Abstract
The aging process in the hippocampus is associated with aberrant epigenetic marks, such as DNA methylation and histone tail alterations. Recent evidence suggests that caloric restriction (CR) can potentially delay the aging process, while upregulation of antioxidants may also have a beneficial effect in this respect. We have recently observed that CR attenuates age-related changes in the levels of the epigenetic molecules DNA methyltransferase 3a, 5-methylcytidine (5-mC) and 5-hydroxymethylcytosine in the mouse hippocampus while overexpression of the antioxidant Cu/Zn superoxide dismutase 1 (SOD1) does not. However, the impact of aging on the levels of histone-modifying enzymes such as histone deacetylase 2 (HDAC2) in the hippocampus has not been studied in much detail. Here, we investigated immunoreactivity (IR) of HDAC2 in three subregions of the hippocampus (dentate gyrus, CA3 and CA1-2) of mice taken from large cohorts of aging wild-type and transgenic mice overexpressing normal human SOD1, which were kept under normal diet or CR from weaning onwards. Independent from the genotype, aging (between 12 and 24 months) increased levels of HDAC2 IR in the hippocampus. Moreover, CR prevented this age-related increase, particularly in the CA3 and CA1-2 subregions, while SOD1 overexpression did not. Quantitative image analyses showed that HDAC2 IR correlated positively with 5-mC IR while these markers were shown to colocalize in the nucleus of hippocampal cells. Together with recent literature reports, these findings suggest that altered levels of epigenetic regulatory proteins including HDAC2 regulate age-related changes in the mouse hippocampus and that CR may prevent these age-related changes.
Keywords: Aging, epigenesis, histone deacetylase 2 (HDAC2), caloric restriction, hippocampus
INTRODUCTION
Several molecular and morphological alterations have been observed in the aging brain and have been associated with cognitive impairment and an increased risk for neurodegenerative disorders [1–4]. Among others, age-associated changes in gene expression involve downregulation of synaptic genes and genes encoding neurotrophins as well as upregulation of genes mediating the immune response [5–7]. Dysfunctional epigenetic regulation of gene expression, especially through DNA (hydroxy)methylation and histone modifications, have recently been implicated with such changes [8]. For example, we have recently reported age-related increases in hippocampal immunoreactivity (IR) of DNA methyltransferase 3a [9], the DNA methylation marker 5-methylcytidine (5-mC) [10] and the DNA hydroxymethylation marker 5-hydroxymethylcytosine (5-hmC) [11].
While caloric restriction (CR) has been shown to increase life span and delay the onset of age-related neurodegeneration in various species [12–16], upregulation of endogenous antioxidant activity is believed to act beneficially by reducing oxidative damage [17–20]. We tested the effects of CR and overexpression of the normal human antioxidant Cu/Zn superoxide dismutase 1 (SOD1) in large cohorts of aging mice (the same mice used in the present study) on the observed age-related changes in hippocampal levels of Dnmt3a, 5mC and 5-hmC and found that they all could be prevented by CR, but not by SOD1 overexpression.
Next to DNA methylation and hydroxymethylation changes, histone tail modifications encompass a wide range of variation in epigenetic regulation. In particular, histone acetylation, mediated by histone acetyltransferases (HATs), and histone deacetylation, mediated by histone deacetylases (HDACs), represent key processes in the regulation of gene expression [8, 21]. Several HDACs, particularly HDAC2, have been implicated in physiological and pathological aging of the brain [22, 23]. HDAC2 belongs to the class I of histone deacetylases that are responsible for the removal of acetyl groups from histone tails, generally promoting transcriptional repression [21]. HDAC2 was recently identified as a negative regulator of memory and synaptic plasticity while age-related memory impairment has been linked with histone acetylation deregulation [22–26]. Moreover, HDAC2 was shown to decrease acetylation and block the expression of genes related to learning and memory, while HDAC2 knockdown reversed synaptic deficits and associated cognitive impairment in the Ck-p25 mouse model of neurodegeneration [27]. In support of this notion, various other studies in rodents have revealed the potentially protective effects of HDAC inhibitors (HDACi) against age-related cognitive impairment and various types of neurodegenerative disorders [25, 28–31]. To date, the exact role of HDAC2 in brain aging is far from being completely understood. Furthermore, it is unknown whether altered levels and/or function of HDAC2 could mediate the beneficial effects of protective strategies such as CR.
Using the same experimental design as in our previous work [9–11] the present study investigated hippocampal IR of HDAC2 in the dentate gyrus (DG), CA3, and CA1-2 of the mouse hippocampus. The aim of the present study was to examine i) the regional and cellular localization of HDAC2 in the hippocampus, ii) the effects of aging, CR and overexpression of SOD1 on the levels of HDAC2 in the hippocampus, and iii) to investigate whether altered levels of HDAC2 IR in the hippocampus correlate with altered levels of 5-mC IR, another marker of DNA methylation that we have investigated in a separate study [10].
MATERIALS AND METHODS
Animals
A total of 48 male mice were used for the present study. These mice were derived from a cohort of 240 aging male mice that consisted of four groups based on genotype and diet: 1) WT (C57Bl6J) mice on CD (WT-CD), 2) SOD1 mice on CD (SOD-CD), 3) WT mice on CR (WT-CR) and SOD1 mice on CR (SOD-CR). Per group, 6 mice were euthanized at 12 and 24 months of age, thus yielding a total of 8 groups of 6 mice each for histological analyses.
Detailed descriptions of the transgenic mice generation, preparation and composition of the diets and weight and survival curves have been reported previously [9, 11, 32]. Briefly, the transgenic mice were generated on a C57Bl6J background and were carrying 7 copies of the entire human SOD1 sequence, inserted into chromosome 3, resulting in an increased enzyme activity in the brain and other tissues [33]. In order to fully monitor the caloric intake, the CD covered approximately 85% of the ad libitum consumption, while the calorie restricted diet entailed a 50% reduction in calories. The animals were housed individually, with ad libitum access to water, on 12/12 hours light/ dark circle kept under standard temperature, humidity, and specified pathogen free (SPF) conditions. All experiments were approved by the Animals Ethics Board of Maastricht University.
