Abstract
Objective
Health-related quality of life (HRQoL) is an important outcome in individuals with a high risk for cardiovascular diseases. We investigated the association of HRQoL and body mass index (BMI) as an indicator for obesity.
Design
Secondary longitudinal analysis of the ORBITAL study, an intervention study which included high-risk cardiovascular primary care patients with hypercholesterolemia and an indication for statin therapy.
Methods
HRQoL was determined with the generic Short Form (SF)-12 health status instrument. Body weight and height were assessed at baseline and at months 6, 12, 18, 24, 30, and 36. We used a linear and a linear mixed-effects regression model to investigate the association between BMI and SF-12 summary scores at baseline as well as between change in BMI and SF-12 summary scores over 3 years. We adjusted for age, sex, smoking status, and in the longitudinal analysis also for the study arm and its interaction term with time.
Results
Of the 7640 participants who completed the baseline questionnaire, 6726 participants (mean age: 61 years) were analyzed. The baseline BMI was inversely associated with physical and mental SF-12 summary scores (β [95% CI] per 1 kg/m2: −0.36 [−0.41; −0.30] and −0.05 [−0.11; −0.00], respectively). A significant association between the change in BMI and physical SF-12 summary scores over time was only present in women (−0.18 [−0.27; −0.09]) and only in obese participants (−0.19 [−0.29; −0.10]). A change in BMI was directly associated with mental SF-12 summary scores (0.12 [0.06; 0.19]) in the total population.
Conclusion
Increases in BMI were associated with decreases in physical HRQoL, particularly in obese individuals and in women. In contrast, the mental HRQoL seemed to increase with increasing BMI over time. Thus, body weight management with respect to the HRQoL should be evaluated differentially by sex and body weight status.
Trial Registration
ClinicalTrials.gov NCT00379249
Introduction
The obesity epidemic is a major public health challenge in an increasing number of countries worldwide [1]. For the individual, the major consequences of obesity include an increased risk of both all-cause and, in particular, cardiovascular mortality [2]–[4]. In addition, obesity is a risk factor for several morbidities, such as type 2 diabetes mellitus [5], [6], and it is associated with psychological disorders, such as depression, and social discrimination [5]–[7].
Obesity is also an important indicator for health-related quality of life (HRQoL) [8]–[37]. HRQoL, which can be classified into physical and mental components, is both a predictor for future health status and an outcome itself. It has been shown to predict premature mortality [38]–[43] and morbidities such as type 2 diabetes mellitus and cardiovascular events [42], [44], [45]. HRQoL as an outcome is especially relevant for individuals with chronic diseases who spend an increasing amount of time living with their disease due to improved survival.
The cross-sectional association between measures of obesity and HRQoL in various populations has been broadly studied [8]–[31], [37]. In most studies, HRQoL was reduced in underweight and obese individuals; typically it was highest for individuals with a body mass index (BMI) between around 18.5 kg/m2 and 25 kg/m2, i.e., normal-weight individuals [8]–[15], [24], [26].
In contrast, only a few studies have investigated the longitudinal association between measures of obesity and HRQoL. In populations with diabetes or hypertension, a higher BMI was a predictor for later decreased physical HRQoL but not for mental HRQoL [32], [33]. Three cohort studies indicated that weight gain over time was linked to an impairment of physical HRQoL [34]–[36]. With the exception of these results, there is a lack of evidence that a change in body weight has an impact on later HRQoL.
In the present study, we analyzed data from patients with a high risk for cardiovascular diseases. We investigated the longitudinal impact of a change in BMI on HRQoL over a 3-year study period. In addition, we analyzed the cross-sectional association between baseline BMI and HRQoL.
Materials and Methods
Study Design
The analyzed patient cohort originated from the ORBITAL (Open Label Primary Care Study: Rosuvastatin Based Compliance Initiatives to Achievements of LDL Goals) study. This study was a randomized controlled trial, registered at www.clinicaltrials.gov with the identifier NCT00379249. The design and results are described in detail elsewhere [46], [47]. The primary aim of this intervention study was to investigate the cost-effectiveness of an adherence-enhancing intervention in patients with hypercholesterolemia at primary care practices. Participants were randomized to receive rosuvastatin therapy alone or together with an adherence program for 1 year, followed by a 2-year observational period. In a secondary longitudinal analysis of this intervention study combining all participant data, we investigated the association between BMI and HRQoL measured at baseline and at six follow-up points within 3 years.
Ethics Statement
The ethics committee of the Charité – Universitätsmedizin Berlin approved the study protocol. All of the participants gave written informed consent before study inclusion.
Study Population
In the ORBITAL study, the participants were enrolled with hypercholesterolemia and an indication for statin therapy from 1961 primary care practices in Germany. The participants had to have low-density lipoprotein cholesterol levels ≥3.0 mmol/l for drug-naïve individuals or ≥3.25 mmol/l for participants who were already on lipid-lowering therapy and with at least one of the following risk factors: diabetes, a history of coronary heart disease or other atherosclerotic disease, or an absolute coronary heart disease risk ≥20% over 10 years according to the Framingham criteria [48].
For the present analyses, we included all of the participants of the ORBITAL study with an available baseline questionnaire. We excluded participants with missing baseline data and those without paired data on BMI, HRQoL, and smoking status for at least one follow-up point. In addition, we excluded participants who were underweight, defined as a BMI <18.5 kg/m2. The size of the subsample of underweight individuals (n = 50) was too small to allow subgroup analyses by BMI category. Also, underweight is often associated with underlying diseases and an increased mortality, especially in the elderly, even after controlling for cancer and cardiovascular diseases [49], [50]. The impact of these diseases on HRQoL and weight-changes is difficult to disentangle and may introduce confounding by indication.
Assessments and Included Variables
At baseline, the participants filled out a standardized self-administered questionnaire. Physicians assessed the participants’ medical history and performed a physical examination. The follow-up data were collected from the participants by postal questionnaires every 6 months during a period of 3 years at the 6-, 12-, 18-, 24-, 30-, and 36-month follow-up points. In addition, a physical examination was performed at the 6- and 12-month follow-up points.
Health-related quality of life (HRQoL)
HRQoL was assessed at baseline and at all of the follow-up points with the standard 4-week recall version 1 of the Short Form-12 (SF-12), a generic health status instrument [51]. The SF-12 includes one or two items from each of the eight health concepts of the instrument SF-36 [51]. The SF-12 allows the calculation of the physical and mental component summary scores. The items selected for the SF-12 summary scales and the scoring algorithms were cross-validated in nine countries [52]. The SF-12 summary scales were calculated directly from the 12 items and were set missing if the respondent was missing any one of the items. Higher physical or mental SF-12 summary scores indicated a better HRQoL.
