Abstract
PURPOSE
Adolescents are often cited as having poor rates of compliance with medical regimens and research protocols. We quantified compliance in a cohort of urban adolescents participating in a complex research protocol in which measures were obtained without direct supervision by research personnel.
METHODS
A total of 54 early adolescents ages 10–13 were asked to wear a vest containing a personal air pollutant exposure monitor for two 24-hour periods and to perform daily peak expiratory flow (PEF) for six consecutive days. Compliance with wearing the vest was measured by comparing accelerometer data from a device within the vest to one worn continuously on the child’s wrist. Daily PEF data were recorded using an electronic meter.
RESULTS
A priori definition of compliance was met by 85% of the adolescents by wearing the exposure monitoring vest and 72% by performing PEF.
CONCLUSIONS
These findings suggest that early adolescents can be compliant with complex research protocols that are needed to help bridge gaps in pediatric asthma research.
Keywords: adolescents, teen compliance, asthma studies, exposure monitoring, peak expiratory flow, accelerometer, objective measurements, wearing compliance
Introduction
Adolescents are often cited as having poor rates of compliance with medical regimens and research protocols compared to other age groups.1–5 Complex and time-consuming regimens are potential reasons for non-compliance.1,3 Reports of successful adolescent participation in cohort studies primarily involve questionnaires, treatment regimens, and/or scheduled visits for study procedures that are supervised by research personnel.6–9 Few health studies of adolescents report objective rates of compliance with acquisition of unsupervised measurements including wearing personal monitoring devices for air pollution exposure assessment10–14 and other outcomes.15–17 With continuing advancements in treatment modalities and research tools, it is critical that research protocols include the participation of adolescents; however, concern for non-compliance with complicated protocols may be a deterrent in designing and conducting such studies.
Peak expiratory flow (PEF) monitoring is a useful tool in the management of chronic asthma to detect asymptomatic decline in lung function, monitor response to treatment, and detect impending asthma exacerbation.18,19 The 2007 National Heart, Lung, and Blood Institute guidelines recommend the use of peak flow monitoring in moderate to severe asthmatics. However, previous studies in children have demonstrated inconsistent short-term compliance (48–80%)20,21 with daily peak flow diaries and monitoring. One study of urban black and Hispanic children ages five to nine years living in New York City (NYC) and Cleveland, OH, reported 48% compliance with daily peak flow monitoring over a three-week period.21 In contrast, a study of white children ages 6–15 years living in the Netherlands reported compliance rates as high as 80% over a one-week period with decreasing rates to 73% over a four-week period.20 Moreover, most of the studies to date focus on the use of peak flow to monitor and guide asthma management. Limited data are available on its use as an independent research tool in an adolescent population of healthy children and those with asthma.
Our goal was to quantify compliance objectively in a birth cohort of early adolescents with and without asthma, participating in an environmental exposure study in which participants conduct multiple measures (wearing equipment for personal black carbon (BC) exposure monitoring, daily PEF, and others) without direct supervision from research personnel.
Methods
Description of cohort
The Columbia Center for Children’s Environmental Health (CCCEH) longitudinal birth cohort comprises African American and Dominican children in NYC, whose mothers were recruited during pregnancy to study health effects of children in an urban, minority population. Demographic characteristics of the CCCEH cohort have been published.22–24 For the purposes of this current study, 10–13-year-old cohort participants were recruited based on physician-diagnosed asthma (target 56% asthmatics). This particular age group was selected because symptoms during early adolescence may mark progression of the disease, and tend to persist into adulthood.25,26 The longitudinal birth cohort study was conducted in accordance with Columbia University Institutional Review Board guidelines, and informed consents and assents from each participant were obtained.
Air pollution monitoring
The children who were studied were asked to wear a vest containing a light-weight (<300 g) microAeth air monitor (AethLabs, San Francisco, CA) for two 24-hour time periods, five days apart (Fig. 1), to measure personal exposure to the environmental pollutant BC. Instructions were to wear the vest continuously during wakeful hours and remove only while sleeping, bathing, or performing vigorous activities if not comfortable. Research assistants visited children’s home on days 0 and 5 to train the children on how to use and wear the personal air pollution monitors and on days 1 and 6 to remove monitors.
