Abstract
Early life history stages of marine organisms are generally thought to be more sensitive to environmental stress than adults. Although most marine invertebrates are broadcast spawners, some species are brooders and/or protect their embryos in egg or capsules. Brooding and encapsulation strategies are typically assumed to confer greater safety and protection to embryos, although little is known about the physico-chemical conditions within egg capsules. In the context of ocean acidification, the protective role of encapsulation remains to be investigated. To address this issue, we conducted experiments on the gastropod Crepidula fornicata. This species broods its embryos within capsules located under the female and veliger larvae are released directly into the water column. C. fornicata adults were reared at the current level of CO2 partial pressure (pCO2) (390 μatm) and at elevated levels (750 and 1400 μatm) before and after fertilization and until larval release, such that larval development occurred entirely at a given pCO2. The pCO2 effects on shell morphology, the frequency of abnormalities and mineralization level were investigated on released larvae. Shell length decreased by 6% and shell surface area by 11% at elevated pCO2 (1400 μatm). The percentage of abnormalities was 1.5- to 4-fold higher at 750 μatm and 1400 μatm pCO2, respectively, than at 390 μatm. The intensity of birefringence, used as a proxy for the mineralization level of the larval shell, also decreased with increasing pCO2. These negative results are likely explained by increased intracapsular acidosis due to elevated pCO2 in extracapsular seawater. The encapsulation of C. fornicata embryos did not protect them against the deleterious effects of a predicted pCO2 increase. Nevertheless, C. fornicata larvae seemed less affected than other mollusk species. Further studies are needed to identify the critical points of the life cycle in this species in light of future ocean acidification.
Introduction
Early life history stages of marine species, including embryos and larvae, are of crucial importance in population dynamics as they ensure dispersion, colonize new areas and sustain populations [1]. Their success in development and final recruitment are essential for the persistence of viable populations. Early stages of marine invertebrates are in general morphologically and ecologically distinct from the adult stage and are generally thought to be more sensitive to environmental stress [1] although, in some cases, they may be more tolerant than adults, e.g. some Antarctic species exposed to warming [2]. In the context of climate change, early development may be affected by various factors, such as temperature increases, hypoxia zones or ocean acidification. Due to the increase in atmospheric pCO2 predicted for the end of the century (from 475 to 1313 μatm according to the Intergovernemental Panel on Climate Change (IPCC)), pH in surface seawaters is likely to decline by 0.06 – 0.32 units [3], leading to a decrease in carbonate ion concentrations (CO3 2−) and a reduction in the calcium carbonate saturation state (Ω) [4]. Due to these changes in seawater carbonate chemistry, ocean acidification is considered a major threat to calcifying marine species, affecting their physiology and impairing their ability to build calcium carbonate shells and skeletons [8], [9], [10], [11], which can ultimately modify their behavior and distribution [5], [6], [7]. Early life stages (embryos, larvae and juveniles) of calcifying species are thus expected to be highly affected by ocean acidification [12], [13], as opposed to non-calcified larvae which are predicted to be more tolerant [14], [15]. This relatively higher vulnerability is likely due to fragile larval skeletons [9] and their high ratio of exposed surface-to-body mass compared to adults [16]. Identifying life history stages that are the most vulnerable to global change is needed to determine bottlenecks for species persistence and addressing their sensitivity to acidification is a major issue in a changing ocean [9].
Responses to near-future (end of century) levels of pCO2 depend on species, populations, habitats and developmental stages [9], [17], [18], [19] and understanding these effects on the early life stages requires taking into account the complete developmental cycle, from egg to juvenile [12]. In particular, the impact of elevated pCO2/decreased pH on early life stages has been investigated in a broad range of species, including corals [20], [21], echinoderms [22], [23], [24], crustaceans [15], [16], [25], mollusks [26], [27], and fish [28], [29]. In mollusks, which have been studied intensively (see refs. [26], [30] for a review), deleterious effects of increased pCO2 have been demonstrated on fertilization success [31], [32], hatching success [33], [34], larval survival [35], [36], growth [37], [38], shell formation [39], [40], development duration [41], [42], and settlement [43], [44].
Most of the species studied are broadcast spawners, which may be considered particularly vulnerable to ocean acidification because fertilization and complete pelagic larval life occur in the water column [9], [45]. Whether alternative reproductive modes are affected in a changing ocean is still poorly documented. Brooding and/or egg laying in egg masses or capsules are typically assumed to confer protection to developing embryos [46], [47]. For example, it has been shown that encapsulated embryos of some gastropod species survive better in conditions of salinity stress than embryos removed from their capsule [48], [49]. A few studies have explored the effects of decreased pH on embryos brooded and/or laid in benthic gelatinous egg masses or in egg capsules in bivalve [50], gastropod [35], [41], [51], [52] or cephalopod [27], [53] mollusks. Depending on the study, reduced pH has different effects that are related to the range of species habitats and the strategy to protect embryos (brooding, egg masses, capsules) studied as well as the source of pH change (pCO2 increase, salinity stress). Encapsulation has been suggested to protect embryos against ocean acidification [41], [54], whereby the buffer capacity of intracapsular fluids may reduce the potential effect of extracapsular elevated pCO2 seawater.