BMI, BMI categories, and weight-change groups
The body weight and height were assessed to the nearest kilogram and centimeter, respectively. They were self-reported by the participants in the baseline questionnaire and in the six follow-up questionnaires. In addition, physicians assessed body weight and height at baseline and at the 6- and 12- month follow-up. We calculated the BMI by dividing the self-reported body weight in kilograms by the baseline physician-reported height in meters squared (kg/m2). In the case of missing data for physician-reported height, the self-reported height at baseline was used. We used self-reported body weight to calculate the BMI allowing for consistency across all assessment points. We applied the World Health Organization (WHO) classification to categorize BMI values into the following BMI categories: underweight (BMI<18.5 kg/m2), normal weight (BMI 18.5 to <25 kg/m2), overweight (BMI 25 to <30 kg/m2), and obese (≥30 kg/m2) [5]. To differentiate between individuals with weight gain, stable weight, and weight loss from baseline to the 36-month follow-up, we classified the study population into three weight-change groups: weight loser (BMI change within 36 months <−0.5 kg/m2), stable weight (BMI change −0.5 to 0.5 kg/m2), and weight gainer (BMI change >0.5 kg/m2). These BMI change cut-offs were based on the cut-offs used in a comparable study on weight-change groups [35].
Smoking status and other covariates
We assessed socio-demographic variables such as age, sex, education level, living situation, and employment status by using standardized self-administered questionnaires at baseline. The living situation indicated whether the participants lived alone or not. Participants were categorized by their employment status according to whether they currently worked in a job or not. The education level was categorized into three levels according to the number of school years needed for the different levels of school graduation: low (≤9 years), middle (10 years), and high (12 to 13 years). Participants’ smoking status was categorized into three categories: current, former, and never smoker. The medical history was assessed at baseline and included diagnosis of diabetes and hypertension, history of myocardial infarction and stroke, as well as history of coronary artery bypass graft (CABG) and percutaneous coronary intervention (PCI) procedures. For each diagnosis or procedure, a binary variable was built. Cardiovascular events and revascularization procedures during follow-up were self-reported by participants in the questionnaires at the six follow-up points. A time-dependent variable, cumulative incidence of myocardial infarction or stroke, was generated by cumulating a dummy variable for the incidence of at least one of these events during the previous 6 months at each follow-up point. The time-dependent variable, cumulative incidence of CABG or PCI, was generated in the same way.
Statistical Analyses
First, we analyzed the relative validity of the BMI that we calculated using the self-reported body weight (hence: self-reported BMI) by comparing it with the BMI calculated using the physician-reported body weight (hence: physician-reported BMI). For the validation analysis, we used all of the available data from the ORBITAL study population at baseline. We calculated the Spearman correlation coefficient to determine the correlation between the self-reported and physician-reported BMI. To identify systematic and BMI-dependent differences between the two assessment methods, we used the Bland-Altman plot [53]. The difference between self-reported and physician-reported BMI was plotted against the average of both. The limits of agreement were defined by the mean difference ±2 standard deviations (SD) as suggested by Bland and Altman [53]. The association of the difference between the two assessment methods with the average of the two methods was tested by regression analysis. We also performed regression analyses to test for an association of the difference between the two assessment methods with the two SF-12 summary scores.
We performed descriptive statistical analysis for the baseline characteristics of the analyzed study population and stratified by BMI category and weight-change group. Baseline SF-12 summary scores were also provided for three age groups (<40 years, 40 to <60 years, and ≥60 years). The arithmetic means (±SD) or percentages were reported.
The cross-sectional association between the baseline BMI and baseline SF-12 summary scores was estimated using a linear regression model, which was adjusted for age, sex, and smoking status at baseline. We adjusted for smoking status as a potential confounder due to its association with both BMI and HRQoL in previous studies [11], [14], [32], [33]. In a further adjusted model, we additionally controlled for the potential confounders: education level, employment status, living situation, diagnosis of diabetes and hypertension, history of myocardial infarction, stroke, and CABG and PCI procedures at baseline.
The longitudinal association between change in BMI and SF-12 summary scores over the 3-year study period was estimated by using a linear mixed-effects regression model (PROC MIXED in SAS 9.3). The physical and mental SF-12 summary scores at baseline and at the six follow-up points were defined as the dependent variables in separate models. The models included the following as independent variables: change in BMI (as a time-dependent variable), baseline BMI, and time (as a categorical variable with seven levels: baseline and six follow-up points). The change in BMI was calculated at each follow-up point by subtracting the BMI value at baseline from the BMI value at the respective follow-up point. The regression coefficients (β) for change in BMI (in kg/m2) were generated in a linear mixed-effects regression model. These models have the advantage to deal with missing values because they use all of the available data from an individual during follow-up. A random statement was included to account for the initial differences between individuals. A repeated statement with a heterogeneous autoregressive covariance structure fitted best (according to Akaike’s information criterion) to account for correlations on the individual level between the repeated measures at baseline and the six follow-up points. This basic model was controlled for age, sex, and smoking status (as a time-dependent variable). Since we analyzed data from an intervention study, we also adjusted the basic model for the study arm (two levels: intervention and control group) and the interaction term time × study arm. A further adjusted model additionally controlled for the education level, employment status, living situation, diagnosis of diabetes and hypertension, history of myocardial infarction, stroke, and CABG and PCI procedures at baseline, with the combined cumulative incidence of myocardial infarction or stroke and the combined cumulative incidence of CABG or PCI procedures within the previous 6 months as time-dependent variables. The random effects parameters of the mixed-effects models are reported for the basic models.
To investigate the possible effect modifications with sex and baseline BMI category, the interaction terms with baseline BMI and change in BMI were separately entered into the basic models of the cross-sectional and longitudinal analyses. In the case of a significant interaction term, post-hoc subgroup analyses were performed and regression coefficients are presented.
All of the tests for significance were two-sided; the significance level was α = 0.05. The statistical analyses were performed using SAS 9.3 (SAS Institute Inc., Cary, North Carolina, US) and the figures were created with SPSS 19 (SPSS Inc., Chicago, Illinois, US). No adjustment for multiple testing was applied.
