Abstract
The incidence, diagnostic landscape, and workload impact of CNS inflammatory diseases other than multiple sclerosis (MS) (CIDOMS) in a tertiary setting is unknown. We describe a retrospective case series of 64 patients identified over a 2-year period (2009–2010) at the Wessex Neurological Centre in the United Kingdom, accounting for 4% of all patients seen at the center. As expected, neurosarcoidosis and neuromyelitis optica (NMO) were the most common diagnoses reached (14% each); other diagnoses singly accounted for <10%. However, the likeliest diagnostic outcome (strikingly, in 25%) was nondiagnosis, despite intensive investigation and a mean follow-up period of 3 years. Undiagnosed patients with CIDOMS represented the largest workload of the neurology center.
The incidence, diagnostic landscape, and workload impact of CNS inflammatory diseases other than multiple sclerosis (MS) (CIDOMS) in a tertiary setting is unknown. We describe a retrospective case series of 64 patients identified over a 2-year period (2009–2010) at the Wessex Neurological Centre in the United Kingdom, accounting for 4% of all patients seen at the center. As expected, neurosarcoidosis and neuromyelitis optica (NMO) were the most common diagnoses reached (14% each); other diagnoses singly accounted for <10%. However, the likeliest diagnostic outcome (strikingly, in 25%) was nondiagnosis, despite intensive investigation and a mean follow-up period of 3 years. Undiagnosed patients with CIDOMS represented the largest workload of the neurology center.
The Wessex Neurological Centre is a typical regional neurologic service with a catchment population of ∼3 million in southern England and on-site expertise in neurology, neurosurgery, neuropathology, neurophysiology, neuroradiology, neuropsychology, neurorehabilitation, and neurophysiotherapy, across which specialist interest in neuroinflammatory disease is adequately represented. The study was approved by the University of Southampton's Ethics and Research Governance. A diagnosis of CIDOMS was made when there was unequivocal evidence of CNS inflammation (with certainty of an inflammatory etiology based on clinical, radiologic, CSF, and other laboratory findings) in the absence of MS or clinically isolated syndrome (CIS). Published diagnostic criteria (or, in their absence, published consensus opinions) were utilized to establish specific CIDOMS diagnoses (tables e-1 and e-2 on the Neurology® Web site at Neurology.org). MS and CIS were diagnosed according to established criteria.1,2 Detailed evaluation was conducted regarding resource utilization, including inpatient episode duration, imaging, invasive procedures, neurophysiology, non-neurology specialist reviews, and laboratory tests. In order to provide a sense of proportion to figures, a similar analysis was performed in patients admitted during the same period whose final diagnosis was MS. Further method details are available online (e-Methods).
From a total of 1,525 patients admitted to the tertiary neurology center, 81 had a working diagnosis of CIDOMS, which was maintained in 64 cases as a final diagnosis. This represents an incidence rate of 11 cases of CIDOMS per million person-years. Seventeen patients with an initial working diagnosis of CIDOMS received a diagnosis of MS by the end of the follow up-period. A breakdown of individual CIDOMS diagnoses is given in table 1 (for more detail, see tables e-1 and e-2).
Table 1.