To study this issue in a non-broadcast-spawner species, we chose the slipper limpet Crepidula fornicata, Linné 1758 (Gastropoda) as our biological model. Native to the northeast American coast, this species was introduced in Europe at the end of the 19th century, primarily via oyster farming [55], and has now become invasive in bays and estuaries where it reaches very high densities of up to several thousands of individuals per m2 [56]. It has a bentho-pelagic life cycle, with a number of original features. Benthic adults form stacks with males at the top and females at the bottom. After internal fertilization, females brood their embryos in egg capsules for 3 to 4 weeks [57], [58]. Capsules are protected between the neck and the propodium of the female parent and attached to the substratum to which the female is fixed [58]. Each female spawns between 28 and 64 capsules, each containing 300 to 500 embryos [57]. At the end of capsular development, veliger larvae are released at a size of about 400 μm in length into the water column where they spend between 2 and 7 weeks [59], [60]. Upon reaching competence (800–1000 μm in length), larvae are able to metamorphose and settle on hard substrata [59], [60].
The objective of this work was to investigate the effects of near-future levels of pCO2 on the development of C. fornicata encapsulated embryos by studying the shell morphology and mineralization level of released larvae. To ensure that the complete development, from the egg to the released larva, occurred under high pCO2, parents were conditioned to the different pCO2 levels before mating occurred.
Methods
Crepidula fornicata adult collection and culture
C. fornicata stacks were collected by SCUBA divers on 30 November 2011, after the end of the reproductive period [61] in Morlaix Bay (northwestern Brittany, France), at the “Barre des Flots” site (3°53.015'W; 48°40.015'N). No specific permissions were required for sampling at the selected location, as it is not privately-owned or protected. Field sampling did not involve endangered or protected species.
After being held 6 weeks in natural ambient unfiltered seawater, C. fornicata adults were randomly distributed into 18 aquarium tanks of 10 L each (adapted from [62]) and reared for 24 weeks (12 January 2012 to 28 June 2012) in three pCO2 treatments selected according to the recommendations of Barry et al. [63]: (1) 390 μatm (pH on the total scale (pHT) = 8.07) as the current pCO2 (control), (2) 750 μatm (pHT = 7.82) and (3) 1400 μatm (pHT = 7.56); the former two pCO2 levels are pessimistic scenarios predicted for the end of the century by the IPCC [3]. The pCO2 was adjusted by bubbling CO2-free air (current control pCO2) or pure CO2 (two elevated pCO2 treatments) in three 100 L header tanks supplied with unfiltered seawater pumped at the foot of the Station Biologique de Roscoff. Each of the three pCO2 treatments had six 10 L replicate aquaria. This was an open system, and CO2-treated seawater from the mixing header tanks was continuously supplied to the 18 aquaria (6 per pCO2 condition), at a rate of 9 L h−1 (i.e. a renewal rate of 90% h−1). Aquaria were placed in a thermostatic bath where temperature was controlled to within ±0.2°C using 150 to 250 W submersible heaters. C. fornicata adults were grown at four successive temperature levels (10, 13, 16 and 19°C) which corresponded to the range of in situ temperatures typically encountered in our study area (Service d’Observation de la Mer et du LITtoral data). Adults were reared for four weeks at each temperature level. Changes in temperature were implemented slowly, with increases of 0.2°C day−1 over a period of two weeks.
pCO2 and temperature were monitored and controlled by an off-line feedback system (IKS Aquastar, Karlsbad, Germany) that regulated the addition of gas in the header tanks and the on/off heater switch in the thermostatic bath. The pH values of the system were adjusted from daily measurements of pHT in each of the 18 aquaria using a pH meter (826 pH mobile, Metrohm AG, Herisau, Switzerland) calibrated using Tris/HCl and 2-aminopyridine/HCl buffers [64]. Slipper limpets were fed three times a week with a mix made from a stock solution of Chaetoceros gracilis (∼15×106 cells mL−1) and Isochrysis affinis galbana (∼26×106 cells mL−1). This algal mix (400 mL) was distributed in each aquarium. Seawater flow was stopped for two hours to allow the limpets to feed.
Seawater parameters were monitored throughout the experiment in each of the 18 aquaria. pHT and temperature were recorded daily. Total alkalinity was measured every four weeks, by 0.01 N HCl potentiometric titration on an automatic titrator (Titroline alpha, Schott SI Analytics, Mainz, Germany) in a 20 mL seawater sample taken from each aquarium. Salinity was also measured every four weeks with a conductimeter (LF 330/ SET, WTW, Weilheim, Germany) and it varied between 34.2±0.1 and 35.1±0.1 over the course of the experiment. The carbonate chemistry of seawater, i.e. dissolved inorganic carbon (DIC), exact pCO2 and saturation state of aragonite (ΩAr) were calculated for each pCO2 and temperature treatment using CO2SYS software [65] with the constants of Mehrbach et al. [66] refitted by Dickson and Millero [67]. Mean values of these parameters are given in Table 1.
Table 1. Seawater parameters.