Tissue Processing
Procedures regarding brain processing have been described in detail previously [9, 32]. In brief, mice were transcardially perfused with tyrode solution and two fixative solutions (4% parafolmaldehyde, 0.9% NaCl, 1% acetic acid and 8% parafolmaldehyde, 0.9% NaCl, 1% acetic acid). Brains were removed and postfixed for 24 hours in 8% parafolmaldehyde (at 4° C), hemisected along the midsagittal line, cryoprotected in sucrose solutions (10, 20, and 30% sucrose in Tris-HCl buffer, 2×12 hours per solution at 4° C) and embedded in Tissue Tek® (Sakura Finetec Europe, Zoeterwoude, The Netherlands). For the present study only the left brain halves were cut serially in 30 µm-thick free-floating coronal sections on a cryostat (type HM 500 OMV, Microm, Walldorf, Germany). For this study, parallel 1:10 series of sections were used and stored for further histological processing [9, 11, 32].
Immunohistochemical Detection of HDAC2
Using previously described protocols one series of sections was stained [10]. A rabbit monoclonal anti-HDAC2 (dilution 1:5000, ab32117, Abcam, Cambridge, UK) was used as a primary and a biotinylated donkey anti-rabbit (dilution 1:200, Jackson Westgrove, PA, USA) as a secondary antibody. The specificity of the commercially available anti-HDAC2 primary antibody has been confirmed previously using short interfering RNA (siRNA) inhibition [34]. In particular this antibody recognizes residues in the C-terminal of the HDAC2 protein.
Double immunofluorescence of HDAC2 and 5-mC was performed using the rabbit monoclonal anti-HDAC2 (dilution 1:5000, Abcam, Cambridge, UK) and a mouse monoclonal anti-5-mC (dilution 1:500; GenWay Biotech, San Diego, CA, USA) as primary antibodies. A donkey anti-rabbit Alexa 488 (dilution 1:200, Invitrogen, Eugene, Oregon, USA) and a donkey anti-mouse biotin (dilution 1:200, Jackson Westgrove, PA, USA) coupled with streptavidin Alexa 594 (dilution 1:400, Invitrogen, Eugene, Oregon, USA) were used for detection. In addition, the sections were counterstained with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI; Sigma Aldrich, Zwijndrecht, The Netherlands).
Analysis of HDAC2 IR
Mean intensity (inversed gray values) and surface area of HDAC2 IR were analyzed as described previously [9–11]. As indicated in (Fig. 1), two images from the CA1-2 region, two images from the CA3 pyramidal layer, and four images from the granule cell layer of the DG were taken at four different bregma levels (−1.58, −1.82, −3.40, −3.52), according to a mouse brain atlas [35], using a 40× objective. Thus, a total of 32 images were taken for every animal, with a digital camera (F-view; Olympus, Tokyo, Japan) connected to an Olympus AX70 brightfield microscope (analySIS; Imaging System, Münster, Germany). The ImageJ software program (version 1.42q, Wayne Rasband, National Institutes of Health, Bestheda, Maryland, USA), was used for the calculation of the mean intensities and surface areas. The mean intensities were calculated in arbitrary units and therefore the differences between groups in these units do not reflect the differences in actual levels of HDAC2 protein. Surface area measurements were corrected for previously reported volume reductions by age and CR [9, 32]. Surface area measurements were corrected by means of multiplying the volume of each subregion of each animal with the corresponding surface area measurement. Intensity measurements are independent of volume or cell number differences and therefore no adjustments were applied.
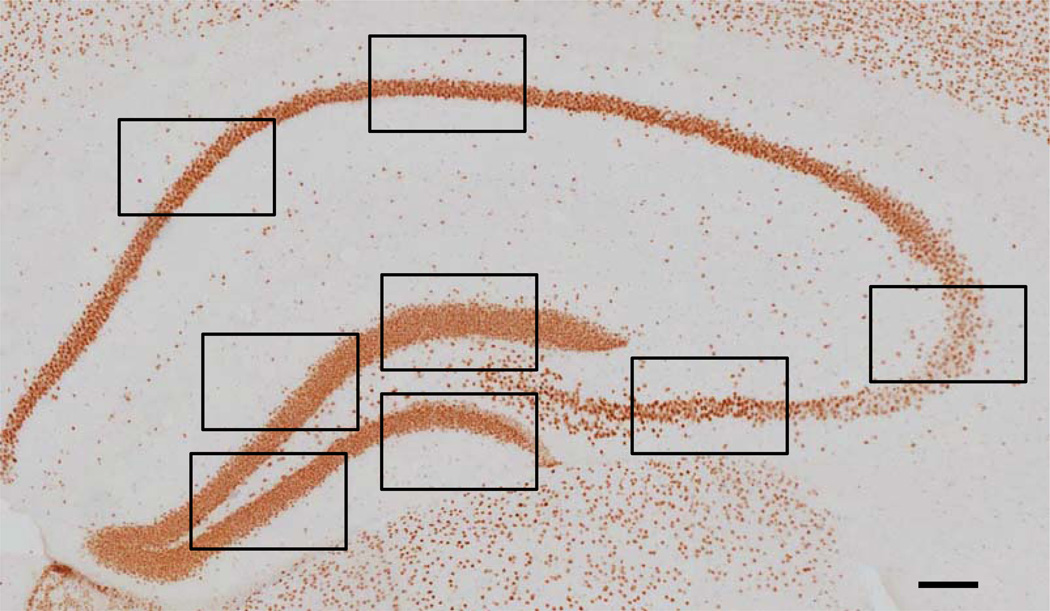
Fig. (1).
Hippocampal HDAC2 IR. Representative image of a hippocampal section stained for HDAC2 (bregma level −1.58). The black boxes indicate where the high-magnification images were taken for the analysis. A total of 32 images per animal (i.e. four images in the dentate gyrus, two in the CA3 and two in the CA1-2 regions, at four different bregma levels) were taken for the analysis (see text for more details). Scale bar = 100 µm.