Results
Study Population
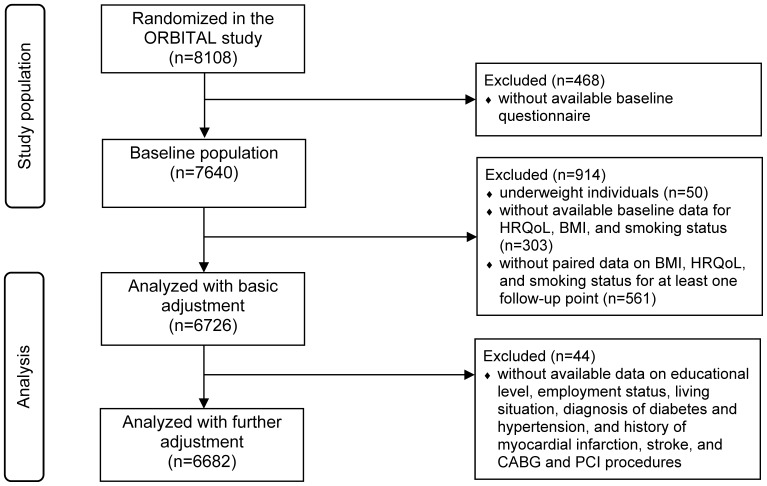
Figure 1 shows the participant flow from randomization in the ORBITAL study to the combined study population included in our analyses. At baseline, 7640 participants completed the self-administered questionnaire. We analyzed 6726 participants in the basic models. In the further adjusted models, 6682 participants could be analyzed because of the missing values of the additional variables. In Table 1 , the baseline characteristics including the SF-12 summary scores of the analyzed study population are presented, which are also stratified by BMI category. At baseline, the mean (±SD) physical SF-12 summary score was 49.7±8.1 in the age group of <40 years, 45.5±10.2 in the age group of 40 to <60 years, and 44.2±9.9 in participants aged ≥60 years. The respective means of the mental SF-12 summary score were 50.6±9.4, 50.4±9.8, and 44.2±9.9.
Figure 1. Participant flow from randomization in the ORBITAL intervention study to analyses of the present study.
Abbreviations: BMI = body mass index, CABG = coronary artery bypass graft, HRQoL = health-related quality of life, ORBITAL = Open Label Primary Care Study Rosuvastatin Based Compliance Initiatives to Achievements of LDL Goals, PCI = percutaneous coronary intervention.
Table 1. Baseline characteristics and SF-12 summary scores of the analyzed study population, stratified by BMI category.
| Variables1 | Totalpopulation | Normal weight (BMI 18.5to <25 kg/m2) | Overweight (BMI 25to <30 kg/m2) | Obese (BMI≥30 kg/m2) |
| No. | 6726 | 1493 | 3288 | 1945 |
| Physical SF-12 summary score (mean±SD)2 | 45±10 | 46±10 | 45±10 | 43±10 |
| Mental SF-12 summary score (mean±SD)2 | 52±9 | 52±9 | 52±9 | 52±10 |
| Age (years, mean±SD) | 61±10 | 61±11 | 61±10 | 60±10 |
| Male (%) | 57 | 51 | 63 | 51 |
| Intervention group (%) | 50 | 51 | 50 | 49 |
| Body mass index (kg/m2, mean±SD) | 28±4 | 23±1 | 27±1 | 33±3 |
| LDL cholesterol (mg/dl, mean±SD) | 170±39 | 173±40 | 171±39 | 168±38 |
| Education level (%)3 | ||||
| Low (≤9 school years) | 64 | 58 | 64 | 68 |
| Middle (10 school years) | 19 | 21 | 19 | 18 |
| High (12 to 13 school years) | 17 | 21 | 16 | 13 |
| Living alone (%) | 19 | 21 | 17 | 20 |
| Actively employed (%) | 33 | 34 | 33 | 31 |
| Smoking status (%) | ||||
| Current smoker | 21 | 27 | 21 | 18 |
| Former smoker | 36 | 27 | 39 | 38 |
| Never smoker | 43 | 46 | 40 | 45 |
| Hypertension (%) | 58 | 46 | 56 | 71 |
| Diabetes (%) | 28 | 17 | 25 | 43 |
| History of myocardial infarction (%) | 16 | 14 | 19 | 14 |
| History of stroke (%) | 7 | 6 | 7 | 7 |
| History of coronary artery bypass graft (%) | 10 | 9 | 12 | 7 |
| History of percutaneous coronaryintervention (%) | 12 | 10 | 13 | 10 |
Abbreviations: LDL = low-density lipoprotein, SD = standard deviation, SF = Short Form.
Percentages may not add up due to rounding.
Score could range between 0 and 100 and was assessed with the SF-12 health status instrument.
Categorization by the number of school years needed for the different levels of school graduation.
Relative Validity of Self-reported BMI
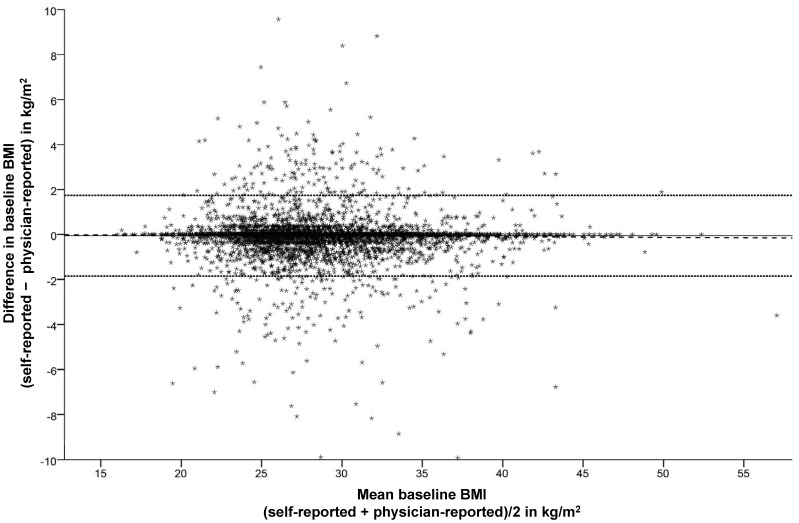
The paired data for the validation of BMI calculated with the self-reported body weight against the physician-reported body weight was available for 7570 participants. The Spearman correlation coefficient for the correlation between the self-reported and physician-reported BMI was r = 0.976 demonstrating a nearly perfect linear relationship between both of the measures. The Bland-Altman analysis indicated that BMI was underestimated on average by −0.1 kg/m2 when calculated with self-reported body weight ( Figure 2 ). The limits of agreement between the two assessment methods ranged from −1.9 to 1.7 kg/m2. The visual analysis of the plot and the results of the regression analysis (β = −0.003, P = 0.190) did not suggest that the difference between the two assessment methods depended on the participants’ BMI, which was calculated as the average of the two assessment methods. Similarly, the difference in BMI measured by the two methods was not associated with the self-reported physical and mental SF-12 summary scores (β = 0.001, P = 0.718 and β = 0.001, P = 0.350, respectively).
Figure 2. Bland Altman plot for baseline BMI calculated from the self-reported and physician-reported body weight.
The bias (mean) between the two methods is marked by the full line (–), the upper and lower limits of agreement (mean ±2 standard deviations) by the dotted line (···) and the regression line by the broken line (− − −). Six observations are outside the axis range. Abbreviation: BMI = body mass index.
