Abstract
The lipid phase transition (LPT) from the fluid liquid crystalline phase to the more rigid gel structure phase that occurs upon exposure to low temperatures can affect physical structure and function of cellular membranes. This study set out to investigate the membrane phase behavior of oocytes of three gorgonian corals; Junceela fragilis, J. juncea and Ellisella robusta,at different developmental stages after exposure to reduced temperatures. Oocytes were chilled to 5°C for 48, 96 or 144 h, and the LPT temperature (LPTT) was determined with Fourier Transform Infrared (FTIR) spectroscopy. The J. fragilis oocytes had a higher LPTT (∼23.0–23.7°C) than those of J. juncea and E. robusta oocytes (approximately 18.3–20.3°C). Upon chilling for 96 h at 5°C, the LPTTs of J. juncea and E. robusta oocytes in the early (18.0±1.0 and 18.3±0.6°C, respectively) and late (17.3±0.6 and 17.7±1.2°C, respectively) stages were significantly lower than those of J. fragilis oocytes (20.3±2.1 and 19.3±1.5°C for the early and late stages, respectively). The LPTTs of early stage gorgonian oocytes was significantly lower than those of late stage oocytes. These results suggest that the LPT of three gorgonian oocytes at different developmental stages may have been influenced by the phospholipid composition of their plasma membranes, which could have implications for their low temperature resistance.
Introduction
Cryopreservation technology applied to the preservation of coral germ cells has recently shown promise as a potential ex situ conservation technique [1]. Low temperature preservation of coral oocytes may ultimate become an essential tool for conservation of genetic resources and regeneration of populations of valuable and endangered coral species, as this life history stage is less susceptible to cryoinjuries [1], [2]. Our previous studies have found that hard coral (Echinopora spp.) and gorgonian coral (Junceella juncea and Junceella fragilis) oocytes had significant levels of chilling tolerance down to 5°C and 0°C; however, these oocytes were susceptible to chilling injuries at sub-zero temperatures. For instance, mitochondrial activities decreased dramatically after four hours of chilling [1], [2].
The microtubules [3], [4], [5], cytoskeletal organization [6], [7], [8], and intracellular organelles [9] of oocytes have all shown to be damaged upon exposure to reduced temperatures. It has been proposed that lipid phase transition (LPT) may have important implications for susceptibility to chilling injury, as LPT can affect cell membrane properties, such as their function and integrity [10], [11], [12]. When cells are chilled below their LPT temperature (LPTT), cell membranes undergo a transition from the liquid to the gel phase [12]. It has been reported that lowering the temperature results in profound changes in the phase transition of lipids of the gametes of various species, and LPT has been found to influence the degree of cryoinjury inpig sperm membranes [13] and shrimp embryos [14], as well as oocytes from humans [12], cattle [10], sheep [15], and zebrafish [16].
Phospholipids are major components of cellular membranes, and the transition from the gel phase to the liquid-crystalline face at low temperatures can cause membrane damage [17]. Our previous studies indicated that phosphatidylethanolamine (PE) and phosphatidylcholine (PC) are the main phospholipids in the membranes of oocytes from two gorgonian coral species; such a high PE and PC content helps to establish a higher surface viscosity and is associated with lower membrane melting temperatures [18]. The fatty acid composition of phospholipids also strongly influences the LPT profile of cellular membranes [15]. For instance, cellular membranes with short chain polyunsaturated fatty acids are more fluid at low temperatures and are more resistant to chilling stress [17], [18], [19], [20].
The fact that a low LPTT is associated with resistance to chilling stress [10], [12] suggests that coral species with greater degrees of cell membrane fluidity will more likely survive the cryopreservation process. Improving our understanding of the LPT mechanism through chilling experiments may therefore help in developing an effective cryopreservation method for chilling-sensitive coral cell types, including oocytes. In this study, we used Fourier transform infrared (FTIR) spectroscopy to investigate the membrane lipid phase behavior of oocytes of different developmental stages from three coral species; Junceela fragilis, J. juncea, and Euplexaura robusta, after low temperature exposure in order to better understand why these oocytes are so sensitive to dramatic reductions in temperature.
