Abstract
Objective:
This cross-sectional study of acute ischemic stroke patients examined relationships between hypoglossal nerve conduction, sleep-disordered breathing (SDB), and its severity.
Methods:
Patients within 7 days of stroke underwent nocturnal respiratory monitoring with the ApneaLink device and hypoglossal nerve conduction studies.
Results:
Eighteen of 52 subjects (35% [95% confidence interval: 22%, 49%]) had an abnormal hypoglossal amplitude and 23 (44% [95% confidence interval: 30%, 59%]) had an abnormal hypoglossal latency. No differences were identified in hypoglossal nerve latency or amplitude between those with (n = 26) and without (n = 26) significant SDB, defined by an apnea-hypopnea index ≥15. However, hypoglossal nerve conduction latency was associated (linear regression p < 0.05) with SDB severity as reflected by the apnea-hypopnea index.
Conclusions:
Acute ischemic stroke patients have a high prevalence of hypoglossal nerve dysfunction. Further studies are needed to explore whether hypoglossal nerve dysfunction may be a cause or consequence of SDB in stroke patients and whether this association can provide further insight into the pathophysiology of SDB in this population.
Sleep-disordered breathing (SDB) is now recognized as an independent risk factor for stroke1 and a predictor of poor stroke outcomes.2 While SDB affects more than half of acute ischemic stroke patients,2 the reasons for this high prevalence are unknown.
Hypoglossal nerve dysfunction has been implicated as a possible contributor to SDB in the general population based on palatal denervation3 and nerve conduction study abnormalities in patients with SDB compared to those without SDB.4 However, hypoglossal nerve dysfunction has not been well studied in stroke patients, in whom a high prevalence of diabetes and other health conditions may make this dysfunction an even more important issue than in the general population. We therefore sought to investigate hypoglossal nerve conduction in stroke patients by SDB status and severity. We hypothesized that hypoglossal nerve distal latency would be more prolonged in stroke patients who have SDB than in those who do not.
METHODS
Inpatient acute ischemic stroke patients at the University of Michigan were eligible if they were 18 years or older and had the onset of stroke within the prior 7 days. Ischemic stroke was defined as an abrupt onset focal neurologic deficit that was associated with acute infarction on MRI or CT or that persisted for more than 24 hours and was thought to be attributable to cerebral ischemia. Patients were excluded if they had previous significant facial, head, or neck cancer, previous face or neck surgery, previous radiation therapy to head or neck, known diagnosis of peripheral neuropathy, any implanted electronic device, known pregnancy, or known diagnosis of sleep apnea.
Standard protocol approvals, registrations, and patient consents.
The study was approved by the University of Michigan Institutional Review Board and required written informed consent.
Comorbidity information was obtained from the medical record. NIH Stroke Scale score was abstracted from the admission note based on standard techniques.5 Signs of dysarthria and tongue motor abnormalities were abstracted from the admission note. Dysphagia was determined from Speech and Language Pathology consultation notes. Every patient who failed a bedside swallow test conducted by a nurse had a Speech and Language Pathology evaluation (n = 20, 38%). Location of infarction and TOAST (Trial of Org 10172 in Acute Treatment) stroke subtype6 were determined by a vascular neurologist after review of the hospitalization record and CT and MRI brain images.
The validated7 ApneaLink device (ResMed, San Diego, CA) was used to monitor nocturnal nasal pressure and oxygen saturation, and validated software analyzed the data (see appendix e-1 on the Neurology® Web site at Neurology.org).7 Significant SDB was defined by an ApneaLink apnea-hypopnea index (AHI) ≥15, based on a median split, and supported by the high sensitivity and specificity with this cutoff.7
Hypoglossal nerve conduction studies were performed with the use of standard, previously reported techniques4,8 by a registered EMG technologist masked to the ApneaLink results. Electrodes adhered to a tongue blade 2 cm apart8 were placed on the dorsal surface of the right side of the tongue, with a ground on the cheek. Because no difference exists between left and right,9 for standardization purposes, the right was selected. Electrical stimulation was performed along the base of the mandible with bipolar percutaneous stimulation set to 0.02-millisecond duration and 100 mA.9 Electrical stimulation duration was increased gradually until a supramaximal compound muscle action potential waveform was attained.