Cross-sectional Association between BMI and HRQoL
Physical SF-12 summary score
On the cross-sectional level, BMI was inversely associated with physical SF-12 summary scores in the basic model, adjusted for age, sex, and smoking status, i.e., physical SF-12 summary scores decreased with increasing BMI ( Table 2 ). The further adjusted model confirmed this association controlling for education level, employment status, living situation, diagnosis of diabetes and hypertension, and history of myocardial infarction, stroke, and CABG and PCI procedures. There was a significant interaction in the basic model between baseline BMI category and baseline BMI with regard to physical SF-12 scores. In overweight and obese participants, BMI (in kg/m2) was inversely associated with physical SF-12 summary scores (β: −0.47, P<0.001 and β: −0.42, P<0.001, respectively). In contrast, in normal-weight participants, BMI was not significantly associated with physical SF-12 summary scores (β: 0.33, P = 0.082). There was no significant interaction between sex and baseline BMI ( Table 2 ).
Table 2. Cross-sectional association of baseline BMI with the mental and physical SF-12 summary scores, stratified by BMI category.
| Population | Association betweenbaseline BMIand SF-12 summaryscore1 | Interaction ofbaseline BMIwith sex | Interaction ofbaseline BMIwith baselineBMI category | |||
| β2 | [95% CI] | P | P | P | ||
| Physical SF-12 summary score | ||||||
| Basic model3 | total | −0.36 | [−0.41; −0.30] | <0.001 | 0.227 | 0.003 |
| Further adjusted model4 | total | −0.35 | [−0.37; −0.32] | <0.001 | ||
| Post-hoc analysis by baselineBMI category3 , 5 | normalweight | 0.33 | [−0.04; 0.70] | 0.082 | ||
| overweight | −0.47 | [−0.71; −0.23] | <0.001 | |||
| obese | −0.42 | [−0.57; −0.28] | <0.001 | |||
| Mental SF-12 summary score | ||||||
| Basic model3 | total | −0.05 | [−0.11; −0.00] | 0.045 | 0.976 | 0.488 |
| Further adjusted model4 | total | −0.05 | [−0.07; −0.03] | <0.001 | ||
Abbreviations: BMI = body mass index, CI = confidence intervals, SF = Short Form.
Stratified regression coefficients are reported only with significant interaction terms in the basic model (P<0.05).
β can be interpreted as difference in SF-12 summary score per increase in baseline BMI of 1 kg/m2.
Basic model included the SF-12 summary score at baseline as the dependent variable and baseline BMI, age, sex, and smoking status at baseline as independent variables, n = 6726.
Further adjusted model consisting of the basic model additionally adjusted for education level, employment status, living situation, diagnosis of diabetes and hypertension, and history of myocardial infarction, stroke, coronary artery bypass grafting, and percutaneous coronary intervention at baseline, n = 6682.
Normal weight (BMI 18.5 to <25 kg/m2), overweight (BMI 25 to <30 kg/m2), and obese (BMI ≥30 kg/m2).
Mental SF-12 summary score
There was a significant albeit small inverse cross-sectional association between BMI and mental SF-12 summary score (β: −0.05, P = 0.045) in the basic model ( Table 2 ). The association remained after further adjustment for education level, employment status, living situation, diagnosis of diabetes and hypertension, and history of myocardial infarction, stroke, and CABG and PCI procedures. The interactions of baseline BMI with baseline BMI category and with sex were not significant ( Table 2 ).
Longitudinal Association between BMI and HRQoL
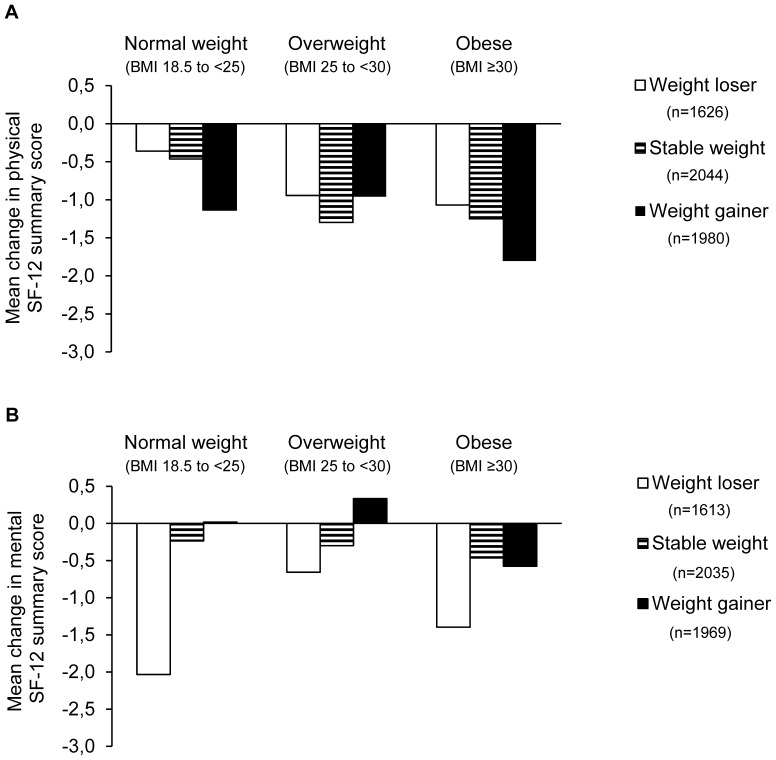
Figure 3 shows the mean changes in physical and mental SF-12 summary scores from baseline to the 36-month follow-up according to baseline BMI category in the three weight-change groups. The mean change (±SD) in BMI during the 36-month follow-up was 0.1±1.7 kg/m2.
Figure 3. Mean changes in the physical (part A) and mental (part B) SF-12 summary scores from baseline to 36-month follow-up according to baseline BMI category in three weight-change groups.
Weight-change groups: weight loser (BMI change over 36 months <−0.5 kg/m2), stable weight (BMI change −0.5 to 0.5 kg/m2), and weight gainer (BMI change >0.5 kg/m2). Abbreviations: BMI = body mass index in kg/m2, SF = Short Form.
Physical SF-12 summary score
The mixed-effects model revealed a significant inverse association between change in BMI and physical SF-12 summary scores over time, adjusted for baseline BMI, age, sex, smoking status, study arm, and the interaction term time × study arm ( Table 3 ). The association remained significant after further adjustment for education level, employment status, living situation, diagnosis of diabetes and hypertension, and history and time-dependent cumulative incidence of myocardial infarction/stroke and CABG/PCI. There were significant interactions between change in BMI with baseline BMI category and with sex. In initially obese participants, an increase in BMI was associated with a reduction in physical SF-12 summary scores over time (β: −0.19, P<0.001), whereas there was no association in normal-weight and overweight participants ( Table 3 ). Stratified analysis by sex showed that the inverse association between change in BMI and physical SF-12 summary scores over time was significant only in women (β: −0.18, P<0.001) ( Table 3 ).