Imaging of HDAC2, 5-mC, and DAPI
Imaging of the triple labeling of HDAC2, 5-mC, and DAPI (Fig. 2) was achieved by collecting 16 µm-thick image stacks consisting of 80 confocal images (0.2 µm apart), made with a 60× objective (Olympus UPlanSApo; numerical aperture [NA] = 1.40, Olympus, Tokyo, Japan) and the SI-SD system, which consists of a modified Olympus BX51 fluorescence microscope with customized spinning disk unit (DSU Olympus), computer controlled excitation and emission filterwheel, three-axis accuracy computer-controlled stepping motor specimen stage (4×4 Grid Encoded stage, LudL Electronic Products, Hawthorne, NY, USA), linear z-axis position encoder (Ludl), ultra-high sensitivity monochrome electron multiplier CCD camera (1000 × 1000 pixels, C9100-02, Hamamatsu Photonics, Hamamatsu City, Japan) and controlling software (Stereo Investigator; MBF Bioscience Williston, VT, USA). Projections and minor corrections in intensity and contrast were made with the Imaris software program (Bitplane AG, Zurich, Switzerland) [9]. Fig. (1) represents a “Virtual Slice” of the hippocampus (montage by collecting a series of contiguous images aligned, stitched and blended together) using a 20× objective (Olympus BX50 with UPlanApo objectives), a motor specimen stage (Ludl) and the Stereo Investigator software (MBF Bioscience).
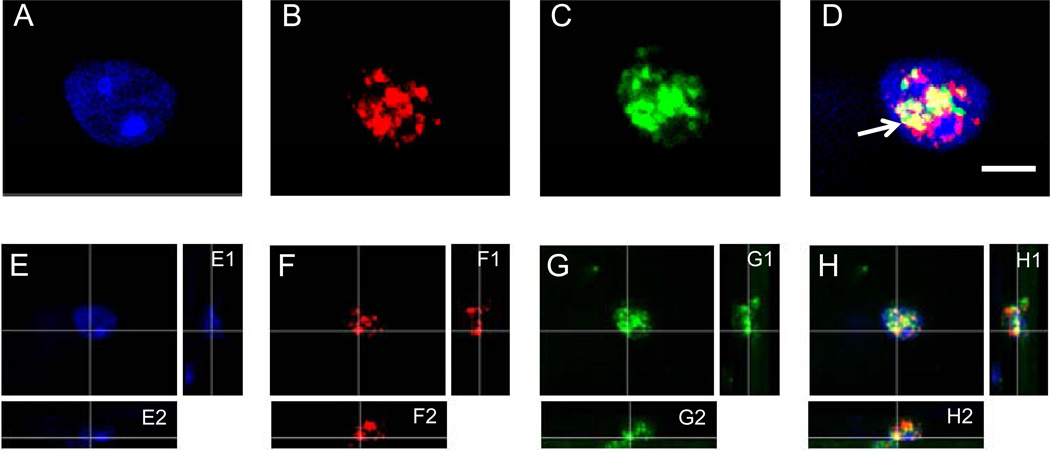
Fig. (2).
HDAC2 and 5-mC immunofluorescence, and DAPI labeling. Representative high magnification images of a nucleus of a cell in a 12-month-old mouse showing DAPI labeling (A and E) and fluorescence labeling of 5-methylcytidine (5-mC) (red) in B and F, and HDAC2 (green) in C and G, at the corresponding location and focal plane, with merged pictures in D and H. All images represent one stack taken with the SI-SD system (see text for more details). Note the white arrow indicates the colocalization between HDAC2 and 5-mC (yellow). Images E–H show a three-dimensional reconstruction of a representative image stack. The crossing lines indicate the position within the X–Y view at which the Y–Z (E1–H1) and X–Z (E2–H2) views were generated. Scale bar = 10 µm.
Statistical Analysis
All data are presented as mean and standard error of the mean (SEM). The general linear model univariate analysis of variance was used for comparisons between groups, controlling for the main and interactive effects of age, genotype and diet (three-way ANOVA). Statistical significance was set at α = 0.05. Pair-wise comparisons were performed with a Bonferroni post-hoc test. Correlation analyses between HDAC2 and 5-mC intensity measurements, that have previously been reported [10], were carried out by calculating the Pearson’s correlation coefficient (rp). The Statistical Package for the Social Sciences, (SPSS 17, SPSS Inc., Chicago, IL, USA) was used for all the statistical calculations. Graphs were built in GraphPad Prism (Version 4, GraphPad Software, San Diego, USA).
RESULTS
Qualitative Analysis of HDAC2 IR
HDAC2 IR was observed in all hippocampal subregions and particularly located in the nuclei of neurons and glial cells. Immunofluorescence labeling of HDAC2 and 5-mC showed that the two markers are colocalized (see yellow in Fig. 2D, 2H). Moreover, in accordance with previous observations, colocalization of 5-mC and DAPI was also observed, while there was no evident colocalization between HDAC2 and DAPI-dense heterochromatic DNA regions (Fig. 2).
