Abstract
Background and Purpose
Azithromycin is a macrolide antibiotic with anti-inflammatory and immunomodulating effects. Long-term azithromycin therapy in patients with chronic lung diseases such as cystic fibrosis has been associated with increased antimicrobial resistance, emergence of hypermutable strains, ototoxicity and cardiac toxicity. The aim of this study was to assess the anti-inflammatory effects of the non-antibiotic azithromycin derivative CSY0073.
Experimental Approach
We compared the effects of CSY0073 with those of azithromycin in experiments on bacterial cultures, Pseudomonas aeruginosa biofilm, lung cells and mice challenged intranasally with P. aeruginosa LPS.
Key Results
In contrast to azithromycin, CSY0073 did not inhibit the growth of P. aeruginosa, Staphylococcus aureus or Haemophilus influenzae and had no effect on an established P. aeruginosa biofilm. Bronchoalveolar lavage (BAL) fluids and lung homogenates collected after the LPS challenge in mice showed that CSY0073 and azithromycin (200 mg·kg−1, i.p.) decreased neutrophil counts at 24 h and TNF-α, CXCL1 and CXCL2 levels in the BAL fluid after 3 h and IL-6, CXCL2 and IL-1β levels in the lung after 3 h compared with the vehicle. However, only azithromycin reduced IL-1β levels in the lung 24 h post LPS challenge. CSY0073 and azithromycin similarly diminished the production of pro-inflammatory cytokines by macrophages, but not lung epithelial cells, exposed to P. aeruginosa LPS.
Conclusions and Implications
Unlike azithromycin, CSY0073 had no antibacterial effects but it did have a similar anti-inflammatory profile to that of azithromycin. Hence, CSY0073 may have potential as a long-term treatment for patients with chronic lung diseases.
Keywords: macrolides, lung inflammation, cystic fibrosis, COPD
Introduction
Azithromycin is a widely used antibiotic belonging to the macrolide family characterized by a highly substituted 15-member macrocyclic lactone ring and it has a broad antimicrobial spectrum. Several clinical studies have shown that azithromycin has beneficial effects in cystic fibrosis (CF) patients, most notably those colonized with Pseudomonas aeruginosa, and it is now used as part of a standard maintenance therapy for CF (Shinkai and Rubin, 2005; Flume et al., 2007; Cai et al., 2011). However, evidence has emerged of that azithromycin also has beneficial effects in patients with chronic airway diseases but no infection, suggesting it has anti-inflammatory and immunomodulating properties (Rubin and Henke, 2004). In a recent study of children and adults with CF but no P. aeruginosa infection, azithromycin for 24 weeks did not improve lung function, but it did decrease the occurrence of pulmonary exacerbations (Saiman et al., 2011). A recent Cochrane Database systematic review indicated that azithromycin has beneficial effects for at least 6 months on lung function, reduces the occurrence of exacerbations, the need for other antibiotics, and weight gain in patients with CF (Southern et al., 2012). In addition to CF, the effects of azithromycin have also been evaluated in other chronic airway diseases such as diffuse panbronchiolitis, chronic obstructive pulmonary disease (COPD), asthma, non-CF bronchiectasis and bronchiolitis obliterans syndrome (Spagnolo et al., 2012). It has also recently been found to be effective in surfactant-associated disorders (Hayes et al., 2012; Thouvenin et al., 2013).
The mechanisms by which azithromycin produces clinical benefits is still unclear (Yousef and Jaffe, 2010). These effects are likely to be independent of its antimicrobial activity and it has been hypothesized that they are the result of a multifactorial effect on inflammation, immune responses and bacterial virulence (Yousef and Jaffe, 2010). In fact, it has been shown that azithromycin is not bactericidal against P. aeruginosa, but decreases the virulence of this pathogen, most notably by acting on its biofilm (Wagner et al., 2005).
However, the long-term use of azithromycin has raised a number of concerns (Spagnolo et al., 2012). Chronic azithromycin therapy has been associated with increased antimicrobial resistance and with the emergence of hypermutable strains, particularly of Staphylococcus aureus and Haemophilus species (Phaff et al., 2006). These resistant or hypermutable pathogens might spread from patients undergoing chronic azithromycin treatment to the general population, decreasing the effectiveness of macrolide treatment. In addition, chronic azithromycin use can result in ototoxicity (Wallace et al., 1994; Albert et al., 2011) and an increased risk of cardiac events (Ray et al., 2012).
One option available to improve the risk/benefit ratio of macrolide therapy in patients with chronic airway diseases, is to produce derivatives of azithromycin that have the anti-inflammatory and immunomodulating effects of macrolides without the antibiotic effects. Recently, a non-antibiotic derivative of azithromycin, CSY0073, was shown to correct the inflammation-driven immune dysfunction seen in animal models of inflammatory bowel disease and arthritis (Mencarelli et al., 2011). Hence, in the present study our objective was to characterize the effects of CSY0073 in a mouse model of acute lung inflammation. To achieve this, we compared the effects of CSY0073 with those of azithromycin in experiments on bacterial cultures, P. aeruginosa biofilm, lung cells and mice challenged intranasally with P. aeruginosa LPS.
Methods
Reagents
LPS (P. aeruginosa 10) and DMSO were from Sigma-Aldrich (Saint-Quentin Fallavier, France) and TNF-α and IL-1β were from Immunotools (Friesoythe, Germany). Azithromycin and CSY0073 (11-(4-dimethylamino-3-hydroxy-6-methyl-tetrahydro-pyran-2-yloxy)-2-ethyl-3,4,10-trihydroxy-3,5,6,8,10,12,14-heptamethyl-1-oxa-6-azacyclopentadecane-13,15 dione) were synthesized as described previously (Mencarelli et al., 2011) and provided by Synovo (Tübingen, Germany).
In vitro effect of CSY0073 and azithromycin on bacterial growth and P. aeruginosa biofilm viability
Effect of CSY0073 on bacterial growth
The antibacterial activities of CSY0073 and azithromycin were evaluated in liquid cultures of P. aeruginosa (PAK strain), S. aureus (Newman strain) and Haemophilus influenza (NTHI 86-028NP strain) as published elsewhere, with a few modifications (Mencarelli et al., 2011). Bacteria were grown overnight in adapted medium [Luria Bertoni (LB) broth for P. aeruginosa and S. aureus and brain heart infusion broth for H. influenzae], then transferred to fresh medium and grown for 4–5 h to midlog phase. Cultures were centrifuged at 3000× g for 15 min and pellets were washed four times with cold PBS. Bacterial pellets were diluted in PBS and the OD600nm was adjusted to 108 CFU·mL−1 in appropriate medium broth. Bacteria were then added to 96-well plates containing vehicle (citric acid; maximal final concentration was 0.01% and did not influence cell viability), CSY0073 or azithromycin. Briefly, drugs were diluted from a 20 mM stock solution in 2% citric acid to obtain concentrations similar to the azithromycin concentrations measured in sputum from patients with CF (Baumann et al., 2004; Wilms et al., 2006). OD600 was recorded at baseline then after 6 h, using an Asys multiplate reader (Biochrom, Cambridge, UK). During incubation, the plates under agitation were maintained at 37°C and 100% relative humidity. Recorded ODs were normalized to the baseline value (no drug) and expressed as percentages [mean OD (drug)/mean OD (baseline) ].
To determine whether the bacteria carrying the most common form of macrolide resistance (erm) exhibit differential sensitivity to CSY0073 and azithromycin, we cultured Bacillus subtilis WT168 carrying the erm-containing plasmid pAM77, as mentioned earlier, and compared growth by monitoring OD600 with and without the plasmid at concentrations of substance up to 10 mM. Data were normalized as mentioned earlier.
