Abstract
Introduction
Preterm birth is a major contributor to neonatal morbidity and mortality and its rate has been increasing over the past two decades. Antidepressant medication use during pregnancy has also been rising, with rates up to 7.5% in the US. The objective was to systematically review the literature to determine the strength of the available evidence relating to a possible association between antidepressant use during pregnancy and preterm birth.
Methods
We conducted a computerized search in PUBMED, MEDLINE and PsycINFO through September 2012, supplemented with a manual search of reference lists, to identify original published research on preterm birth rates in women taking antidepressants during pregnancy. Data were independently extracted by two reviewers, and absolute and relative risks abstracted or calculated. Our a priori design was to group studies by level of confounding adjustment and by timing of antidepressant use during pregnancy; we used random-effects models to calculate summary measures of effect.
Results
Forty-one studies met inclusion criteria. Pooled adjusted odds ratios (95% CI) were 1.53 (1.40–1.66) for antidepressant use at any time and 1.96 (1.62–2.38) for 3rd trimester use. Controlling for a diagnosis of depression did not eliminate the effect. There was no increased risk [1.16 (0.92–1.45)] in studies that identified patients based on 1st trimester exposure. Sensitivity analyses demonstrated unmeasured confounding would have to be strong to account for the observed association.
Discussion
Published evidence is consistent with an increased risk of preterm birth in women taking antidepressants during the 2nd and 3rd trimesters, although the possibility of residual confounding cannot be completely ruled out.
Introduction
Preterm birth is a major clinical problem throughout the world. It is the leading cause of infant mortality: approximately 75% of all perinatal deaths occur among preterm infants [1]. It is also a major contributor to both short- and long-term morbidity: surviving infants are at increased risk of health problems ranging from neurodevelopmental disabilities such as cerebral palsy and mental retardation to other chronic health problems such as asthma [2]. Although the risk is highest in very preterm infants (<32 gestational weeks), it has been well documented that moderate (32–33 gestational weeks) and mild (34 to 36 gestational weeks) preterm birth infants are also at increased risk for neonatal and post-neonatal mortality and morbidity [3]–[6]. Rates of preterm birth have been increasing over the past two decades and it is a major public health concern [7], with costs to society that have been estimated to be as high as $26.2 billion per year in the US [7], and £939 million per year in the UK [8]. It has been reported that two thirds of these costs are incurred for the care of babies born moderately prematurely [8].
In many developed countries, the use of antidepressant medications has increased sharply between 1996 and 2005, and now surpasses antihypertensives as the most commonly prescribed drug class in ambulatory care [9]. During this same time period, rates of antidepressant use during pregnancy have increased approximately 4-fold, with reported rates of up to 3–6% in Europe [10]–[12] and up to 8% in the US [13], [14].
Numerous studies, of varying size and quality, have examined the effects of antidepressant medication use on pregnancy outcomes, including preterm birth. They differ in terms of the timing of the antidepressant exposure during pregnancy and adjustment for potential confounding variables, including lifestyle factors, co-morbidities, and the severity of the underlying psychiatric illness. The extent to which such differences contribute to variability in findings remains to be elucidated. The objective of this review was to determine the strength of the available evidence relating to a possible association between antidepressant use during pregnancy and preterm birth, and to assess this relationship in terms of (1) the timing of the antidepressant use studied, and (2) attempts to control for the possible confounding effects of depression itself.
Methods
To identify all available studies on the topic of antidepressant medication use during pregnancy and preterm delivery, we performed a computerized search in PUBMED, MEDLINE, and PsycINFO using the key words: (“antidepressant*” or “tricyclic antidepressant*” or “selective serotonin reuptake inhibitor*” or “serotonin-norepinephrine reuptake inhibitor*”) and (“preterm birth*” or “preterm deliver*” or “pregnanc*” or “pregnancy complication*”). The databases were searched from their inception through September 12, 2012. Reference lists of selected articles were also searched to identify additional studies that reported on preterm births and antidepressant exposure.
Studies were included if they identified a group of pregnant women exposed to antidepressants at some point during their pregnancy as well as a comparison group, and reported on preterm birth rates, irrespective of whether preterm birth was a pre-specified study endpoint or one of several pregnancy characteristics reported. No restrictions were imposed on study size or design.
Once relevant studies were identified, for each study two investigators with clinical and epidemiologic expertise (ACU, KFH) independently abstracted preterm birth rates in the group(s) exposed to antidepressants and in the comparator group(s). When these were not expressed as percentages in the manuscript, they were calculated. Relative risks (expressed as odds ratios in all studies) were also taken directly from the manuscript. Whenever available, preference was given to relative risks adjusted for potential confounding variables. When relative risks were not reported in the manuscript, we estimated the unadjusted odds ratio and its corresponding 95% confidence limits (Wald method) based on the available information. When relative risks were only presented graphically, we contacted the authors to obtain the corresponding numerical estimates. Types of antidepressants used, numbers of users and other pertinent exposure information (e.g., time and duration), and potential confounders accounted for were also retrieved. Any discrepancies in data abstraction were resolved by consensus between the reviewers.
A funnel plot was examined for evidence of publication bias [15]. Between-study heterogeneity was examined using the Cochran Q and I2 tests [16]. We used a random-effects meta-analysis model to calculate summary measures of effect while accounting for heterogeneity across studies [17]. Because of the critical importance of (1) the timing of antidepressant use during pregnancy, and (2) the potential for confounding by the presence of depression itself (confounding by indication), we had determined a priori to group studies by timing of antidepressant exposure and level of confounding adjustment. We chose this approach to address the inherent issues of clinical and methodological diversity [18]. Finally, we conducted a sensitivity analysis to identify the strength of the residual confounding that would be necessary to fully explain the estimated association between antidepressant medication use and preterm birth [19].
Results
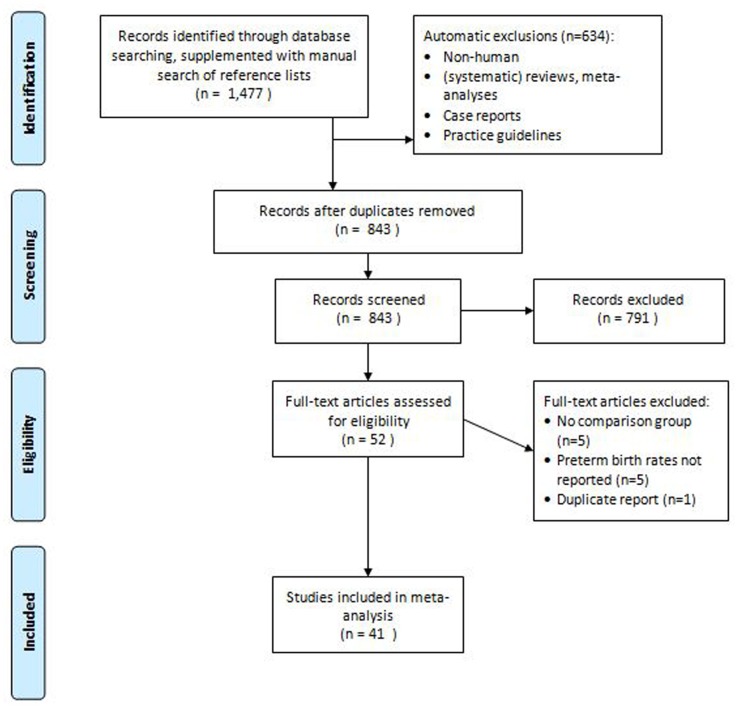
After an initial screen of the 1,477 studies identified through database searches, we identified 52 studies that met our predetermined criteria as possibly assessing the association between antidepressant use during pregnancy and preterm birth [20]–[67] [68]–[71]. Five studies reported on preterm births in antidepressant-exposed pregnancies but had no comparison group, and were excluded [20]–[24]. In five other studies a comparison was made between patients treated with antidepressants and untreated controls, but preterm birth rates were not reported [25]–[29]. One study used the same cohort [67] for which preterm birth rates had previously been reported [57]. (Figure 1).
Figure 1. Study selection flowchart.