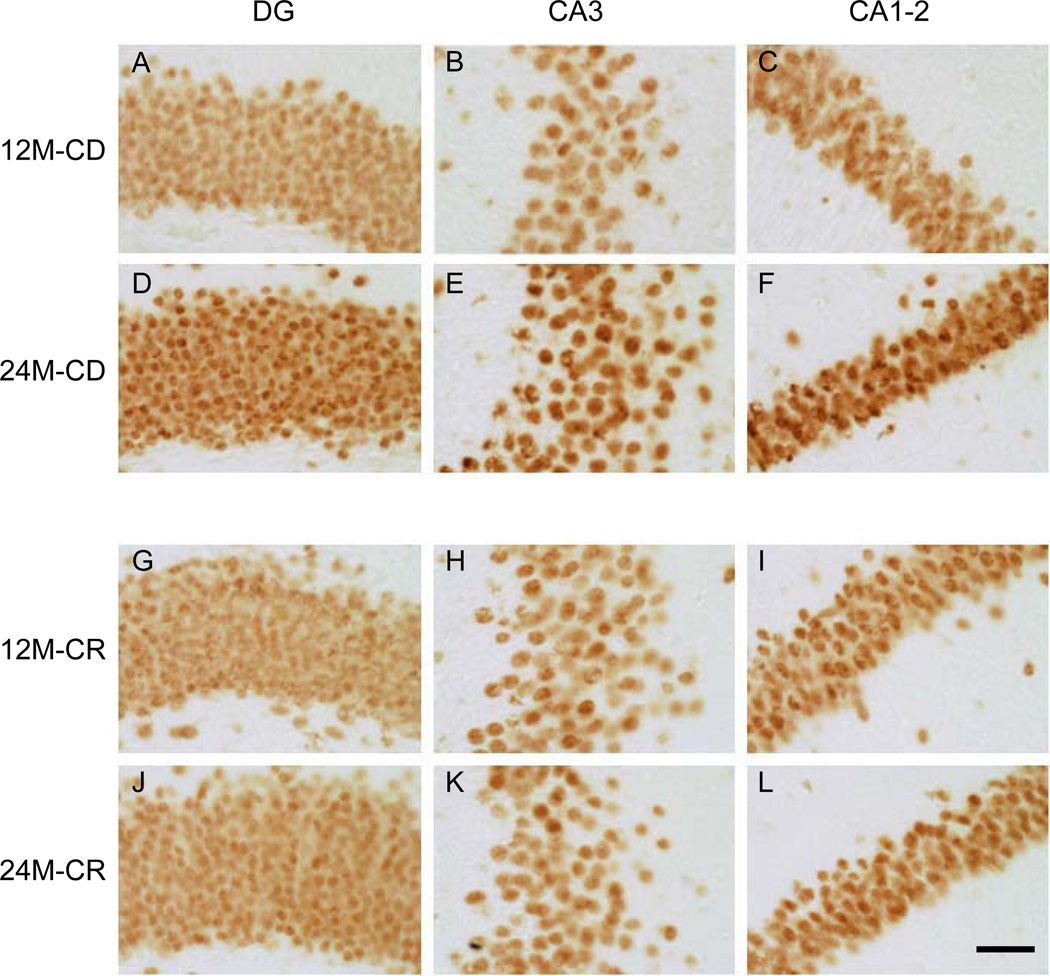
Qualitative analysis, by inspection, of HDAC2 IR suggested an increase of HDAC2 IR from 12- to 24-month-old in CD mice in the DG, CA3 and CA1-2 hippocampal subregions, which was not observed in the CR mice (Fig. 3). Image analyses were performed to quantify these observations.
Fig. (3).
Representative images of HDAC2 IR. High magnification representative images of the hippocampal regions DG, CA3, and CA1-2. A–C represent a 12-month-old mouse on CD, D– F represent a 24-month-old CD mouse. G–I, represent a 12-month-old mouse on CR, and J–L a 24-month-old CR mouse. Note: An increase in HDAC2 IR is observed from 12 to 24 months, more pronounced in CD mice, while no differences are observed in the CA3 region of 12- and 24-month-old CR mice. The images were taken with a 40× objective. Scale bar = 50 µm.
Quantitative Analysis of HDAC2 IR
HDAC2 IR Intensity
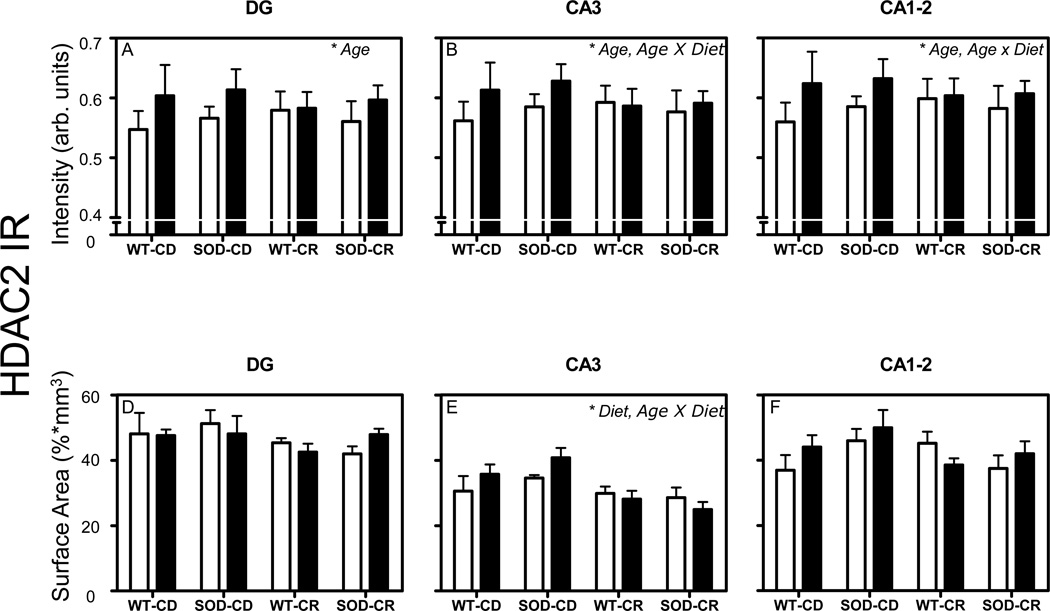
General linear model univariate analysis of variance showed an increase of HDAC2 IR intensity with aging from 12- to 24-month-old mice in all investigated regions (p = 0.001 for DG, p = 0.008 for CA3, and p = 0.001 for CA1-2; Fig. 4A–C). Furthermore, significant age × diet interactions were observed in the CA3 (p = 0.026) and CA1-2 (p = 0.045) regions (Fig. 4B–C). While no significant interaction between age and CR was found for HDAC2 IR in DG, it is interesting to note i) that 24-month-old mice fed with CR showed a similar mean value of HDAC2 IR in DG compared to 12-month-old mice fed with CR while the mean HDAC2 IR in 24-month-old mice fed with CD was higher than the mean HDAC2 IR in 12-month-old mice fed with CD, and ii) that the standard error of mean for HDAC2 IR in DG was relatively large compared to the data for CA3 and CA1-2. No significant effect of the SOD1 genotype was observed in any of the cases. In addition, post-hoc Bonferroni tests showed a significant increase of HDAC2 intensity from 12-to 24-month old WT mice (+11,2 %, p = 0.049).
Fig. (4).
HDAC2 intensity and surface area. Mean and standard error of means of mean intensity value measurements of HDAC2 IR (A–F). Pooled data from the 4 groups of 12-month-old (white bars) and 24-month-old mice (black bars) are represented separately for DG (A, D), CA3 (B, E), and CA1-2 (C, F). Volume corrections were applied for the surface area occupied by HDAC2 IR measurements. Significant effects (p < 0.05 in all cases) in each analysis are indicated with an asterisk in the top right corner of each graph. AU= arbitrary units.