Effect of CSY0073 on P. aeruginosa established biofilm
AZM has been reported to inhibit P. aeruginosa biofilm production (Mulet et al., 2009; Fernandez-Olmos et al., 2012), and we investigated this effect with both azithromycin and CSY0073. We used three P. aeruginosa strains, PAO1, PAK and PA14. In vitro biofilms were grown for 24 h on UV-sterilized PVC 96-well plates (Thermo Scientific, Rochester, NY, USA) as previously described (Bernier et al., 2013). Overnight cultures were diluted in LB to an OD of 0.05 at 595 nm, and 100 μL of this dilution was placed in each well, in triplicate. After 24 h, each well was washed with PBS to remove planktonic bacteria then treated for 24 h with CSY0073 or azithromycin at concentrations of 0.5, 5, and 50 μM in LB [concentrations chosen in keeping with earlier studies (Mulet et al., 2009) ]. Citric acid 0.005% in LB served as the control. Then, each well was washed twice with PBS to remove planktonic bacteria. After vigorous manual resuspension, excess compounds and surviving bacteria (colony-forming units, CFU) were quantified in each well by serial dilution and plating. Data are expressed as % survival representing viable cells after 24 h of treatment compared with untreated biofilm. Untreated controls (baseline) represent 48 h biofilms in which fresh medium was added after 24 h, so the % of surviving CFU can be different from 100% (Bernier et al., 2013).
In vivo study in LPS-challenged mice
C57BL/6 mice were supplied by the Centre d'Elevage R. Janvier, Le Genest Saint-Isle, France and used at 8 weeks of age. Mice were fed normal mouse chow and water ad libitum and were reared and housed under standard conditions with air filtration. Mice were cared for in accordance with Pasteur Institute guidelines in compliance with the European Animal Welfare regulations. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). Seven/eight mice were used per group. Vehicle (V: DMSO, final concentration was 5% v v−1), azithromycin or CSY0073 (200 mg·kg−1) was injected i.p. (500 μL) as described previously (Bosnar et al., 2012). Azithromycin and CSY0073 were first dissolved in vehicle and diluted in 0.5% (w v−1) methylcellulose (Sigma-Aldrich) (Mencarelli et al., 2011). Two hours later, animals were treated intranasally under general anaesthesia, induced by the administration of a mixture of ketamine (40 mg·kg−1) and xylazine (8 mg·kg−1) i.m. Parameters used to monitor anaesthetic depth were reflexes, chest wall movements and mucous membrane colour. Each animal received an intranasal instillation (25 μL in each nostril) of LPS solution (100 μg·kg−1 equivalent) in 50 μL of PBS.
The inflammatory lung response was assessed by evaluating bronchoalveolar lavage (BAL) fluid and lung homogenates at 3 and 24 h. BAL was obtained as described previously (Guillot et al., 2008), using 2 mL of PBS (four 0.5-mL aliquots). Total cell counts in BAL fluids were determined using a Coulter counter (Beckman, Roissy, France), and differential cell counts were determined with 200 cells after cells had been subjected to cytospin centrifugation and stained with Diff-Quick (Thermo Fisher Scientific, Pittsburgh, PA, USA). Lungs collected at 3 and 24 h were homogenized in 1 mL PBS. Supernatants of BAL fluid and lung homogenates were stored at −80°C for cytokine elisas, performed as described below.
Studies of cells exposed to LPS
In a previous study done in a mouse model, azithromycin inhibited the inflammatory response of alveolar macrophages to intranasal LPS but did not have a similar effect on respiratory epithelial cells (Bosnar et al., 2009). We therefore compared the effects of azithromycin and CSY0073 on these two cell types.
Alveolar macrophages from C57BL/6J mice were isolated by BAL as described previously (Guillot et al., 2008). The cells were pretreated (as indicated in the figure legends) with vehicle (citric acid, maximal concentration 0.005%) or increasing doses of azithromycin or CSY0073, before LPS (1 μg·mL−1).
We used three types of respiratory epithelial cells: A549 adenocarcinoma human alveolar basal epithelial cells stably transfected with NFκB-luciferase (A549/NFκB-luc, Panomics, Fremont, CA, USA), BEAS-2B normal human bronchial-epithelium cells, and human primary differentiated bronchial cells (MucilAir™, Epithelix, Geneva, Switzerland). A549/NFκB-luc and BEAS-2B cells were cultured as previously described (Guillot et al., 2004) then seeded at 1 × 105 cells per well on 96-well plates (TPP, Techno Plastic Products, Trasadingen, Switzerland). BEAS-2B cells were pretreated with AZM or CSY0073 (10 and 20 μM), before or concomitantly with LPS (1 μg mL−1). The A549/NFκB-luc cells were stimulated for 6 h with LPS, TNF-α 20 ng mL−1, or IL-1β 0.1 ng mL−1 to allow an assessment of CSY0073 effects on IL-8 release and NF-κB activation. The MucilAir™ cells were isolated from the bronchi of healthy individuals and cultured at an air-liquid interface for 3 weeks until differentiation. The cells were then cultured in MucilAir™ culture medium as recommended by the manufacturer and pretreated at the basal side 24 h with AZM or CSY0073 (20 μM, 700 μL), before apical LPS exposure (10 μg mL−1, 50 μL). Citric acid (maximal concentration 0.002%) was used as the vehicle.
elisa
Concentrations of human IL-8 and murine TNF-α, CXCL1 (KC), CXCL2 (macrophage inflammatory protein-2), IL-1β, IL-12p40 and IL-6 were measured in cell culture supernatants, BAL fluids and lung homogenates supernatants according to the manufacturer's instructions (Duoset, R&D Systems, Minneapolis, MN, USA). The 3,3′,5,5′-tetramethylbenzidine (TMB) substrate was from Cell Signaling Technology (Danvers, MA, USA).
Statistical analysis
Differences among groups were assessed for statistical significance using Prism 6.00 software (GraphPad Software, La Jolla, CA, USA) as noted in the figure legends. A P value < 0.05 was taken to indicate statistical significance. ANOVA was used to compare quantitative variables between several groups. Correction for multiple testing was either done by the Bonferroni (all groups compared) or Dunnett's method (groups compared vs. a control condition).
Results
CSY0073 had no in vitro bacteriostatic activity or inhibitory effect on the biofilm P. aeruginosa
CSY0073 did not inhibit the growth of P. aeruginosa (Figure 1A), S. aureus (Figure 1B), or H. influenzae (Figure 1C). As expected, AZM inhibited the growth of all three pathogens, H. influenzae being the most susceptible.
Figure 1.