Figure 1.
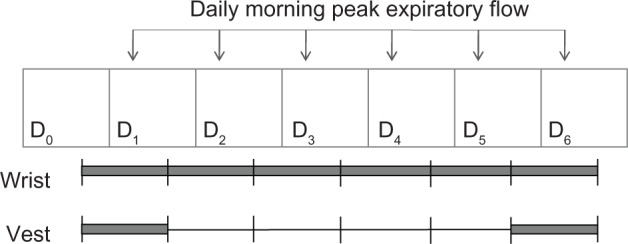
Timeline of study procedures. Study procedures took place over six consecutive 24-hour monitoring periods. Participants were asked to wear an Actical accelerometer to measure activity level on the wrist for six consecutive 24-hour periods. They were also asked to wear a vest, into which a similar accelerometer was built in, for two 24-hour monitoring periods, five days apart (from the start of each monitoring period). Daily morning PEF was measured on days 1–6. Each gray bar represents one 24-hour monitoring period. D0 = day 0.
PEF measurements
The children who were studied were asked to perform morning PEF once daily (Fig. 1) using a portable, electronic meter (Microlife, Clearwater, FL). On day 0, children received instruction and proper PEF technique was demonstrated. Children were asked to repeat the maneuver three times every morning for the remaining six days. The PEF monitor’s internal device recorded the daily best of three PEF measurements, date, and time.
Determination of compliance and compensation
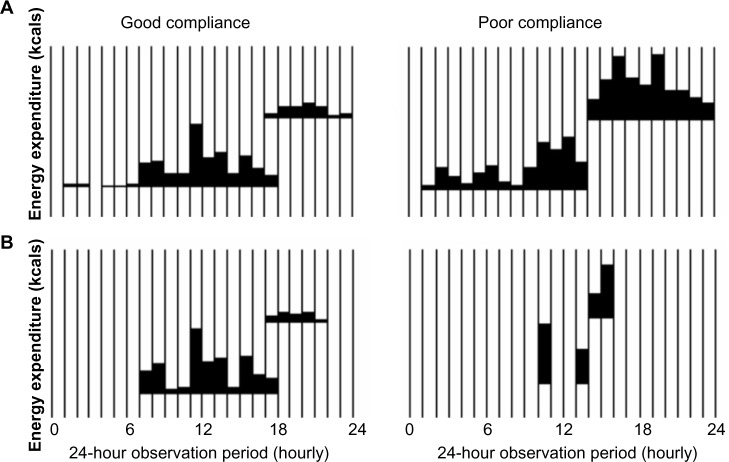
The air pollution (BC) monitoring vest contained an accelerometer (Actical, Philips Respironics, Bend, OR). A similar waterproof accelerometer was worn continuously on the child’s wrist via a hospital wrist band for six consecutive days and could not be removed by the child, thus representing a standard measurement of movement throughout the day. The accelerometers independently measured and recorded movement and energy expenditure at one-minute intervals, and data were displayed graphically by the program’s software. Integrated measures of gross motor activity were compared between the two accelerometers and assessed for similar movement patterns on days 1 and 6 (Fig. 2). If movement patterns between the wrist and the vest accelerometers were concordant, as illustrated in the panel on the left of Figure 2, the child was determined to be compliant. After each 24-hour period, the child also was queried by questionnaire regarding the times the vest was not worn for verification of missing data.
Figure 2.
Representation of good and poor compliance with wearing vest for monitoring. Data from wrist and vest accelerometers were visually inspected by research assistants and compared to assess for discordant movement. On the left, good compliance is represented by concordant movement of the wrist (A) and vest (B) accelerometers as opposed to poor compliance on the right demonstrating large gaps in vest activity.
A priori, participants were considered compliant if there was less than two hours of discordant data (motion detected on wrist but not vest accelerometer). If greater than two hours of discordant data was detected, questionnaires were examined and hours of discordant data were recalculated to exclude time while child was participating in vigorous activities and thus not wearing the vest.