| Temperature | pHT | pCO2 | AT | DIC | ΩAr | ||||||||
| (°C) | (μatm) | (μEq kg−1) | (μmol C kg−1) | ||||||||||
| n (except AT) | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | |
| 10°C | |||||||||||||
| 390 μatm | 23 | 9.7 | 0.2 | 8.14 | 0.01 | 323 | 7 | 2365 | 2 | 2138 | 4 | 2.47 | 0.04 |
| 750 μatm | 23 | 9.8 | 0.2 | 7.82 | 0.01 | 729 | 19 | 2369 | 2 | 2270 | 4 | 1.33 | 0.03 |
| 1400 μatm | 23 | 9.5 | 0.2 | 7.55 | 0.03 | 1487 | 75 | 2377 | 3 | 2366 | 11 | 0.78 | 0.08 |
| 13°C | |||||||||||||
| 390 μatm | 27 | 12.9 | 0.2 | 8.12 | 0.02 | 356 | 25 | 2418 | 2 | 2167 | 8 | 2.76 | 0.07 |
| 750 μatm | 27 | 13.0 | 0.1 | 7.81 | 0.01 | 781 | 20 | 2416 | 2 | 2304 | 3 | 1.48 | 0.03 |
| 1400 μatm | 27 | 12.8 | 0.1 | 7.53 | 0.01 | 1557 | 43 | 2422 | 2 | 2405 | 4 | 0.82 | 0.02 |
| 16°C | |||||||||||||
| 390 μatm | 28 | 15.9 | 0.1 | 8.08 | 0.01 | 376 | 11 | 2379 | 5 | 2127 | 5 | 2.80 | 0.05 |
| 750 μatm | 28 | 16.1 | 0.1 | 7.82 | 0.00 | 748 | 8 | 2369 | 5 | 2238 | 2 | 1.66 | 0.01 |
| 1400 μatm | 28 | 16.0 | 0.1 | 7.55 | 0.01 | 1492 | 19 | 2380 | 5 | 2345 | 2 | 0.94 | 0.01 |
| 19°C | |||||||||||||
| 390 μatm | 23 | 18.4 | 0.5 | 8.02 | 0.01 | 550 | 10 | 2391 | 2 | 2152 | 5 | 2.70 | 0.05 |
| 750 μatm | 23 | 18.6 | 0.5 | 7.77 | 0.01 | 858 | 19 | 2395 | 3 | 2266 | 4 | 1.68 | 0.04 |
| 1400 μatm | 23 | 18.4 | 0.5 | 7.51 | 0.01 | 1652 | 41 | 2394 | 3 | 2359 | 4 | 0.96 | 0.03 |
Legend: Mean parameters of carbonate chemistry in each pCO2 treatment at each temperature level. The pH on the total scale (pHT) was measured daily and total alkalinity (AT) was measured every 4 weeks. Other parameters were calculated with the CO2SYS software [65]. pCO2: CO2 partial pressure; DIC: dissolved inorganic carbon; Ω Ar: saturation state of aragonite.
Larvae collection
In C. fornicata, at the end of the embryonic development, the capsule membrane splits and veliger larvae are released in seawater. To prevent released larvae from escaping from their source aquarium, 200 μm mesh size nets covered the overflow outlet of each aquarium. Offspring presence was checked visually every day to collect larvae within 24 h post-hatching. When present, larvae were collected by pouring aquarium seawater on a 200 μm mesh sieve, rinsed with seawater and preserved in 96% ethanol.
Pools of larvae from adults acclimated to the different pCO2 levels since January were collected from the different pCO2 conditions at the temperature level of 19°C between 8 and 24 June 2011. Only samples with enough intact larvae were used. Thus two viable samples per pCO2 condition were studied.
Morphological variables
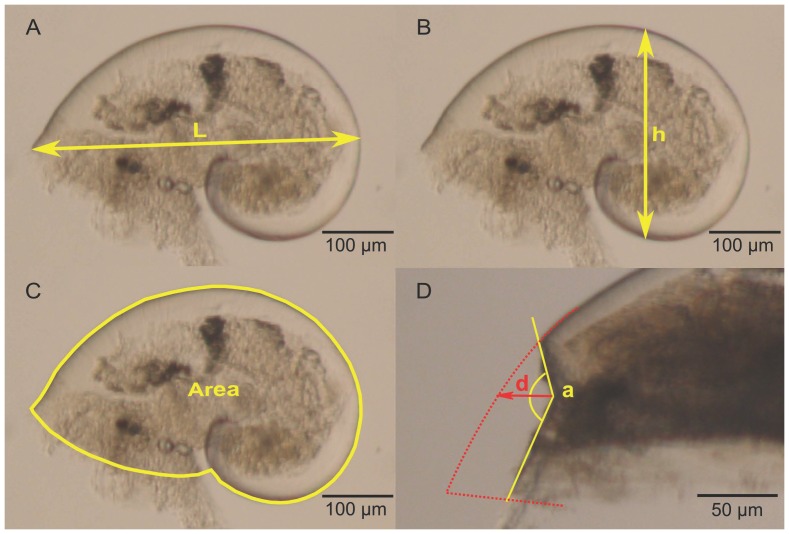
Morphological measurements were performed on a random subsample of 40 larvae when possible or at least 20 larvae from each of the 6 larval pools. Larvae with unbroken shells were isolated in sterile, flat-bottom, 96-well plates and preserved in pure glycerol as described in Auzoux-Bordenave et al. [68]. Each larva was placed on its right side and photographed under light microscopy using an Olympus Camedia C-7070 camera attached to an Olympus SZX 12 dissecting microscope. Pictures were taken without autofocus at ×90 magnification. Maximum length, height and projected surface area of the left side (Figures 1A, B, and C, respectively) were measured by analyzing images with ImageJ software [69], after calibration with a stage micrometer.
Figure 1. Morphological variables.
All measurements were taken on larvae lying on their right side. A: maximum shell length (L) (in μm); B: maximum shell height (h) (in μm); C: projected surface area of the left side (in mm2); D: deformity index, a is the angle of the abnormality (in degrees) and d, the depth of the abnormality (in μm).
Abnormalities
In each subsample used for morphological measurements, veliger larvae with abnormal shells were counted and the percentage of abnormal larvae was estimated per pCO2 treatment. To be considered as a shell abnormality and not as a broken shell, deformities had to be devoid of fracture lines. A “deformity index” was calculated to quantify the intensity of the shell deformity. It was defined as the ratio between the angle formed by the abnormality and its “depth”, which is the distance between the theoretical curve of the shell and the forest point (extreme point) of the deformity (Figure 1D).