Forty-one studies [30]–[66], [68]–[71], published between 1993 and 2012, were identified that met entry criteria as reporting on preterm birth rates in a group exposed to antidepressants versus a control group (Table 1). All were observational cohort studies; not surprisingly, no randomized controlled trials have been performed on this topic. Most studies (n = 21) were prospective in nature, four were bi-directional with some women included during pregnancy and some post-delivery, and the remaining 16 were retrospective (i.e., participants were identified after delivery). The majority of the retrospective studies (n = 14) used administrative healthcare utilization databases. Nine studies recruited patients through Teratogen Information Services, 17 recruited participants from clinics, physician offices and other referrals, and the remaining 15 studies used population-based electronic healthcare databases (with and without linkage to birth registries). The studies ranged in size from 44 to 1,618,255 participants. As expected, the studies using electronic healthcare databases were much larger (median: 199,547 participants) than those using other approaches (median: 290 participants).
Table 1. Characteristics of 41 studies evaluating the association between antidepressant medication use during pregnancy and preterm birth.
| Author | Year | Study type | Exposure | Total N | Preterm birth rate | OR | 95% CI | ||||
| Exposed | Reference | ||||||||||
| Calderon-Margalit [30] | 2009 | Clinic-based prosp | Psychotropics,including SRI | 2,793 | 12.1% | 9.4% | Any time | 1.21 | (0.67; 2.21) | ||
| T2/3 | 4.79 | (1.66; 13.9) | |||||||||
| Casper [31] | 2003 | Clinic-based prosp/retrosp | SRI | 44 | 3.2% | 7.7% | Any time | 0.40 | (0.02; 6.93) | ||
| Casper [32] | 2011 | Clinic-based prosp/retrosp | SRI | 55 | 9.8% | 0.0% | undefined | ||||
| Chambers [33] | 1996 | TIS prosp | fluoxetine | 388 | 14.3% | T1/2 | 4.1% | T3 vs. T1/2 | 4.80 | (1.10; 20.80) | |
| control | 5.9% | ||||||||||
| Colvin [34] | 2011 | Population-based healthdataset, linked tobirth registry | SRI | 96,698 | 11.5% | 8.0% | Any time | 1.43 | (1.24; 1.65) | ||
| Costei [35] | 2002 | TIS prosp | paroxetine | 109 | 20.0% | 37.0% | T3 vs. (T1/2or unexp) | 6.50 | (1.37; 30.91) | ||
| Davis [36] | 2007 | Population-based healthdataset | SRI, TCA | 81,527 | SRI | 9.4% | 6.6% | SRI - any time | 1.45 | (1.25; 1.68) | |
| TCA | 1.1% | TCA - any time | 1.67 | (1.25; 2.22) | |||||||
| Diav-Citrin [37] | 2008 | TIS prosp | paroxetine,fluoxetine | 1,953 | paroxetine | 8.7% | 6.4% | paroxetine –T1&beyond | 1.38 | (0.91; 2.10) | |
| fluoxetine | 9.0% | fluoxetine –T1&beyond | 1.44 | (0.90; 2.32) | |||||||
| Djulus [38] | 2006 | TIS prosp | mirtazapine,other ADs | 312 | mirtazapine | 9.6% | 1.9% | mirtazapine | 5.43 | (1.16; 25.41) | |
| other AD | 6.7% | other AD | 3.68 | (0.75; 18.15) | |||||||
| Einarson [39] | 2010 | TIS prosp | all ADs | 1,856 | 8.8% | 5.4% | 1.70 | (1.18; 2.45) | |||
| Einarson [63] | 2011 | TIS prosp | all ADs | 267 | >1 AD | 12.4% | 4.5% | >1 AD | 3.13 | (0.95; 10.31) | |
| 1 AD | 10.1% | 1 AD | 2.39 | (0.71; 8.07) | |||||||
| El Marroun [64] | 2012 | Population-based prosp | SRI | 7,696 | SRI | 10.1% | 5.1% | SRI | 2.14 | (1.08; 4.25) | |
| Depression,no tmt | 6.3% | Depression,no tmt | 1.10 | (0.77; 1.59) | |||||||
| Ericson [40] | 1999 | National birth registry | all ADs | 281,728 | notprovided | T1&beyond | 1.43 | (1.14; 1.80) | |||
| Ferreira [41] | 2007 | Clinic-based retro | SRI,venlafaxine | 166 | 27.6% | 8.9% | T3 | 2.40 | (0.9; 6.3) | ||
| Gavin [42] | 2009 | Prosp | ADs, otherpsychoactivemeds | 3,019 | notprovided | no depression | 1.40 | (0.8; 2.4) | |||
| depression | 1.40 | (0.7; 2.7) | |||||||||
| Grzeskowiak [68] | 2012 | Clinc-based retro | SRI | 33,791 | 24.9% | psych illness | 11.8% | SRI late vs.psych illness | 2.68 | (1.83; 3.93) | |
| no psych illness | 10.7% | SRI late vs.no psych illness | 2.46 | (1.75; 3.50) | |||||||
| Hayes [70] | 2012 | Population-based healthdataset | All ADs | 228,876 | ≥3 Rx filled | 14.6% | no depression | 13.5% | ≥3 Rx vs.no Rx, 1st trim | 1.11 | (0.94; 1.32) |
| depression | 13.5% | ≥3 Rx vs. noRx, 2nd trim | 2.33 | (1.96; 2.86) | |||||||
| ≥3 Rx vs. noRx, 3rd trim | 0.20 | (0.15; 0.26) | |||||||||
| Kallen [43] | 2004 | National birth registry | all ADs | 563,656 | 10.3% | 5.1% | all ADs - late | 1.96 | (1.6; 2.41) | ||
| SRI - late | 2.06 | (1.58; 2.69) | |||||||||
| TCA - late | 2.50 | (1.87; 3.34) | |||||||||
| Kieler [62] | 2012 | Population-based healthdataset | SRI | 1,618,255 | 5.4% | 3.8% | 1.44 | (1.37; 1.51) | |||
| Klieger-Grossman [61] | 2011 | TIS prosp | Escitalopram,other SRI | 637 | escitalopram | 8.9% | 4.2% | escitalopram –T1&beyond | 2.21 | (0.98; 5.00) | |
| other SRI | 4.7% | other SRI –T1&beyond | 1.12 | (0.44; 2.81) | |||||||
| Latendresse [44] | 2011 | Clinic-based prosp | SRI | 100 | 30.8% | 5.7% | 11.70 | (2.2; 60.7) | |||
| Lennestal [45] | 2007 | National birth registry | SRI, SNRI/NRI | 860,215 | SNRI/NRI | 9.1% | 4.4% | SNRI/NRI –early | 1.60 | (1.19; 2.15) | |
| SRI | 6.7% | SSRI - early | 1.24 | (1.11; 1.39) | |||||||
| Lewis [46] | 2010 | Clinic-based prosp | SRI, SNRI,NaSSA | 54 | 14.8% | 3.7% | continuous exposure | 4.52 | (0.47; 43.41) | ||
| Lund [47] | 2009 | Clinic-based prosp,linked to birth registry | SRI | 57,001 | 8.8% | psych illness | 5.0% | SRI any time vs.psych illness | 2.05 | (1.28; 3.31) | |
| no psych illness | 4.9% | SRI any time vs.no psych illness | 2.02 | (1.29; 3.16) | |||||||
| Maschi [48] | 2008 | Clinic-based prosp | SRI, TCA | 1,400 | any time | 6.5% | 2.9% | any time | 2.31 | (1.14; 4.63) | |
| throughoutpregnancy | 10.3% | 2.6% | throughoutpregnancy | 4.35 | (1.31; 14.07) | ||||||
| Mulder [49] | 2011 | Clinic-based prosp | SRI | 263 | 8.3% | psych illness | 5.4% | SRI vs.psych illness | 1.59 | (0.32; 7.87) | |
| no psych illness | 0.0% | SRI vs.no psych illness | undefined | ||||||||
| Nordeng [65] | 2012 | Population-based,linked to birth registry | all ADs,SRI | 63,395 | Any AD | 6.6% | 4.5% | Any AD | 1.21 | (0.87; 1.69) | |
| SRI | 6.5% | SRI | 1.28 | (0.9; 1.84) | |||||||
| Depression | 1.13 | (1.03; 1.25) | |||||||||
| Oberlander [50] | 2006 | Population-based healthdataset | SRI | 199,547 | 9.0% | depression | 6.5% | SRI vs.depression: unadjusted | 1.42 | (1.17; 1.72) | |