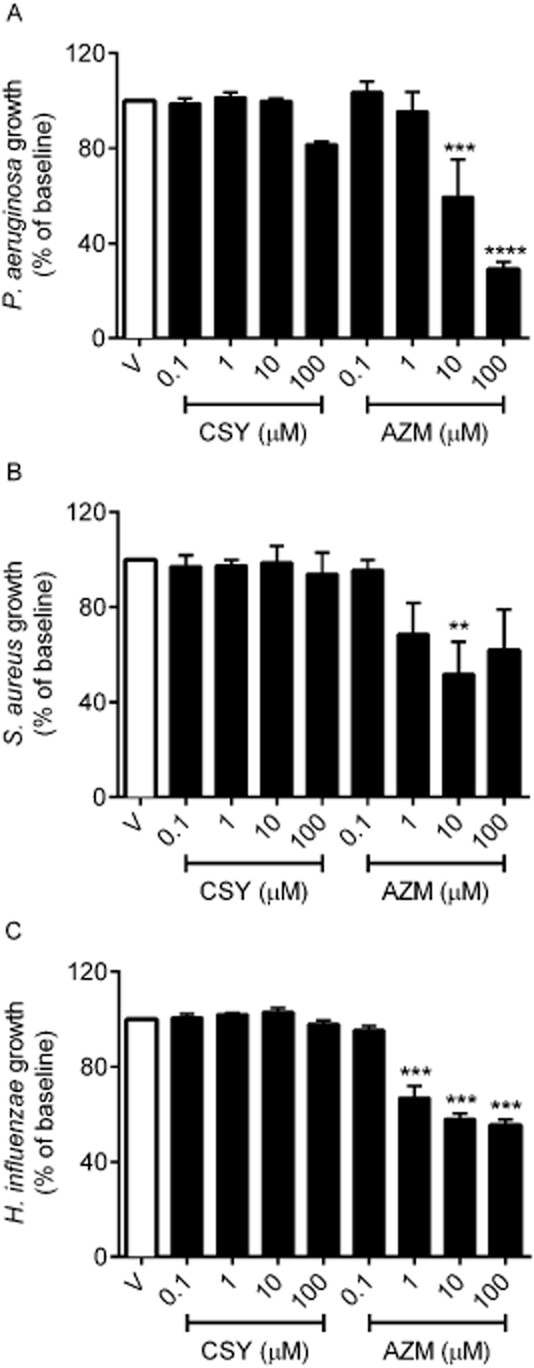
CSY0073 does not inhibit the growth of Pseudomonas aeruginosa, Staphylococcus aureus or Haemophilus influenzae. The antibacterial activities of increasing concentrations (0.1–100 μM) of CSY0073 (CSY) and AZM were determined in liquid cultures of P. aeruginosa (PAK) (A), S. aureus (Newman) (B), and H. influenza (NTHI) (C) strains. The drugs and pathogens were incubated for 6 h and the OD was then measured. Data are mean ± SEM values (% of the baseline) of four independent experiments. Statistical analysis was done by use of anova followed by Dunnett's multiple comparison test. **P < 0.01, ***P < 0.001 and ****P < 0.0001. Each value was compared with the control (V: citric acid).
We assessed the activity of CSY against 24 h biofilms by quantification of the surviving CFU after a 24 h treatment, as stated in the methods section. CSY0073 had no significant effect on established biofilms produced by any of the three P. aeruginosa strains tested, whereas AZM exhibited a dose-dependent effect with all three strains (Figure 2).
Figure 2.
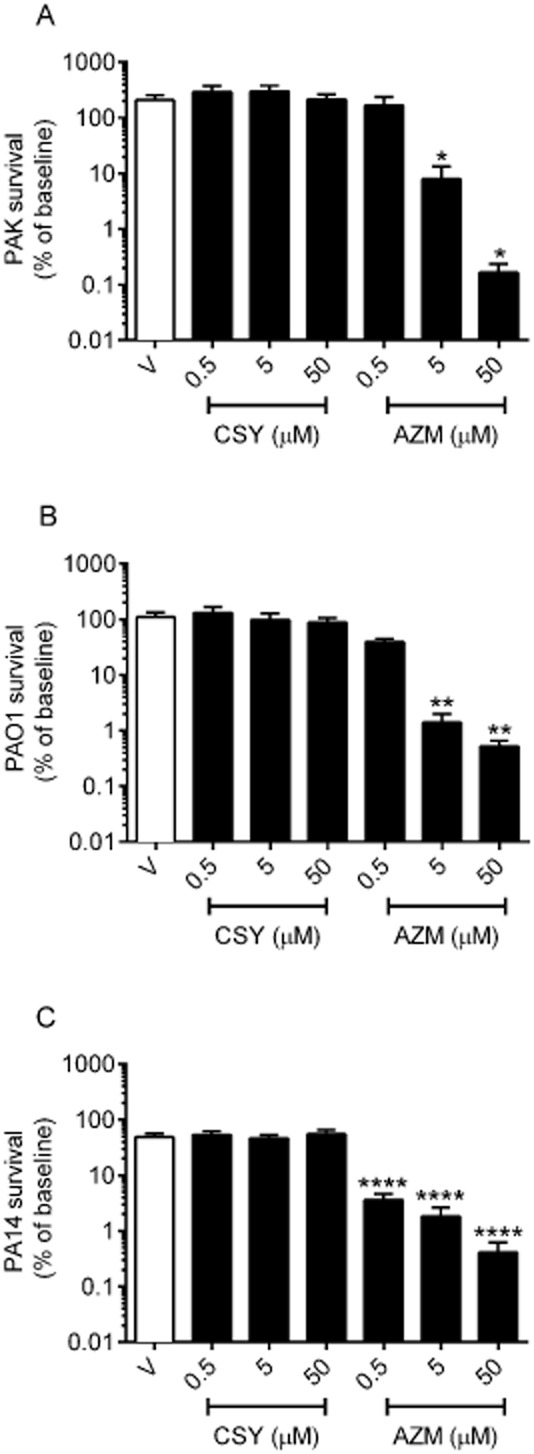
CSY0073 has no effect on an established Pseudomonas aeruginosa biofilm. P. aeruginosa PAK (A), PAO1 (B), and PA14 (panel C) in vitro biofilms were exposed for 24 h to increasing concentrations (0.5–50.0 μM) of CSY0073 (CSY) or AZM. Data are mean ± SEM values (%) of at least three independent experiments performed in triplicate. Statistical analysis was done by use of anova followed by Dunnett's multiple comparison test. *P < 0.05, **P < 0.01 and ****P < 0.0001. Each value was compared with the control (V: citric acid).
To determine whether organisms carrying a resistance to macrolides have any advantage in the presence of the agents, we examined the response of B. subtilis carrying an erm mutation (WT168 pAM77) to both CSY0073 and AZM (Figure 3A). Because of the extent of resistance, concentrations of azithromycin in the mM range were needed to observe any effects, whereas the wild-type (WT168) strain responds to azithromycin in the 1–10 μM range (not illustrated). Complete inhibition of B. subtilis growth was reached with approximately 0.4 mM azithromycin, whereas CSY0073, in the range of 1 to 3 mM, reduced growth by less than 50% (Figure 3A). When concentrations up to 10 mM were tested, WT168 B. subtilis tolerated CSY0073 to a slightly greater extent than the strain carrying the erm gene on pAM77 (Figure 3B). These data suggest that CSY0073 provides no selective advantage for treating erm-carrying strains of bacteria.
Figure 3.