Table 3. Longitudinal association of change in BMI with the mental and physical SF-12 summary scores during the 3-year follow-up, stratified by sex and BMI category.
| Population | Association between changein BMI and SF-12 summaryscore during follow-up1 | Interaction of changein BMIwith sex | Interaction of changein BMI withbaseline BMIcategory | |||
| β2 | [95% CI] | P | P | P | ||
| Physical SF-12 summary score | ||||||
| Basic model3 | total | −0.09 | [−0.15; −0.03] | 0.004 | 0.015 | 0.033 |
| Further adjusted model4 | total | −0.09 | [−0.16; −0.03] | 0.003 | ||
| Post-hoc analysis by sex3 | women | −0.18 | [−0.27; −0.09] | <0.001 | ||
| men | −0.01 | [−0.09; −0.07] | 0.821 | |||
| Post-hoc analysis by baselineBMI category3 , 5 | normalweight | 0.00 | [−0.15; 0.16] | 0.959 | ||
| overweight | −0.02 | [−0.12; 0.07] | 0.632 | |||
| obese | −0.19 | [−0.29; −0.10] | <0.001 | |||
| Mental SF-12 summary score | ||||||
| Basic model3 | total | 0.12 | [0.06; 0.19] | <0.001 | 0.484 | 0.089 |
| Further adjusted model4 | total | 0.13 | [0.06; 0.19] | <0.001 | ||
Abbreviations: BMI = body mass index, CI = confidence intervals, SF = Short Form.
Stratified regression coefficients are reported only in the case of significant interaction terms in the basic model (P<0.05).
β can be interpreted as the change in SF-12 summary score per increase in BMI change of 1 kg/m2 over time.
Basic model included the SF-12 summary score at all time points as the dependent variable and baseline BMI, change in BMI from baseline to all follow-up points, age, sex, study arm, interaction term time × study arm, and time-dependent smoking status as independent variables, n = 6726.
Further adjusted model consisting of the basic model additionally adjusted for education level, employment status, living situation, diagnosis of diabetes and hypertension, and history and time-dependent cumulative incidence of myocardial infarction/stroke and coronary artery bypass grafting/percutaneous coronary intervention within the previous 6 months, n = 6682.
Normal weight (BMI 18.5 to <25 kg/m2), overweight (BMI 25 to <30 kg/m2), and obese (BMI ≥30 kg/m2).
As estimated in the basic mixed-effects model, the variance in baseline physical SF-12 summary score between individuals (random effects parameter for the intercept) was 66.1 (standard error [SE]: 1.3). The variance between the individuals by the seven time points (random effects for the different time points) ranged from 27.4 (SE: 0.6) at the 12-month follow-up to 41.3 (SE: 0.8) at the 36-month follow-up. The heterogeneous autoregressive variance parameter was 0.20 (SE: 0.01).
Mental SF-12 summary score
With regard to the mental SF-12 summary score, an increase in BMI by 1 kg/m2 was associated with a higher mental SF-12 summary score by 0.12 score units (P<0.001) in the basic model ( Table 3 ). The association was similar after further adjustment for education level, employment status, living situation, diagnosis of diabetes and hypertension, and history and time-dependent cumulative incidence of myocardial infarction/stroke and CABG/PCI. There was no significant interaction between change in BMI and baseline BMI category or sex ( Table 3 ).
The random effects parameter for the baseline mental SF-12 summary score was 52.6 (SE: 1.1). The random effects for the different time points ranged from 30.7 (SE: 0.7) at the 12-month follow-up to 47.6 (SE: 0.7) at the 36-month follow-up. The heterogeneous autoregressive variance parameter was 0.15 (SE: 0.01).
Discussion
In the present study, we investigated the cross-sectional and the longitudinal association between BMI and HRQoL over 3 years in individuals with a high risk for cardiovascular diseases. In the cross-sectional analyses, BMI was inversely associated with physical and mental HRQoL, measured by the SF-12 health status instrument. The longitudinal analyses showed that change in BMI was inversely related to physical HRQoL in women and in obese individuals. In contrast, change in BMI was directly associated with mental HRQoL over time.
Cross-sectional Association between BMI and HRQoL
Numerous cross-sectional studies have affirmed that compared to normal-weight individuals, obese individuals are more likely to have a poorer level of physical HRQoL [8]–[17], [22], [25], [30], [31], [34], [37]. Our study results confirmed that BMI is inversely associated with physical SF-12 summary score. In addition, the cross-sectional association seemed to be non-linear. In overweight and obese individuals, the physical SF-12 summary score decreased with increasing BMI whereas no such association was found in normal-weight individuals. Several studies identified the highest levels of physical HRQoL among normal-weight adults [8]–[11], [13], [16], [17], [30], [54]. However, other studies showed significantly poorer physical HRQoL only in obese but not in overweight individuals [14], [15], [31], [37]. Studies that modeled a non-linear relationship identified different peak values of physical HRQoL at BMI-ranges from <20 to about 30 kg/m2 [10], [26], [29], [54].
The literature is less consistent in reporting an association between BMI and mental HRQoL. In several studies, obesity was related to the physical but not to the mental HRQoL components [11]–[13], [16]–[18], [22], [34], [37], [54]. Other studies showed an association between obesity and the mental HRQoL [8], [10], [14], [26], [31], [55], whereas the association was often much weaker compared to the physical components [11], [12], [16]. Our study confirmed a weak but significant inverse cross-sectional relationship between BMI and mental HRQoL. Several studies indicated a non-linear association between BMI and mental HRQoL [8], [10], [12], [26], [54]. According to our data, there was no significant interaction between baseline BMI and BMI category, which did not hint at a non-linear association between BMI and mental SF-12 summary score.
Longitudinal Association between Changes in BMI and HRQoL
Weight gain, indicated by an increase in BMI, was associated with decreased physical HRQoL over time in women and in obese individuals. Up to now, only a few studies have investigated the longitudinal association between change in body weight measures and HRQoL over time [34]–[36]. Cameron et al. showed in a population-based study in Australia that weight gain over 5 years was associated with an impaired physical HRQoL as measured by the SF-36 questionnaire [34]. Similar to our results, this negative impact of additional weight gain over time was greatest in the obese participants and tended to be stronger in women compared to men. This difference by gender was also observed in a British cohort study with middle-aged adults that combined retrospective and prospective assessments of body weight with a median follow-up time of 49 years [36]. Women, but not men, in the highest average weight gain tertile were more likely to have poor physical functioning compared to their counterparts in the lowest tertile [36]. Fine et al. analyzed the association of weight loss and weight gain over 4 years with HRQoL in middle- to older-aged women of the Nurses’ Health Study [35]. In this study, weight gain was associated with a decreased physical function score in all age and BMI groups. Weight loss, on the other hand, was associated with a slight improvement in physical HRQoL among overweight and obese women [35]. The descriptive analysis of our data also indicated that compared to weight loss, weight gain is more strongly associated with a decrease in physical HRQoL in obese individuals. To conclude, there seems to be an adverse impact of weight gain on physical HRQoL, especially in obese individuals and in women.