| SRI vs.depression: adjusted | 1.11 | (0.75; 1.64) | |||||||||
| no depression | 5.9% | SRI vs.no depression | 1.59 | (1.33; 1.91) | |||||||
| Pastuszak [51] | 1993 | TIS prosp | fluoxetine | 170 | 7.1% | 8.2% | 0.85 | (0.27; 2.63) | |||
| Pearson [52] | 2007 | Clinic-based retro | SRI, TCA | 252 | 10.7% | 10.1% | 1.07 | (0.45; 2.50) | |||
| Reis [53] | 2010 | National birth registry | all ADs | 1,062,190 | SRI | 7.4% | 5.5% | 1.46 | (1.31; 1.63) | ||
| SNRI | 10.0% | 1.98 | (1.49; 2.63) | ||||||||
| TCA | 11.1% | 2.36 | (1.89; 2.94) | ||||||||
| Roca [66] | 2011 | Clinic-based prosp/retro | SRI | 252 | 13.1% | 4.8% | exp vs. unexp | 3.44 | (1.3; 9.11) | ||
| high vs. low dose | 5.53 | (1.46; 20.93) | |||||||||
| Rurak [54] | 2011 | Clinic-based prosp | SRI | 74 | 13.8% | 2.2% | 7.04 | (0.75; 66.50) | |||
| Simon [55] | 2002 | Population-basedhealth dataset | SRI, TCA | 788 | SRI | 10.3% | 3.2% | 4.38 | (1.57; 12.22) | ||
| TCA | 10.0% | 5.3% | 1.86 | (0.83; 4.17) | |||||||
| Sivojelezova [56] | 2005 | TIS prosp | SRI | 396 | any time;50% T1–T3 | 8.0% | 3.8% | 2.20 | (0.81; 5.96) | ||
| Suri [57] | 2007 | Prosp (clinic and otherreferrals) | All ADs | 90 | >50% pregnancy | 14.3% | depression | 0.0% | depression history | notdefined | |
| no depression | 5.3% | no depression | 3.00 | (0.34; 26.19) | |||||||
| Toh [58] | 2009 | Retro | SRI,non-SRI | 5,961 | non-SRI | 15.3% | 7.3% | non-SRI | 2.23 | (1.02; 4.88) | |
| SRI - all | 8.9% | SRI - all | 1.12 | (0.64; 1.95) | |||||||
| SRI - continuersbeyond T1 | 10.5% | SRI - continuersbeyond T1 | 1.27 | (0.59; 2.76) | |||||||
| SRI –discontinuers | 7.5% | SRI - discontinuers | 1.01 | (0.47; 2.19) | |||||||
| Wen [59] | 2006 | Population-based healthdataset | SRI | 4,850 | 19.3% | 12.0% | 1.57 | (1.28; 1.92) | |||
| Wisner [60] | 2009 | Prosp (clinic and otherreferrals) | SRI | 238 | 15.5% | depression | 13.9% | SRI vs. depression | 1.14 | (0.36; 3.56) | |
| no depression | 6.1% | SRI vs. no depression | 2.82 | (1.08; 7.37) | |||||||
| Wogelius [71] | 2006 | Population-based healthdataset | SRI | 151,831 | SRI - early | 7.2% | 5.0% | 1.48 | (1.17; 1.87) | ||
| SRI –early & late | 8.8% | 1.83 | (1.33; 2.54) | ||||||||
| Yonkers [69] | 2012 | Prosp (clinic and otherreferrals) | SRI | 2,654 | SRI –depression | 16.4% | depression | 10.2% | SR depr vs. nodepression | 1.51 | (0.60; 3.80) |
| SRI –no depression | 11.3% | no depression | 7.8% | SRI no depr vs. nodepression | 1.50 | (0.94; 2.40) | |||||
| Depression vs. nodepression | 0.86 | (0.44; 1.70) | |||||||||
Abbreviations: Prosp = Prospective cohort; Retro = Retrospective cohort; depr = depression; AD = antidepressant; SRI = serotonin reuptake inhibitor; TCA = tricyclic antidepressant; NaSSA = Noradrenergic and specific serotonergic antidepressants; SNRI = Serotonin–norepinephrine reuptake inhibitors; NRI = noradrenaline reuptake inhibitors; T = trimester; exp = exposed; TIS = Teratogen Information Service.
All but one study defined preterm birth as an infant born before 37 weeks’ gestation, in accord with the WHO definition. Maschi et al considered infants born before 36 weeks of gestation as premature [48]. Most studies evaluated the association between selective serotonin-reuptake inhibitors (SSRI) and preterm birth (n = 22), but some also evaluated other antidepressants such as tricyclic antidepressants (TCA) and serotonin–norepinephrine reuptake inhibitors (SNRI) (n = 17). Two studies evaluated the effect of a variety of psychotropic medications, including antidepressants.
Table 2 presents the findings from the heterogeneity tests, along with the summary effect estimates from the meta-analysis. Medium to high heterogeneity was found across studies that adjusted for potential confounding factors (I2∶46 to 85%), but not across studies that provided unadjusted estimates (I2<25%). We conducted a random-effects meta-analysis of the adjusted estimates given the consistency in the direction of the effects [18].
Table 2. Effect of antidepressant medication use during pregnancy on preterm birth: meta-analysis results.
| Level of adjustment | Timing ofexposure | Number of individualstudy estimates | Summary OR(95% CI) | Heterogeneity | ||
| Qdf | (P value) | I2(95% uncertaintyinterval)(4) | ||||
| Unadjusted | Early(1) | 8 | 1.57 (1.30–1.90) | 8.097 | (0.324) | 13.5 (0.0–56.2) |
| Any time | 4 | 1.44 (1.34–1.56) | 2.873 | (0.411) | 0.0 (0.0–84.0) | |
| Adjusted for potential confounders(3) | Early | 8 | 1.16 (0.92–1.45) | 46.477 | (<0.001) | 84.9 (72.1–91.9) |
| Late(2) | 12 | 1.96 (1.62–2.38) | 69.2611 | (<0.001) | 84.1 (73.8–90.4) | |
| Any time | 17 | 1.53 (1.40–1.66) | 19.7216 | (0.233) | 18.9 (0.0–54.3) | |
| Adjusted for psychiatric illness | ||||||
| Controls with psychiatric illness | All combined | 12 | 1.61 (1.26–2.05) | 20.4711 | (0.039) | 46.3 (0.0–72.5) |
| Controls without psychiatric illness | All combined | 7 | 1.88 (1.48–2.40) | 7.466 | (0.280) | 19.6 (0.0–63.2) |
Typically 1st trimester; some women continued during pregnancy, others discontinued.
Typically 3rd trimester.
Factors varied between studies, but typically included maternal age, smoking, alcohol use, parity, and history of prematurity or miscarriage.
Values of I2 are percentages (% of variance explained). 95% uncertainty intervals are calculated as proposed by Higgins and Thompson [89].
Unadjusted Estimates
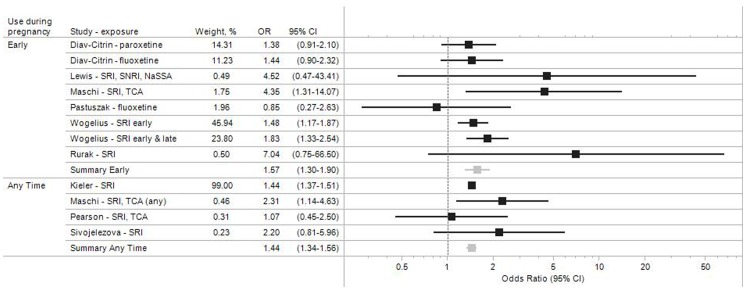
Figure 2 summarizes the results for the 9 studies that did not account for potential confounding factors, either by design or because preterm birth rates were not pre-specified study endpoints. The category ‘Early’ includes studies in which women were known to have taken antidepressants early in pregnancy, typically in the first trimester. Some of these women continued antidepressant medication use during pregnancy whereas others discontinued. Studies classified under ‘Any time’ are those in which women were considered exposed irrespective of the specific time during pregnancy that medication was used, and studies where the timing was not specified. If multiple exposures were analyzed in a given study (e.g., estimate for paroxetine and fluoxetine [37]), they have all been included to ensure completeness of the evidence presented.