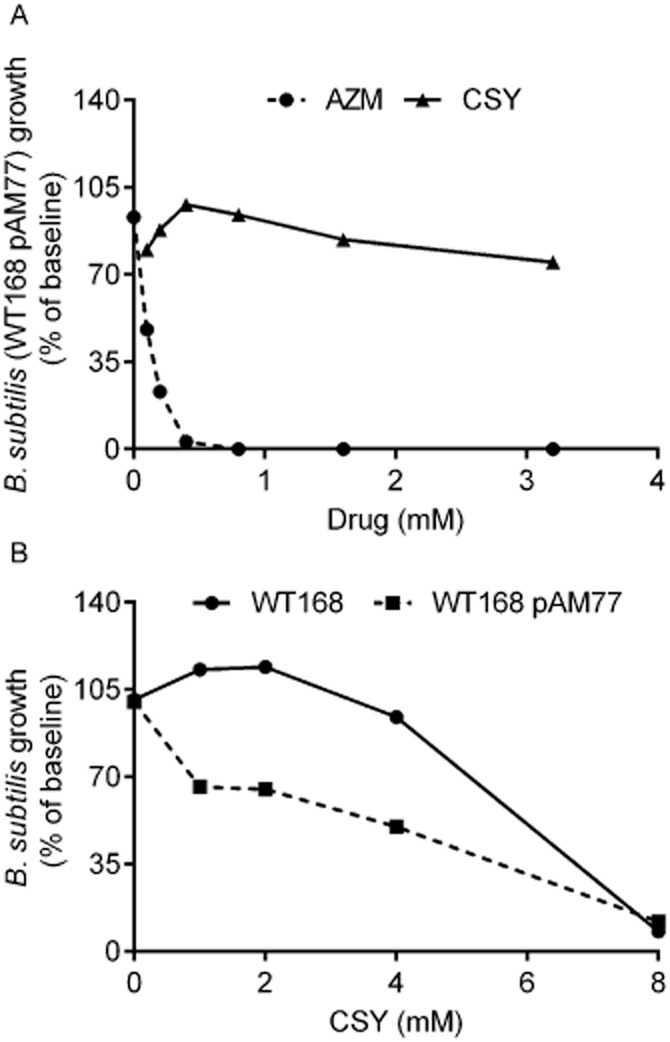
CSY0073 does not inhibit the growth of Bacillus subtilis WT168 or the corresponding strain carrying the erm resistance gene. The antibacterial activity of increasing concentrations (0–3 mM) of CSY0073 (CSY) or AZM was determined in liquid cultures using B. subtilis (WT168 pAM77) (A). The anti-bacterial activity of increasing concentrations (0–10 mM) of CSY0073 on B. subtilis (WT168) or B. subtilis (WT168 pAM77) was determined in liquid cultures (B). Data are mean values for two independent experiments with three replicates in each.
CSY0073 attenuated acute lung inflammation in mice challenged with P. aeruginosa LPS
Total and differential cell counts and pro-inflammatory cytokine levels in BAL fluids collected from C57BL/6J mice 3 and 24 h after intranasal instillation of P. aeruginosa LPS or PBS were compared in the groups given CSY0073, azithromycin or vehicle (V: DMSO). As expected, after PBS instillation, the vast majority of cells in the BAL fluid were macrophages (Figure 4A). In BAL fluids obtained 3 h after the LPS challenge, the macrophage and neutrophil counts were similar in the CSY0073, azithromycin and vehicle groups, and were not significantly different from those obtained after PBS instillation. The BAL fluids obtained after 24 h showed an increase in neutrophil counts (Figure 4B) and a decrease in macrophages numbers. Compared with vehicle, CSY0073 significantly diminished the 24-hour neutrophil count, by about 23% and azithromycin reduced it by about 63%, which was significantly different from the CSY0073 group (Figure 4B). None of the drugs had any effect on the macrophages counts at 3 h and 24 h.
Figure 4.
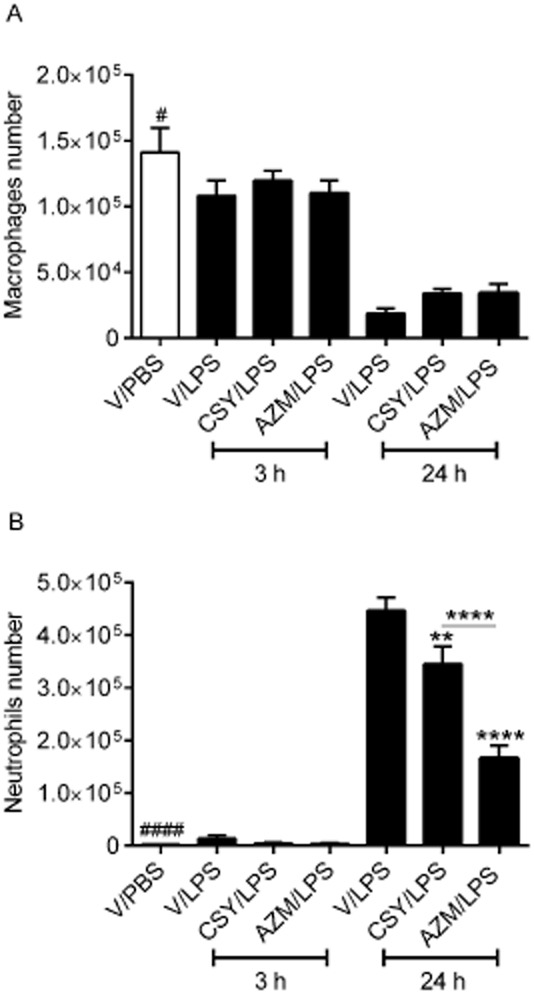
CSY0073 inhibits airway neutrophil recruitment induced by Pseudomonas aeruginosa LPS. Counts of (A) macrophages and (B) neutrophils 3 and 24 h after challenge with 100 μg·kg−1 LPS or PBS. Mice were pretreated 2 h before the challenge with vehicle (V: DMSO), CSY0073 (CSY) or AZM. Data are mean ± SEM of 7–8 mice per group. Statistical analysis was done for each time point, 3 h (*) or 24 h (#), by use of anova followed by Bonferroni's multiple comparison test. ***P < 0.001 and ****P < 0.0001.
LPS challenge (V/LPS: positive control) induced a transient but marked inflammatory response as shown by the increases in 3 h BAL fluid levels of IL-6 (Figure 5A), TNF-α (Figure 5B), CXCL1 (Figure 5C) and CXCL2 (Figure 5D), compared with V/PBS (negative control). Both azithromycin and CSY0073 decreased the levels of TNF-α (Figure 5B), CXCL1 (Figure 5C) and CXCL2 (Figure 5D) compared with vehicle. LPS did not increase the release of IL-1β (Figure 5E) or IL-12p40 (Figure 5F) compared with PBS at 3 h, whereas BAL fluids from 24 h LPS-challenged mice (Figure 5) contained higher levels of IL-6 (Figure 5A), IL-1β (Figure 5E) and IL-12p40 (Figure 5F) compared with PBS. Only azithromycin reduced the BAL IL-1β levels from 24 h LPS-challenged mice.
Figure 5.