Results
LPT of Coral Oocytes
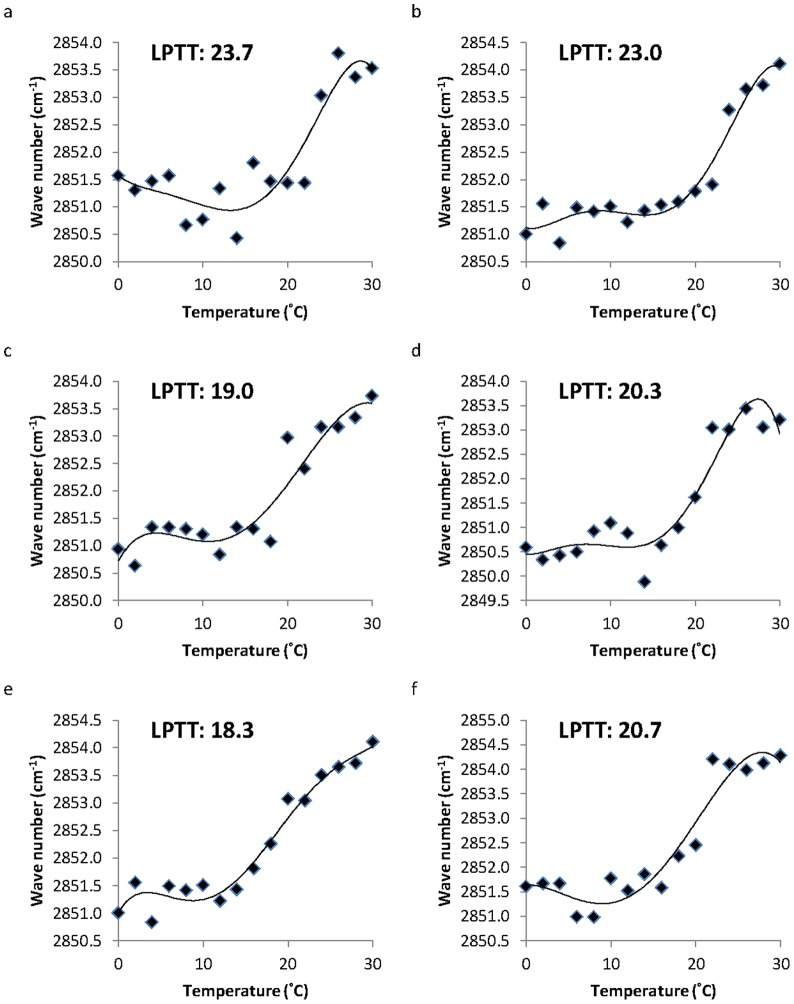
The LPT of three gorgonian oocyte membranes from the liquid crystalline to the gel phase revealed a different LPTT at different developmental stages (Fig. 1). The J. fragilis oocytes demonstrated LPTTs ranging from 23.0±0.6 to 23.7±0.7°C. In contrast, J. juncea and E. robusta oocytes were found to have LPTTs of approximately 18.3±0.7 and 20.3±0.7°C, respectively (Fig. 1). The LPT occurred at a significantly lower temperature in the early stage oocytes of J. juncea and E. robusta (p<0.05 for each species), whilst there were no significant differences in the LPTT between the early and late stage oocytes of J. fragilis (p>0.05, Fig. 1). The membrane LPTT in the early stage oocytes of J. fragilis, J. juncea, and E. robusta were 23.7±0.7°C, 19±0.6°C, and 18.3±0.7°C (Fig. 1a, 1c, and 1e, respectively), respectively, while the late stage oocyte LPTTs were 23±0.6°C, 20.3±0.7°C, and 20.3±0.3°C, respectively (Fig. 1b, 1d, and 1f, respectively).
Figure 1. The lipid phase transition of Juncea fragilis, J. juncea, and Ellisella robusta oocytes at early (1a, c, and e, respectively) and late (1b, d, and f, respectively) stages, as determined from a Fourier Transform Infrared analyzer.
The inflection point of the lipid phase transition curve from liquid crystalline to gel phase represents the the lipid phase transition temperature.
Inter-specific Differences in Coral Oocyte LPT
The LPTTs of oocyte membranes of J. juncea and E. robusta were significantly lower after 96 h of exposure to 5°C than those of oocytes of J. fragilis (p<0.05, Fig. 2a and 2b). The LPTT values were significantly lower for early stage oocytes of J. juncea and E. robusta at all chilling exposure periods than those of J. fragilis oocytes (Fig. 2a). The LPTT in the late stage oocytes of J. fragilis was 19.3±1.5°C after chilling at 5°C for 144 h, significantly less than that of controls incubated at 25C for this same length of time (23.0±2.0°C, Tukey’s HSD, p<0.05). Membrane LPTTs of J. juncea and E. robusta late stage oocytes were 18.7±1.2 and 18.0±1.0°C, respectively, after chilling for 96 h (Fig. 2), a decrease of nearly 2°C in comparison to the respective controls (21.0±2.0 and 20.7±1.2°C, respectively).
