 μg/m3 and 250 μg/m3). In addition, the same analysis was performed using the time-weighted average of PM2.5 concentrations.
Human subject considerations
Prior to enrollment and data collection, mothers provided separate informed consent for their children's participation in the birth cohort and in the substudy on indoor exposure to particulate matter. The study protocol was reviewed and approved by institutional review boards at the ICDDR,B (Dhaka, Bangladesh); the University of Virginia (Charlottesville, Virginia); Johns Hopkins University (Baltimore, Maryland); and the US Centers for Disease Control and Prevention (Atlanta, Georgia).
RESULTS
A total of 265 children were enrolled in the birth cohort through April 2009. Three children had left the study by April, so 262 children were eligible for the air-quality monitoring study and were enrolled. Complete baseline information and PM2.5 measurements were available for 257 (98%) of the 262 children enrolled in the air monitoring study, and these children were included in the analysis (Table 1). Cohort children were between the ages of 1 week and 16 months (median, 9 months) when the air-quality monitoring began. Children were breastfed for a median of 30 months, and 36% had low birth weight (i.e., <2,500 g). The study children lived in crowded conditions, with a median of 1.8 m2 of floor space per person. Six percent of the children's families burned only biomass (including wood, bamboo, and paper) for cooking, but 52% occasionally burned biomass when their natural gas or electricity supply was interrupted. The median time-weighted average PM2.5 concentration in the children's sleeping spaces was 127 μg/m3 (interquartile range, 88–194). PM2.5 concentrations were over 100 μg/m3 for a median of 5.3 hours per day (interquartile range, 4.0–6.9) (Table 1). Results of additional analyses showing the seasonal distribution and primary determinants of PM2.5 concentrations in these children's homes are reported elsewhere (10).
Table 1.
Baseline Characteristics of Children Enrolled in the Mirpur Birth Cohort (n = 257) and Their Households, Dhaka, Bangladesh, 2008–2011
| Characteristic | No. | % | Median | Interquartile Rangea |
|---|---|---|---|---|
| Child or Mother | ||||
| Male sex | 137 | 53 | ||
| Weighed <2,500 g within 72 hours of birth | 93 | 36 | ||
| Mean weight-for-age z score from birth to age 6 monthsb,c | −1.4 | −1.9 to −0.8 | ||
| Duration of breastfeeding, months | 30 | 23–35 | ||
| Duration of exclusive breastfeeding, months | 4 | 2–6 | ||
| Experienced ALRI during the first 2 years of life | 169 | 66 | ||
| Age at first ALRI, months | 8 | 3 to >24 | ||
| Highest level of formal education completed by mother | ||||
| No formal education | 92 | 36 | ||
| Elementary school | 92 | 36 | ||
| Middle school | 69 | 27 | ||
| High school | 4 | 2 | ||
| Household | ||||
| No. of household members | 5 | 4–6 | ||
| Living space floor area, m2 | 9.6 | 7.8–11.3 | ||
| No. of people per m2 of floor space | 1.8 | 1.4–2.4 | ||
| No. of external windows and doors in the home | 2 | 1–3 | ||
| Ownership of a cell phone | 173 | 67 | ||
| Ownership of a television | 160 | 62 | ||
| Cookstove located inside the home | 84 | 33 | ||
| Type of cooking fuel used in the home | ||||
| Only clean-burning fuels | 107 | 42 | ||
| Only biomass fuels | 16 | 6 | ||
| Primarily clean fuels but sometimes biomass | 134 | 52 | ||
| Usually burning kerosene in the home for any purpose | 119 | 46 | ||
| Tobacco smoking inside the home | 72 | 28 | ||
| No. of particulate matter measurements per household | 12 | 11–12 | ||
| Daily duration of PM2.5 concentrations >100 µg/m3, hours | 5.3 | 4.0–6.9 | ||
| Time-weighted mean PM2.5 concentration, µg/m3 b | 127 | 88–194 | ||
Abbreviations: ALRI, acute lower respiratory infection; PM2.5, particulate matter less than or equal to 2.5 µm in diameter.
a 25th–75th percentiles.
b The mean value was calculated for each individual; the median value for the entire cohort is presented.
c Normal range for z scores.