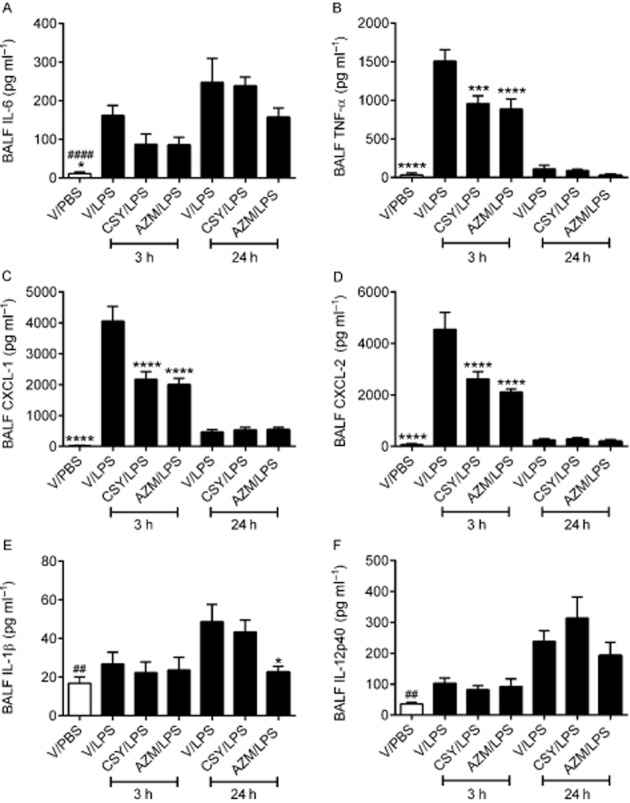
CSY0073 inhibits the production of TNF-α, CXCL1 and CXCL2 in airways induced by Pseudomonas aeruginosa LPS. (A) IL-6 (B) TNF-α, (C) CXCL1, (D) CXCL2, (E) IL-1β and (F) IL-12p40 in bronchoalveolar lavage fluid (BALF) 3 and 24 h after challenge with 100 μg·kg−1 LPS or PBS. Mice were pretreated for 2 h with vehicle (V: DMSO), CSY0073 (CSY) or AZM. Data are mean ± SEM for 7–8 mice per group. Statistical analysis was done for each time point, 3 h (*) or 24 h (#) with anova followed by Bonferroni's multiple comparison test. **P < 0.01, ***P < 0.001 and ****P < 0.0001.
Supernatants of lung homogenates from 3 h LPS-challenged mice (Figure 6) contained higher levels of IL-6 (Figure 6A), TNF-α (Figure 6B), CXCL1 (Figure 6C), CXCL2 (Figure 6D), IL-1β (Figure 6E) and IL-12p40 (Figure 6F) compared with PBS. Among these cytokines, only IL-6, CXCL2 and IL-1β were decreased by azithromycin and CSY0073 compared with vehicle. Supernatants of lung homogenates from 24 h LPS-challenged mice (Figure 6) contained higher levels of IL-1β (Figure 6E) and IL-12p40 (Figure 6F) compared with PBS, but only azithromycin reduced this increased IL-1β level.
Figure 6.
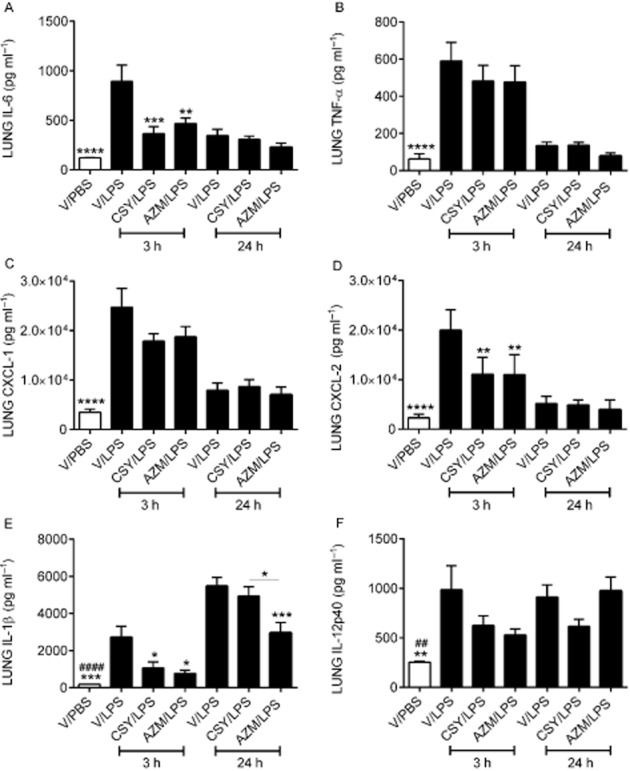
CSY0073 inhibits the production of IL-6, TNF-α, CXCL1 and CXCL2 induced in lung tissue by Pseudomonas aeruginosa LPS. (A) IL-6 (B) TNF-α, (C) CXCL1, (D) CXCL2, (E) IL-1β and (F) IL-12p40 in lung homogenate supernatants 3 and 24 h after challenge with 100 μg·kg−1 LPS or PBS. Mice were pretreated for 2 h with vehicle (V: DMSO), CSY0073 (CSY) or AZM. Data are the mean ± SEM for 7–8 mice per group. Statistical analysis was done for each time point, 3 h (*) or 24 h (#) with ANOVA followed by Bonferroni's multiple comparison test. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.001.
CSY0073 inhibited alveolar macrophage, but not lung epithelial cell, inflammation induced by P. aeruginosa LPS
Stimulation of primary alveolar macrophages with P. aeruginosa LPS resulted in increased IL-1β (Figure 7A), IL-12p40 (Figure 7B), CXCL1 (Figure 7C), IL-6 (Figure 7D) and TNF-α production (Figure 7E). Both azithromycin and CSY0073 dose-dependently decreased the production of IL-1β and IL-6 by primary murine alveolar macrophages (Figure 7A) compared with vehicle. Both drugs also decreased CXCL1, but this effect was not dose-dependent (Figure 7C, D). Neither drug affected the production of TNF-. We observed that low concentrations of CSY0073 (10 and 20 μM) moderately increased IL-12p40 production by the macrophages.
Figure 7.
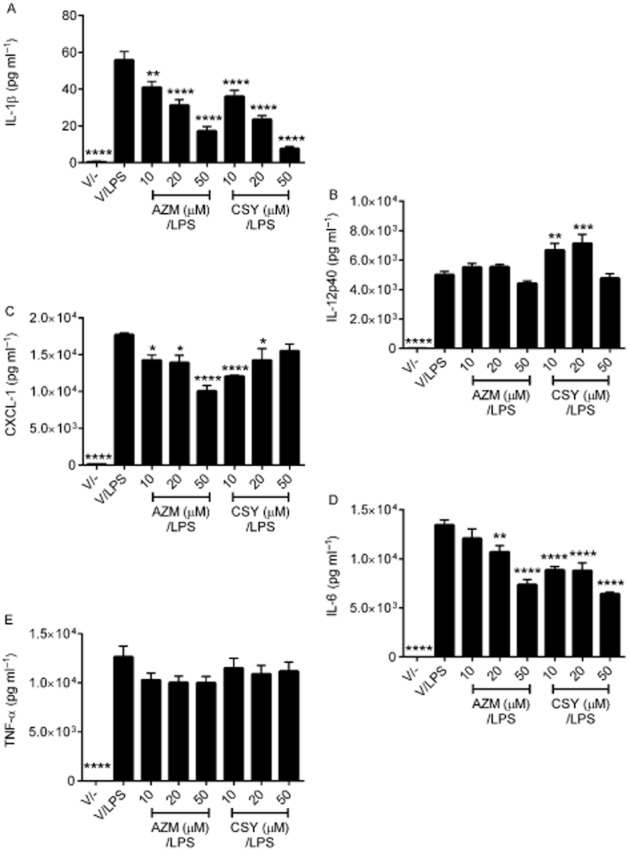
CSY0073 inhibits the production of IL-1β, CXCL1 and IL6 by primary alveolar macrophages stimulated with Pseudomonas aeruginosa LPS. Alveolar macrophages from bronchoalveolar lavage fluid (BALF) from C57BL/6 mice were pretreated for 24 h with vehicle (V: citric acid), CSY0073 (CSY) or AZM (10–50 μM) then stimulated with 1 μg·mL−1 LPS for 24 h. IL-1β (A), IL12-p40 (B), CXCL1 (C), IL-6 (D), and TNF-α (E) were measured in the supernatants. Data are mean ± SEM of three independent experiments performed in triplicate. Statistical analysis was done with anova followed by Dunnett's multiple comparison test. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.001.