Consistent with previous reports,27 a participant was considered compliant with PEF monitoring if he/she had at least three out of six morning PEF data (50%) over the six-day period.
Reimbursement was given to the child ($25 gift card) and parent ($100 cash) for study participation, independent of compliance. During consent and assent, the child was informed that he/she could earn an additional $25 gift card for good compliance by wearing the vest. No additional reimbursement was given for compliance with PEF.
Results
Compliance with personal BC exposure measure (vest)
Of the 54 participants studied, 48 (89%) were compliant with wearing the vest during the first 24-hour time period and 46 (85%) were compliant with both 24-hour time periods. There was no difference in compliance at either time period when stratified by diagnosis of asthma, sex, race/ethnicity, or maternal high school education at time of birth (P > 0.05), as summarized in Table 1.
Table 1.
Demographic characteristics.
| ALL (N = 54) |
ASTHMA (N = 31) |
NO ASTHMA (N = 23) |
P VALUE* | |
|---|---|---|---|---|
| Age mean (standard deviation) | 13.3 (0.60) | 13.1 (0.71) | 13.5 (0.31) | 0.17 |
| Male | 25 (46%) | 14 (45%) | 11 (48%) | 0.85 |
| Race/ethnicity | 0.67 | |||
| Dominican | 30 (56%) | 18 (58%) | 12 (52%) | |
| African American | 24 (44%) | 13 (42%) | 11 (48%) | |
| Maternal high school degree at time of participant’s birth** | 33 (67%) | 20 (77%) | 13 (57%) | 0.13 |
Notes:
Using Pearson’s chi-square test, no difference was noted between asthma and non-asthma groups at a significance level of 0.05.
Missing data of five children.
Compliance with PEF
Morning PEF measurements were recorded at least 50% of the time (three of six measurements) in 39 (72%) of the participants (Fig. 3). Non-asthmatic children appeared to be more compliant than asthmatic children (87 vs. 61%, P = 0.04). There was no significant difference in compliance with PEF when stratified by sex, race/ethnicity, or maternal high school degree at time of birth.
Figure 3.
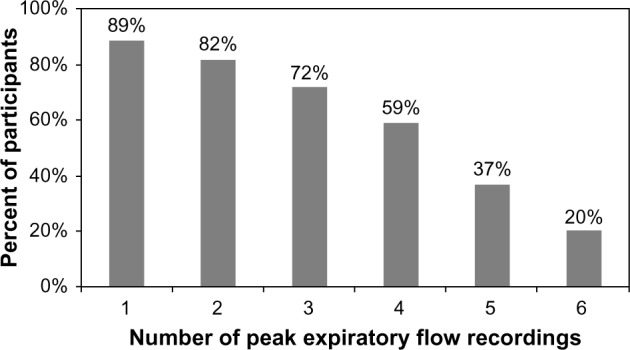
Cumulative percentage of compliant morning PEF recordings. Compliance was defined as performing morning PEF at least three days (50%). Number of PEF recording was plotted against percentage of participants, n = 54.
Discussion
We found that among the 54 early adolescents studied, a majority (85%) appropriately complied with instructions to wear a vest containing a device for personal BC monitoring for two 24-hour periods. The majority (72%) also performed at least three morning PEF measures during a six-day observation period. These results suggest that urban teens were able to comply with complex research protocols in the absence of direct supervision from research personnel.