Figure 2. The lipid phase transition temperature (LPTT) of Juncea fragilis, J. juncea, and Euplexaura. robusta oocytes at early (a) and late (b) developmental stages after chilling at 5°C for up to 144 h in filtered seawater.
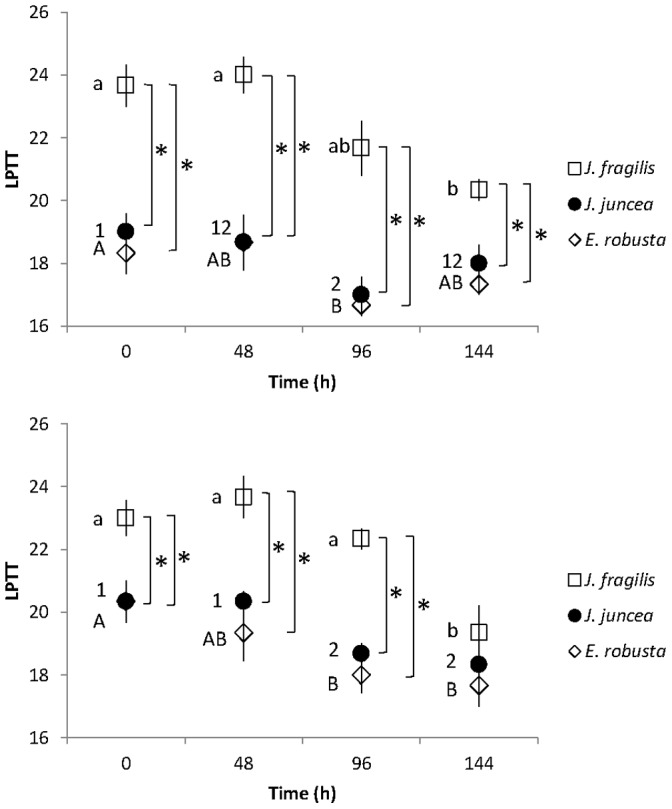
Error bars indicate standard error of the means. Astrices represent significant differences (p<0.05) between LPTTs of J. fragilis, J. juncea, and E. robusta oocytes at the same chilling time period.
Effect of Chilling on the LPT of Coral Oocytes at Different Developmental Stages
The LPTTs of J. fragilis, J. juncea, and E. robusta oocytes at different developmental stages were measured after chilling at 5°C for up to 144 h, and the results are shown in Figure 3. The chilled oocytes of J. juncea and E. robusta had a significantly lower LPTT after 96 h of exposure to5°C than those of control group at all stages (p<0.05, Fig. 3b and 3c), whilst LPTTs of oocyte membranes of J. fragilis were not significantly lower than time 0 controls until 144 h of chilling exposure had occurred (p<0.05, Fig. 3a). The LPTT values of J. juncea and E. robusta oocytes in the early and late stages were significantly lower (18.0±1.0 and 17.3±0.6°C, 18.3±0.6 and 17.7±1.2°C) after 96 h chilling at 5°C compared to those obtained from J. fragilis oocytes (20.3±2.1 and 19.3±1.5°C for early and late stage oocytes, respectively, p<0.05). Oocyte membranes of J. juncea and E. robusta showed a less well-defined LPTT value in the early and late stages (p>0.05, Fig. 3). The lowest LPTT (16.7±0.6°C was obtained in the early stage ooctyes of E. robusta after chilling at 5°C for 144 h (Fig. 3c). There were no statistical differences in the LPTTs between the early and late stage oocytes of any of the three coral species at any sampling time (Fig. 3).
Figure 3. The lipid phase transition temperature (LPTT) of early and late stages oocytes of Juncea fragilis (a), J. juncea (b), and Ellisella robusta.