Stimulation of bronchial epithelial cells, both primary (Figure 8A) and BEAS-2B cell line (Figure 8B) with P. aeruginosa LPS resulted in increased IL-8 production. Neither CSY0073 nor azithromycin decreased IL-8 levels of primary cells compared with vehicle (Figure 8A). Similarly, with BEAS2-B cells, IL-8 levels were unaffected by azithromycin or CSY0073 given before or concomitantly with LPS (Figure 8B).
Figure 8.
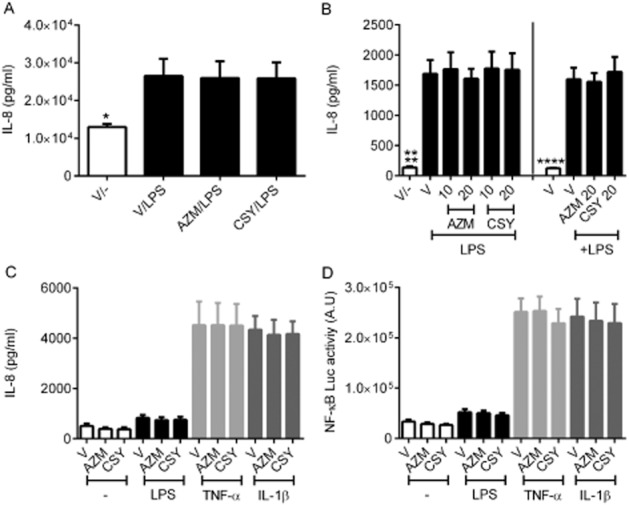
CSY0073 fails to decrease IL-8 production or NF-κB activation in lung respiratory epithelial cells. (A) MucilAir™ primary air-liquid lung epithelial cells were pretreated for 24 h at the basal side with vehicle (V: citric acid) 20 μM of CSY0073 (CSY) or AZM then stimulated at the apical side with 50 μL of Pseudomonas aeruginosa LPS 10 μg·mL−1 for 24 h. Data are the mean ± SEM of one experiment performed in quadriplicate (four different patients). Statistical analysis was with Student's t-test. (B) BEAS-2B cells were treated for 24 h with V, CSY0073 (CSY) or AZM. Treatment with these drugs was started 24 h before or concomitantly with the 24 h P. aeruginosa LPS 1 μg·mL−1 treatment. Data are the mean ± SEM of three experiments performed in triplicate. (C) and (D). (C) A549/NF-κB-luc cells were pretreated for 24 h with V, 20 μM of CSY0073 (CSY) or AZM then exposed for 6 h to 1 μg·mL−1 P. aeruginosa LPS, 10 nM TNF-α, or 0.1 nM IL-1β. IL-8 was measured in the supernatants and (D) NF-κB luciferase activity in the cell lysates. Data are the mean ± SEM of five experiments performed in triplicate. Statistical analysis was done with anova followed by Bonferroni's multiple comparisons test (B, C, and D). Each value was compared with the positive control condition (V for all three stimuli). *P < 0.05 and ****P < 0.0001.
We then used A549/NFκB-luc cells to evaluate effects on IL-8 release and NFκB-activation. Stimulation of A549/NFκB-luc cells by LPS, TNF-α and IL-1β resulted in an increased IL-8 production (Figure 8C) associated with NF-κB activation (Figure 8D), compared with untreated cells. We observed that, neither azithromycin nor CSY0073 decreased IL-8 production or NFκB activation for any of the inflammatory agents tested.
Discussion and conclusions
Our results show that CSY0073, in contrast to azithromycin, has no bacteriostatic activity against P. aeruginosa, S. aureus or H. influenza, three pathogens that often colonize the airways of patients with CF or other chronic respiratory tract diseases. In clinical practice, the main issue is whether a therapy will positively select for organisms that are specifically or multi-resistant to antibacterial agents. Given that there was no obvious effect of CSY0073 on various growth forms of P. aeruginosa, this requirement appears to be met. Moreover, the observation that B. subtilis tolerated CSY0073 to a slightly greater extent than the strain carrying the erm gene on pAM77 suggest that CSY0073 provides no selective advantage for erm carrying strains. Consequently, the increase in antimicrobial resistance reported with long-term azithromycin use would be unlikely to occur with CSY0073 (Spagnolo et al., 2012). The established biofilm produced by three P. aeruginosa strains (PAO1, PAK and PA14) was partly destroyed by azithromycin but was unaffected by CSY0073. It is hard to speculate whether this loss of anti-biofilm activity would be detrimental for patients. Indeed, a decrease in mucoid P. aeruginosa from sputum samples from CF patients has been observed and may be attributable to the anti-biofilm activity of AZM (Hansen et al., 2005). However, metanalyses of clinical studies with subgroup analysis, with or without chronic P. aeruginosa has not provided sufficient evidence to promote azithromycin therapy for a particular group of patients (Southern et al., 2012).
Similar to azithromycin, CSY0073 was able to attenuate the in vivo inflammatory lung response to LPS, as shown by the nearly 25% decrease in neutrophil numbers recruited in BAL fluid and decreases in pro-inflammatory cytokine levels in BAL fluid (IL-6, TNF-α, CXCL1 and CXCL2) and lung homogenates (IL-6, IL-1β and CXCL-1) from LPS-challenged mice. IL-12-p40 levels were not affected suggesting that only specific intracellular signalling pathways are affected by these drugs. However, azithromycin was more effective than CSY0073 at reducing neutrophil recruitment, decreasing it by nearly 63%. The effects of azithromycin observed in the present study are consistent with those obtained previously in a similar acute LPS challenge model (Bosnar et al., 2009): they observed decreased BAL fluid neutrophil counts and levels of pro-inflammatory mediators, including IL-1β, CXCL-2, TNF-α and IL-6, in lung homogenate in the presence of azithromycin. In the present study, the difference in the effects of the two drugs at reducing neutrophil influx in the airways may be explained by the inability of CSY0073 to strongly reduce IL-1β, as observed in the lung tissue. Indeed, it was recently suggested that the azithromycin-induced reduction in neutrophil recruitment induced by LPS is strongly dependent on IL-1-β levels (Bosnar et al., 2010). This difference in efficacy may also be attributable to the administration route, treatment time and/or the dose chosen. In our in vivo mouse experiments, we used a 2 h azithromycin pretreatment at a dose of 200 mg·kg−1i.p., as in an earlier study (Bosnar et al., 2012). In this earlier study, based on an oral bioavailability of 35–55%, it was estimated that the effective dose of azithromycin after i.p. administration is 200–300 mg·kg−1. This dose is lower than that used in a previous similar acute LPS model where azithromycin 600 mg·kg−1 administered p.o. was the effective dose (Bosnar et al., 2009); this is substantially higher than the azithromycin dose recommended for the treatment of chronic lung diseases such as CF (500 mg day-1) (Flume et al., 2007). In this context, chronic azithromycin therapy at a much lower dose, 10 mg·kg−1 day−1, in Cftr mice (F508del homozygous) challenged with LPS was successful in attenuating neutrophil recruitment and cytokine (CXCL2 and TNF-α) production in the airways (Legssyer et al., 2006). Therefore, more studies need to be performed to determine the effects of chronic treatment with CSY0073 at lower oral doses.