Our analyses showed that the mental SF-12 summary score was directly associated with BMI over time. The descriptive analysis indicated that weight loss, rather than weight gain, was associated with a decrease in mental HRQoL, especially among normal-weight individuals. Such an association was also reported by Fine et al. in the Nurses’ Health Study [35]. They found that losing 2 to 9 kg of body weight was associated with a decrease in the mental HRQoL, especially in women ≥65 years of age [35]. On the other hand, weight gain >9 kg was also associated with a reduction in the mental health score, but only in overweight and obese women. The study by Cameron et al. did not find any association between change in body weight over 5 years and mental HRQoL [34].
Explanations for the decreasing mental HRQoL with decreasing BMI remain speculative. Fine et al. suggested unintentional weight loss as a reason for the association in women in the Nurses’ Health Study [35]. Unintentional weight loss can be a symptom of an underlying disease such as undiagnosed cancer and psychiatric problems that may also have an impact on HRQoL [56]. A disease that was shown to be more strongly correlated with the mental rather than the physical HRQoL is depression [57]. Because depression is considered to be a main cause of unintentional weight loss in the elderly [58], it could be an underlying disease causing weight loss and reduced mental HRQoL.
Limitations
A major limitation of the present analyses is that BMI and HRQoL were self-reported which may introduce measurement bias. Thus, we validated self-reported BMI with physician-reported BMI. The results indicated that underreporting by participants was on average very low. Because the difference between the two measurement methods neither depended on the baseline BMI nor on the SF-12 summary scores, our data does not support a differential measurement bias by these factors. However, calculated changes in BMI over time may be imprecise because of the high individual imprecision of the self-reported BMI. This may reduce the power to show differences in HRQoL associated with changes in BMI.
Our study population consisted of primary care patients with hypercholesterolemia and an indication for statin therapy, and thus is not representative for the general population. However, the age-specific physical and mental SF-12 summary scores were similar to the German general population in the age group of ≥60 years, whereas in the younger age groups (<40 years and 40 to <60 years) both summary scores were slightly lower [59].
Another limitation is the linear modeling of the associations between BMI and HRQoL. To detect a possible non-linearity, we tested for an interaction of baseline BMI with BMI category and, in consequence, stratified the analyses. Our results suggest non-linear associations between BMI and physical HRQoL. To facilitate the interpretation of the results, we had decided to use the BMI categories according to the WHO classification. Other models without BMI categorization may result in a better model fit.
A strength of our longitudinal analysis was the application of the mixed-effects model which used all available data of the baseline and the six follow-up points by multilevel modeling. This method also considered individuals with missing data at some follow-up points. Thus, selection bias by the exclusion of participants with missing data could be reduced.
Clinical Relevance
We found a significant longitudinal association between BMI and physical HRQoL. With each increase in BMI due to weight gain the physical SF-12 summary score decreased over time in the overall study population. Stratified analysis revealed that this inverse association was definite in obese individuals and in women. In obese participants, for example, each increase in BMI by 1 kg/m2 was associated with a decreased physical SF-12 summary score by 0.19 units over 3 years. The clinical relevance of the observed association may thus be limited during the 3-year follow-up period. However, considering weight gain over the lifespan the clinical relevance might increase over the long term.
A beneficial impact of weight change would become relevant if population groups are identified for which interventions for weight loss or weight maintenance have the potential to increase or maintain HRQoL. Maciejewski et al. found in their review and meta-analysis that interventions for weight loss can improve HRQoL but study results were inconsistent [54]. In weight-loss interventions, it can be difficult to distinguish between the effect of the reduced body weight on HRQoL and the effect of the components of the program such as increased physical activity or improved diet. Two studies investigating this difference showed that the individual’s weight loss could not completely but partly explain the beneficial intervention effect on physical HRQoL [55], [56].
We observed that weight gain was associated with a decrease in physical HRQoL in women but not in men. This result was in accordance with two of the three longitudinal studies [34], [36] in which the impact of weight gain on physical HRQoL was more pronounced in women compared to men. The third longitudinal study included only women [35]. Maciejewski et al. could not analyze the effect of weight loss interventions on HRQoL in their meta-analysis for the subgroup of women [60]. Statistical pooling of the data was not possible as the original data was unavailable to the authors. Thus, weight loss interventions may be especially appropriate in the target group of obese women but more research is needed to investigate gender differences in the association between BMI and HRQoL.
Conclusion
Physical HRQoL decreases with increasing BMI in overweight and obese individuals. Mental HRQoL seems to be slightly poorer in individuals with a higher BMI. Weight gain over time was associated with an impairment of the physical HRQoL. In contrast, weight loss seems to result in a reduced mental HRQoL. The negative impact of weight gain on the physical HRQoL seems to be specifically relevant in obese individuals and in women, and these could be target groups for preventive and therapeutic interventions that aim at improved HRQoL. An adverse effect of weight loss on mental HRQoL over time needs to be replicated in further studies considering factors such as unintentional weight loss.
Funding Statement
The original intervention study was supported by an unconditional grant from AstraZeneca. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The present analysis was supported exclusively by the Berlin School of Public Health, Charité University Medical Center Berlin.