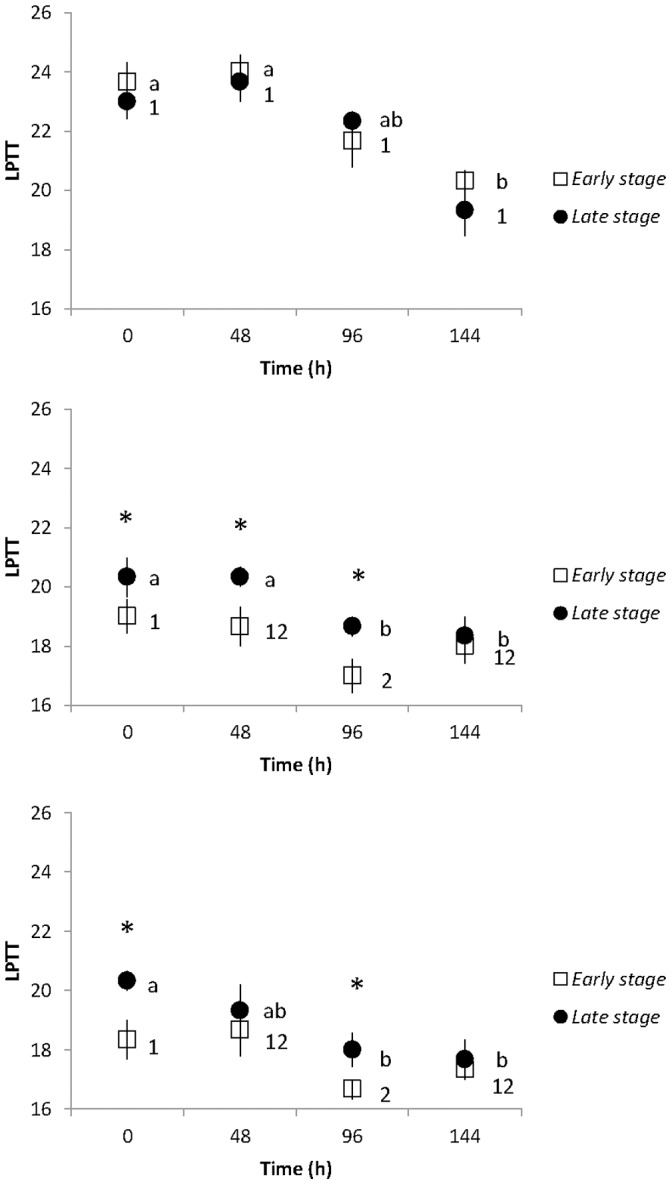
Error bars indicate standard error of the means. Astrices represent significant difference between early and late stage at the same chilling time period (p<0.05).
Discussion
Many studies have suggested that chilling damage is a major obstacle for successful cryopreservation of gametes, and cell membranes are thought to be amongst the most sensitive cellular structures to temperature decreases [1], [2], [14], [21], [22]. Chilling damage has been directly correlated with thermal LPT [11], [14], [22]. For instance, the LPT from the fluid liquid crystalline phase to the more rigid gel phase [23] is associated with chilling injury in mammalian sperm [11], [13] and fish oocytes [22]. Species whose lipid membranes demonstratelower LPTTs have typically been found to better resist cold stress [10], [12]. Our current results showed a significant difference between the LPTT values between three gorgonian coral oocytes. The phase transition temperature of J. fragilis oocytes was significantly higher than oocytes of J. juncea and E. robusta, and this may indicate that the former are more prone to chilling injury.
Phospholipids are the major lipid component of cellular membrane, and the fatty acids within the phospholipids strongly influence the membrane properties [12], [24], [25]. Fatty acids are abundant in the phospholipid fraction of oocytes from cattle [24], [26], pigs [27], sheep [28], and some coral species [14], [18], [29], providing a valuable energy source [29], [30], or as precursors for the elongation of long chain fatty acids [31], [32] influencing reproduction and oocyte membrane fluidity [24], [33]. Oocyte membrane fluidity is influenced by temperature alterations between seasons that may be due to changes in fatty acid composition [24], [34], [35]. The LPT is also strongly affected by the fatty acid composition of the membrane [25]. Seasonal studies of bovine oocytes have found that oocyte membranes are composed of more saturated fatty acids during the summer whereas they contain mainly mono- and polyunsaturated fatty acids during the winter months [24]. This resulted in LPTT decreases of 6°C from the summer to the winter [24].
In the ascidian Halocynthia aurantium, temperature-induced changes in the phase transition temperature of phospholipids decreased by 10°C in both the summer and winter [34], [35]. In the present study, the LPTT of three gorgonian coral oocytes occurred over a broad temperature range, from 16°C to 23°C, which suggests the presence of a variety of different types of fatty acids in the membranes as well as a high concentration of long chain fatty acids. We have also found that J. fragilis oocytes were extremely sensitive to chilling. The LPTT of J. fragilis oocytes dropped to 3.5°C lower than controls after chilling at 5°C for 144 h. On the other hand, J. juncea and E. robusta oocytes showed a lower transition temperature at approximately 2°C under the same condition and yet did not cause damage to the membranes. Presumably, damage was avoided because the J. juncea and E. robusta oocytes had lower LPTT value, so the injury that would result normally was avoided.