Number, percentage, and biennial total resource use of patients with CIDOMS by their final diagnostic categories
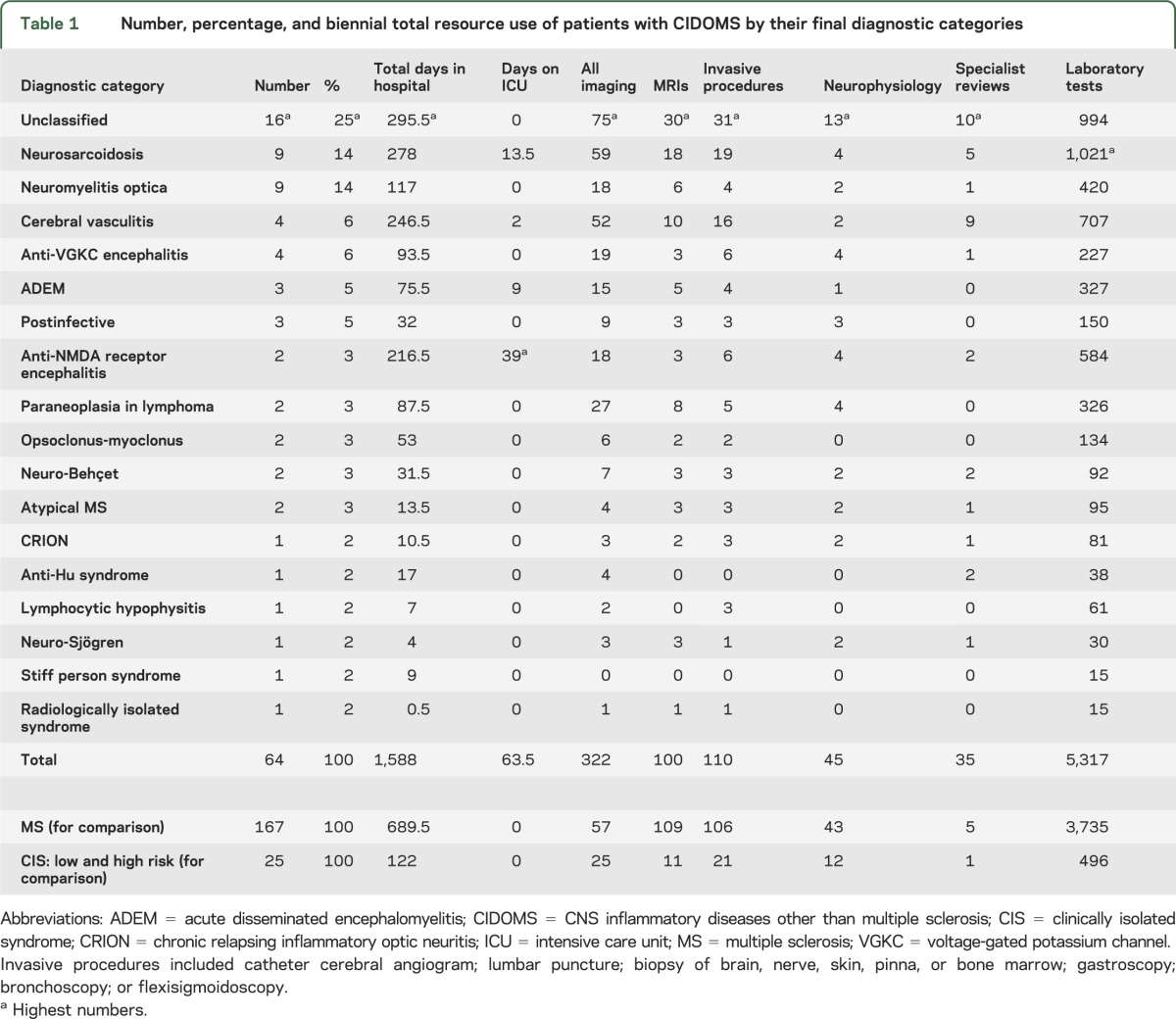
Despite their small number (n = 64) in comparison with MS patients (n = 167), patients with CIDOMS required disproportionately longer inpatient stays, more intensive care, and larger numbers of investigations (see table 1 and figure e-1, p < 0.001 across all categories). Naturally, this resulted in higher costs (£520,409.18 vs £259,941.51, i.e., twice as much, p < 0.0001, see table e-3 and figure e-2). Among patients with CIDOMS, those without a diagnosis represented the largest workload of the neurology center, since they collectively needed the longest inpatient stay and the greatest number of investigations (see table 1).