References
- 1. Aranceta J, Moreno B, Moya M, Anadon A (2009) Prevention of overweight and obesity from a public health perspective. Nutr Rev 67 Suppl 1S83–88. [DOI] [PubMed] [Google Scholar]
- 2. Flegal KM, Kit BK, Orpana H, Graubard BI (2013) Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA 309: 71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, et al. (2009) Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 373: 1083–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lenz M, Richter T, Muhlhauser I (2009) The morbidity and mortality associated with overweight and obesity in adulthood: a systematic review. Dtsch Arztebl Int 106: 641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization (2000) Obesity: preventing and managing the global epidemic. Report of a WHO consultation. Geneva, Switzerland: World Health Organization. i-xii, 253 p. [PubMed] [Google Scholar]
- 6. Kumanyika SK, Obarzanek E, Stettler N, Bell R, Field AE, et al. (2008) Population-based prevention of obesity: the need for comprehensive promotion of healthful eating, physical activity, and energy balance: a scientific statement from American Heart Association Council on Epidemiology and Prevention, Interdisciplinary Committee for Prevention (formerly the expert panel on population and prevention science). Circulation 118: 428–464. [DOI] [PubMed] [Google Scholar]
- 7. Onyike CU, Crum RM, Lee HB, Lyketsos CG, Eaton WW (2003) Is obesity associated with major depression? Results from the Third National Health and Nutrition Examination Survey. Am J Epidemiol 158: 1139–1147. [DOI] [PubMed] [Google Scholar]
- 8. Doll HA, Petersen SE, Stewart-Brown SL (2000) Obesity and physical and emotional well-being: associations between body mass index, chronic illness, and the physical and mental components of the SF-36 questionnaire. Obes Res 8: 160–170. [DOI] [PubMed] [Google Scholar]
- 9. Bottone FG Jr, Hawkins K, Musich S, Cheng Y, Ozminkowski RJ, et al. (2013) The relationship between body mass index and quality of life in community-living older adults living in the United States. J Nutr Health Aging 17: 495–501. [DOI] [PubMed] [Google Scholar]
- 10. Brown WJ, Dobson AJ, Mishra G (1998) What is a healthy weight for middle aged women? Int J Obes Relat Metab Disord 22: 520–528. [DOI] [PubMed] [Google Scholar]
- 11. Yan LL, Daviglus ML, Liu K, Pirzada A, Garside DB, et al. (2004) BMI and health-related quality of life in adults 65 years and older. Obes Res 12: 69–76. [DOI] [PubMed] [Google Scholar]
- 12. Heo M, Allison DB, Faith MS, Zhu S, Fontaine KR (2003) Obesity and quality of life: mediating effects of pain and comorbidities. Obes Res 11: 209–216. [DOI] [PubMed] [Google Scholar]
- 13. Sach TH, Barton GR, Doherty M, Muir KR, Jenkinson C, et al. (2007) The relationship between body mass index and health-related quality of life: comparing the EQ-5D, EuroQol VAS and SF-6D. Int J Obes (Lond) 31: 189–196. [DOI] [PubMed] [Google Scholar]
- 14. Ford ES, Moriarty DG, Zack MM, Mokdad AH, Chapman DP (2001) Self-reported body mass index and health-related quality of life: findings from the Behavioral Risk Factor Surveillance System. Obes Res 9: 21–31. [DOI] [PubMed] [Google Scholar]
- 15. Jia H, Lubetkin EI (2005) The impact of obesity on health-related quality-of-life in the general adult US population. J Public Health (Oxf) 27: 156–164. [DOI] [PubMed] [Google Scholar]
- 16. Katz DA, McHorney CA, Atkinson RL (2000) Impact of obesity on health-related quality of life in patients with chronic illness. J Gen Intern Med 15: 789–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Larsson U, Karlsson J, Sullivan M (2002) Impact of overweight and obesity on health-related quality of life–a Swedish population study. Int J Obes Relat Metab Disord 26: 417–424. [DOI] [PubMed] [Google Scholar]
- 18. Le Pen C, Levy E, Loos F, Banzet MN, Basdevant A (1998) “Specific” scale compared with “generic” scale: a double measurement of the quality of life in a French community sample of obese subjects. J Epidemiol Community Health 52: 445–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lean ME, Han TS, Seidell JC (1998) Impairment of health and quality of life in people with large waist circumference. Lancet 351: 853–856. [DOI] [PubMed] [Google Scholar]
- 20. Lean ME, Han TS, Seidell JC (1999) Impairment of health and quality of life using new US federal guidelines for the identification of obesity. Arch Intern Med 159: 837–843. [DOI] [PubMed] [Google Scholar]
- 21. Minet Kinge J, Morris S (2010) Socioeconomic variation in the impact of obesity on health-related quality of life. Soc Sci Med 71: 1864–1871. [DOI] [PubMed] [Google Scholar]
- 22. Han TS, Tijhuis MA, Lean ME, Seidell JC (1998) Quality of life in relation to overweight and body fat distribution. Am J Public Health 88: 1814–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wee HL, Cheung YB, Loke WC, Tan CB, Chow MH, et al. (2008) The association of body mass index with health-related quality of life: an exploratory study in a multiethnic Asian population. Value Health 11 Suppl 1S105–114. [DOI] [PubMed] [Google Scholar]
- 24. Groessl EJ, Kaplan RM, Barrett-Connor E, Ganiats TG (2004) Body mass index and quality of well-being in a community of older adults. Am J Prev Med 26: 126–129. [DOI] [PubMed] [Google Scholar]
- 25. Franco OH, Wong YL, Kandala NB, Ferrie JE, Dorn JM, et al. (2012) Cross-cultural comparison of correlates of quality of life and health status: the Whitehall II Study (UK) and the Western New York Health Study (US). Eur J Epidemiol 27: 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Finkelstein MM (2000) Body mass index and quality of life in a survey of primary care patients. J Fam Pract 49: 734–737. [PubMed] [Google Scholar]
- 27. Ul-Haq Z, Mackay DF, Fenwick E, Pell JP (2012) Impact of metabolic comorbidity on the association between body mass index and health-related quality of life: a Scotland-wide cross-sectional study of 5,608 participants. BMC Public Health 12: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Herman KM, Hopman WM, Vandenkerkhof EG, Rosenberg MW (2012) Physical activity, body mass index, and health-related quality of life in Canadian adults. Med Sci Sports Exerc 44: 625–636. [DOI] [PubMed] [Google Scholar]
- 29. Hunger M, Schunk M, Meisinger C, Peters A, Holle R (2012) Estimation of the relationship between body mass index and EQ-5D health utilities in individuals with type 2 diabetes: Evidence from the population-based KORA studies. J Diabetes Complications 26: 413–418. [DOI] [PubMed] [Google Scholar]
- 30. Huang IC, Frangakis C, Wu AW (2006) The relationship of excess body weight and health-related quality of life: evidence from a population study in Taiwan. Int J Obes (Lond) 30: 1250–1259. [DOI] [PubMed] [Google Scholar]