Of the 257 children, 169 (66%) experienced their first ALRI before 2 years of age. Ten percent of the children experienced an ALRI by 1.7 months of age, 25% by 3.3 months of age, and 50% by 8.5 months of age (Figure 1). Ninety-three percent (157/169) of the children had physician-observed tachypnea during their first ALRI, and 52% (88/169) had chest in-drawing (Table 2). No hospitalizations or deaths from ALRI were observed among cohort study children.
Figure 1.
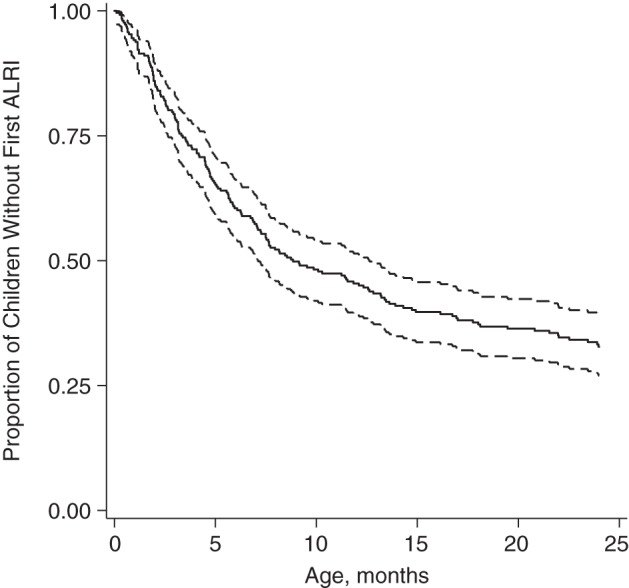
Proportion of children who had not yet experienced an acute lower respiratory infection (ALRI) through 2 years of age in the Mirpur Birth Cohort (n = 257), Dhaka, Bangladesh, 2008–2011. Dashed lines show the 95% confidence interval.
Table 2.
Signs and Symptoms Experienced by Children in the Mirpur Birth Cohort During Their First Acute Lower Respiratory Infection (n = 169), Dhaka, Bangladesh, 2008–2011
| Sign or Symptom | No. | % |
|---|---|---|
| Total | 169 | 100 |
| Mother-reported: | ||
| Cough | 168 | 99 |
| Fever | 157 | 93 |
| Difficulty breathing | 97 | 57 |
| Reluctance/inability to eat | 50 | 30 |
| Physician-observed: | ||
| Tachypneaa | 157 | 93 |
| Crepitations | 124 | 73 |
| Chest in-drawing | 88 | 52 |
a Defined by age: ≥60 breaths/minute for children aged <2 months, ≥50 breaths/minute for children aged 2–11 months, and ≥40 breaths/minute for children aged 12–23 months.
Children exposed to PM2.5 concentrations above 100 µg/m3 for 5.3 or more hours per day (the median value) experienced their first ALRI at a median of 3.8 months of age. For children exposed to concentrations above 100 µg/m3 for less than 5.3 hours per day, the median age at first ALRI was 8.5 months (Figure 2). In bivariate analyses, each 1-hour increase in the number of hours for which mean PM2.5 concentrations exceeded 100 µg/m3 was associated with a 13% decrease in children's age at first ALRI (95% confidence interval (95% CI): 4, 21; P = 0.005) (Table 3). Children who lived in households with both a television and a cell phone were 60% older (95% CI: 5, 142; P = 0.028) when they first experienced ALRI than children whose families did not have these assets. In multivariate analysis, each hour that PM2.5 concentrations exceeded 100 µg/m3 was associated with a 12% decrease (95% CI: 2, 21; P = 0.021) in children's age at first ALRI, after adjustment for potential confounders. Indoor PM2.5 concentration was the only exposure that was independently associated with children's age at first ALRI (Table 3). Sensitivity analyses showed similar estimates of relative age at first ALRI, regardless of the PM2.5 exposure metric used (see Web Tables 1–3, available at http://aje.oxfordjournals.org/).
Figure 2.