Several studies have addressed the lack of adherence to complicated medical regimens in adolescents, particularly with asthma medications.1,3,28 These suggest that early adolescents are unfavorable subjects for research studies. However, few publications to date have assessed formally compliance with non-treatment protocols such as wearing exposure monitoring equipment. Based on our findings, we argue that this age group can comply with complicated research protocols. The rate of compliance with peak flow recordings in this present study was consistent with that of Redline and colleagues who monitored electronic peak flow measurements in 58 African American and Hispanic children ages five to nine years in NYC and Cleveland, OH and found 52% missing peak flow recordings over a three-week period.21 This group, much like ours, consisted of urban minority children, though the age ranges differed. Though literature supporting the exact age when children are more or less likely to comply with medical or research regimens is sparse, Jonnason and colleagues reported decreased compliance with inhaled budesonide for treatment of asthma in 10–16-year olds compared to those of 7–9-year olds. The authors suggested that stronger parental supervision in the younger age group could be a potential cause for this difference, though this hypothesis was not explicitly measured.28
Increased compliance with wearing the vest compared to obtaining daily morning PEF measurements could be related to reimbursement for good compliance with the former but not the later procedure. In a meta-analysis review of 39 studies that offered reimbursements for primarily psychological outcome measures, Jenkins and colleagues found a positive correlation between financial reimbursement and the number of measures conducted but not necessarily quality of measures.29 Based on our findings and that of Jenkins and colleagues, financial reimbursement for good compliance should be considered in future studies to increase compliance rates of adolescents with research protocols. Alternately, the higher compliance with vest versus PEF may be attributed to the lack of repetitive action required for wearing equipment. As Buston and Wood reported, adolescents often cited forgetfulness as a barrier to compliance;3 thus, early adolescents could be more likely to forget to perform daily repetitive PEF maneuvers. Future studies also should consider the frequency and duration of study procedures to improve compliance of adolescents with research protocols.
We acknowledge that our cohort is a highly selected sample of early adolescents who have participated in research studies since birth and provided assent. Therefore, our findings may differ in a population of adolescents who have not participated previously in research studies. Interestingly, we found slightly higher compliance with PEF monitoring in non-asthmatic compared to those of asthmatic adolescents. As Winnick et al. report, duration of therapy has been associated with decreased compliance over time and though data are limited in children, long-term compliance with peak flow monitoring is as low as 33–63% in adults.27 Perhaps, the asthmatic children in our study have been previously prescribed daily peak flow monitoring and thus, were less likely to comply with this measure than the non-asthmatic children.
The level of compliance with the environmental asthma study protocol in this early adolescent population was remarkable given the lack of direct supervision by research assistants during these tasks. Disease phenotypes including asthma often vary by age and thus, should be well characterized and studied across the spectrum of age groups. For example, adolescents with asthma symptoms that did not remit from earlier childhood may be more likely to have disease in adulthood;25,26 little is known about the mechanisms behind these phenotypic differences. Likewise, disease phenotypes vary by socioeconomic status, and asthma morbidity and mortality rates are the highest in inner-city neighborhoods.30 Our findings are highly significant and demonstrate that early adolescents living in urban environments can comply with complicated research protocols in the study of environmental exposure and asthma. Future studies should not neglect this population based on concerns of adolescent non-compliance as their participation in asthma research is important to improving our understanding of the pathogenesis of childhood asthma.
Footnotes
Author Contributions
RLM, SNC, and FP conceived and designed the experiments. SLD and KHJ analyzed the data. SLD wrote the first draft of the manuscript. CF, KHJ, RLM, and SNC contributed to the writing of the manuscript. DT and EG agreed with manuscript results and conclusions. SLD, RLM, and SNC jointly developed the structure and arguments for the paper. SLD, CF, KHJ, DT, EG, FP, RLM, and SNC made critical revisions and approved the final version. All authors reviewed and approved the final manuscript.
ACADEMIC EDITOR: Hussein D. Foda, Editor in Chief
FUNDING: We would like to acknowledge the following NIH funding sources: 2R01ES13163–06 A1, 3R01ES013163–07S1, P50ES015905, 5P01ES09600/EPA RD-83214101, and P30ES009089.
COMPETING INTERESTS: Author(s) disclose no potential conflicts of interest.
DISCLOSURES AND ETHICS
As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests.