Figure 2. Study-specific and pooled odds ratio estimates for antidepressant medication during pregnancy and preterm birth.
Studies that did not adjust for other risk factors.
In these unadjusted analyses, the pooled odds ratio for the risk of preterm birth following antidepressant use in pregnancy was 1.57 (95% CI 1.30–1.90) for early exposure, and 1.44 (1.34–1.56) for exposure any time during pregnancy (Figure 2, Table 2). With the exception of two studies [51], [52], the unadjusted point estimates for all other studies suggest that antidepressant medication use during pregnancy may be associated with an increased risk of preterm delivery, but the effects are estimated imprecisely in many studies, as evidenced by the width of the 95% confidence intervals.
Estimates Adjusted for Potential Confounders
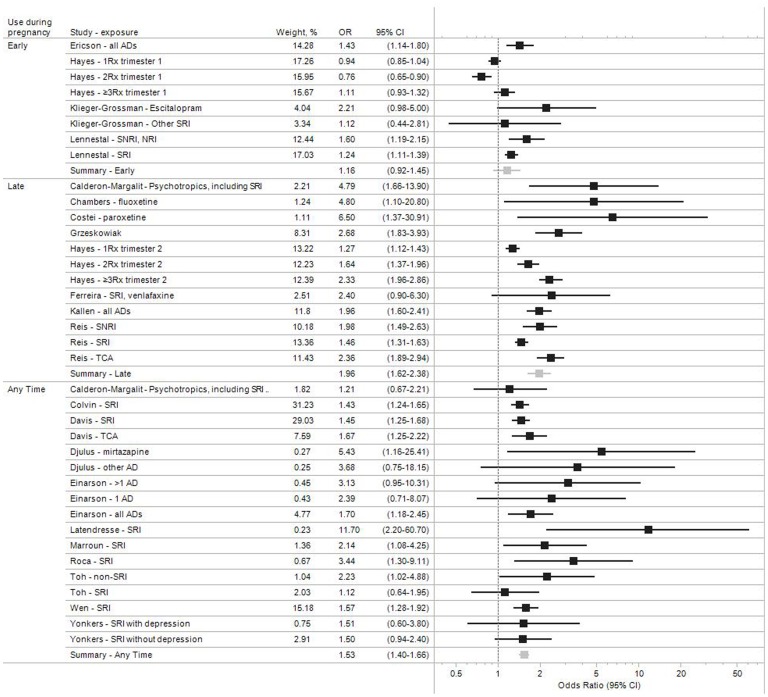
The adjusted odds ratios for the 22 studies which accounted for potential confounding variables are shown in Figure 3. The potential confounding factors adjusted for varied between studies, but typically included maternal age, smoking, alcohol use, parity, and history of prematurity or miscarriage.
Figure 3. Study-specific and pooled odds ratio estimates for antidepressant medication during pregnancy and preterm birth.
Studies that adjusted for other risk factors.
In addition to the previously defined ‘Early’ and ‘Any Time” categories defining the timing of antidepressant use, Figure 3 also includes a ‘Late’ category which comprises studies in which women were known to use antidepressant medications late in pregnancy, generally in the third trimester. Results suggest an increased risk of preterm birth for all exposure-outcome combinations, with four exceptions. Calderon-Margalit and colleagues [30] found an increased risk for use of SSRIs late in pregnancy (aOR, 95%CI = 4.79, 1.66–13.90), but a much weaker association for exposure at any time (1.21, 0.67–2.21). Toh et al [58] estimated a positive association for non-SSRI antidepressants (2.23, 1.02–4.88), but not for SSRIs (1.12, 0.64–1.95). Klieger-Grossman et al [61] found a positive association for escitalopram (2.21, 0.98–5.00), but not for all other antidepressants combined (1.12, 0.44–2.81). Hayes et al [70] observed a positive association and a dose-response relation with second trimester exposure, but not with first trimester exposure.
In general, associations appeared stronger for antidepressant use known to have occurred late in pregnancy (pooled aOR, 95% CI: 1.16, 0.92–1.45 for early exposure; 1.53, 1.40–1.67 for exposure at any time; and 1.96, 1.62–2.38 for late exposure) (Figure 3, Table 2).
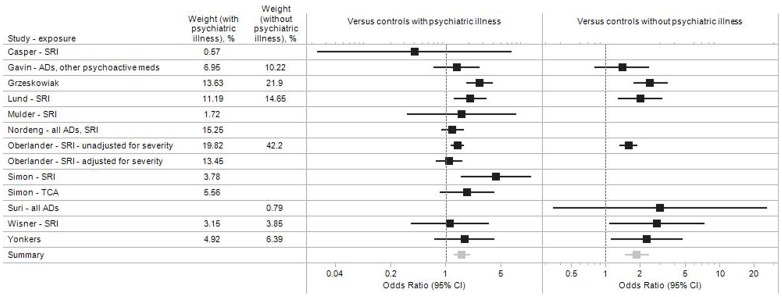
Estimates Adjusted for Psychiatric Illness
A major concern about the validity of studies assessing the effect of antidepressant medications on preterm birth is the potential for confounding by indication. It has been hypothesized that the underlying depression and its severity, or the behaviors potentially associated with depression (e.g., smoking, alcohol intake, nutritional changes), rather than antidepressant medication, may themselves increase the risk of preterm birth [72]. A few studies tried to address this concern directly by using as a comparator group women with a diagnosis of depression or other psychiatric illnesses who did not use antidepressant medications during their pregnancy, or by adjusting for the presence of a psychiatric diagnosis (Figure 4). Most of these 11 studies nonetheless found an increased risk of preterm birth associated with antidepressant medication use, resulting in a pooled OR of 1.61 (95% CI 1.26–2.05) for antidepressant users compared to women with psychiatric illness but no antidepressant use, versus 1.88 (1.48–2.40) compared to women without psychiatric illness or antidepressant use (Figure 4, Table 2). Oberlander et al [50] found an increased risk of preterm birth for women taking antidepressant medication compared to untreated women with a depression diagnosis (1.42, 1.17–1.72), but the association was much attenuated in a subgroup matched on depression severity (1.12, 0.75–1.64). Wisner and colleagues [60] found an increased risk for women on SSRI treatment (OR = 2.82), which was similar in magnitude to the increased risk seen in women with a depression diagnosis who were untreated (OR = 2.48), both compared to untreated women without depression. There were substantial differences, however, between SSRI users and non-users in terms of socio-economic status, alcohol use, and history of preterm birth, with SSRI users having consistently worse histories in these domains. These differences likely contributed to the equally high preterm birth rate observed among women with untreated depression (>20%), and were not accounted for in the analyses which only adjusted for age and race.
Figure 4. Adjusted study-specific and pooled odds ratio estimates for antidepressant medication during pregnancy and preterm birth.
Subset of studies that account for the underlying psychiatric illness.
A few studies indirectly addressed the issue of confounding by depression by comparing preterm birth rates in women who continued vs. those who discontinued their antidepressant medication during pregnancy. Comparing women who continued antidepressant use through the third trimester with those who discontinued, Chambers and colleagues estimated an OR of 4.8 for fluoxetine [33]. Toh et al observed an increased risk of preterm birth in women who continued SSRI treatment beyond the first trimester (OR = 1.27), but not in those who discontinued their medication before the end of the first trimester (OR = 1.01), although the CI were wide and largely overlapped (Table 1) [58].