Surface Area of HDAC2 IR
General linear model univariate analysis of variance of HDAC2 IR surface area revealed a significant main effect of diet (p = 0.001) and an age × diet interaction (p = 0.046) in the CA3 region only (Fig. 4E). Again, no significant effect of the SOD1 genotype was observed in any of the cases. In addition, post-hoc Bonferroni tests showed a significant decrease of HDAC2 surface area from 24-month-old SOD CD mice to 24-month-old SOD CR mice (−38, 8%, p = 0.017).
HDAC2 IR and 5-mC IR Correlation Analysis
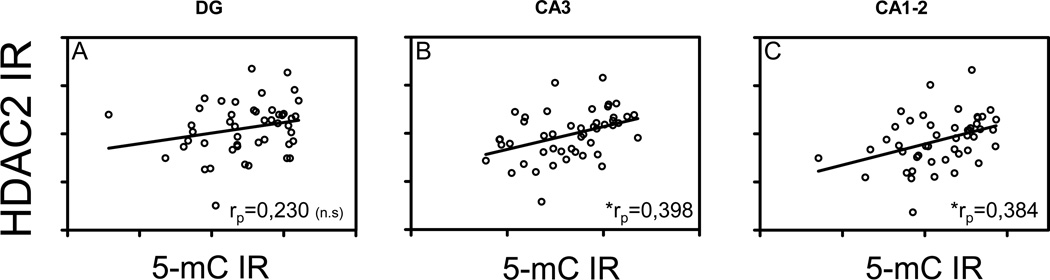
Correlation analyses between intensities in 5-mC IR and HDAC2 IR showed a positive linear correlation in the CA3: (rp = 0.398, p = 0.006) and the CA1-2 (rp = 0.384, p = 0.008) regions (Fig. 5B–C), but not in the DG (rp = 0.230, p = 0.120; Fig. 5A)
Fig. (5).
HDAC2 and 5-mC correlation analysis. Correlation analysis of HDAC2 and 5-mC IR. Significant Spearman’s correlation coefficients are noted in the bottom right of each graph.
DISCUSSION
Qualitative analysis of HDAC2 IR showed nuclear HDAC2 IR in all hippocampal subregions, and suggested an age-related increase. Quantitative analyses indicated that HDAC2 IR indeed increased with aging, in particular in mice fed with CD, but not in mice under CR. No effects of the SOD1 genotype were found. HDAC2 IR correlated positively with 5-mC IR in the CA3 and CA1-2 subregions, and nuclear colocalization of these two markers was observed. However, HDAC2 IR was also observed in nuclear compartments devoid of 5-mC IR. The present study is the first to describe age-related changes in HDAC2 levels in the mouse hippocampus, and to describe the effects of CR and SOD1 overexpression.
Age-Related Increase in HDAC2 IR
The quantitative analyses showed a significant increase in HDAC2 IR with aging from 12 to 24 months in all investigated hippocampal subregions of mice fed with CD. The mechanism behind the increased levels of HDAC2 with aging is not yet clear, but it has been reported that HDAC2 is recruited at sites of increased DNA damage as part of the DNA damage response mechanisms [36]. Because increased DNA damage is a hallmark of aging [2, 37], it is possible that increased levels of HDAC2 are a secondary response to other age-related changes such as DNA damage. Alternatively, increased levels of HDAC2 may be associated with age-related dysregulation of histone acetylation, which has been demonstrated to contribute to age-related memory impairments [22, 25]. Peleg et al. reported that age-related memory impairment of 16-month-old mice is linked with impaired hippocampal H4K12 acetylation and associated expression of learning-related genes [25]. Overexpression of HDAC2 in mice (specifically in neurons) was shown to result in decreased spine density, synaptic number, and synaptic plasticity as well as impaired memory formation, while knocking out HDAC2 resulted in opposite effects [26]. Furthermore, increased cortical and hippocampal acetylation and methylation of histones H3 and H4 has been linked with improved memory after environmental enrichment in the CK-p25 transgenic mouse model of neurodegeneration [24]. This mouse model expresses the p25 activating subunit of cyclin-dependent kinase 5 in the basal forebrain and exhibits hyperphosporylation of tau and neuronal loss [24]. Furthermore, increased HDAC2 levels have been observed in the CA1 subregion of this model, which were associated with down-regulation of expression of genes associated with memory and synaptic plasticity, such as Arc, Bdnf and GluR1 [27]. Meanwhile, short-hairpin-RNA-mediated knockdown of HDAC2 reversed the downregulation of such genes, improved synaptic plasticity and eliminated the neurodegeneration-associated cognitive decline observed in the Ck-p25 transgenic mice [27]. Increased HDAC2 levels were further observed in the hippocampus of 5XFAD transgenic mice, which carry five familial mutations associated with Alzheimer’s disease (AD), while HDAC2 levels increased in primary hippocampal neuronal cultures after exposure to AD-related neurotoxic insults, i.e. H2O2 and Aβ oligomers [27]. On the other hand, contrasting results have been reported in the human AD brain, where Graff et al. reported increased HDAC2 levels in the entorhinal cortex and hippocampus, while Mastroeni et al. reported decreased levels of HDAC2 in the entorhinal cortex, when comparing AD patients to non-demented controls [27, 38, 39]. Our findings of increased hippocampal HDAC2 with aging are in line with recent reports of increased HDAC2 levels in the hippocampus of aged (22–23 month-old) rats [40]. Zeng and colleagues further demonstrated that increased HDAC2 and decreased histone acetylation were associated with decreased expression of brain-derived neurotrophic factor (BDNF), age-associated deficits in long-term synaptic plasticity and loss of dendritic spines, while HDAC inhibition or activation of trkB receptors could ameliorate these effects. Age-related decreases in acetylation of histone H3 at lysines 9 and 14, particularly of genes related to neuronal function, has furthermore been reported in the human prefrontal cortex [41]. Moreover, an age-related decrease in acetylation of H4 has been shown in neuronal cell culture studies from wild-type mice, while the opposite pattern was observed in neurons cultured from 3xTg-AD mice [42]. Altogether, these findings suggest a causal contribution of aberrant histone acetylation and increased levels of HDAC2 to age-related changes in brain function. Clearly, such data provide evidence for the role of epigenetic mechanisms on mediating complex gene-environment interactions leading to the development of age-related neurodegeneration [43]. Evidence from the literature, such as the Latent Early-life Associated Regulation, (LEARn) model paradigm further suggest that these changes might already be primed at early developmental stages of life [44, 45].