Shell mineralization
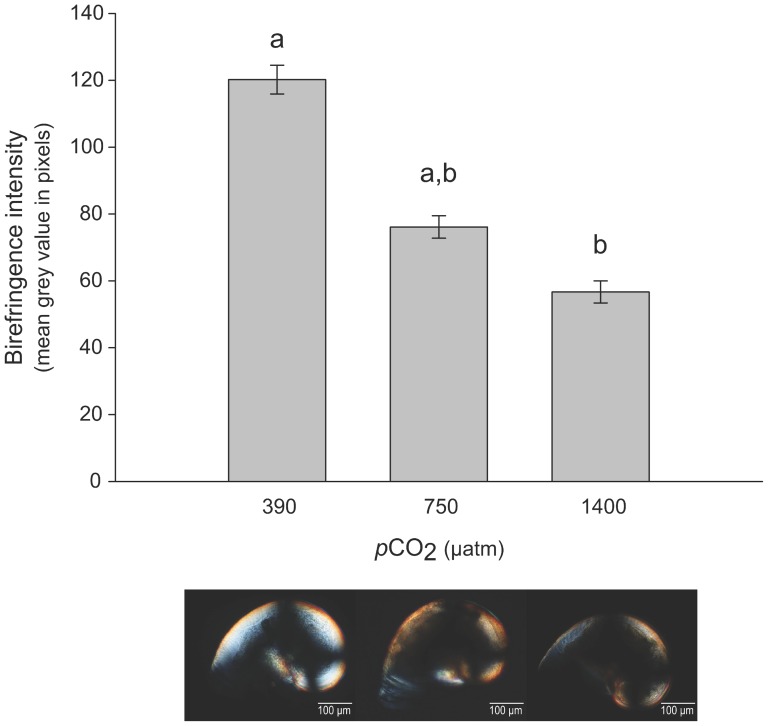
For each pCO2 treatment, 5 to 8 larvae were randomly chosen among the previous subsamples and observed under polarized light to determine birefringence patterns with an Olympus dissecting microscope equipped with polarizing filters. All polarized images were acquired with an Olympus camera at ×100 magnification with 40 ms light exposition. Birefringence under polarized light is due to the mineral phase composing the shell [40], [68], [70], [71]. In the absence of mineralized structures, there is no birefringence and the picture looks totally black. Under identical light conditions, areas appearing more birefringent contain a much larger proportion of crystalline calcium carbonate [70], [72]. The intensity of birefringence of each shell was used as a proxy for mineralization level for the three pCO2 treatments. It was quantified from pictures by using ImageJ software [69]. Pictures of polarized shells were first transformed into grayscale images. A mean gray value (in pixels) was determined for each birefringent zone. All birefringent zones of the shell were compiled to obtain a global mean gray value, giving the intensity of the birefringence of the whole shell.
Statistics
All statistical analyses were performed using the free software R 2.15.0 version [73]. Normality and homoscedasticity of the data were first checked using Shapiro and Levene tests, respectively. Due to the non-normality and heterogeneity of variance, the influence of pCO2 on morphological variables, deformity indices and birefringence intensity was analyzed using the non-parametric Kruskal-Wallis test followed by the Dunn post-hoc test [74]. A Chi-squared (χ2) test followed by G-tests (likelihood-ratio test) [75] were used to compare percentages of anomaly between the three pCO2 conditions.
Results
Morphological variables
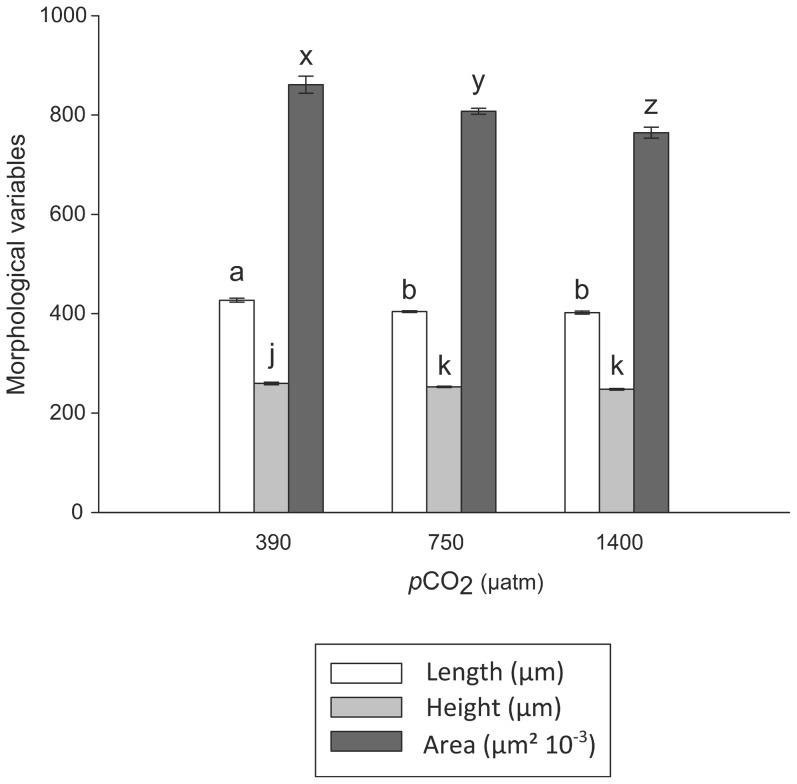
pCO2 significantly affected length, height and surface area of the hatched larvae (Figure 2, Table 2). These morphological variables are related to each other and were generally influenced in the same way by pCO2. Length and height were the highest at 390 μatm and significantly decreased with increased pCO2. Larvae collected at 750 and 1400 μatm pCO2 showed a decrease of 5.3% and 5.9% in length, respectively, and 2.6% and 4.5% in height, respectively, compared to control larvae (390 μatm). Similarly, the greatest shell surface area was observed at 390 μatm pCO2, but then significantly decreased by 6.2% and 11.2% at 750 and 1400 μatm pCO2, respectively.