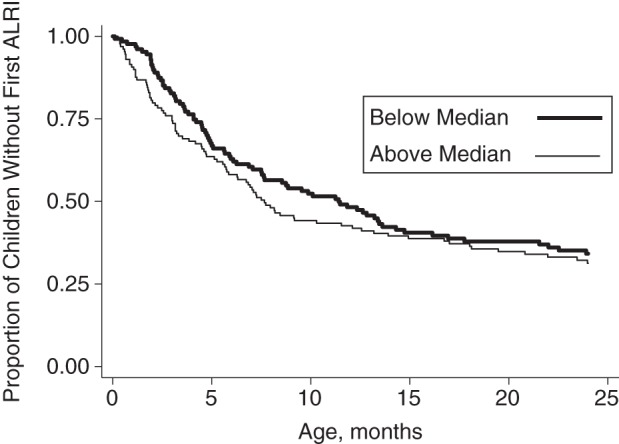
Proportions of children above (>100 µg/m3 for ≥5.3 hours/day) and below (>100 µg/m3 for <5.3 hours/day) the median level of exposure to particulate matter less than or equal to 2.5 µm in diameter who had not yet experienced an acute lower respiratory infection (ALRI) through 2 years of age in the Mirpur Birth Cohort (n = 257), Dhaka, Bangladesh, 2008–2011.
Table 3.
Crude and Adjusted Relative Ages at First Acute Lower Respiratory Infection According to Child and Household Characteristics (Generalized Gamma Distribution Models) in the Mirpur Birth Cohort (n = 257), Dhaka, Bangladesh, 2008–2011
| Child or Household Characteristic and Increment of Change | Relative Age at First ALRI |
|||||
|---|---|---|---|---|---|---|
| Crude Age, months | 95% CI | P Value | Adjusted Age, months | 95% CI | P Value | |
| Weighed <2,500 g within 72 hours of birth (yes/no) | 0.67 | 0.44, 1.04 | 0.076 | 0.71 | 0.42, 1.19 | 0.192 |
| Male sex (yes/no) | 0.69 | 0.45, 1.04 | 0.097 | 0.70 | 0.47, 1.06 | 0.093 |
| 1-unit increase in mean weight-for-age z score | 1.17 | 0.92, 1.50 | 0.203 | 0.99 | 0.74, 1.32 | 0.935 |
| Each additional month of breastfeeding | 1.01 | 0.98, 1.03 | 0.617 | 1.01 | 0.99, 1.03 | 0.406 |
| Each additional person per m2 | 1.01 | 0.80, 1.28 | 0.939 | 0.91 | 0.27, 2.23 | 0.460 |
| Mother had more than elementary school education (yes/no) | 1.38 | 0.86, 2.23 | 0.185 | 1.03 | 0.63, 1.68 | 0.902 |
| Ownership of both a cell phone and a television (yes/no) | 1.60 | 1.05, 2.42 | 0.028 | 1.32 | 0.85, 2.03 | 0.213 |
| At least 1 household member smoked cigarettes indoors (yes/no) | 0.81 | 0.51, 1.29 | 0.384 | |||
| 1-hour increase in indoor PM2.5 concentrations >100 µg/m3 | 0.87 | 0.79, 0.96 | 0.005 | 0.88 | 0.79, 0.98 | 0.021 |
| Additional model parameters | ||||||
| Scale | −0.40 | −0.96, 0.15 | ||||
| Shape | 1.61 | 1.43, 1.82 | ||||
Abbreviations: ALRI, acute lower respiratory infection; CI, confidence interval; PM2.5, particulate matter less than or equal to 2.5 µm in diameter.
DISCUSSION
Half of all children in this study experienced their first ALRI by 8 months of age, and each hour that PM2.5 concentrations exceeded 100 µg/m3 was associated with a 12% decrease in a child's age at first ALRI. These results suggest that reducing indoor exposure to PM2.5 in this low-income urban setting could increase the average age at which children experienced their first ALRI and therefore could decrease the severity of these infections (6–8).