Sensitivity Analyses
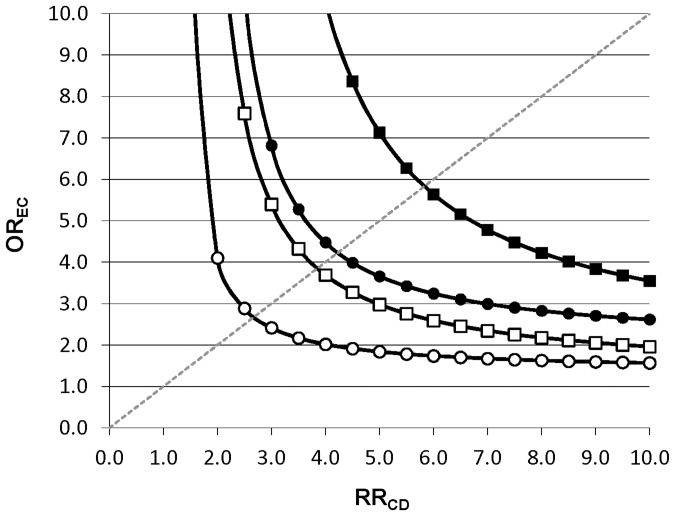
Figure 5 displays the strength of the association between a potential unmeasured confounder and the exposure (OREC) and the outcome (RRCD) that would be required to fully explain the observed increased rate of preterm birth associated with antidepressant medication use during pregnancy (depression-adjusted OR = 1.61) if in truth no such increase existed. For an unmeasured confounder present in 25% of the population, relative risks ≥4 linking the hypothetical confounder to both antidepressant medication use and preterm birth would need to be present to fully explain the observed association, assuming 8% of pregnant women are exposed to antidepressants [14]. For a confounder present in just 5% of the population, relative risks >5.5 would be needed. For an apparent association of 1.26 (lower bound of the 95% CI for the depression-adjusted OR), the required strength would be >2.5 for an unmeasured confounder present in at most 25% of the population.
Figure 5. Sensitivity analysis of residual confounding (Rule-out approach).
Example for estimated OR = 1.61 (depression adjusted point estimate) and OR = 1.26 (lower 95% bound of depression adjusted estimate) for different levels of confounder prevalence (▪ Pc = 0.05, OR = 1.61; •Pc = 0.25, OR = 1.61; □ Pc = 0.05, OR = 1.26; ○Pc = 0.25, OR = 1.26). Each line splits the area into two. The upper right area represents all combinations of OREC and RRCD that would create confounding by an unmeasured factor strong enough to move the point estimate of OR to the null (OR = 1) or beyond. The area to the lower left represents all parameter combinations that would not be able to move the estimated OR to the null.
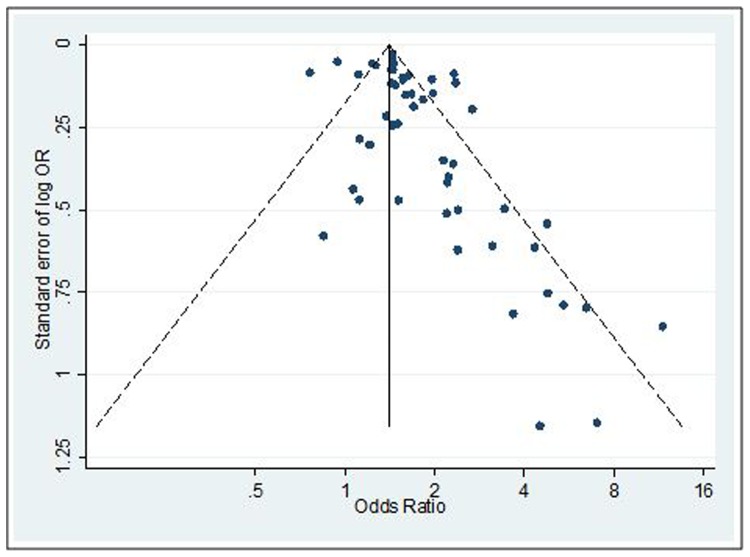
Visual inspection of the funnel plot reveals some asymmetry, suggesting smaller studies with negative associations might be under-represented in the literature (Figure 6). It should be noted, however, that funnel plot asymmetry need not result from bias [15].
Figure 6. Funnel plot with pseudo 95% confidence limits.
Discussion
This systematic review of the literature concerning the relationship between antidepressant use and preterm birth identified 41 observational studies. Findings suggest the risk of preterm birth is increased in women treated with antidepressant medications during pregnancy, with pooled odds ratio estimates ranging between 1.16 (95% CI 0.92–1.45) and 1.96 (95% CI 1.62–2.38). The associations were stronger for antidepressant use later in pregnancy. Adjusting for a diagnosis of depression in most cases did not eliminate the effect, although the strength of the observed associations was somewhat attenuated. Sensitivity analyses demonstrated that very strong risk factors of preterm birth that are fairly imbalanced among exposure groups and independent of the adjusted confounders must be unmeasured and uncontrolled to explain the observed associations. Although it would be unlikely to miss such a strong single confounder, it is conceivable that several weaker confounders could have acted together to account for the apparent effect. Our findings are consistent with those from an earlier study which evaluated the association between prenatal antidepressant exposure and a range of adverse pregnancy and delivery outcome [73].
Preterm birth is a major problem worldwide [74]. The rate of antidepressant use during pregnancy has steadily increased over time in many industrialized nations, from less than 1% of women exposed in the early 90s to 3–6% in 2006 in Europe [10], [12], and to 7.5% in 2008 in the US [13]. It is therefore essential to determine if antidepressant use increases the risk of preterm birth. Studies in this area, however, are complicated due to several issues.
First, antidepressant exposure in many pregnancies is not a “yes or no” phenomenon. Many women stop antidepressants when they discover they are pregnant, resulting in first trimester exposure only. Others stop, but may restart the medications later in the pregnancy. Still other patients do not take the medications for much of the pregnancy but start in the third trimester. The issue of classifying exposure is therefore complex. Several studies, for example, have been performed by Teratogen Information Services (TIS). Women who called the TIS and reported that they were taking antidepressants were classified as “exposed” in those studies. The advantages of this design include the fact that exposure is determined prior to outcome, eliminating recall bias, and a control group is readily identifiable–women who contact the TIS with exposure to another medication. Yet, there are also major drawbacks to this approach. Most women who contact TIS are concerned about the use of antidepressants during pregnancy; therefore many of them may not continue with the medication throughout the pregnancy. If exposure to antidepressants in the second and third trimesters is more likely to be associated with preterm birth (and available evidence suggests that this might be the case) then TIS studies that classify women as exposed solely on the basis of first trimester exposure would likely underestimate the association between antidepressant use and preterm delivery. Studies that rely on electronic healthcare databases, on the other hand, contain detailed information on filled prescriptions for antidepressant medications during the entire pregnancy. Automated pharmacy dispensing information is usually seen as the gold standard of drug exposure compared to self-reported information [75] or prescribing records in outpatient medical records [76]. Patient recall bias is not an issue in healthcare utilization databases since all data recording is independent of a patient’s memory or agreement to participate in a research study [77]–[80]. However, filling a prescription does not necessarily guarantee that it was ingested, which could result in some misclassification. Such misclassification of exposure which is independent of the outcome status is likely to bias results towards the null (i.e. attenuate the association between antidepressant use and preterm birth.).
A major concern is the potential for confounding by the underlying depression and its severity, and associated poor health behaviors (e.g., nutrition, smoking, drug and alcohol use). More severely depressed women may be more likely to take antidepressants during pregnancy, and it has been suggested that it may be the depression itself that is causing the preterm birth and not the medication. Several of the studies in this systematic review made efforts to control for maternal depression and these studies continued to show increased rates of preterm birth in the antidepressant exposed pregnancies. The majority of studies we reviewed did not find increased preterm birth rates in depressed women unexposed to medication. However, despite these studies’ attempts to control for depression, it is likely that women with a diagnosis of depression who opt to continue treatment during pregnancy are inherently different from women with a depression diagnosis who discontinue treatment during pregnancy. It is therefore questionable whether these studies completely addressed confounding by indication severity. Nevertheless, the available data on whether depression itself is associated with preterm birth is inconsistent [81], and expert review panels have concluded that there is no clear association between depression and preterm birth. The Institute of Medicine reviewed this question and concluded that “Overall, recent prospective studies on depression do not suggest a strong pattern for depression as a general risk for preterm delivery, consistent with the results of earlier studies” [7]. A 2009 AAP/ACOG review similarly concluded: “Available data neither support nor refute a link between MDD [major depressive disorder] and these outcomes [preterm delivery and gestational age]” [82]. Despite the weak evidence to support the independent association between depression and adverse pregnancy outcomes, such as preterm birth, the prior belief is strong among some investigators [83].