Early studies conducted on the cryosensitivity of late stage oocytes found that the main cellular damage occurred due to meiotic spindle disorganization followed by microtubule depolymerization [36], [37]. Oocytes at later developmental stages are extremely sensitive to chilling temperatures, with the predominant damage having been documented to occur at the plasma membrane [10]. However, immature, early stag oocytes are arrested in prophase of the first meiotic division and do not contain any polymerized microtubules [38], [39], [40]. Our previous study showed that late stage coral oocytes were more susceptible to cryoinjury than early stage oocytes [1]. Early stage J. juncea and E. robusta oocytes had lower LPTT value than those of J. fragilis at all stages, indicating that the microtubular organization of early stage oocytes may be less prone to chilling damage.
The composition of phospholipids in cellular membrane has also been linked to chilling sensitivity of coral oocytes [18], [20]. Our previous studies have suggested that PE and PC are the more abundant phospholipids in early stage oocytes, and they collectively help to create a higher membrane surface viscosity [18], [20]. On the basis of previous reports and our present findings, we suggest that changes in the LPT behavior of coral oocyte membranes of three species at different developmental stages may be a function of changes in phospholipid composition at different stages of oogenesis.
The present study membrane phase behavior of two gorgonian coral oocytes was identified, which induced the lethality after chilling for the oocytes. The present study has showed evidence of changes in oocyte membrane fluidity with depth. In fact, as water pressure increases with increasing depth, temperature decreases more abruptly in deeper water. Our previous studies have demonstrated that the higher concentration of phospholipid fatty acid in J. juncea oocytes increased the probability of membrane fluidity with depth [20]. As a result, the oocytes from the three gorgonian coral showed significant differences in LPTT and sensitivity with increasing depth.
The phospholipid fatty acid composition of coral oocyte membranes is influenced by the presence of dinoflagellate endosymbionts (genus Symbiodinium) [18]. It was demonstrated that enriching diets with polyunsaturated fatty acid changes the fatty acid profile of ewe oocytes, resulting in a reduction of oocyte chilling susceptibility [15]. Studies on the phase behavior of lipids of marine invertebrate (H. aurantium) cells have also found that the change in phase behavior of phospholipids is relevant to the decrease of saturated to polyunsaturated fatty acid ratios [34]. The present study revealed that J. juncea and E. robusta oocytes had lower LPTTs than oocytes of J. fragilis. It is possible that J. fragilis oocytes inherit dinoflagellates and so contain less unsaturated fatty acids than J. juncea and E. robusta oocytes, the latter two of which carry no symbiotic dinoflagellates. Our present study also found that membranes of J. juncea and E. robusta oocytes exhibited altered physical properties of their membranes and decreased in the LPTT (20.7–16.7°C), whilst J. fragilis had a higher (LPTT) value (approximately 23°C); this may indicate that J. juncea and E. robusta oocytes are more resistance to chilling injury.
Herein the LPT of coral oocytes was reported for the first time, and our findings confirm preliminary results from our previous studies that LPT and chilling sensitivity are associated with the phospholipid composition of the membrane [18], [20]. In this study on the LPT of gorgonian oocytes, higher LPTT values were observed in J. juncea oocytes, and this may be relate to their deeper natural habitat and their higher level of phospholipids. Our previous works have suggested that higher levels of PE and PC are found in early stage coral oocytes and create a higher surface viscosity that increases membrane fluidity [18].The present study also showed that LPTT value of early stage oocytes was significantly lower than that of late stage oocytes. In fact, the oocytes membranes in the three different gorgonian species demonstrated different LPTTs at different developmental stages, which may be due to changes in the phospholipid composition of their plasma membranes that occur over the course of their maturation. This current study of the LPT behavior of gorgonian coral oocytes demonstrates that the LPTT is a critical parameter to assess in gauging whether or not a coral can be successfully cryopreserved.
Materials and Methods
Ethics Statement: these corals are not regulated under Taiwanese law and the coral collection was approved by Kenting National Park.
Collection of J. Fragilis, J. Juncea, and E. Robusta
Three gorgonian corals; J. fragilis, J. juncea and E. robusta were collected by SCUBA divers from reefs within Nanwan Bay, Kenting National Park, Taiwan (21°56′N, 120°44′E) during between July and September 2013. The J. fragilis colonies were located on the seaward reef slopes at a depth range of 3–5 m, whilst J. juncea and E. robusta communities were collected from below 20 m. Three specimens of each species were cut into ∼60-cm branches with surgical scissors. After fragmentation, the coral branches were transporter in 200 L containers filled with seawater and taken to the Coral Husbandry Center of Taiwan’s National Museum of Marine Biology and Aquarium (NMMBA). Once at NMMBA, the coral branches were transporters to flow-through seawater tanks (4 tons) maintained at 25°C for further processing.