A randomized controlled trial of the introduction of improved cookstoves to reduce indoor exposure to particulate matter in Guatemala found that the intervention was associated with a decrease in the incidence of severe pneumonia episodes but not in the incidence of all pneumonia (29). Our study findings provide one possible explanation for the results of the Guatemala study: The reduction in PM2.5 exposure from the intervention may have increased the age at which children first experienced ALRI, reducing the severity but not the incidence of all episodes. Our study findings are limited by our measurements of PM2.5 exposure. Children were enrolled as they were born into the community from January 2008 through April 2009, but our measurements of particulate matter did not begin until May 2009. Therefore, our exposure measurements did not temporally overlap with the time at which most children experienced their first ALRI. Rather than a time-varying measure of exposure to PM2.5 in the home, our measurements were combined into a crude, yearly average metric of PM2.5 concentrations in children's homes. Nevertheless, we observed a strong association between PM2.5 exposure and age at first ALRI, and since we do not suspect that our misclassification of children's exposure was related to our measurement of age at first ALRI, the magnitude of the true effect may be higher than what we report (30). In addition, the threshold cutoff we used (100 µg/m3) was arbitrary; however, it was preferable to time-weighted average measurements because of the lower limit of detection of our monitors. Despite these limitations in our measurements, they are probably more useful than other proxy measurements of PM2.5 exposure in this community, considering that PM2.5 concentrations were high in these households, even for homes that used electric or natural gas cookstoves. Previous analyses suggested that sources external to the household, such as smoke from neighbors' homes or ambient pollution (10, 31), could be contributing to exposure in these homes.
We defined ALRI according to criteria suggested by the World Health Organization, based on clinical assessments conducted by study physicians when children appeared at the study clinic for care. This definition is useful because it means that our results are comparable to those of other studies in low-income settings where these clinical criteria are most frequently used. Children in our cohort were visited frequently in their homes to identify the onset of respiratory symptoms and were quickly referred to free, high-quality medical care at the nearby study clinic. This study design is a strength for measuring age at first ALRI, but since children received care so quickly, their clinical illnesses were probably less severe than would have occurred without this intervention. One important indicator of disease severity is hospitalization, but children in our cohort with relatively more severe disease may not have received care at hospitals because they had access to free care nearby. In addition, physicians may have been more comfortable treating children on an outpatient basis, rather than referring them to hospitals, since they knew that the children would be followed up closely at home after the clinic visit. Thus, one important limitation of this study is that we were unable to investigate severity of ALRI, particularly hospitalization, as an outcome. Some children who were referred to the clinic may not have actually visited the study clinic; although study staff reported that this was rare, we did not routinely collect data on how many children's mothers did not comply with our referrals. We provided free health care to all study children and the study clinic was near their homes, so this probably reduced the possibility that some mothers would comply with referrals more than others.
Our study suggests that increased exposure to PM2.5 in this community puts children at risk for developing ALRI at a younger age. Therefore, interventions to reduce indoor air pollution, particularly for neonates and young infants, could be effective in increasing the age at which children experience their first ALRI in this and similar communities. This could, in turn, reduce the severity of first ALRI in the age group most likely to die from these infections (5, 7, 8). Our study findings are probably generalizable to other settings where exposure to PM2.5 has been associated with increased risk of ALRI among young children. Efforts to reduce reliance on biomass burning may improve indoor air quality in many households, but interventions to reduce particulate matter exposure in households that use cleaner-burning fuels also deserve further investigation.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Centre for Communicable Diseases, International Centre for Diarrhoeal Disease Research, Bangladesh, Dhaka, Bangladesh (Emily S. Gurley, Nusrat Homaira, Rashidul Haque, Stephen P. Luby, Eduardo Azziz-Baumgartner); Department of Epidemiology, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland (Emily S. Gurley, Henrik Salje, William J. Moss); Department of Environmental Health, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland (Patrick Breysse); Department of Social and Preventive Medicine, School of Public Health and Health Professions, University at Buffalo, State University of New York, Buffalo, New York (Pavani K. Ram); Departments of Microbiology and Pathology, School of Medicine, University of Virginia, Charlottesville, Virginia (William A. Petri, Jr.); Influenza Division, Centers for Disease Control and Prevention, US Department of Health and Human Services, Atlanta, Georgia (Joseph Bresee, Eduardo Azziz-Baumgartner); and Global Disease Detection Branch, Centers for Disease Control and Prevention, US Department of Health and Human Services, Atlanta, Georgia (Stephen P. Luby).