Figure 2. Morphological variables.
Mean shell length, height and left surface area (± SE) in the different pCO2 treatments. Different letters above bars indicate significant differences between treatments (p<0.05, Dunn post-hoc test), n = 51 to 92.
Table 2. Effect of pCO2 on morphological variables, abnormality indices and intensity of birefringence.
| Kruskal-Wallis Test | |||
| df | H | p | |
| Length | 2 | 37.353 | < 0.001 |
| Height | 2 | 16.235 | < 0.001 |
| Surface area | 2 | 30.106 | < 0.001 |
| Abnormality index | 2 | 6.046 | 0.049 |
| Intensity of birefringence | 2 | 14.562 | < 0.001 |
Legend: Summary of the non-parametric Kruskal-Wallis tests testing the effect of pCO2 on each morphological variable, abnormality index and birefringence intensity.
Abnormalities
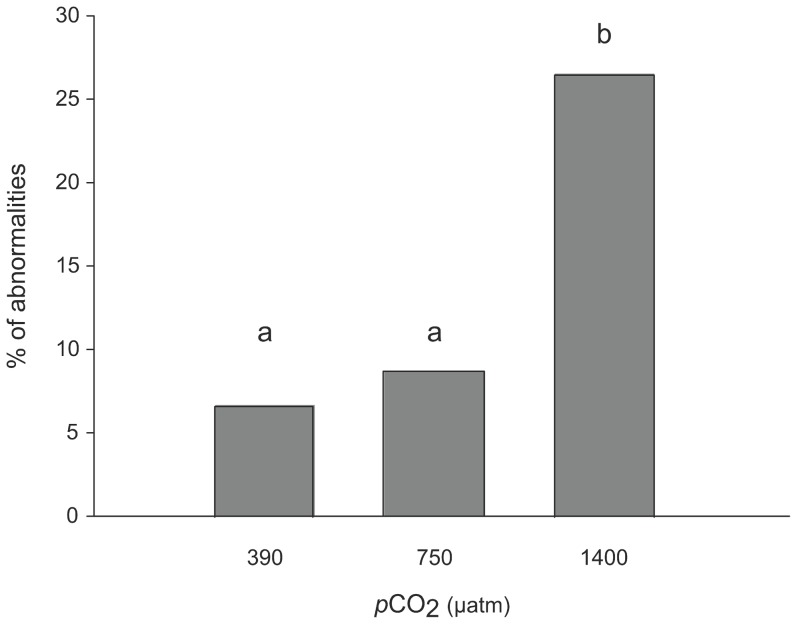
Abnormalities in larvae were observed as notches located close to the shell aperture (Figure 3). The percentage of abnormal larvae increased with increased pCO2 and ranged from 6.7 to 26.5% (Figure 4; χ2 test, p<0.05). Abnormalities were 1.5- and 4-fold at pCO2 levels of 750 and 1400 μatm, respectively, than at 390 μatm. Furthermore, different intensities of shell abnormalities were observed with variation in notch acuteness. The deformity index varied between 0.03 (390 μatm pCO2) and 0.17 (1400 μatm pCO2). Although the Kruskal-Wallis test showed a marginally significant pCO2 effect (p = 0.049; Table 2), the pairwise Dunn post-hoc test did not detect significant differences between the three pCO2 treatments (p > 0.05).
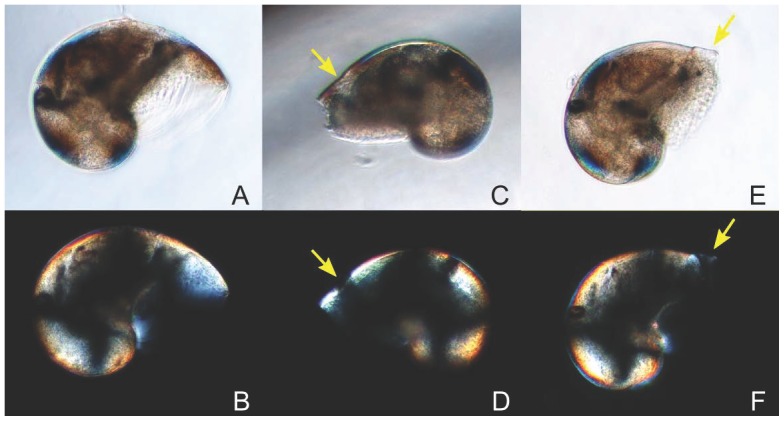
Figure 3. Shell abnormalities.
Different intensities of shell abnormalities observed among samples. A, B, C, and D show whole larvae whereas E, F, G, and H show the detail of their respective abnormalities.
Figure 4. Percentage of abnormalities in the different pCO2 conditions.
Different letters above bars indicate significant differences between treatments (G-test, p<0.05).
Among the abnormal larvae observed under polarized microscopy (see below), some showed abnormalities which appeared less birefringent, and even not mineralized, as revealed by the lack of birefringence in these parts of the shell (Figure 5).
Figure 5. Shell abnormalities under normal (A, C, and E) and polarized (B, D, and F) light.