Oocyte Isolation
Coenchyme tissues from coral branches were excised with sterile scalpels and transferred directly to 6-well tissue culture dishes containing 2 ml filtered (0.4 μm) seawater (FSW; 35 psu). Oocytes were mechanically separated from the coenchyme tissue with sterile forceps followed by aspiration with a pipette under a dissecting microscope (Olympus, SZ51, Japan). The oocytes were washed three times with FSW and then stored in FSW at 25°C for further processing. The developmental stages of the gorgonian oocytes were determined based on the classification scheme of Lin et al. 2011 [1]. Briefly, oocyte diameters were measured under the microscope with an ocular micrometer (Olympus, C31, Japan). The oocytes in the early stage were in the range of 50–200 μm, whereas late stage oocytes ranged from 200 to 350 μm.
Effect of Chilling on the LPT of Coral Oocytes
The effect of chilling on the LPT of coral oocytes at different developmental stages was investigated. Oocytes in each test tube (1.5 mL) were placed in a low temperature bath (Dry bath, CB-1502, Medclub Scientific CO., LTD., Taiwan) at 5°C, and the oocytes were allowed to chill for 0, 48, 96, and 144 h. After chilling, oocytes were warmed in a water bath at 25°C and washed twice in with FSW. Control oocytes were kept in 35 psu FSW at 25°C. The total number of oocytes in each test tube was quantified under the light microscope (Olympus, C31, Japan).
Measurement of Membrane Phase Transition
The membrane phase transition of oocytes was determined with a Bruker-Tensor 27 FTIR spectrometer connected to a Bruker FTIR Hyperion 2000 microscope (Ettlingen, D-76275, Germany). The mercury-cadmium telluride detector within the microscope was cooled down with liquid nitrogen before measurement of the samples. Oocytes were positioned on a silicon wafer which was then placed in a temperature controlled IR stage (Linkam, FTIR 600, United Kingdom) and measured under the reflectance mode under the microscope. The sample temperature was controlled with a thermoelectric cooler at the range of 30 to 0°C at 2°C intervals and to within 0.1°C at each temperature by microprocessor – controlled cooling system (Linkam, PE95/T95, UK) equipped with temperature control and capture software (Linkam, Linksys 32, United Kingdom). At each temperature, a total of 128 scans at a resolution of 4 cm−1 were collected for each oocyte.
The properties of the membranes were determined from the temperature influence on the methylene (CH2) asymmetric stretching vibration frequency, which is particularly sensitive to the conformational order of the lipid acyl chain; briefly, there is a lower wave number in gel phase membranes compared to those of the more fluid lamellar phase. All the spectral analyses, operation qualification test for improving the quality of the images, and reference and sample measurements for attenuated total reflectance calculations following the FTIR studies were performed with OPUS spectroscopy software (Bruker, Version 7.0, Germany) on a PC computer. The membrane LPTT curve from liquid crystalline to the gel phase was determined by statistical analysis.
Statistical Analysis
Each treatment in the experiment contained three replicates, and experiments were repeated at least three times. The data were analyzed with SPSS software (Version 17.0; SPSS Inc., Chicago, IL, USA). The data were checked for normal distribution based on a modification of the Kolmogorov-Smirnov one-sample test and homogeneity of variance was determined with Levene’s test. In all statistical tests used, p<0.05 was considered to be significant. Results are presented as means ± SEM.
Acknowledgments
The authors express their deepest appreciation to Dr. Anderson Mayfield for valuable comments and for proofreading this manuscript.