This study was funded by the US Centers for Disease Control and Prevention (grant U01/CI000628-02) and the US National Institutes of Health (grant 5R01 AI043596).
The ICDDR,B acknowledges with gratitude the commitment of the Centers for Disease Control and Prevention and the National Institutes of Health to its research efforts. We appreciate the efforts of study clinic staff and field-workers and the statistical assistance of Jaynal Abedin and Yushuf Sharker.
Conflict of interest: none declared.
REFERENCES
- 1.Black RE, Cousens S, Johnson HL, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375(9730):1969–1987. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 2.Brooks WA, Goswami D, Rahman M, et al. Influenza is a major contributor to childhood pneumonia in a tropical developing country. Pediatr Infect Dis J. 2010;29(3):216–221. doi: 10.1097/INF.0b013e3181bc23fd. [DOI] [PubMed] [Google Scholar]
- 3.Zaman K, Baqui AH, Yunus M, et al. Acute respiratory infections in children: a community-based longitudinal study in rural Bangladesh. J Trop Pediatr. 1997;43(3):133–137. doi: 10.1093/tropej/43.3.133. [DOI] [PubMed] [Google Scholar]
- 4.El Arifeen S. Child health and mortality. J Health Popul Nutr. 2008;26(3):273–279. [PMC free article] [PubMed] [Google Scholar]
- 5.National Institute of Population Research and Training; Mitra and Associates; and Macro International. Bangladesh Demographic and Health Survey 2004. Dhaka, Bangladesh, Calverton, MD: National Institute of Population Research and Training; Mitra and Associates; and Macro International; 2005. [Google Scholar]
- 6.Izurieta HS, Thompson WW, Kramarz P, et al. Influenza and the rates of hospitalization for respiratory disease among infants and young children. N Engl J Med. 2000;342(4):232–239. doi: 10.1056/NEJM200001273420402. [DOI] [PubMed] [Google Scholar]
- 7.Djelantik IG, Gessner BD, Sutanto A, et al. Case fatality proportions and predictive factors for mortality among children hospitalized with severe pneumonia in a rural developing country setting. J Trop Pediatr. 2003;49(6):327–332. doi: 10.1093/tropej/49.6.327. [DOI] [PubMed] [Google Scholar]
- 8.Graham NM. The epidemiology of acute respiratory infections in children and adults: a global perspective. Epidemiol Rev. 1990;12:149–178. doi: 10.1093/oxfordjournals.epirev.a036050. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. WHO Air Quality Guidelines for Particulate Matter, Ozone, Nitrogen Dioxide and Sulfur Dioxide: Global Update 2005. Summary of Risk Assessment. Geneva, Switzerland: World Health Organization; 2006. [Google Scholar]
- 10.Gurley ES, Salje H, Homaira N, et al. Seasonal concentrations and determinants of indoor particulate matter in a low-income community in Dhaka, Bangladesh. Environ Res. 2013;121:11–16. doi: 10.1016/j.envres.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dasgupta S, Huq M, Khaliquzzaman M, et al. Indoor air quality for poor families: new evidence from Bangladesh. Indoor Air. 2006;16(6):426–444. doi: 10.1111/j.1600-0668.2006.00436.x. [DOI] [PubMed] [Google Scholar]
- 12.Dasgupta S, Huq M, Khaliquzzaman M, et al. Who suffers from indoor air pollution? Evidence from Bangladesh. Health Policy Plan. 2006;21(6):444–458. doi: 10.1093/heapol/czl027. [DOI] [PubMed] [Google Scholar]
- 13.Campbell H. Indoor air pollution and acute lower respiratory infections in young Gambian children. Health Bull (Edinb) 1997;55(1):20–31. [PubMed] [Google Scholar]
- 14.Gurley ES, Homaira N, Salje H, et al. Indoor exposure to particulate matter and the incidence of acute lower respiratory infections among children: a birth cohort study in urban Bangladesh. Indoor Air. 2013;23(5):379–386. doi: 10.1111/ina.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith KR, Samet JM, Romieu I, et al. Indoor air pollution in developing countries and acute lower respiratory infections in children. Thorax. 2000;55(6):518–532. doi: 10.1136/thorax.55.6.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ezzati M, Kammen D. Indoor air pollution from biomass combustion and acute respiratory infections in Kenya: an exposure-response study. Lancet. 2001;358(9282):619–624. doi: 10.1016/s0140-6736(01)05777-4. [DOI] [PubMed] [Google Scholar]