A-B are pictures of a normal larva presenting the characteristic black cross of birefringence. C-D and E-F show examples of abnormalities observed among the samples, with arrows indicating less calcified zones.
Shell mineralization
Pictures taken under polarized light (Figure 6) suggest that the intensity of the birefringence decreased with increased in pCO2. The measure of birefringence intensity using the mean gray values estimated for each shell clearly confirmed this relationship (Table 2). Mineralization was greatest at 390 μatm pCO2, intermediate at 750 μatm pCO2 and lowest at 1400 μatm pCO2 (Figure 6).
Figure 6. Birefringence intensity.
Mean gray value (in pixel) of the shells under polarized light in the different pCO2 treatments (± SE). Bars with different letters are significantly different (Dunn post-hoc test, p<0.05,), n = 5 to 8 larvae per condition. Pictures taken under polarized light (below the graph) show birefringent patterns in the different pCO2 treatments.
Discussion
In our study, the effects of elevated pCO2 were integrated from embryo formation and throughout embryonic stages up until the release of veliger larvae. The integration of pCO2 effects across all developmental stages, from fertilization to settlement and beyond, is particularly instructive because early life stages may respond to environmental stressors in a different way than adults. Acute exposures of early life stages have shown various effects in growth or feeding performances [76], [77], but these may not represent field conditions. Results obtained from acclimation to high pCO2 across different life stages often differ from those arising from acute exposure of a given stage. For example, it has been shown that oyster D-veliger larvae grown from eggs fertilized at elevated pCO2 were more drastically affected than those first produced at ambient pCO2 and then reared later (embryo stages) at high pCO2 [78]. Keeping the parents under different pCO2 conditions before fertilization and until larval release allowed embryos develop entirely under a given level of stress. To our knowledge, only Dupont et al. [79] on sea urchins, Parker et al. [80] on mollusks and Vehmaa et al. [81] on copepods acclimated adults to high pCO2 during reproductive conditioning before studying larvae in the same pCO2 conditions.
pCO2 effects were first investigated on the shell morphology of the hatched larvae. The size (length, height and surface area) of the released larvae decreased with increased pCO2. Length and height were not significantly different between the pCO2 levels of 750 and 1400 μatm, whereas the shell surface area progressively decreased with increased pCO2 from 390 to 1400 μatm. Although in some rare cases, elevated pCO2 does not affect larval morphology and growth [82], [83], a correlation between high pCO2 and smaller size has been demonstrated in most bivalve and gastropod species studied to date (see review in Table 3), with pCO2 sometimes affecting the shape of the larval shell [41]. As observed here for length and height (ca. –5% in the two high pCO2 treatments), shell lengths of Crassostrea gigas veliger larvae are smaller under elevated pCO2, but are similar in conditions of pH lowered by 0.4 and 0.7 pH units, a range corresponding to our pH values [32]. Smaller size and delayed shell growth can be attributed to problems in shell deposition, delayed periostracum formation and/or increased shell dissolution, as hypothesized by Watson et al. [84].
Table 3. Literature review of reports of high pCO2 effects on morphological variables in mollusk larvae.
| Reproductive mode | Species (veliger stage) | Measured parameter | pH conditions | pCO2 conditions (μatm) | % decrease due to pCO2 | Study |
| Broadcast spawner | Crassostrea gigas | shell length | pHNBS 7.4 | 2268 | decrease | [40] |
| Broadcast spawner | Crassostrea gigas | shell length | pHNBS7.8 | 1000 | 16% | [78] |
| Broadcast spawner | Crassostrea gigas | shell length and height | pHNBS 7.76 – 7.37 | 1386 – 3573 | 10.6% | [32] |
| Broadcast spawner | Crassostrea gigas | shell area | pHNBS 7.7 – 7.4 | 1497 – 2386 | 18.7 – 29% | [101] |
| Broadcast spawner | Crassostrea virginica | shell length | pHT 7.84 – 7.49 | ≈ 650 – 1500 | 16.7% | [38] |
| Broadcast spawner | Crassostrea virginica | shell area | pHNBS 7.76 | 840 | 16% | [94] |
| Broadcast spawner | Saccostrea glomerata | shell length | - | 1000 | 22% | [31] |
| Broadcast spawner | Saccostrea glomerata | shell length | pHNBS7.8 | 1000 | 34% | [78] |
| Broadcast spawner | Saccostrea glomerata | shell length | pHNBS7.9 | 856 | 31.6 – 1.3% | [80] |
| Broadcast spawner | Saccostrea glomerata | shell length | pHNBS 7.8 – 7.6 | 508.8 – 775.6 | 8.7 – 6.3% | [84] |
| Broadcast spawner | Saccostrea glomerata | shell height | pHNBS 7.8 – 7.6 | 508.8 – 775.6 | 7.5 – 5.1% | [84] |
| Broadcast spawner | Mytilus californianus | shell area | pHNBS 7.75 | 970 | 5 – 7% | [37] |
| Broadcast spawner | Mytilus edulis | shell length | pHNBS 7.8 | 1200 | 4.5 – 6% | [39] |
| Broadcast spawner | Mytilus edulis | shell thickness | pHNBS 7.8 | 1200 | 12% | [39] |
| Broadcast spawner | Mytilus edulis | shell area | pHNBS 7.6 | 1388 – 1493 | 7 – 8% | [16] |
| Broadcast spawner | Mytilus galloprovincialis | shell length and height | pHNBS 7.4 | 2000 | 26 – 20% | [86] |
| Broadcast spawner | Argopecten irradians | shell length | pHT 7.84 – 7.49 | ≈ 650 – 1500 | 50% | [38] |
| Broadcast spawner | Argopecten irradians | shell diameter | pHT (?) 7.5 | 1500 | 30.7% | [43] |
| Broadcast spawner | Argopecten irradians | shell thickness | pHT (?) 7.5 | 1500 | 43% | [43] |
| Broadcast spawner | Argopecten irradians | shell length | pHSW 7.39 | 1987 | 18.9 – 7.5% | [102] |