Statistical methods.
With the use of a 2-sample design and the assumption of an α of 0.05 and 80% power, approximately 17 patients in each group would be required to determine a difference of 0.7 second in hypoglossal distal latency. This assumes a mean of 2.19 with an SD of 0.7. We therefore planned to recruit at least 20 subjects with and 20 subjects without SDB.
A Wilcoxon rank sum test was used to compare the hypoglossal nerve distal latency (primary outcome) and amplitude, in those with and without SDB. Hypoglossal amplitude and latency, considered abnormal if they exceeded the 95% confidence interval (CI) of published normative data elicited using similar techniques,8 were calculated along with binomial 95% CI using the exact method, and compared by SDB status using χ2 tests and by infarction location (brainstem vs not brainstem) using a Fisher exact test. The square of Spearman ρ rank correlation was calculated to assess the correlation between nerve conduction findings and AHI. Because the results were significant for latency, the association between AHI and hypoglossal nerve conduction latency was also explored with linear regression. Because model diagnostics suggested possible nonadherence to linear regression assumptions when latency was treated continuously, it was also modeled using quartiles (as dummy variables), and in quartiles treated linearly (1–4). Assessment was made for overly influential observations (using S+’s “lm.influence”) and one was identified. Regression analyses were repeated with this single observation removed. Spotfire S+ 8.1 for Windows (TIBCO Software Inc., Palo Alto, CA) was used for all analyses.
RESULTS