- 17.Bautista LE, Correa A, Baumgartner J, et al. Indoor charcoal smoke and acute respiratory infections in young children in the Dominican Republic. Am J Epidemiol. 2009;169(5):572–580. doi: 10.1093/aje/kwn372. [DOI] [PubMed] [Google Scholar]
- 18.Haque R, Ali IM, Sack RB, et al. Amebiasis and mucosal IgA antibody against the Entamoeba histolytica adherence lectin in Bangladeshi children. J Infect Dis. 2001;183(12):1787–1793. doi: 10.1086/320740. [DOI] [PubMed] [Google Scholar]
- 19.Homaira N, Luby SP, Petri WA, et al. Incidence of respiratory virus-associated pneumonia in urban poor young children of Dhaka, Bangladesh, 2009–2011. PLoS One. 2012;7(2):e32056. doi: 10.1371/journal.pone.0032056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. Management of the Child With a Serious Infection of Severe Malnutrition: Guidelines for Care at the First-Referral Level in Developing Countries. Geneva, Switzerland: Department of Child and Adolescent Health and Development, United Nations Children's Fund; 2000. [Google Scholar]
- 21.Taylor JA, Del Beccaro M, Done S, et al. Establishing clinically relevant standards for tachypnea in febrile children younger than 2 years. Arch Pediatr Adolesc Med. 1995;149(3):283–287. doi: 10.1001/archpedi.1995.02170150063011. [DOI] [PubMed] [Google Scholar]
- 22.Smith KR, Dutta K, Chengappa C, et al. Monitoring and evaluation of improved biomass cookstove programs for indoor air quality and stove performance: conclusions from the Household Energy and Health Project. Energy Sustain Dev. 2007;11(2):5–18. [Google Scholar]
- 23.Chowdhury Z, Edwards RD, Johnson M, et al. An inexpensive light-scattering particle monitor: field validation. J Environ Monit. 2007;9(10):1099–1106. doi: 10.1039/b709329m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edwards R, Smith KR, Kirby B, et al. An inexpensive dual-chamber particle monitor: laboratory characterization. J Air Waste Manag Assoc. 2006;56(6):789–799. doi: 10.1080/10473289.2006.10464491. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- 26.Cox C, Chu H, Schneider MF, et al. Parametric survival analysis and taxonomy of hazard functions for the generalized gamma distribution. Stat Med. 2007;26(23):4352–4374. doi: 10.1002/sim.2836. [DOI] [PubMed] [Google Scholar]
- 27.Dherani M, Pope D, Mascarenhas M, et al. Indoor air pollution from unprocessed solid fuel use and pneumonia risk in children aged under five years: a systematic review and meta-analysis. Bull World Health Organ. 2008;86(5):390–398. doi: 10.2471/BLT.07.044529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.United Nations Children's Fund and World Health Organization. Low Birthweight: Country, Regional and Global Estimates. New York, NY: United Nations Children's Fund; 2004. [Google Scholar]
- 29.Smith KR, McCracken J, Weber M, et al. Effect of reduction in household air pollution on childhood pneumonia in Guatemala (RESPIRE): a randomised controlled trial. Lancet. 2011;378(9804):1717–1726. doi: 10.1016/S0140-6736(11)60921-5. [DOI] [PubMed] [Google Scholar]
- 30.Jurek AM, Greenland S, Maldonado G, et al. Proper interpretation of non-differential misclassification effects: expectations vs observations. Int J Epidemiol. 2005;34(3):680–687. doi: 10.1093/ije/dyi060. [DOI] [PubMed] [Google Scholar]
- 31.Salje H, Gurley ES, Homaira N, et al. Impact of neighborhood biomass cooking patterns on episodic high indoor particulate matter concentrations in clean fuel homes in Dhaka, Bangladesh [published online ahead of print September 23, 2013] Indoor Air. 2013 doi: 10.1111/ina.12065. ( doi:10.1111/ina.12065) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