The definition of CIDOMS did not include CIS, and it may be argued that cases of CIS with low risk of conversion to MS (with normal MRI and CSF1) may turn out to have CIDOMS. None of the patients with a diagnosis of CIS converted to a diagnosis of CIDOMS in this study, but this may need longer follow-up, as illustrated by a recent case series.3 However, categorization of low-risk CIS with CIDOMS and high-risk CIS with MS maintains nondiagnosis as the most common outcome in patients with CIDOMS (16 out of 78, i.e., 21%), and maintains patients with CIDOMS as still more resource-intensive than patients with MS (data not shown).
Two undiagnosed patients with CIDOMS had a clinical phenotype that resembled NMO spectrum disorder, though not typical (last 2 cases in table e-2). Restriction of anti-aquaporin antibodies to the CSF,4 anti-aquaporin-4 antibody assay sensitivity,5,6 and antimyelin oligodendrocyte glycoprotein antibodies7 may be explanations. Yet again, a sensitivity analysis excluding these patients did not change conclusions.
Collectively CIDOMS are common and consist of up to 25% of the neurologic practice pertaining to inflammatory CNS disorders. The data highlight the importance of education regarding the diagnosis and treatment of these disorders. The striking finding that one-quarter of CIDOMS remained undiagnosed means that, in the absence of a diagnosis of neurosarcoidosis or NMO, an unclassified inflammatory disease is eventually more likely than other rarer diagnoses, which singly accounted for fewer than 10%. Broader serologic testing for individually rare antibodies and discovery of novel biomarkers should facilitate more rapid diagnoses in unclassified cases. Meanwhile, in the absence of a diagnosis, consensus guidelines to help recognition of antibody vs T-cell–mediated neuroinflammatory disorders will enable a rational empirical approach to treatment based on the likely underlying pathophysiologic mechanism.
Supplementary Material
Footnotes
Supplemental data at Neurology.org
Author contributions: Dr. Street: data acquisition, data analysis, drafting of the manuscript, and review of manuscript for important intellectual content. Dr. Halfpenny: review of the manuscript for important intellectual content. Dr. Galea: data acquisition, data analysis, drafting of the manuscript, review of the manuscript for important intellectual content, and study supervision.
Study funding: Supported by the Wellcome Trust/Academy of Medical Sciences (starter grant to I.G.) and the University of Southampton's Faculty of Medicine (D.S.).
Disclosure: D. Street reports no disclosures relevant to the manuscript. C. Halfpenny has received expenses to attend meetings from Biogen-Idec, Merck-Serono, and Teva-UK. I. Galea has received expenses to attend meetings from Teva-UK, has received commercial research grant support from Merck-Serono and IQ products, and holds research grants from the MS Society, Association of British Neurologists, Wessex Medical Research, Medical Research Council, and the Engineering, and Physical Sciences Research Council, UK. Go to Neurology.org for full disclosures.
This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.Miller DH, Chard DT, Ciccarelli O. Clinically isolated syndromes. Lancet Neurol 2012;11:157–169 [DOI] [PubMed] [Google Scholar]
- 2.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurne A, Isikay IC, Karlioguz K, et al. A clinically isolated syndrome: a challenging entity: multiple sclerosis or collagen tissue disorders: clues for differentiation. J Neurol 2008;255:1625–1635 [DOI] [PubMed] [Google Scholar]
- 4.McKeon A, Pittock SJ, Lennon VA. CSF complements serum for evaluating paraneoplastic antibodies and NMO-IgG. Neurology 2011;76:1108–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fujihara K, Leite MI. Seronegative NMO: a sensitive AQP4 antibody test clarifies clinical features and next challenges. Neurology 2013;80:2176–2177 [DOI] [PubMed] [Google Scholar]
- 6.Marignier R, Bernard-Valnet R, Giraudon P, et al. Aquaporin-4 antibody-negative neuromyelitis optica: distinct assay sensitivity-dependent entity. Neurology 2013;80:2194–2200 [DOI] [PubMed] [Google Scholar]
- 7.Kitley J, Woodhall M, Waters P, et al. Myelin-oligodendrocyte glycoprotein antibodies in adults with a neuromyelitis optica phenotype. Neurology 2012;79:1273–1277 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