REFERENCES
- 1.Dinwiddie R, Muller WG. Adolescent treatment compliance in asthma. J R Soc Med. 2002;95(2):68–71. doi: 10.1258/jrsm.95.2.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song M, Omar HA. Discovering the complexities of adolescent compliance to treatment. Int J Adolesc Med Health. 2009;21(1):3–8. doi: 10.1515/ijamh.2009.21.1.3. [DOI] [PubMed] [Google Scholar]
- 3.Buston KM, Wood SF. Non-compliance amongst adolescents with asthma: listening to what they tell us about self-management. Fam Pract. 2000;17(2):134–8. doi: 10.1093/fampra/17.2.134. [DOI] [PubMed] [Google Scholar]
- 4.Winnick S, Lucas DO, Hartman AL, Toll D. How do you improve compliance? Pediatrics. 2005;115(6):e718–e724. doi: 10.1542/peds.2004-1133. [DOI] [PubMed] [Google Scholar]
- 5.Reznik M, Ozuah PO. Measurement of inhaled corticosteroid adherence in inner-city, minority children with persistent asthma by parental report and integrated dose counter. J Allergy. 2012;2012:570850. doi: 10.1155/2012/570850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kattan M, Kumar R, Bloomberg GR, et al. Asthma control, adiposity, and adipokines among inner-city adolescents. J Allergy Clin Immunol. 2010;125(3):584–92. doi: 10.1016/j.jaci.2010.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jung KH, Hsu SI, Yan B, et al. Childhood exposure to fine particulate matter and black carbon and the development of new wheeze between ages 5 and 7 in an urban prospective cohort. Environ Int. 2012;45:44–50. doi: 10.1016/j.envint.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andersson M, Hedman L, Bjerg A, Forsberg B, Lundback B, Ronmark E. Remission and persistence of asthma followed from 7 to 19 years of age. Pediatrics. 2013;132(2):e435–e442. doi: 10.1542/peds.2013-0741. [DOI] [PubMed] [Google Scholar]
- 9.Zeiger RS, Szefler SJ, Phillips BR, et al. Response profiles to fluticasone and montelukast in mild-to-moderate persistent childhood asthma. J Allergy Clin Immunol. 2006;117(1):45–52. doi: 10.1016/j.jaci.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 10.Buonanno G, Marks GB, Morawska L. Health effects of daily airborne particle dose in children: direct association between personal dose and respiratory health effects. Environ Pollut. 2013;180:246–50. doi: 10.1016/j.envpol.2013.05.039. [DOI] [PubMed] [Google Scholar]
- 11.Spira-Cohen A, Chen LC, Kendall M, Lall R, Thurston GD. Personal exposures to traffic-related air pollution and acute respiratory health among Bronx schoolchildren with asthma. Environ Health Perspect. 2011;119(4):559–65. doi: 10.1289/ehp.1002653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kinney PL, Chillrud SN, Ramstrom S, Ross J, Spengler JD. Exposures to multiple air toxics in New York City. Environ Health Perspect. 2002;110(Suppl 4):539–46. doi: 10.1289/ehp.02110s4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sax SN, Bennett DH, Chillrud SN, Ross J, Kinney PL, Spengler JD. A cancer risk assessment of inner-city teenagers living in New York City and Los Angeles. Environ Health Perspect. 2006;114(10):1558–66. doi: 10.1289/ehp.8507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chillrud SN, Epstein D, Ross JM, et al. Elevated airborne exposures of teenagers to manganese, chromium, and iron from steel dust and New York City’s subway system. Environ Sci Technol. 2004;38(3):732–7. doi: 10.1021/es034734y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campbell N, Prapavessis H, Gray C, McGowan E, Rush E, Maddison R. The Actiheart in adolescents: a doubly labelled water validation. Pediatr Exerc Sci. 2012;24(4):589–602. doi: 10.1123/pes.24.4.589. [DOI] [PubMed] [Google Scholar]