Correlation and Colocalization of HDAC2 and 5-mC IR
Previously, our group has reported an age-related increase in levels of the DNA methylation marker 5-mC (which was prevented by CR) [10]. The observed positive correlations between the previously reported 5-mC and HDAC2 IR (as described in the present study) in the CA3 and CA1-2 suggest that the increased levels of HDAC2 might be closely connected to the increased levels of DNA methylation. The lack of correlation between 5-mC and HDAC2 IR in the DG could be explained by the high variation of HDAC2 IR in this subregion and might be associated with the presence of proliferating cells in the DG, the epigenetic programming of which might be different from the aging post-mitotic neurons [9]. The positive correlations between 5-mC and HDAC2 IR may be explained by the fact that HDACs can be recruited by DNA methylation marks and associated methyl-CpG binding proteins in order to induce chromatin remodeling and silence transcription [46, 47].
Three-dimensional image analyses showed that HDAC2 IR colocalized with 5-mC IR. However, HDAC2-IR was also localized in nuclear locations devoid of 5-mC IR. In addition, we observed that HDAC2 IR did not overlap with dense DAPI heterochromatic regions. These findings suggest that HDAC2, first as part of methyl-binding protein complexes, is associated with methylated DNA in the open euchromatin, and second can also act independent of DNA methylation marks, in order to deacetylate histone tails of active genes and induce transcriptional silencing.
Caloric Restriction Prevents Age-Related Increase of HDAC2 IR
Age-related increases of HDAC2 IR in the CA3 and CA1-2 regions were significantly attenuated by CR. 24-month-old CD mice showed a marked increase of HDAC2 IR compared to 12-month-old CD mice, while no significant age-related difference was observed for CR mice. The prevention of this age-dependent effect may be associated with the aging-retardation properties of CR and its impact on gene expression [6, 15, 32]. Moreover, the beneficial effects of CR have been linked with the upregulation of sirtuins, which belong to the class III HDACs [48–50], a finding that further supports a major role for chromatin-modifying enzymes such as HDACs in mediating the effects of CR on gene expression.
SOD1 Overexpression Does Not Affect HDAC2 IR
Transgenic overexpression of SOD1 did not have any effect on HDAC2 IR, which is in line with our previous findings on hippocampal volumes, Dnmt3a IR, 5-mC IR and 5-hmC IR [9–11, 32]. Either SOD1 overexpression does not directly affect these epigenetic marks, or, alternatively, it could be possible that the effects of SOD1 are more pronounced in other tissues than the brain. Then again, it could be speculated that the experimental design, husbandry, position effects promoters used as well as the actual levels of SOD1 over expression and other antioxidants in this model where not sufficient to induce such changes [32, 33, 51].
CONCLUSIONS
The present findings indicate that the level of HDAC2 increases in the aging mouse hippocampus while CR prevents such an effect. Considering the conservative nature of the IR analyses, the biological impact of the observed changes of HDAC2 levels should be further investigated. Additional work is required to clarify whether the activity of HDAC2 is altered during aging and affected by CR. It will also be important to investigate which specific genes are targeted by HDAC2 and whether their histone acetylation status and gene transcription are altered. As HDACi have been shown to improve age-associated memory impairment and counteract the effects of HDAC2 in mice, additional research is needed to explore the therapeutic potential of HDACi. Importantly, it is to be expected that HDAC inhibition will be accompanied by considerable side effects, given the fundamental role of HDACs in neuronal development, adult neurogenesis and DNA damage response [36, 52–54]. Evidently, more research on the role of epigenetic modifications and their moderators is of crucial importance to decipher fully the role of epigenetic dysregulation in age-associated neurodegeneration and may provide novel targets for prevention or treatment of age-related dysfunction.
ACKNOWLEDGEMENTS
Funds have been provided by the Internationale Stichting Alzheimer Onderzoek (ISAO), grant number 09552, and the Netherlands Organization for Scientific Research (NWO, Veni Award 916.11.086) to B.P.F. Rutten, by the ISAO, grant numbers 07551 and 11532, to D.L.A. van den Hove and by a Marie Curie Host Fellowship Grant MC-EST 020589 EURON to L. Chouliaras. P.R. Hof is supported by NIH grant P50 AG05138. Fig. (2) was generated with an SI-SD system (MBF Bioscience), which was obtained by NWO grant number 91106003.
Footnotes
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflicts of interest.