Some limitations of this review, resulting from limitations in the source data, should be noted. The studies included were heterogeneous in terms of design, size, exposure assessment, timing and nature of the exposure, and confounding adjustment. For example, in some studies, women classified as exposed were only those taking medication throughout the pregnancy, and controls were those who stopped antidepressant use before the second trimester, while in others the “exposed” were patients taking an antidepressant in the first trimester, who may have stopped by the second trimester. We tried to address this by presenting the results separately by level of adjustment and timing of the exposure, but assignment of studies to these categories is somewhat subjective, and heterogeneity within categories remains. Although we would have liked to simultaneously investigate the effects of some of these factors through meta-regression, this was not feasible due to an insufficient number of studies. Given that all but one study defined preterm birth as a dichotomous outcome (<37 gestational weeks), we were not able to examine the strength of the association between antidepressant medication use and very-, moderate- and mild-preterm birth respectively. Nevertheless, even late preterm birth is a significant contributor to poor neonatal outcomes [3]–[6]. Likewise, the available evidence did not permit evaluation of the association with specific types of prematurity [83].
The most rigorous method for determining an association between antidepressant medication and preterm birth would be a randomized controlled trial, and some have argued for this [84]. Yet, numerous studies are now available that suggest antidepressant use during pregnancy may be associated with spontaneous abortion [85], birth defects [86], persistent pulmonary hypertension of the newborn [87], and newborn behavioral syndrome [88]. Whether it would be ethical to randomize depressed women to a medication arm with these possible effects is open for debate. Since it is unlikely a randomized trial will ever be conducted and since causality can never be ‘proven’ in the absence of trial data, we need to decide at what point the evidence is sufficiently strong to warrant informing patients, providers, and the public about the potential risks, so that they can be weighed against any expected benefits in a given patient.
In conclusion, the findings from our review of the literature are consistent with an association between antidepressant use during pregnancy and preterm birth, although the possibility of residual confounding by depression severity cannot be completely ruled out based on the available evidence. Counseling of pregnant women must take into consideration the clinical circumstances of a given patient, the strength of the available evidence on the risks and benefits (i.e., avoidance of risks associated with untreated depression), and alternatives to medication use during pregnancy. While our study findings cannot prove causality, they reinforce the notion that antidepressants should not be used by pregnant women in the absence of a clear need that cannot be met through alternative approaches.
Supporting Information
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist.
(DOC)
Acknowledgments
We would like to acknowledge the contributions of Jessica L. Bauer MD, MS (Beth Israel Medical Center, Albert Einstein College of Medicine, New York, NY) and Wesley Eddings, PhD (Division of Pharmacoepidemiology, Brigham and Women’s Hospital, Boston, MA) in the preparation of this manuscript.
This study was presented at the 29th International Conference on PharmacoEpidemiology and Therapeutic Risk Management, Montreal, August 25–28, 2013.
Funding Statement
There are no current funding sources for this study. Krista Huybrechts is supported by a career development grant K01MH099141 from the National Institute of Mental Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Slattery MM, Morrison JJ (2002) Preterm delivery. The Lancet 360: 1489–1497. [DOI] [PubMed] [Google Scholar]
- 2. Goyal N, Fiks A, Lorch S (2011) Association of late-preterm birth with asthma in young children: practice-based study. Pediatrics 128: e830–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shapiro-Mendoza CK, Tomashek KM, Kotelchuck M, Barfield W, Nannini A, et al. (2008) Effect of late-preterm birth and maternal medical conditions on newborn morbidity risk. Pediatrics 121: e223–232. [DOI] [PubMed] [Google Scholar]
- 4. Engle WA, Tomashek KM, Wallman C (2007) “Late-preterm” infants: a population at risk. Pediatrics 120: 1390–1401. [DOI] [PubMed] [Google Scholar]
- 5. Leone A, Ersfeld P, Adams M, Schiffer PM, Bucher HU, et al. (2012) Neonatal morbidity in singleton late preterm infants compared with full-term infants. Acta Paediatr 101: e6–10. [DOI] [PubMed] [Google Scholar]
- 6. Kramer MS, Demissie K, Yang H, Platt RW, Sauve R, et al. (2000) The contribution of mild and moderate preterm birth to infant mortality. Fetal and Infant Health Study Group of the Canadian Perinatal Surveillance System. JAMA 284: 843–849. [DOI] [PubMed] [Google Scholar]
- 7.Institute of Medicine (US) Committee on Understanding Premature Birth and Assuring Healthy Outcomes; Behrman RE, Butler AS, editors. Preterm Birth: Causes, Consequences, and Prevention. Washington (DC): National Academies Press (US); 2007. [PubMed]
- 8. Mangham LJ, Petrou S, Doyle LW, Draper ES, Marlow N (2009) The cost of preterm birth throughout childhood in England and Wales. Pediatrics 123: e312–327. [DOI] [PubMed] [Google Scholar]
- 9. Olfson M, Marcus SC (2009) National patterns in antidepressant medication treatment. Arch Gen Psychiatry 66: 848–856. [DOI] [PubMed] [Google Scholar]
- 10. Petersen I, Gilbert RE, Evans SJ, Man SL, Nazareth I (2012) Pregnancy as a major determinant for discontinuation of antidepressants: an analysis of data from The Health Improvement Network. J Clin Psychiatry 72: 979–985. [DOI] [PubMed] [Google Scholar]
- 11. Bakker MK, Kolling P, van den Berg PB, de Walle HEK, de Jong van den Berg LTW (2008) Increase in use of selective serotonin reuptake inhibitors in pregnancy during the last decade, a population-based cohort study from the Netherlands. Br J Clin Pharmacol 65: 600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Munk-Olsen T, Gasse C, Laursen TM (2012) Prevalence of antidepressant use and contacts with psychiatrists and psychologists in pregnant and postpartum women. Acta Psychiatr Scand 125: 318–324. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell AA, Gilboa SM, Werler MM, Kelley KE, Louik C, et al.. (2011) Medication use during pregnancy, with particular focus on prescription drugs: 1976–2008. Am J Obstet Gynecol 205: 51 e51–58. [DOI] [PMC free article] [PubMed]
- 14. Huybrechts KF, Palmsten K, Mogun H, Kowal M, Avorn J, et al. (2013) National trends in antidepressant medication treatment among publicly insured pregnant women. Gen Hosp Psychiatry 35: 265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hedges L, Olkin I (1985) Statistical Methods for Meta-analysis. Boston, Mass: Academic Press.
- 16.Borenstein M, Hedges L, Higgins J, Rothstein H (2009) Introduction to Meta-Analysis: John Wiley & Sons, Ltd.
- 17. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 18.Deeks JJ, Higgins JPT, Altman DG (2008) Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Chichester (UK): John Wiley & Sons.