Funding Statement
This research was supported by funds from the National Science Council (NSC 102-2313-B-291 -002). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lin C, Zhang T, Kuo FW, Tsai S (2011) Studies on oocytes chilling sensitivity in the context of ATP response of two gorgonian coral species (J. juncea and J. fragilis). Cryo Lett 32: 141–147. [PubMed] [Google Scholar]
- 2. Lin C, Tsai S (2012) The effect of chilling and cryoprotectants on hard coral (Echinopora spp.) oocytes during short-term low temperature preservation. Theriogenology 77: 1257–1261. [DOI] [PubMed] [Google Scholar]
- 3. Albertini DF (1992) Cytoplasmic microtubular dynamics and chromatin organization during mammalian oogenesis and oocyte maturation. Mutat Res 296: 57–58. [DOI] [PubMed] [Google Scholar]
- 4. Albertini DF, Eppig JJ (1995) Unusual cytoskeletal chromatin configurations in mouse oocytes that are atypical in meiotic progression. Dev Genet 16: 13–19. [DOI] [PubMed] [Google Scholar]
- 5. Bou G, Liu LQ, Zheng Z, Tian JT, Kong QR, et al. (2009) Effect of chilling on porcine germinal vesicle stage oocytes at the subcellular level. Cryobiology 59: 54–58. [DOI] [PubMed] [Google Scholar]
- 6. Wu B, Tong J, Leibo SP (1999) Effect of cooling germinal vesicle- stage bovine oocytes on meiotic spindle formation following in vitro maturation. Mol Reprod Dev 54: 388–395. [DOI] [PubMed] [Google Scholar]
- 7. Zenes MT, Bielecki R, Casper RF, Leibo SP (2001) Effects of chilling to 0 degrees C on the morphology of meiotic spindles in human metaphase II oocyte. Fertil Steril 75: 769–777. [DOI] [PubMed] [Google Scholar]
- 8. Songsasen N, Yu IJ, Ratterree MS, VandeVoort CA, Leibo SP (2002) Effect of chilling on the organization of tubulin and chromosomes in rhesus monkey oocytes. Fertil Sterill 77: 818–825. [DOI] [PubMed] [Google Scholar]
- 9. Sathananthan AH, Kirby C, Trounson A, Philipators D, Shaw J (1992) The effects of cooling mouse oocytes. J Assist Reprod Gent 9: 139–148. [DOI] [PubMed] [Google Scholar]
- 10. Arav A, Zeron Y, Leslie SB, Behboodi E, Anderson GB, et al. (1996) Phase transition temperature and chilling sensitivity of bovine oocytes. Cryobiology 33: 589–599. [DOI] [PubMed] [Google Scholar]
- 11. Arav A, Pearl M, Zeron Y (2000) Does lipid explain chilling sensitivity and membrane lipid phase transition of spermatozoa and oocyte? Cry Lett 21: 179–186. [PubMed] [Google Scholar]
- 12. Ghetler T, Yavin S, Shalgi R, Arav A (2005) The effect of chilling on membrane lipid phase transition in human oocytes and zygotes. Hum Reprod 20: 3385–9. [DOI] [PubMed] [Google Scholar]
- 13. Drobnis EZ, Crowe LM, Berger T, Anchordoguy TJ, Overstreet JW, et al. (1993) Cold shock damage is due to lipid phase transition in cell membranes: a demonstration using sperm as a model. J Exp Zool 265: 432–509. [DOI] [PubMed] [Google Scholar]
- 14. Lin C, Han CC, Tsai S (2013) Effect of thermal injury on embryos of banded coral shrimp (Stenopus hispidus) under hypothermal conditions. Cryobiology 66: 3–7. [DOI] [PubMed] [Google Scholar]
- 15. Zeron Y, Sklan D, Arav A (2002a) Effect of polyunsaturated fatty acid supplementation on biophysical parameters and chilling sensitivity of ewe oocytes. Mol Reprod Dev 61: 271–278. [DOI] [PubMed] [Google Scholar]
- 16. Peral M, Arav A (2000) Chilling sensitivity in zebra fish (Brachydanio rerio) oocytes is related to lipid phase transition. Cryo Lett 21: 171–178. [PubMed] [Google Scholar]
- 17. Quinn PJ (1985) A lipid-phase separation model of low temperature damage to biological membranes. Cryobiology 22: 128–146. [DOI] [PubMed] [Google Scholar]
- 18.Lin C, Wang LH, Fan TY, Kuo FW (2012) Lipid content and composition during the oocyte development of two gorgonian coral species in relation to low temperature preservation. PLoS ONE 7(7), e38689. doi: 10.1371/journal.- pone.0038689. [DOI] [PMC free article] [PubMed]
- 19. White IG (1993) Lipids and calcium uptake of sperm in relation to cold shock and preservation: a review. Reprod Fertil Dev 5: 639–658. [DOI] [PubMed] [Google Scholar]