REFERENCES
- 1.Hof PR, Morrison JH. The aging brain: morphomolecular senescence of cortical circuits. Trends Neurosci. 2004;2710:607–613. doi: 10.1016/j.tins.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 2.Rutten BP, Schmitz C, Gerlach OH, Oyen HM, de Mesquita EB, Steinbusch HW, et al. The aging brain: accumulation of DNA damage or neuron loss? Neurobiol Aging. 2007;281:91–98. doi: 10.1016/j.neurobiolaging.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 3.Dickstein DL, Kabaso D, Rocher AB, Luebke JI, Wearne SL, Hof PR. Changes in the structural complexity of the aged brain. Aging Cell. 2007;63:275–284. doi: 10.1111/j.1474-9726.2007.00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.VanGuilder HD, Farley JA, Yan H, Van Kirk CA, Mitschelen M, Sonntag WE, et al. Hippocampal dysregulation of synaptic plasticity-associated proteins with age-related cognitive decline. Neurobiol Dis. 2011;431:201–212. doi: 10.1016/j.nbd.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berchtold NC, Cribbs DH, Coleman PD, Rogers J, Head E, Kim R, et al. Gene expression changes in the course of normal brain aging are sexually dimorphic. Proc Natl Acad Sci U S A. 2008;10540:15605–15610. doi: 10.1073/pnas.0806883105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee CK, Weindruch R, Prolla TA. Gene-expression profile of the ageing brain in mice. Nat Genet. 2000;253:294–297. doi: 10.1038/77046. [DOI] [PubMed] [Google Scholar]
- 7.Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, et al. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;4296994:883–891. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- 8.Chouliaras L, Rutten BP, Kenis G, Peerbooms O, Visser PJ, Verhey F, et al. Epigenetic regulation in the pathophysiology of Alzheimer's disease. Prog Neurobiol. 2010;904:498–510. doi: 10.1016/j.pneurobio.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Chouliaras L, van den Hove DL, Kenis G, Dela Cruz J, Lemmens MA, van Os J, et al. Caloric restriction attenuates age-related changes of DNA methyltransferase 3a in mouse hippocampus. Brain Behav Immun. 2011;254:616–623. doi: 10.1016/j.bbi.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 10.Chouliaras L, van den Hove DL, Kenis G, Keitel S, Hof PR, van Os J, et al. Prevention of age-related changes in hippocampal levels of 5-methylcytidine by caloric restriction. Neurobiol Aging. 2012;33(8):1672–1681. doi: 10.1016/j.neurobiolaging.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chouliaras L, van den Hove DL, Kenis G, Keitel S, Hof PR, Van Os J, et al. Age-related increase in levels of 5-hydroxymethylcytosine in mouse hippocampus is prevented by caloric restriction. Curr Alzheimer Res. 2012;9(5):536–544. doi: 10.2174/156720512800618035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chouliaras L, Sierksma AS, Kenis G, Prickaerts J, Lemmens MA, Brasnjevic I, et al. Gene-environment interaction research and transgenic mouse models of Alzheimer's disease. Int J Alzheimers Dis. 2010 doi: 10.4061/2010/859101. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;3255937:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halagappa VK, Guo Z, Pearson M, Matsuoka Y, Cutler RG, Laferla FM, et al. Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer's disease. Neurobiol Dis. 2007;261:212–220. doi: 10.1016/j.nbd.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 15.Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;2735271:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katewa SD, Kapahi P. Dietary restriction and aging. Aging Cell. 2009;92:105–112. doi: 10.1111/j.1474-9726.2010.00552.x. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borg J, London J. Copper/zinc superoxide dismutase overexpression promotes survival of cortical neurons exposed to neurotoxins in vitro. J Neurosci Res. 2002;702:180–189. doi: 10.1002/jnr.10404. [DOI] [PubMed] [Google Scholar]
- 18.Cadet JL, Sheng P, Ali S, Rothman R, Carlson E, Epstein C. Attenuation of methamphetamine-induced neurotoxicity in copper/zinc superoxide dismutase transgenic mice. J Neurochem. 1994;621:380–383. doi: 10.1046/j.1471-4159.1994.62010380.x. [DOI] [PubMed] [Google Scholar]
- 19.Cardozo-Pelaez F, Song S, Parthasarathy A, Epstein CJ, Sanchez-Ramos J. Attenuation of age-dependent oxidative damage to DNA and protein in brainstem of Tg Cu/Zn SOD mice. Neurobiol Aging. 1998;194:311–316. doi: 10.1016/s0197-4580(98)00067-0. [DOI] [PubMed] [Google Scholar]
- 20.Rutten BP, Steinbusch HW, Korr H, Schmitz C. Antioxidants and Alzheimer's disease: from bench to bedside (and back again) Curr Opin Clin Nutr Metab Care. 2002;56:645–651. doi: 10.1097/00075197-200211000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Kouzarides T. Chromatin modifications and their function. Cell. 2007;1284:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Stilling RM, Fischer A. The role of histone acetylation in age-associated memory impairment and Alzheimer's disease. Neurobiol Learn Mem. 2011;961:19–26. doi: 10.1016/j.nlm.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Fischer A, Sananbenesi F, Mungenast A, Tsai LH. Targeting the correct HDAC(s) to treat cognitive disorders. Trends Pharmacol Sci. 2010;3112:605–617. doi: 10.1016/j.tips.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH. Recovery of learning and memory is associated with chromatin remodelling. Nature. 2007;4477141:178–182. doi: 10.1038/nature05772. [DOI] [PubMed] [Google Scholar]
- 25.Peleg S, Sananbenesi F, Zovoilis A, Burkhardt S, Bahari-Javan S, Agis-Balboa RC, et al. Altered histone acetylation is associated with age-dependent memory impairment in mice. Science. 2010;3285979:753–756. doi: 10.1126/science.1186088. [DOI] [PubMed] [Google Scholar]
- 26.Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, Gao J, et al. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 2009;4597243:55–60. doi: 10.1038/nature07925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graff J, Rei D, Guan JS, Wang WY, Seo J, Hennig KM, et al. An epigenetic blockade of cognitive functions in the neurodegenerating brain. Nature. 2012;4837388:222–226. doi: 10.1038/nature10849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Govindarajan N, Agis-Balboa RC, Walter J, Sananbenesi F, Fischer A. Sodium Butyrate Improves Memory Function in an Alzheimer's Disease Mouse Model When Administered at an Advanced Stage of Disease Progression. J Alzheimers Dis. 2011;26(1):187–197. doi: 10.3233/JAD-2011-110080. [DOI] [PubMed] [Google Scholar]
- 29.Ricobaraza A, Cuadrado-Tejedor M, Perez-Mediavilla A, Frechilla D, Del Rio J, Garcia-Osta A. Phenylbutyrate ameliorates cognitive deficit and reduces tau pathology in an Alzheimer's disease mouse model. Neuropsychopharmacology. 2009;347:1721–1732. doi: 10.1038/npp.2008.229. [DOI] [PubMed] [Google Scholar]