- 19. Schneeweiss S (2006) Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiology and Drug Safety 15: 291–303. [DOI] [PubMed] [Google Scholar]
- 20. Cohen LS, Heller VL, Bailey JW, Grush L, Ablon JS, et al. (2000) Birth outcomes following prenatal exposure to fluoxetine. Biol Psychiatry 48: 996–1000. [DOI] [PubMed] [Google Scholar]
- 21. Goldstein DJ (1995) Effects of third trimester fluoxetine exposure on the newborn. J Clin Psychopharmacol 15: 417–420. [DOI] [PubMed] [Google Scholar]
- 22. Malm H, Klaukka T, Neuvonen PJ (2005) Risks associated with selective serotonin reuptake inhibitors in pregnancy. Obstet Gynecol 106: 1289–1296. [DOI] [PubMed] [Google Scholar]
- 23. McElhatton PR, Garbis HM, Elefant E, Vial T, Bellemin B, et al. (1996) The outcome of pregnancy in 689 women exposed to therapeutic doses of antidepressants. A collaborative study of the European Network of Teratology Information Services (ENTIS). Reprod Toxicol 10: 285–294. [DOI] [PubMed] [Google Scholar]
- 24. Oberlander TF, Warburton W, Misri S, Aghajanian J, Hertzman C (2008) Effects of timing and duration of gestational exposure to serotonin reuptake inhibitor antidepressants: population-based study. Br J Psychiatry 192: 338–343. [DOI] [PubMed] [Google Scholar]
- 25. Kulin NA, Pastuszak A, Sage SR, Schick-Boschetto B, Spivey G, et al. (1998) Pregnancy outcome following maternal use of the new selective serotonin reuptake inhibitors: a prospective controlled multicenter study. JAMA 279: 609–610. [DOI] [PubMed] [Google Scholar]
- 26. Nulman I, Rovet J, Stewart DE, Wolpin J, Gardner HA, et al. (1997) Neurodevelopment of children exposed in utero to antidepressant drugs. N Engl J Med 336: 258–262. [DOI] [PubMed] [Google Scholar]
- 27. Nulman I, Rovet J, Stewart DE, Wolpin J, Pace-Asciak P, et al. (2002) Child development following exposure to tricyclic antidepressants or fluoxetine throughout fetal life: a prospective, controlled study. Am J Psychiatry 159: 1889–1895. [DOI] [PubMed] [Google Scholar]
- 28. Suri R, Altshuler L, Hendrick V, Rasgon N, Lee E, et al. (2004) The impact of depression and fluoxetine treatment on obstetrical outcome. Arch Womens Ment Health 7: 193–200. [DOI] [PubMed] [Google Scholar]
- 29. Zeskind PS, Stephens LE (2004) Maternal selective serotonin reuptake inhibitor use during pregnancy and newborn neurobehavior. Pediatrics 113: 368–375. [DOI] [PubMed] [Google Scholar]
- 30. Calderon-Margalit R, Qiu C, Ornoy A, Siscovick DS, Williams MA (2009) Risk of preterm delivery and other adverse perinatal outcomes in relation to maternal use of psychotropic medications during pregnancy. Am J Obstet Gynecol 201: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Casper RC, Fleisher BE, Lee-Ancajas JC, Gilles A, Gaylor E, et al. (2003) Follow-up of children of depressed mothers exposed or not exposed to antidepressant drugs during pregnancy. J Pediatr 142: 402–408. [DOI] [PubMed] [Google Scholar]
- 32. Casper RC, Gilles AA, Fleisher BE, Baran J, Enns G, et al. (2011) Length of prenatal exposure to selective serotonin reuptake inhibitor (SSRI) antidepressants: effects on neonatal adaptation and psychomotor development. Psychopharmacology (Berl) 217: 211–219. [DOI] [PubMed] [Google Scholar]
- 33. Chambers CD, Johnson KA, Dick LM, Felix RJ, Jones KL (1996) Birth outcomes in pregnant women taking fluoxetine. N Engl J Med 335: 1010–1015. [DOI] [PubMed] [Google Scholar]
- 34. Colvin L, Slack-Smith L, Stanley FJ, Bower C (2011) Dispensing patterns and pregnancy outcomes for women dispensed selective serotonin reuptake inhibitors in pregnancy. Birth Defects Res A Clin Mol Teratol 91: 142–152. [DOI] [PubMed] [Google Scholar]
- 35. Costei AM, Kozer E, Ho T, Ito S, Koren G (2002) Perinatal outcome following third trimester exposure to paroxetine. Arch Pediatr Adolesc Med 156: 1129–1132. [DOI] [PubMed] [Google Scholar]
- 36. Davis RL, Rubanowice D, McPhillips H, Raebel MA, Andrade SE, et al. (2007) Risks of congenital malformations and perinatal events among infants exposed to antidepressant medications during pregnancy. Pharmacoepidemiol Drug Saf 16: 1086–1094. [DOI] [PubMed] [Google Scholar]
- 37. Diav-Citrin O, Shechtman S, Weinbaum D, Wajnberg R, Avgil M, et al. (2008) Paroxetine and fluoxetine in pregnancy: a prospective, multicentre, controlled, observational study. Br J Clin Pharmacol 66: 695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Djulus J, Koren G, Einarson TR, Wilton L, Shakir S, et al. (2006) Exposure to mirtazapine during pregnancy: a prospective, comparative study of birth outcomes. J Clin Psychiatry 67: 1280–1284. [DOI] [PubMed] [Google Scholar]
- 39. Einarson A, Choi J, Einarson TR, Koren G (2010) Adverse effects of antidepressant use in pregnancy: an evaluation of fetal growth and preterm birth. Depress Anxiety 27: 35–38. [DOI] [PubMed] [Google Scholar]
- 40. Ericson A, Kallen B, Wiholm B (1999) Delivery outcome after the use of antidepressants in early pregnancy. Eur J Clin Pharmacol 55: 503–508. [DOI] [PubMed] [Google Scholar]
- 41. Ferreira E, Carceller AM, Agogue C, Martin BZ, St-Andre M, et al. (2007) Effects of selective serotonin reuptake inhibitors and venlafaxine during pregnancy in term and preterm neonates. Pediatrics 119: 52–59. [DOI] [PubMed] [Google Scholar]
- 42. Gavin AR, Holzman C, Siefert K, Tian Y (2009) Maternal depressive symptoms, depression, and psychiatric medication use in relation to risk of preterm delivery. Womens Health Issues 19: 325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kallen B (2004) Neonate characteristics after maternal use of antidepressants in late pregnancy. Arch Pediatr Adolesc Med 158: 312–316. [DOI] [PubMed] [Google Scholar]
- 44. Latendresse G, Ruiz RJ (2011) Maternal corticotropin-releasing hormone and the use of selective serotonin reuptake inhibitors independently predict the occurrence of preterm birth. J Midwifery Womens Health 56: 118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lennestal R, Kallen B (2007) Delivery outcome in relation to maternal use of some recently introduced antidepressants. J Clin Psychopharmacol 27: 607–613. [DOI] [PubMed] [Google Scholar]
- 46. Lewis AJ, Galbally M, Opie G, Buist A (2010) Neonatal growth outcomes at birth and one month postpartum following in utero exposure to antidepressant medication. Aust N Z J Psychiatry 44: 482–487. [DOI] [PubMed] [Google Scholar]
- 47. Lund N, Pedersen LH, Henriksen TB (2009) Selective serotonin reuptake inhibitor exposure in utero and pregnancy outcomes. Arch Pediatr Adolesc Med 163: 949–954. [DOI] [PubMed] [Google Scholar]
- 48. Maschi S, Clavenna A, Campi R, Schiavetti B, Bernat M, et al. (2008) Neonatal outcome following pregnancy exposure to antidepressants: a prospective controlled cohort study. BJOG 115: 283–289. [DOI] [PubMed] [Google Scholar]
- 49. Mulder EJH, Ververs FF, de Heus R, Visser GHA (2011) Selective serotonin reuptake inhibitors affect neurobehavioral development in the human fetus. Neuropsychopharmacology 36: 1961–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Oberlander TF, Warburton W, Misri S, Aghajanian J, Hertzman C (2006) Neonatal outcomes after prenatal exposure to selective serotonin reuptake inhibitor antidepressants and maternal depression using population-based linked health data. Arch Gen Psychiatry 63: 898–906. [DOI] [PubMed] [Google Scholar]
- 51. Pastuszak A, Schick-Boschetto B, Zuber C, Feldkamp M, Pinelli M, et al. (1993) Pregnancy outcome following first-trimester exposure to fluoxetine (Prozac). JAMA 269: 2246–2248. [PubMed] [Google Scholar]
- 52. Pearson KH, Nonacs RM, Viguera AC, Heller VL, Petrillo LF, et al. (2007) Birth outcomes following prenatal exposure to antidepressants. J Clin Psychiatry 68: 1284–1289. [DOI] [PubMed] [Google Scholar]
- 53. Reis M, Kallen B (2010) Delivery outcome after maternal use of antidepressant drugs in pregnancy: an update using Swedish data. Psychol Med 40: 1723–1733. [DOI] [PubMed] [Google Scholar]
- 54. Rurak D, Lim K, Sanders A, Brain U, Riggs W, et al. (2011) Third trimester fetal heart rate and Doppler middle cerebral artery blood flow velocity characteristics during prenatal selective serotonin reuptake inhibitor exposure. Pediatr Res 70: 96–9101. [DOI] [PubMed] [Google Scholar]