- 31. Hassan MK, Joshi AV, Madhavan SS, Amonkar MM (2003) Obesity and health-related quality of life: a cross-sectional analysis of the US population. Int J Obes Relat Metab Disord 27: 1227–1232. [DOI] [PubMed] [Google Scholar]
- 32. Maatouk I, Wild B, Herzog W, Wesche D, Schellberg D, et al. (2012) Longitudinal predictors of health-related quality of life in middle-aged and older adults with hypertension: results of a population-based study. J Hypertens 30: 1364–1372. [DOI] [PubMed] [Google Scholar]
- 33. Maatouk I, Wild B, Wesche D, Herzog W, Raum E, et al. (2012) Temporal predictors of health-related quality of life in elderly people with diabetes: results of a German cohort study. PLoS One 7: e31088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cameron AJ, Magliano DJ, Dunstan DW, Zimmet PZ, Hesketh K, et al. (2012) A bi-directional relationship between obesity and health-related quality of life: evidence from the longitudinal AusDiab study. Int J Obes (Lond) 36: 295–303. [DOI] [PubMed] [Google Scholar]
- 35. Fine JT, Colditz GA, Coakley EH, Moseley G, Manson JE, et al. (1999) A prospective study of weight change and health-related quality of life in women. JAMA 282: 2136–2142. [DOI] [PubMed] [Google Scholar]
- 36. Stafford M, Hemingway H, Marmot M (1998) Current obesity, steady weight change and weight fluctuation as predictors of physical functioning in middle aged office workers: the Whitehall II Study. Int J Obes Relat Metab Disord 22: 23–31. [DOI] [PubMed] [Google Scholar]
- 37. Yancy WS Jr, Olsen MK, Westman EC, Bosworth HB, Edelman D (2002) Relationship between obesity and health-related quality of life in men. Obes Res 10: 1057–1064. [DOI] [PubMed] [Google Scholar]
- 38. Landman GW, van Hateren KJ, Kleefstra N, Groenier KH, Gans RO, et al. (2010) Health-related quality of life and mortality in a general and elderly population of patients with type 2 diabetes (ZODIAC-18). Diabetes Care 33: 2378–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. ter Horst R, Markou AL, Noyez L (2012) Prognostic value of preoperative quality of life on mortality after isolated elective myocardial revascularization. Interact Cardiovasc Thorac Surg 15: 651–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Osthus TB, Preljevic VT, Sandvik L, Leivestad T, Nordhus IH, et al. (2012) Mortality and health-related quality of life in prevalent dialysis patients: Comparison between 12-items and 36-items short-form health survey. Health Qual Life Outcomes 10: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Osthus TB, von der Lippe N, Ribu L, Rustoen T, Leivestad T, et al. (2012) Health-related quality of life and all-cause mortality in patients with diabetes on dialysis. BMC Nephrol 13: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kato N, Kinugawa K, Seki S, Shiga T, Hatano M, et al. (2011) Quality of life as an independent predictor for cardiac events and death in patients with heart failure. Circ J 75: 1661–1669. [DOI] [PubMed] [Google Scholar]
- 43. Zuluaga MC, Guallar-Castillon P, Lopez-Garcia E, Banegas JR, Conde-Herrera M, et al. (2010) Generic and disease-specific quality of life as a predictor of long-term mortality in heart failure. Eur J Heart Fail 12: 1372–1378. [DOI] [PubMed] [Google Scholar]
- 44. Myint PK, Surtees PG, Wainwright NW, Luben RN, Welch AA, et al. (2007) Physical health-related quality of life predicts stroke in the EPIC-Norfolk. Neurology 69: 2243–2248. [DOI] [PubMed] [Google Scholar]
- 45. Tapp RJ, O'Neil A, Shaw JE, Zimmet PZ, Oldenburg BF (2010) Is there a link between components of health-related functioning and incident impaired glucose metabolism and type 2 diabetes? The Australian Diabetes Obesity and Lifestyle (AusDiab) study. Diabetes Care 33: 757–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Willich SN, Muller-Nordhorn J, Sonntag F, Voller H, Meyer-Sabellek W, et al. (2004) Economic evaluation of a compliance-enhancing intervention in patients with hypercholesterolemia: design and baseline results of the Open Label Primary Care Study: Rosuvastatin Based Compliance Initiatives To Achievements of LDL Goals (ORBITAL) study. Am Heart J 148: 1060–1067. [DOI] [PubMed] [Google Scholar]
- 47. Willich SN, Englert H, Sonntag F, Voller H, Meyer-Sabellek W, et al. (2009) Impact of a compliance program on cholesterol control: results of the randomized ORBITAL study in 8108 patients treated with rosuvastatin. Eur J Cardiovasc Prev Rehabil 16: 180–187. [DOI] [PubMed] [Google Scholar]
- 48. Anderson KM, Wilson PW, Odell PM, Kannel WB (1991) An updated coronary risk profile. A statement for health professionals. Circulation 83: 356–362. [DOI] [PubMed] [Google Scholar]
- 49. Flegal KM, Graubard BI, Williamson DF, Gail MH (2007) Impact of smoking and preexisting illness on estimates of the fractions of deaths associated with underweight, overweight, and obesity in the US population. Am J Epidemiol 166: 975–982. [DOI] [PubMed] [Google Scholar]
- 50. Flegal KM, Graubard BI, Williamson DF, Gail MH (2005) Excess deaths associated with underweight, overweight, and obesity. JAMA 293: 1861–1867. [DOI] [PubMed] [Google Scholar]
- 51. Ware J Jr, Kosinski M, Keller SD (1996) A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 34: 220–233. [DOI] [PubMed] [Google Scholar]
- 52. Gandek B, Ware JE, Aaronson NK, Apolone G, Bjorner JB, et al. (1998) Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol 51: 1171–1178. [DOI] [PubMed] [Google Scholar]
- 53. Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1: 307–310. [PubMed] [Google Scholar]
- 54. Brown WJ, Mishra G, Kenardy J, Dobson A (2000) Relationships between body mass index and well-being in young Australian women. Int J Obes Relat Metab Disord 24: 1360–1368. [DOI] [PubMed] [Google Scholar]
- 55. Moriel G, Roscani MG, Matsubara LS, Cerqueira AT, Matsubara BB (2010) Quality of life in patients with severe and stable coronary atherosclerotic disease. Arq Bras Cardiol 95: 691–697. [DOI] [PubMed] [Google Scholar]
- 56. Hernandez JL, Riancho JA, Matorras P, Gonzalez-Macias J (2003) Clinical evaluation for cancer in patients with involuntary weight loss without specific symptoms. Am J Med 114: 631–637. [DOI] [PubMed] [Google Scholar]
- 57.Kocalevent RD, Hinz A, Brahler E (2013) Standardization of the depression screener Patient Health Questionnaire (PHQ-9) in the general population. Gen Hosp Psychiatry. [DOI] [PubMed]
- 58. McMinn J, Steel C, Bowman A (2011) Investigation and management of unintentional weight loss in older adults. BMJ 342: d1732. [DOI] [PubMed] [Google Scholar]
- 59.Bullinger M, Kirchberger IGH (1998) SF-36. Fragebogen zum Gesundheitszustand. Handbuch für die deutschsprachige Fragebogenversion. Göttingen: Hogrefe Verlag für Psychologie.
- 60. Maciejewski ML, Patrick DL, Williamson DF (2005) A structured review of randomized controlled trials of weight loss showed little improvement in health-related quality of life. J Clin Epidemiol 58: 568–578. [DOI] [PubMed] [Google Scholar]