- 30.Sinn DI, Kim SJ, Chu K, Jung KH, Lee ST, Song EC, et al. Valproic acid-mediated neuroprotection in intracerebral hemorrhage via histone deacetylase inhibition and transcriptional activation. Neurobiol Dis. 2007;262:464–472. doi: 10.1016/j.nbd.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 31.Petri S, Kiaei M, Kipiani K, Chen J, Calingasan NY, Crow JP, et al. Additive neuroprotective effects of a histone deacetylase inhibitor and a catalytic antioxidant in a transgenic mouse model of amyotrophic lateral sclerosis. Neurobiol Dis. 2006;221:40–49. doi: 10.1016/j.nbd.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 32.Rutten BP, Brasnjevic I, Steinbusch HW, Schmitz C. Caloric restriction and aging but not overexpression of SOD1 affect hippocampal volumes in mice. Mech Ageing Dev. 2010;1319:574–579. doi: 10.1016/j.mad.2010.08.002. 2010. [DOI] [PubMed] [Google Scholar]
- 33.Epstein CJ, Avraham KB, Lovett M, Smith S, Elroy-Stein O, Rotman G, et al. Transgenic mice with increased Cu/Zn-superoxide dismutase activity: animal model of dosage effects in Down syndrome. Proc Natl Acad Sci U S A. 1987;8422:8044–8048. doi: 10.1073/pnas.84.22.8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weichert W, Roske A, Niesporek S, Noske A, Buckendahl AC, Dietel M, et al. Class I histone deacetylase expression has independent prognostic impact in human colorectal cancer: specific role of class I histone deacetylases in vitro and in vivo. Clin Cancer Res. 2008;146:1669–1677. doi: 10.1158/1078-0432.CCR-07-0990. [DOI] [PubMed] [Google Scholar]
- 35.Franklin KB, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- 36.Miller KM, Tjeertes JV, Coates J, Legube G, Polo SE, Britton S, et al. Human HDAC1 and HDAC2 function in the DNA-damage response to promote DNA nonhomologous end-joining. Nat Struct Mol Biol. 2010;179:1144–1151. doi: 10.1038/nsmb.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rutten BP, Korr H, Steinbusch HW, Schmitz C. The aging brain: less neurons could be better. Mech Ageing Dev. 2003;1243:349–355. doi: 10.1016/s0047-6374(03)00002-2. [DOI] [PubMed] [Google Scholar]
- 38.Mastroeni D, Grover A, Delvaux E, Whiteside C, Coleman PD, Rogers J. Epigenetic changes in Alzheimer's disease: decrements in DNA methylation. Neurobiol Aging. 2010;3112:2025–2037. doi: 10.1016/j.neurobiolaging.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mastroeni D, McKee A, Grover A, Rogers J, Coleman PD. Epigenetic differences in cortical neurons from a pair of monozygotic twins discordant for Alzheimer's disease. PLoS One. 2009;48:e6617. doi: 10.1371/journal.pone.0006617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeng Y, Tan M, Kohyama J, Sneddon M, Watson JB, Sun YE, et al. Epigenetic enhancement of BDNF signaling rescues synaptic plasticity in aging. J Neurosci. 2011;3149:17800–17810. doi: 10.1523/JNEUROSCI.3878-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Tang B, Dean B, Thomas EA. Disease- and age-related changes in histone acetylation at gene promoters in psychiatric disorders. Transl Psychiatry. 2011;1:e64. doi: 10.1038/tp.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walker MP, Laferla FM, Oddo SS, Brewer GJ. Reversible epigenetic histone modifications and Bdnf expression in neurons with aging and from a mouse model of Alzheimer's disease. Age (Dordr) 2012 doi: 10.1007/s11357-011-9375-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caspi A, Moffitt TE. Gene-environment interactions in psychiatry: joining forces with neuroscience. Nat Rev Neurosci. 2006;77:583–590. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- 44.Lahiri DK, Maloney B. The "LEARn" (Latent Early-life Associated Regulation) model integrates environmental risk factors and the developmental basis of Alzheimer's disease, and proposes remedial steps. Exp Gerontol. 2010;454:291–296. doi: 10.1016/j.exger.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lahiri DK, Maloney B, Zawia NH. The LEARn model: an epigenetic explanation for idiopathic neurobiological diseases. Mol Psychiatry. 2009;1411:992–1003. doi: 10.1038/mp.2009.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Y, Ng HH, Erdjument-Bromage H, Tempst P, Bird A, Reinberg D. Analysis of the NuRD subunits reveals a histone deacetylase core complex and a connection with DNA methylation. Genes Dev. 1999;1315:1924–1935. doi: 10.1101/gad.13.15.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dobosy JR, Selker EU. Emerging connections between DNA methylation and histone acetylation. Cell Mol Life Sci. 2001;585-6:721–727. doi: 10.1007/PL00000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bordone L, Cohen D, Robinson A, Motta MC, van Veen E, Czopik A, et al. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell. 2007;66:759–767. doi: 10.1111/j.1474-9726.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- 49.Chen D, Bruno J, Easlon E, Lin SJ, Cheng HL, Alt FW, et al. Tissue-specific regulation of SIRT1 by calorie restriction. Genes Dev. 2008;2213:1753–1757. doi: 10.1101/gad.1650608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wakeling LA, Ions LJ, Ford D. Could Sirt1-mediated epigenetic effects contribute to the longevity response to dietary restriction and be mimicked by other dietary interventions? Age (Dordr) 2009;314:327–341. doi: 10.1007/s11357-009-9104-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Przedborski S, Jackson-Lewis V, Kostic V, Carlson E, Epstein CJ, Cadet JL. Superoxide dismutase, catalase, and glutathione peroxidase activities in copper/zinc-superoxide dismutase transgenic mice. J Neurochem. 1992;585:1760–1767. doi: 10.1111/j.1471-4159.1992.tb10051.x. [DOI] [PubMed] [Google Scholar]
- 52.Montgomery RL, Hsieh J, Barbosa AC, Richardson JA, Olson EN. Histone deacetylases 1 and 2 control the progression of neural precursors to neurons during brain development. Proc Natl Acad Sci U S A. 2009;10619:7876–7881. doi: 10.1073/pnas.0902750106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Akhtar MW, Raingo J, Nelson ED, Montgomery RL, Olson EN, Kavalali ET, et al. Histone deacetylases 1 and 2 form a developmental switch that controls excitatory synapse maturation and function. J Neurosci. 2009;2925:8288–8297. doi: 10.1523/JNEUROSCI.0097-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jawerka M, Colak D, Dimou L, Spiller C, Lagger S, Montgomery RL, et al. The specific role of histone deacetylase 2 in adult neurogenesis. Neuron Glia Biol. 2010;62:93–107. doi: 10.1017/S1740925X10000049. [DOI] [PubMed] [Google Scholar]