In alveolar macrophages, CSY0073 had a similar anti-inflammatory profile to that of azithromycin in our study. With primary alveolar macrophages exposed to P. aeruginosa LPS, IL-1β, IL-6 and CXCL1 were decreased similarly with CSY0073 and azithromycin, whereas neither drug diminished the levels of TNF-α or IL-12p40. IL12p40 was slightly increased by CSY0073; however, this is unlikely to have any effect in vivo because IL12p40 levels in BAL fluids and supernatants of whole lung were not affected. Interestingly, primary CF alveolar macrophages from mice have an exaggerated cytokine production, which is reduced by azithromycin (Meyer et al., 2009). Also, recently, using a conditional inactivation of Cftr in myeloid-derived cells, it has been demonstrated that myeloid cells, like alveolar macrophages, contribute to the pathophysiological phenotype of the CF lung (Bonfield et al., 2012). Thus, according to these studies and our in vitro data with CSY0073, future investigations using CF myeloid cells from mice should be done in order to better reflect any benefits this drug may have on the physiopathology of CF.
In contrast to their other anti-inflammatory effects, in our study neither CSY0073 nor azithromycin had an anti-inflammatory action in lung epithelial cells or on IL-8 production. IL-8 is one of the main cytokines produced by the respiratory epithelium and is involved in neutrophil recruitment in various chronic lung diseases. Azithromycin has been shown to have variable effects on the inflammatory response of respiratory epithelial cells (Shinkai et al., 2006; Gavilanes et al., 2009; Saint-Criq et al., 2012). Neither azithromycin nor CSY0073 affected IL-8 production by primary differentiated air-liquid bronchial cells or lung epithelial cell lines (BEAS-2B, A549/NF-κB-luc) in our study, in keeping with earlier studies of epithelial cells from CF patients (Gavilanes et al., 2009; Saint-Criq et al., 2012). However, azithromycin has been reported to upregulate IL-8 expression by primary human bronchial cells (Shinkai et al., 2006). In comparison to our results with primary air-liquid differentiated cells, this discrepancy may be explained by the differentiation state of the primary cells (not air-liquid differentiated) as well as the LPS serotype and dose (E. coli 011:B4, 100 μg·mL−1) that were used. For example, azithromycin failed to decrease the murine functional analogues of IL-8, CXCL1 and CXCL2, produced by primary tracheal epithelial cells from Cftr (F508del homozygous) mice in response to LPS (Gavilanes et al., 2009).
Several new macrolides are under development as potential treatments for patients with chronic lung diseases and limited treatment options, such as CF and COPD (Bosnar et al., 2012; Kobayashi et al., 2013; Tarran et al., 2013). In clinical studies of patients with CF and COPD, the mechanisms associated with the beneficial action of azithromycin (increased weight, reduction of exacerbations) are thought to be due to an anti-inflammatory action, but this has not been conclusively demonstrated (Albert et al., 2011; Southern et al., 2012). CSY0073 may help to elucidate the mechanisms by which azithromycin produces these clinical benefits in that it will not modulate bacterial growth. The emergence of resistance to azithromycin is an undesirable outcome of its broader use and, according to various authorities, should be prevented by not using it as a general anti-inflammatory drug (Albert et al., 2012).
Our data suggest that the azithromycin analogue CSY0073, despite having no effect on bacteria, has potential as a therapeutic for attenuating lung inflammation. Moreover, CSY0073 is unlikely to contribute to the emergence of bacterial-resistant strains. CSY0073 is in development for both CF and COPD with the objective of determining whether its anti-inflammatory effects alone are sufficient to reproduce the clinical benefits associated with azithromycin.
Acknowledgments
We thank Amruta Joshi-Datar, Isabelle Ventre, Alain Filloux and Lhousseine Touqui who generously provided the bacterial strains used in this study. LG received a grant from the French cystic-fibrosis non-profit organization Vaincre la mucoviscidose, a Legs Poix grant from the publicly funded source Chancellerie des Universités de Paris and a grant from Aviesan ITMO (Institut Multi-Organismes) Immunologie Hématologie Pneumologie (IHP). DL received a grant from the AXA Research Fund.
Glossary
- AZM
azithromycin
- BAL
bronchoalveolar lavage
- CF
cystic fibrosis
- COPD
chronic obstructive pulmonary disease
- LB
Luria Bertoni broth
Statement of conflicts of interest
MB is a shareholder of Synovo GmbH, which holds US patents in the field of compounds such as US7767797. GG and UH are employed by Synovo GmbH.
References
- Albert RK, Connett J, Bailey WC, Casaburi R, Cooper JA, Jr, Criner GJ, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689–698. doi: 10.1056/NEJMoa1104623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert RK, Connett J, Woodruff PG. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;367:773–774. doi: 10.1056/NEJMc1207269. author reply 775. [DOI] [PubMed] [Google Scholar]
- Baumann U, King M, App EM, Tai S, Konig A, Fischer JJ, et al. Long term azithromycin therapy in cystic fibrosis patients: a study on drug levels and sputum properties. Can Respir J. 2004;11:151–155. doi: 10.1155/2004/747841. [DOI] [PubMed] [Google Scholar]
- Bernier SP, Lebeaux D, DeFrancesco AS, Valomon A, Soubigou G, Coppee JY, et al. Starvation, together with the SOS response, mediates high biofilm-specific tolerance to the fluoroquinolone ofloxacin. PLoS Genet. 2013;9:e1003144. doi: 10.1371/journal.pgen.1003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfield TL, Hodges CA, Cotton CU, Drumm ML. Absence of the cystic fibrosis transmembrane regulator (Cftr) from myeloid-derived cells slows resolution of inflammation and infection. J Leukoc Biol. 2012;92:1111–1122. doi: 10.1189/jlb.0412188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosnar M, Bosnjak B, Cuzic S, Hrvacic B, Marjanovic N, Glojnaric I, et al. Azithromycin and clarithromycin inhibit lipopolysaccharide-induced murine pulmonary neutrophilia mainly through effects on macrophage-derived granulocyte-macrophage colony-stimulating factor and interleukin-1beta. J Pharmacol Exp Ther. 2009;331:104–113. doi: 10.1124/jpet.109.155838. [DOI] [PubMed] [Google Scholar]
- Bosnar M, Cuzic S, Bosnjak B, Nujic K, Ergovic G, Marjanovic N, et al. Azithromycin inhibits macrophage interleukin-1beta production through inhibition of activator protein-1 in lipopolysaccharide-induced murine pulmonary neutrophilia. Int Immunopharmacol. 2010;11:424–434. doi: 10.1016/j.intimp.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Bosnar M, Kragol G, Kostrun S, Vujasinovic I, Bosnjak B, Bencetic Mihaljevic V, et al. N′-substituted-2′-O,3′-N-carbonimidoyl bridged macrolides: novel anti-inflammatory macrolides without antimicrobial activity. J Med Chem. 2012;55:6111–6123. doi: 10.1021/jm300356u. [DOI] [PubMed] [Google Scholar]