Seventy-two patients consented for participation. Of the 52 subjects who had both nerve conduction and ApneaLink studies completed, median age was 55 years (interquartile range: 47, 68), 28 (54%) were male, and median body mass index was 30 kg/m2 (27, 34). Baseline findings (table 1) did not differ between subjects with and without SDB, with the exception that hypertension was more prevalent in the SDB group (23 [88%] vs 16 [62%], p = 0.03). Infarctions were located exclusively in the brainstem (12%), cerebellum (6%), cortex (8%), subcortical region (19%), and the mixed cortical and subcortical (37%) regions; the remainder were multifocal (19%). The median AHI was 15 (5, 30) overall, and 5 (3, 10) in the no SDB group (n = 26) and 31 (20, 42) in the SDB group (n = 26).
Table 1.
Baseline characteristics of acute stroke patients enrolled, by sleep-disordered breathing category
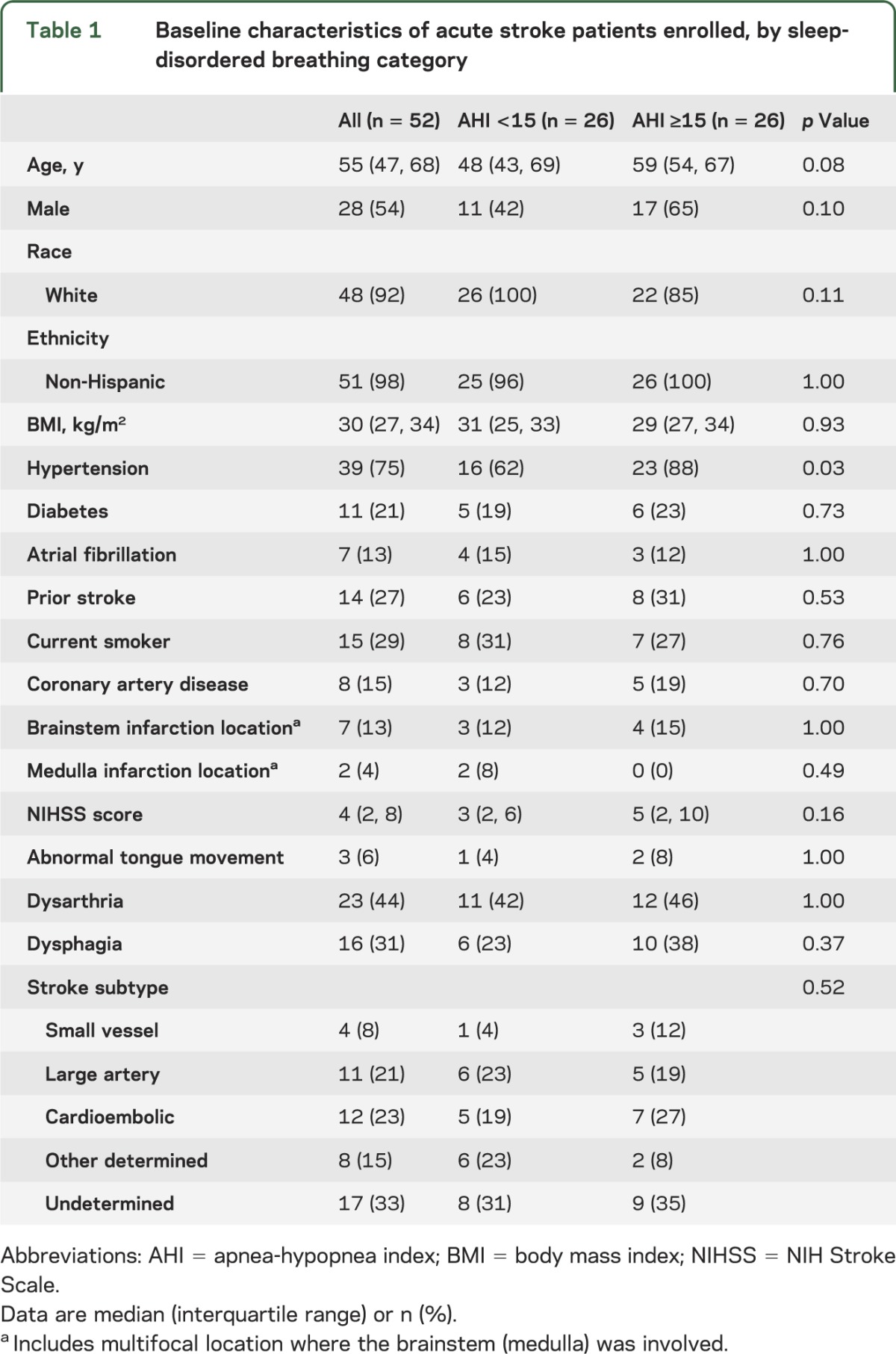
No differences were identified in median hypoglossal latency or amplitude between the 2 groups (table 2). Overall, 32 subjects (62% [95% CI: 47%, 75%]) had an abnormal hypoglossal amplitude or latency. Eighteen of the subjects (35% [95% CI: 22%, 49%]) had an abnormal hypoglossal nerve amplitude and 23 (44% [95% CI: 30%, 59%]) had an abnormal latency, with no difference in proportion abnormal by SDB status in amplitude (35% no SDB vs 35% SDB, p = 1.00). Abnormal latency was found nonsignificantly more often in those with (54%) vs those without (35%, p = 0.16) SDB. In those with and without brainstem infarction, the prevalence of abnormal amplitude (43% brainstem, 33% no brainstem; p = 0.68) and latency (43% brainstem, 47% no brainstem; p = 0.44) did not differ.
Table 2.