| Broadcast spawner | Pecten maximus | shell length | pHNBS(?) 7.51 | 1627 | 10% | [85] |
| Broadcast spawner | Pecten maximus | shell height | pHNBS(?) 7.51 | 1627 | 5% | [85] |
| Broadcast spawner | Macoma balthica | shell length | pHNBS 7.7 – 7.2 | 1700 – 4400 | 4.3 – 8.5% | [103] |
| Broadcast spawner | Macoma balthica | shell length | pHNBS 7.8 – 7.5 | - | 16.2 – 16.9% | [42] |
| Broadcast spawner | Mercenaria mercenaria | shell length | pHT 7.84 – 7.49 | ≈ 650 – 1500 | 22.7% | [38] |
| Broadcast spawner | Mercenaria mercenaria | shell diameter | pHT (?) 7.5 | 1500 | 25.5% | [43] |
| Broadcast spawner | Mercenaria mercenaria | shell thickness | pHT (?) 7.5 | 1500 | 43.0% | [43] |
| Broadcast spawner | Haliotis discus hannai | shell length | pHT (?) 7.71 | 1050 | 2.50% | [33] |
| Broadcast spawner | Haliotis discus hannai | shell length | pHNBS 7.6 – 7.3 | 1224.6 – 2543.6 | decrease | [34] |
| Broadcast spawner | Haliotis kamtschatkana | shell length | pHNBS(?) 8.07 | 800 | 5% | [36] |
| Brooding in pallial cavity | Ostrea chilensis | shell thickness | pHNBS(?) 7.7 – 7.0 | - | no change | [50] |
| Brooding in pallial cavity | Ostrea lurida | shell growth | pHT 7.76 | 1000 | 5 – 14% | [104] |
| Egg masses | Littorina obtusata | shell length | pHNBS(?) 7.6 | - | 10% | [41] |
| Egg masses | Stylocheilus striatus | shell area | pHNBS (?) 7.6 | - | 24 – 36% | [52] |
| Egg | Sepia officinalis | total weight | pHT 7.84 – 7.60 | 750 – 1430 | no change | [53] |
| Encapsulation + brooding | Crepipatella dilatata | shell thickness | pHNBS (?) 6 | - | 30% | [51] |
| Encapsulation + brooding | Crepidula fornicata | shell length | pHT 7.8 – 7.6 | 750 – 1400 | 5.3 – 10.7% | Present study |
| Encapsulation + brooding | Crepidula fornicata | shell height | pHT 7.8 – 7.6 | 750 – 1400 | 2.6 – 13.1% | Present study |
| Encapsulation + brooding | Crepidula fornicata | shell area | pHT 7.8 – 7.6 | 750 – 1400 | 6.2 – 19.8% | Present study |
pHNBS: pH on the NBS scale; pHT: pH on the total scale; pHSW: pH on the seawater scale.
Such processes may lead to developmental abnormalities and to an increase in their frequency under elevated pCO2. Some (7%) C. fornicata larvae in the control group (390 μatm pCO2) showed mild shell abnormalities in the form of a notch close to the aperture. The frequency of this abnormality increased under high pCO2, being 1.5-fold more frequent at 750 μatm pCO2 and reaching 26% at 1400 μatm pCO2. The intensity of shell abnormality, estimated using the deformity index, did not vary significantly with increasing pCO2, although more pronounced shell deformities were detected at the highest pCO2 condition (1400 μatm). The occurrence of abnormal shells is a common response in mollusk larvae exposed to elevated pCO2. In bivalves for example, abnormalities can occur as shell hinge and edge deformities [85], irregular-shaped shells [40] or protruding mantles [86]. The frequency of abnormalities can reach 40% of shell deformities in Pecten maximus larvae reared at 1250 μatm pCO2 [85], and even 90% of abnormal D-veligers in Saccostrea glomerata at 1000 μatm pCO2 [31]. In gastropods, larval shells are considered abnormal when shells are too small to fully cover the soft body [34] or when dissolution zones are observed at the edge of the aragonitic larval shell [33], with frequencies of abnormality ranging from 20% in Haliotis discus hannai at 1650 μatm pCO2 [33] to 40% in Haliotis kamtschatkana at 800 μatm pCO2 [36]. At extremely high pCO2 (> 1700 μatm), some abalone larvae are even unable to precipitate a calcareous shell [36], [44].
Such abnormalities may be due to different processes: (i) the production of amorphous CaCO3 may be affected by damage to embryonic ectodermic cells and/or (ii) seawater corrosion may induce shell dissolution, affecting the strength and calcification of some parts of the shell [32]. Here, the mineralization level of larval shells was investigated at each pCO2 level by observing the veliger aragonitic shell under polarized light [70]. The characteristic dark cross observed in each larval shell indicated a radial arrangement of aragonite crystals [72] and did not have been considered as non-crystalline zones. The intensity of birefringence was used as a proxy for mineralization because increases in birefringence reflect increases in crystalline structure and calcification of the shell. Observed under polarized light, abnormalities appeared less birefringent than the rest of the shell, suggesting that deformities were likely less calcified as proposed by Barros et al. [32]
The birefringence intensity of the larval shells decreased with increased pCO2, and was significantly lower at 1400 μatm pCO2. This drop in birefringence revealed a decrease in calcification, which may be related to a less mineralized matrix [87], or more likely to a reduction in shell thickness [72]. Our data did not allow us to discriminate between these two possibilities, but previous studies have already reported a decrease in shell thickness under high pCO2 in bivalve larvae. For example, using scanning electron microscopy measurements, Gazeau et al. [39] showed a decrease in thickness of 12% in Mytilus edulis larvae at 745 μatm pCO2. Talmage and Gobler [43] report a decrease in thickness of Mercenaria mercenaria (–43%) and Argopecten irradians (– 47.5%) larval shells after 17 days of development at 1500 μatm pCO2, which was associated with an impact on the integrity and the connectedness of the hinge structure. A decrease of 5.7% in shell thickness of brooded larvae of the oyster Ostrea chilensis has also been observed following a decrease in pH (down to 6.56) within the mother’s pallial cavity due to valve closure under salinity stress [50].