- Cai Y, Chai D, Wang R, Bai N, Liang BB, Liu Y. Effectiveness and safety of macrolides in cystic fibrosis patients: a meta-analysis and systematic review. J Antimicrob Chemother. 2011;66:968–978. doi: 10.1093/jac/dkr040. [DOI] [PubMed] [Google Scholar]
- Fernandez-Olmos A, Garcia-Castillo M, Maiz L, Lamas A, Baquero F, Canton R. In vitro prevention of Pseudomonas aeruginosa early biofilm formation with antibiotics used in cystic fibrosis patients. Int J Antimicrob Agents. 2012;40:173–176. doi: 10.1016/j.ijantimicag.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Flume PA, O'Sullivan BP, Robinson KA, Goss CH, Mogayzel PJ, Jr, Willey-Courand DB, et al. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2007;176:957–969. doi: 10.1164/rccm.200705-664OC. [DOI] [PubMed] [Google Scholar]
- Gavilanes X, Huaux F, Meyer M, Lebecque P, Marbaix E, Lison D, et al. Azithromycin fails to reduce increased expression of neutrophil-related cytokines in primary-cultured epithelial cells from cystic fibrosis mice. J Cyst Fibros. 2009;8:203–210. doi: 10.1016/j.jcf.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Guillot L, Medjane S, Le-Barillec K, Balloy V, Danel C, Chignard M, et al. Response of human pulmonary epithelial cells to lipopolysaccharide involves Toll-like receptor 4 (TLR4)-dependent signaling pathways: evidence for an intracellular compartmentalization of TLR4. J Biol Chem. 2004;279:2712–2718. doi: 10.1074/jbc.M305790200. [DOI] [PubMed] [Google Scholar]
- Guillot L, Carroll SF, Homer R, Qureshi ST. Enhanced innate immune responsiveness to pulmonary Cryptococcus neoformans infection is associated with resistance to progressive infection. Infect Immun. 2008;76:4745–4756. doi: 10.1128/IAI.00341-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen CR, Pressler T, Koch C, Hoiby N. Long-term azitromycin treatment of cystic fibrosis patients with chronic Pseudomonas aeruginosa infection; an observational cohort study. J Cyst Fibros. 2005;4:35–40. doi: 10.1016/j.jcf.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Hayes D, Jr, Lloyd EA, Fitch JA, Bush A. ABCA3 transporter deficiency. Am J Respir Crit Care Med. 2012;186:807. doi: 10.1164/ajrccm.186.8.807a. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Wada H, Rossios C, Takagi D, Higaki M, Mikura S, et al. A novel macrolide solithromycin exerts superior anti-inflammatory effect via NF-kappaB inhibition. J Pharmacol Exp Ther. 2013;345:76–84. doi: 10.1124/jpet.112.200733. [DOI] [PubMed] [Google Scholar]
- Legssyer R, Huaux F, Lebacq J, Delos M, Marbaix E, Lebecque P, et al. Azithromycin reduces spontaneous and induced inflammation in DeltaF508 cystic fibrosis mice. Respir Res. 2006;7:134. doi: 10.1186/1465-9921-7-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mencarelli A, Distrutti E, Renga B, Cipriani S, Palladino G, Booth C, et al. Development of non-antibiotic macrolide that corrects inflammation-driven immune dysfunction in models of inflammatory bowel diseases and arthritis. Eur J Pharmacol. 2011;665:29–39. doi: 10.1016/j.ejphar.2011.04.036. [DOI] [PubMed] [Google Scholar]
- Meyer M, Huaux F, Gavilanes X, Brule van den S, Lebecque P, Lo Re S, et al. Azithromycin reduces exaggerated cytokine production by M1 alveolar macrophages in cystic fibrosis. Am J Respir Cell Mol Biol. 2009;41:590–602. doi: 10.1165/rcmb.2008-0155OC. [DOI] [PubMed] [Google Scholar]
- Mulet X, Macia MD, Mena A, Juan C, Perez JL, Oliver A. Azithromycin in Pseudomonas aeruginosa biofilms: bactericidal activity and selection of nfxB mutants. Antimicrob Agents Chemother. 2009;53:1552–1560. doi: 10.1128/AAC.01264-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phaff SJ, Tiddens HA, Verbrugh HA, Ott A. Macrolide resistance of Staphylococcus aureus and Haemophilus species associated with long-term azithromycin use in cystic fibrosis. J Antimicrob Chemother. 2006;57:741–746. doi: 10.1093/jac/dkl014. [DOI] [PubMed] [Google Scholar]
- Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881–1890. doi: 10.1056/NEJMoa1003833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin BK, Henke MO. Immunomodulatory activity and effectiveness of macrolides in chronic airway disease. Chest. 2004;125(2 Suppl):70S–78S. doi: 10.1378/chest.125.2_suppl.70s. [DOI] [PubMed] [Google Scholar]
- Saiman L, Anstead M, Mayer-Hamblett N, Lands LC, Kloster M, Hocevar-Trnka J, et al. Effect of azithromycin on pulmonary function in patients with cystic fibrosis uninfected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA. 2011;303:1707–1715. doi: 10.1001/jama.2010.563. [DOI] [PubMed] [Google Scholar]
- Saint-Criq V, Ruffin M, Rebeyrol C, Guillot L, Jacquot J, Clement A, et al. Azithromycin fails to reduce inflammation in cystic fibrosis airway epithelial cells. Eur J Pharmacol. 2012;674:1–6. doi: 10.1016/j.ejphar.2011.10.027. [DOI] [PubMed] [Google Scholar]
- Shinkai M, Rubin BK. Macrolides and airway inflammation in children. Paediatr Respir Rev. 2005;6:227–235. doi: 10.1016/j.prrv.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Shinkai M, Foster GH, Rubin BK. Macrolide antibiotics modulate ERK phosphorylation and IL-8 and GM-CSF production by human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L75–L85. doi: 10.1152/ajplung.00093.2005. [DOI] [PubMed] [Google Scholar]
- Southern KW, Barker PM, Solis-Moya A, Patel L. Macrolide antibiotics for cystic fibrosis. Cochrane Database Syst Rev. 2012;(11) doi: 10.1002/14651858.CD002203.pub4. CD002203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spagnolo P, Fabbri LM, Bush A. Long-term macrolide treatment for chronic respiratory disease. Eur Respir J. 2012;42:239–251. doi: 10.1183/09031936.00136712. [DOI] [PubMed] [Google Scholar]
- Tarran R, Sabater JR, Clarke TC, Tan CD, Davies CM, Liu J, et al. Nonantibiotic macrolides prevent human neutrophil elastase-induced mucus stasis and airway surface liquid volume depletion. Am J Physiol Lung Cell Mol Physiol. 2013;304:L746–L756. doi: 10.1152/ajplung.00292.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thouvenin G, Nathan N, Epaud R, Clement A. Diffuse parenchymal lung disease caused by surfactant deficiency: dramatic improvement by azithromycin. BMJ Case Rep. 2013;2013 doi: 10.1136/bcr-2013-009988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner T, Soong G, Sokol S, Saiman L, Prince A. Effects of azithromycin on clinical isolates of Pseudomonas aeruginosa from cystic fibrosis patients. Chest. 2005;128:912–919. doi: 10.1378/chest.128.2.912. [DOI] [PubMed] [Google Scholar]
- Wallace MR, Miller LK, Nguyen MT, Shields AR. Ototoxicity with azithromycin. Lancet. 1994;343:241. doi: 10.1016/s0140-6736(94)91030-8. [DOI] [PubMed] [Google Scholar]
- Wilms EB, Touw DJ, Heijerman HG. Pharmacokinetics of azithromycin in plasma, blood, polymorphonuclear neutrophils and sputum during long-term therapy in patients with cystic fibrosis. Ther Drug Monit. 2006;28:219–225. doi: 10.1097/01.ftd.0000195617.69721.a5. [DOI] [PubMed] [Google Scholar]
- Yousef AA, Jaffe A. The role of azithromycin in patients with cystic fibrosis. Paediatr Respir Rev. 2010;11:108–114. doi: 10.1016/j.prrv.2009.12.003. [DOI] [PubMed] [Google Scholar]