Nerve conduction study results by sleep-disordered breathing category
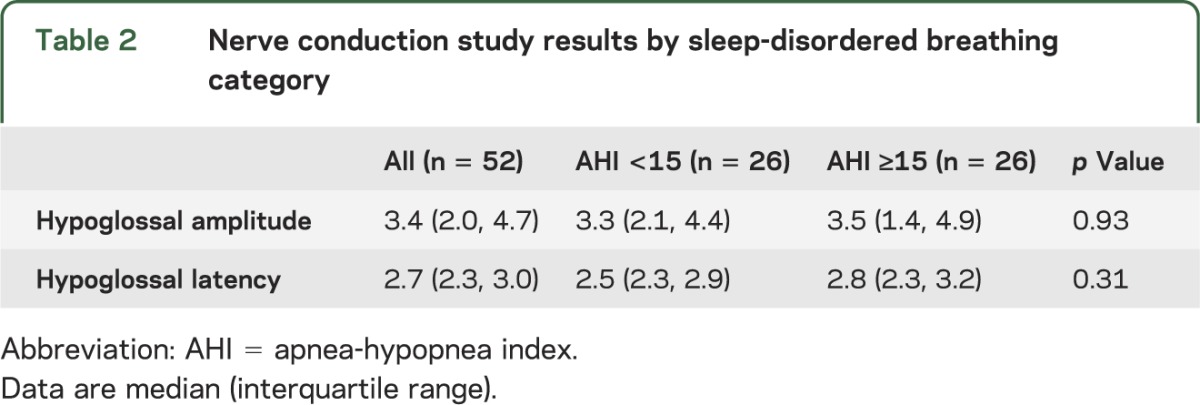
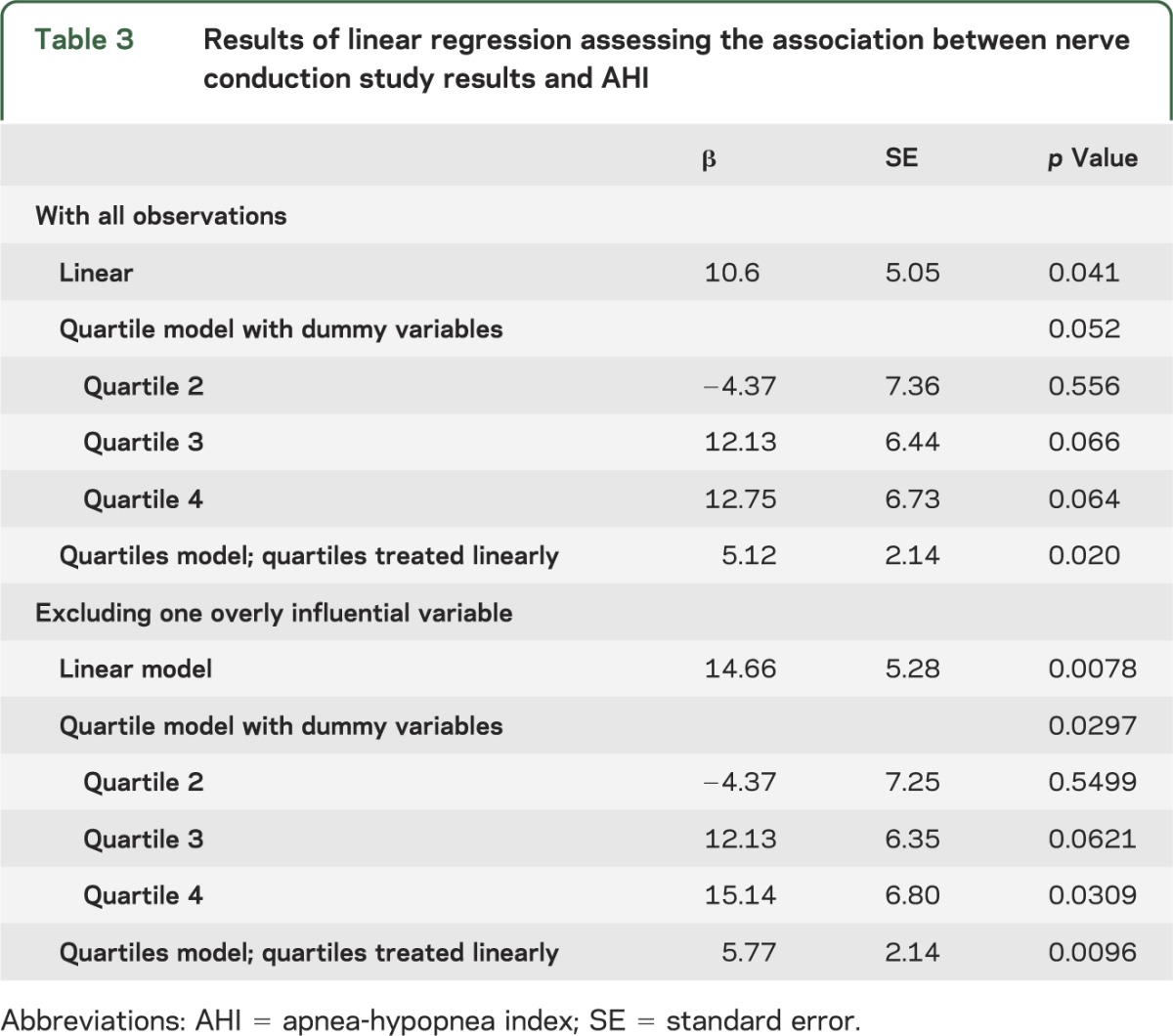
The AHI was correlated with hypoglossal latency (ρ2 = 7.7 × 10−2, p = 0.046) but not with hypoglossal nerve amplitude (ρ2 = 6.6 × 10−4, p = 0.856). Linear regression showed that hypoglossal nerve conduction latency was positively associated with AHI in all 3 models (table 3). With the single overly influential observation removed, findings were strengthened, again with positive associations found in all models (table 3).