- 20.Lin C, Wang LH, Meng PJ, Chen CS, Tsai S (2013) Lipid content and composition of oocytes from five coral species: potential implications for future cryopreservation efforts. PLoS ONE 8(2), e57823. doi:10.1371/journal.pone.0057823. [DOI] [PMC free article] [PubMed]
- 21. Watson PF, Morris GJ (1987) Cold shock injury in animal cells. Symp Soc Exp Biol 41: 311–340. [PubMed] [Google Scholar]
- 22. Tsai S, Rawson DM, Zhang T (2009) Studies on chilling sensitivity of early stage zebrafish (Danio rerio) ovarian follicles. Cryobiology 58: 279–286. [DOI] [PubMed] [Google Scholar]
- 23. Crowe JH, Hoekstra FA, Crowe LM, Anchordoguy TJ, Drobnis E (1989) lipid phase transitions measured in intact cell with Fourier transform infrared spectroscopy. Cryobiology 27: 76–84. [DOI] [PubMed] [Google Scholar]
- 24. Zeron Y, Ocheretny A, Kedar O, Borochov A, Sklan K, et al. (2001) Seasonal changes in bovine fertility: relation to developmental competence of oocytes, membrane properties and fatty acid composition of follicles. Reproduction 121: 447–454. [PubMed] [Google Scholar]
- 25. Zeron Y, Tomczak M, Crowe J, Arav A (2002b) The effect of liposomes on thermotropic membrane phase transitions of bovine spermatozoa and oocytes: implications for reducing chilling sensitivity. Cryobiology 45: 143–152. [DOI] [PubMed] [Google Scholar]
- 26. Kim JY, Kinoshita M, Ohnishi M, Fukui Y (2001) Lipid and fatty acid analysis of fresh and frozen-thawed immature and in vitro matured bovine oocytes. Reproduction 122: 131–138. [PubMed] [Google Scholar]
- 27. Homa ST, Racowsky C, McGaughey RW (1986) Lipid analysis of immature pig oocytes. J Reprod Fertil 77: 425–434. [DOI] [PubMed] [Google Scholar]
- 28. Coull GD, Speake BK, Staines ME, Boradbent PJ, McEvoy TG (1998) Lipid and fatty acid composition of zona-intact sheep oocytes. Theriogenology 49: 179. [Google Scholar]
- 29. Grottoli AG, Rodrigues LJ, Juarez C (2004) Lipids and stable carbon isotopes in two species of Hawaiian corals, Porites compressa and Montipora verrucosa, following a bleaching event. Mar Biol 145: 621–631. [Google Scholar]
- 30. Thompson JG (1996) Defining the requirements for bovine embryos culture. Theriogenology 45: 27–40. [Google Scholar]
- 31. Imbs A, Demidkova D, Latypov Y, Pham L (2007) Application of fatty acids for chemotaxonomy of reefbuilding corals. Lipids 42: 1035–1046. [DOI] [PubMed] [Google Scholar]
- 32. Treignier C, Grover R, Ferrier-pages C, Tolosa I (2008) Effect of light and feeding on the fatty acid and sterol composition of zooxanthellae and host tissue isolated from the scleractinian coral Turbinaria reniformis . Limnol Oceanogr 53: 2702–2710. [Google Scholar]
- 33.Ulrich K (1994) Comparative animal biochemistry. Springer-Verlag.
- 34. Sanina NM, Kostetsky EY (2001) Seasonal changes in thermotropic behavior of phosphatidylcholine and phohphatidylethanolamine in different organs of the ascidian Halocynthia aurantium . Comp Biochem Physiol B 128: 295–305. [DOI] [PubMed] [Google Scholar]
- 35. Sanina NM, Kostetsky EY (2002) Thermotropic hehavior of major phospholipids from marine invertebrates: changes with warm-acclimation and seasonal acclimatization. Comp Biochem Physiol B 133: 143–153. [DOI] [PubMed] [Google Scholar]
- 36. Amna RR, Park JE (1994) Effects of cooling and rewarming on the meiotic spindle and chromosomes of in vitro-matured bovine oocytes. Biol Reprod 50: 103–110. [DOI] [PubMed] [Google Scholar]
- 37. Sathananthan AH, Trounson A, Freeman L, Brady T (1988) The effects of cooling human oocytes. Hum Reprod 3: 968–977. [DOI] [PubMed] [Google Scholar]
- 38. Pielak RM, Gaysinskaya VA, Cohen WD (2003) Cytoskeletal events preceding polar body formation in activated Spisula eggs. Biol Bull 205: 192–193. [DOI] [PubMed] [Google Scholar]
- 39. Pielak RM, Gaysinskaya VA, Cohen WD (2004) Formation and function of polar body contractile ring in Spisula . Dev Biol 269: 421–432. [DOI] [PubMed] [Google Scholar]
- 40. Pang Z, Chang Y, Sun H, Yu J (2010) Role of animal pole protuberance and microtubules during meiosis in sea cucumber Apostichopus japonicas oocytes. Chin J Oceanol Limnol 28: 533–541. [Google Scholar]