- 55. Simon GE, Cunningham ML, Davis RL (2002) Outcomes of prenatal antidepressant exposure. Am J Psychiatry 159: 2055–2061. [DOI] [PubMed] [Google Scholar]
- 56. Sivojelezova A, Shuhaiber S, Sarkissian L, Einarson A, Koren G (2005) Citalopram use in pregnancy: prospective comparative evaluation of pregnancy and fetal outcome. Am J Obstet Gynecol 193: 2004–2009. [DOI] [PubMed] [Google Scholar]
- 57. Suri R, Altshuler L, Hellemann G, Burt VK, Aquino A, et al. (2007) Effects of antenatal depression and antidepressant treatment on gestational age at birth and risk of preterm birth. Am J Psychiatry 164: 1206–1213. [DOI] [PubMed] [Google Scholar]
- 58. Toh S, Mitchell AA, Louik C, Werler MM, Chambers CD, et al. (2009) Antidepressant use during pregnancy and the risk of preterm delivery and fetal growth restriction. J Clin Psychopharmacol 29: 555–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wen SW, Yang Q, Garner P, Fraser W, Olatunbosun O, et al. (2006) Selective serotonin reuptake inhibitors and adverse pregnancy outcomes. Am J Obstet Gynecol 194: 961–966. [DOI] [PubMed] [Google Scholar]
- 60. Wisner KL, Sit DKY, Hanusa BH, Moses-Kolko EL, Bogen DL, et al. (2009) Major depression and antidepressant treatment: impact on pregnancy and neonatal outcomes. Am J Psychiatry 166: 557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Klieger-Grossmann C, Weitzner B, Panchaud A, Pistelli A, Einarson T, et al. (2012) Pregnancy Outcomes Following Use of Escitalopram: A Prospective Comparative Cohort Study. Journal of Clinical Pharmacology 52: 766–770. [DOI] [PubMed] [Google Scholar]
- 62. Kieler H, Artama M, Engeland A, Ericsson Ãr, Furu K, et al. (2012) Selective serotonin reuptake inhibitors during pregnancy and risk of persistent pulmonary hypertension in the newborn: population based cohort study from the five Nordic countries. Bmj 344: d8012. [DOI] [PubMed] [Google Scholar]
- 63. Einarson A, Choi J, Koren G, Einarson T (2011) Outcomes of infants exposed to multiple antidepressants during pregnancy: results of a cohort study. J Popul Ther Clin Pharmacol 18: e390–396. [PubMed] [Google Scholar]
- 64. El Marroun H, Jaddoe VWV, Hudziak JJ, Roza SJ, Steegers EAP, et al. (2012) Maternal Use of Selective Serotonin Reuptake Inhibitors, Fetal Growth, and Risk of Adverse Birth Outcomes. Arch Gen Psychiatry 69: 706–714. [DOI] [PubMed] [Google Scholar]
- 65. Nordeng H, van Gelder M, Spigset O, Koren G, Einarson A, et al. (2012) Pregnancy outcome after exposure to antidepressants and the role of maternal depression: results from the Norwegian mother and child cohort study. J Clin Psychopharmacol 32: 186–194. [DOI] [PubMed] [Google Scholar]
- 66. Roca A, Garcia-Esteve L, Imaz ML, Torres A, Hernandez S, et al. (2011) Obstetrical and neonatal outcomes after prenatal exposure to selective serotonin reuptake inhibitors: The relevance of dose. Journal of affective disorders 135: 208–215. [DOI] [PubMed] [Google Scholar]
- 67. Suri R, Hellemann G, Stowe Z, Cohen L, Aquino A, et al. (2011) A prospective, naturalistic, blinded study of early neurobehavioral outcomes for infants following prenatal antidepressant exposure. J Clin Psychiatry 72: 1002–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Grzeskowiak LE, Gilbert AL, Morrison JL (2012) Neonatal outcomes after late-gestation exposure to selective serotonin reuptake inhibitors. J Clin Psychopharmacol 32: 615–621. [DOI] [PubMed] [Google Scholar]
- 69. Yonkers KA, Norwitz ER, Smith MV, Lockwood CJ, Gotman N, et al. (2012) Depression and Serotonin Reuptake Inhibitor Treatment as Risk Factors for Preterm Birth. Epidemiology 23: 677–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hayes RM, Wu P, Shelton RC, Cooper WO, Dupont WD, et al.. (2012) Maternal antidepressant use and adverse outcomes: a cohort study of 228,876 pregnancies. Am J Obstet Gynecol 207: 49 e41–49. [DOI] [PMC free article] [PubMed]
- 71. Wogelius P, Norgaard M, Gislum M, Pedersen L, Munk E, et al. (2006) Maternal use of selective serotonin reuptake inhibitors and risk of congenital malformations. Epidemiology 17: 701–704. [DOI] [PubMed] [Google Scholar]
- 72. Grote NK, Bridge JA, Gavin AR, Melville JL, Iyengar S, et al. (2010) A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry 67: 1012–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ross LE, Grigoriadis S, Mamisashvili L, Vonderporten EH, Roerecke M, et al. (2013) Selected pregnancy and delivery outcomes after exposure to antidepressant medication: a systematic review and meta-analysis. JAMA Psychiatry 70: 436–443. [DOI] [PubMed] [Google Scholar]
- 74. Blencowe H, Cousens S, Oestergaard M, Chou D, Moller A, et al. (2012) National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systmetic analysis and implications. The Lancet 479: 2162–2172. [DOI] [PubMed] [Google Scholar]
- 75. West S, Savitz D, Koch G, Strom B, Guess H, et al. (1995) Recall accuracy for prescription medications: self-report compared with database information. American Journal of Epidemiology 142: 1103–1112. [DOI] [PubMed] [Google Scholar]
- 76. West S, Strom B, Freundlich B, Normand E, Koch G, et al. (1994) Completeness of prescription recording in outpatient medical records from a health maintenance organization. Journal of Clinical Epidemiology 47: 165–167. [DOI] [PubMed] [Google Scholar]
- 77. Fowles J, Lawthers A, Weiner J, Garnick D, Petrie D, et al. (1995) Agreement between physicians’ office records and Medicare Part B claims data. Health Care Financ Rev 16: 189–199. [PMC free article] [PubMed] [Google Scholar]
- 78. Romano PS, Mark DH (1994) Bias in the coding of hospital discharge data and its implications for quality assessment. Med Care 32: 81–90. [DOI] [PubMed] [Google Scholar]
- 79. Glynn R, Monane M, Gurwitz J, Choodnovskiy I, Avorn J (1999) Agreement between drug treatment data and a discharge diagnosis of diabetes mellitus in the elderly. American Journal of Epidemiology 149: 541–549. [DOI] [PubMed] [Google Scholar]
- 80. Fisher ES, Whaley FS, Krushat WM, Malenka DJ, Fleming C, et al. (1992) The accuracy of Medicare’s hospital claims data: progress has been made, but problems remain. Am J Public Health 82: 243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Grigoriadis S, VonderPorten EH, Mamisashvili L, Tomlinson G, Dennis CL, et al. (2013) The impact of maternal depression during pregnancy on perinatal outcomes: a systematic review and meta-analysis. J Clin Psychiatry 74: e321–341. [DOI] [PubMed] [Google Scholar]
- 82. Yonkers KA, Wisner KL, Stewart DE, Oberlander TF, Dell DL, et al. (2009) The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Obstet Gynecol 114: 703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Palmsten K, Hernandez-Diaz S (2012) Can nonrandomized studies on the safety of antidepressants during pregnancy convincingly beat confounding, chance, and prior beliefs? Epidemiology 23: 686–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Coverdale JH, McCullough LB, Chervenak FA (2008) The ethics of randomized placebo-controlled trials of antidepressants with pregnant women: a systematic review. Obstet Gynecol 112: 1361–1368. [DOI] [PubMed] [Google Scholar]
- 85. Nakhai-Pour H, Broy P, Bérard A (2010) Use of antidepressants during pregnancy and the risk of spontaneous abortion. CMAJ 182: 1031–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Pedersen LH, Henriksen TB, Vestergaard M, Olsen J, Bech BH (2009) Selective serotonin reuptake inhibitors in pregnancy and congenital malformations: population based cohort study. Bmj 339: b3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Grigoriadis S, Vonderporten EH, Mamisashvili L, Tomlinson G, Dennis CL, et al. (2014) Prenatal exposure to antidepressants and persistent pulmonary hypertension of the newborn: systematic review and meta-analysis. Bmj 348: f6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sanz EJ, De-las-Cuevas C, Kiuru A, Bate A, Edwards R (2005) Selective serotonin reuptake inhibitors in pregnant women and neonatal withdrawal syndrome: a database analysis. Lancet 365: 482–487. [DOI] [PubMed] [Google Scholar]
- 89. Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist.
(DOC)