Table 3.
Results of linear regression assessing the association between nerve conduction study results and AHI
DISCUSSION
These novel prospective data advance physiologic understanding of SDB in acute ischemic stroke patients. Although we did not confirm our hypothesis that hypoglossal nerve conduction latency is more prolonged in poststroke patients with SDB than in those without SDB, prolongation of hypoglossal nerve conduction latencies was associated with SDB severity. The association of conduction latency with the continuous variable (AHI) but not a dichotomized outcome (based on an AHI ≥15) suggests that the added statistical power provided by the continuous variable may have been necessary to identify the association. Hypoglossal nerve dysfunction was highly prevalent in stroke patients; subjects in this study had more than double the prevalence of abnormal hypoglossal amplitudes or latencies compared with non-SDB controls from a prior study4 in which the same normative values were applied.8 Although the reasons for hypoglossal nerve dysfunction in some stroke patients without significant SDB are unclear, given the known association between hypoglossal nerve dysfunction and SDB in the general sleep apnea population,4 the high prevalence of hypoglossal mononeuropathy identified may still relate to the high prevalence of SDB in stroke patients. Perhaps traumatic injury to the hypoglossal nerve due to chronic vibration from prestroke snoring accounts for the high prevalence. Prestroke snoring and poststroke AHI are known to have little association,10 and snoring was not objectively measured in the current study.
One previous study reported hypoglossal nerve conduction in only 8 ischemic stroke patients and found none with an abnormality.11 Our findings may differ by chance because of the small sample size used in the previous study, although differences in study populations could also provide an explanation.
The main limitation of the current study was use of the ApneaLink rather than polysomnography to assess SDB. However, portable respiratory devices are much more practical in the acute stroke hospitalization setting,12 and the ApneaLink has been well validated against polysomnography (see appendix e-1).7 Although we were not able to adjust for age and sex because of the small numbers, their association specifically with hypoglossal nerve conduction is not established. Furthermore, we did not collect data on an age-, sex-, and comorbidity-matched control group without SDB or stroke. Therefore, we are unable to test directly whether the high prevalence of hypoglossal dysfunction or association between AHI and hypoglossal dysfunction is specific to stroke. Because the number of brainstem infarctions in our sample was small, we had limited ability to investigate differences between hypoglossal nerve function by infarction location. However, our data suggested no difference in prevalence of abnormal hypoglossal nerve conduction when the brainstem was involved.
The current study demonstrated that ischemic stroke patients have a high prevalence of hypoglossal nerve dysfunction. Longitudinal tests of hypoglossal nerve conduction poststroke and studies that include polysomnography may be needed to determine whether hypoglossal nerve conduction studies could be a useful tool to study the pathophysiology of SDB evolution in stroke patients, and to explore further the reasons for a high prevalence of hypoglossal neuropathy in stroke patients.
Supplementary Material
GLOSSARY
- AHI
apnea-hypopnea index
- CI
confidence interval
- SDB
sleep-disordered breathing
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
D. Brown and R. Chervin: drafting/revising the manuscript for content, including medical writing for content, study concept or design, analysis or interpretation of data. J. Wolfe, R. Hughes, and M. Concannon: drafting/revising the manuscript for content, including medical writing for content, acquisition of data. L. Lisabeth: analysis or interpretation of data. K. Gruis: drafting/revising the manuscript for content, including medical writing for content, study concept or design, analysis or interpretation of data.
STUDY FUNDING
Supported in part by K23 NS051202 and K23 NS055200.