- 16.Ho V, Simmons RK, Ridgway CL, et al. Is wearing a pedometer associated with higher physical activity among adolescents? Prev Med. 2013;56(5):273–7. doi: 10.1016/j.ypmed.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gow RM, Borghese MM, Honeywell CR, Colley RC. Activity intensity during free-living activities in children and adolescents with inherited arrhythmia syndromes: assessment by combined accelerometer and heart rate monitor. Circ Arrhythm Electrophysiol. 2013;6(5):939–45. doi: 10.1161/CIRCEP.113.000514. [DOI] [PubMed] [Google Scholar]
- 18.Feldman JM, Kutner H, Matte L, et al. Prediction of peak flow values followed by feedback improves perception of lung function and adherence to inhaled corticosteroids in children with asthma. Thorax. 2012;67(12):1040–5. doi: 10.1136/thoraxjnl-2012-201789. [DOI] [PubMed] [Google Scholar]
- 19.Cowie RL, Revitt SG, Underwood MF, Field SK. The effect of a peak flow-based action plan in the prevention of exacerbations of asthma. Chest. 1997;112(6):1534–8. doi: 10.1378/chest.112.6.1534. [DOI] [PubMed] [Google Scholar]
- 20.Kamps AW, Roorda RJ, Brand PL. Peak flow diaries in childhood asthma are unreliable. Thorax. 2001;56(3):180–2. doi: 10.1136/thorax.56.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Redline S, Wright EC, Kattan M, Kercsmar C, Weiss K. Short-term compliance with peak flow monitoring: results from a study of inner city children with asthma. Pediatr Pulmonol. 1996;21(4):203–10. doi: 10.1002/(SICI)1099-0496(199604)21:4<203::AID-PPUL1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 22.Miller RL, Garfinkel R, Horton M, et al. Polycyclic aromatic hydrocarbons, environmental tobacco smoke, and respiratory symptoms in an inner-city birth cohort. Chest. 2004;126:1071–8. doi: 10.1378/chest.126.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller RL, Garfinkel R, Lendor C, et al. Polycyclic aromatic hydrocarbon metabolite levels and pediatric asthma-related outcomes in an inner city. Pediatr Allergy Immunol. 2010;21:260–7. doi: 10.1111/j.1399-3038.2009.00980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perera FP, Rauh V, Tsai W-Y, et al. Effects of transplacental exposure to environmental pollutants on birth outcomes in a multiethnic population. Environ Health Perspect. 2003;111(2):201–5. doi: 10.1289/ehp.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rhodes HL, Thomas P, Sporik R, Holgate ST, Cogswell JJ. A birth cohort study of subjects at risk of atopy: twenty-two-year follow-up of wheeze and atopic status. Am J Respir Crit Care Med. 2002;165(2):176–80. doi: 10.1164/ajrccm.165.2.2104032. [DOI] [PubMed] [Google Scholar]
- 26.Guerra S, Wright AL, Morgan WJ, Sherrill DL, Holberg CJ, Martinez FD. Persistence of asthma symptoms during adolescence: role of obesity and age at the onset of puberty. Am J Respir Crit Care Med. 2004;170(1):78–85. doi: 10.1164/rccm.200309-1224OC. [DOI] [PubMed] [Google Scholar]
- 27.Cote J, Cartier A, Malo JL, Rouleau M, Boulet LP. Compliance with peak expiratory flow monitoring in home management of asthma. Chest. 1998;113(4):968–72. doi: 10.1378/chest.113.4.968. [DOI] [PubMed] [Google Scholar]
- 28.Jonasson G, Carlsen KH, Sodal A, Jonasson C, Mowinckel P. Patient compliance in a clinical trial with inhaled budesonide in children with mild asthma. Eur Respir J. 1999;14(1):150–4. doi: 10.1034/j.1399-3003.1999.14a25.x. [DOI] [PubMed] [Google Scholar]
- 29.Jenkins GD, Jr, Mitra A, Gupta N, Shaw JD. Are financial incentives related to performance? A meta-analytic review of empirical research. J Appl Psychol. 1998;83(5):777–87. [Google Scholar]
- 30.Claudio L, Stingone JA, Godbold J. Prevalence of childhood asthma in urban communities: the impact of ethnicity and income. Ann Epidemiol. 2006;16(5):332–40. doi: 10.1016/j.annepidem.2005.06.046. [DOI] [PubMed] [Google Scholar]