DISCLOSURE
D. Brown serves as an editorial board member of Neurology and Stroke, is funded by NIH grants R01 NS062675, R01 HL098065, R01 NS070941, and R18 HS017690, received research support from the Blue Cross Blue Shield of Michigan Foundation and Michigan Department of Community Health, and receives research support from the University of Michigan for stroke-related research. R. Chervin has conducted research funded by the NIH and the Michael J. Fox Foundation; was involved with unrestricted educational gifts to the University of Michigan from Philips Respironics and Fisher Paykel; serves on boards of directors for the American Academy of Sleep Medicine, American Sleep Medicine Foundation, American Board of Sleep Medicine, Associated Professional Sleep Societies, and International Pediatric Sleep Association; has served on the advisory boards for the nonprofit Sweet Dreamzzz and the National Heart, Lung, and Blood Institute; has consulted for Proctor & Gamble, MC3, and Zansors; serves as a section editor for UpToDate; is editing a book for Cambridge University Press; and has produced copyrighted material, patents, and patents pending, owned by the University of Michigan, for screening, diagnosis, and treatment of sleep disorders. J. Wolfe, R. Hughes, and M. Concannon report no disclosures relevant to the manuscript. L. Lisabeth is funded by NIH grants R01 NS38916, R01 NS062675, R01 HL098065, and R01 NS070941. K. Gruis has received research support from the NIH K23 NS055200 as well as the Amyotrophic Lateral Sclerosis and Muscular Dystrophy Associations. Since the original submission of the manuscript, Dr. Gruis changed positions and now works for Pfizer on conditions unrelated to the topics of this manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Yaggi HK, Concato J, Kernan WN, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 2005;353:2034–2041 [DOI] [PubMed] [Google Scholar]
- 2.Turkington PM, Allgar V, Bamford J, Wanklyn P, Elliott MW. Effect of upper airway obstruction in acute stroke on functional outcome at 6 months. Thorax 2004;59:367–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Svanborg E. Impact of obstructive apnea syndrome on upper airway respiratory muscles. Respir Physiol Neurobiol 2005;147:263–272 [DOI] [PubMed] [Google Scholar]
- 4.Ramchandren S, Gruis KL, Chervin RD, et al. Hypoglossal nerve conduction findings in obstructive sleep apnea. Muscle Nerve 2010;42:257–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams LS, Yilmaz EY, Lopez-Yunez AM. Retrospective assessment of initial stroke severity with the NIH Stroke Scale. Stroke 2000;31:858–862 [DOI] [PubMed] [Google Scholar]
- 6.Adams HJ, Bendixen B, Kappelle L, et al. Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial. Stroke 1993;24:35–41 [DOI] [PubMed] [Google Scholar]
- 7.Erman MK, Stewart D, Einhorn D, Gordon N, Casal E. Validation of the ApneaLink™ for the screening of sleep apnea: a novel and simple single-channel recording device. J Clin Sleep Med 2007;3:387–392 [PMC free article] [PubMed] [Google Scholar]
- 8.Campos A, Barona R, Escudero J, Montalt J, Escudero M. Hypoglossal nerve conduction study by transcranial magnetic stimulation in normal subjects. Otolaryngol Head Neck Surg 1995;112:520–525 [DOI] [PubMed] [Google Scholar]
- 9.Redmond MD, Di Benedetto M. Hypoglossal nerve conduction in normal subjects. Muscle Nerve 1988;11:447–452 [DOI] [PubMed] [Google Scholar]
- 10.Bassetti CL, Milanova M, Gugger M. Sleep-disordered breathing and acute ischemic stroke: diagnosis, risk factors, treatment, evolution, and long-term clinical outcome. Stroke 2006;37:967–972 [DOI] [PubMed] [Google Scholar]
- 11.Muellbacher W, Artner C, Mamoli B. Motor evoked potentials in unilateral lingual paralysis after monohemispheric ischaemia. J Neurol Neurosurg Psychiatry 1998;65:755–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown DL, Chervin RD, Kalbfleisch JD, et al. Sleep Apnea Treat After Stroke (SATS) Trial: is it feasible? J Stroke Cerebrovasc Dis 2013;22:1216–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