The decrease in larval size and mineralization level of the shell may be due to reduced CaCO3 saturation or hypercapnic suppression of metabolic pathways involved in the calcification process [9]. Very little is known about the conditions occurring during intracapsular development and how acidified seawater can affect encapsulated embryos. Previous studies have shown that egg capsules of some gastropods, including C. fornicata, are permeable to water and ions (e.g. [48], [71], [88]) and it can be assumed that the capsule wall in C. fornicata is almost impermeable to gas because of its low O2 conductance [89]. Under “normal” conditions, embryos of C. fornicata will progressively be exposed to hypoxia [89] and hypercapnia via their respiration. This may lead to low intracapsular pH, as reported in other gastropod species [90], without altering development [57]. Under elevated pCO2, diffusion of more protons (H+) from external seawater to the intracapsular medium may alter intracapsular carbonate chemistry, thus enhancing metabolic acidosis. Similar acidosis can be observed under low salinity stress. For example, a decrease in pH to 6.4 recorded within the pallial cavity of the calyptraeid Crepipatella dilatata [47],[51] led to the partial shell decalcification of the brooded encapsulated embryos [51]. This decalcification may cause the release of some carbonate ions (CO3 2−), which could bind to free H+ to form bicarbonate ions (HCO3 −), thus buffering the intracapsular acidosis and limiting the drastic pH effects on larval metabolism. This potential buffering role, in combination with a decrease in the CaCO3 saturation state, is likely to affect shell mineralization and calcification. Such processes have been suggested to buffer acidosis resulting from anaerobiosis in C. fornicata [91], and may also explain our observations. Alternative mechanisms that can decrease the intracapsular acidosis, such as the active excretion of H+ out of the capsule through a proton pump, as shown in the cephalopod Sepia officinalis [92], need to be investigated.
Altogether, our results show that, despite the potential protective role provided by encapsulation and brooding, elevated seawater pCO2 affected the shells of the released larvae in C. fornicata. Embryos of C. fornicata were affected by high pCO2 during their intracapsular development. However, the overall low abnormality rate and low decrease in size suggested they were likely less affected than other mollusk early life stages. The natural exposure of embryos to low intracapsular pH as demonstrated in cephalopod eggs (pH on the seawater scale of the perivitalline fluid of ca. 7.35 at 16°C [53]) and gastropod capsules (pH of the intracapsular fluid lower than 7 [90]) could confer to C. fornicata larvae some resilience to elevated pCO2 levels. Indeed, it has been shown that bivalves naturally exposed to high pCO2 conditions in their habitat (due to high levels of benthic respiration or to seawater naturally enriched in CO2) are less affected by ocean acidification than other mollusk species [93], [94]. Further studies are however needed to determine the pH of the intracapsular fluid in C. fornicata, and how it will be affected under future scenarios of ocean acidification.
The effects of elevated pCO2 observed on C. fornicata larvae released from capsules suggest critical ecological consequences for their subsequent planktonic life and benthic settlement. Production of smaller larvae with weaker shell strength may increase vulnerability of larvae to predation and physical damages [37]. Furthermore, larvae physiologically stressed during their development by various abiotic factors may delay metamorphosis and settlement [94], staying longer in the water column which lead them to be more exposed to predators and diseases [94], [95], [96]. In addition, reduced size in early developmental stages may affect the juvenile survivorship and fitness [97], [98]. Given these consequences on the early life stages of C. fornicata, pCO2 may influence its invasion dynamics in its introduction range via reproductive success, larval survival and dispersal, and settlement success. Further studies are required to fully understand the interactions between climate change and biological invasions [99], [100]. In particular, more studies on early life stages and particularly the transition processes between them (e.g. metamorphosis) are needed to identify the potential tipping points, the demographic bottlenecks and the global resistance of non-native species in the context of ocean acidification.
Acknowledgments
The authors thank the “Marine Operations and Services Department” at the Station Biologique de Roscoff for underwater sampling. We also thank the “Multicellular Marine Models” staff for providing microalgae and their help for building the aquarium system. We are grateful to Stéphanie Auzoux-Bordenave and Nathalie Wessel for hosting us at the Station de Biologie Marine de Concarneau and for their help in acquiring the polarized light images. We also thank Frédérique Viard and Thomas Broquet for helpful discussions at various stages of this work. We are grateful to the editor, Pauline Ross, and the two anonymous reviewers for their helpful and constructive comments which greatly improved this manuscript.
Funding Statement
This work was supported by the CALCAO project, which received funding from the Region Bretagne, and contributed to the ‘‘European Project on Ocean Acidification’’ (EPOCA) funded by the European Community’s Seventh Framework Programme (FP7/2007-2013) under grant agreement n° 211384. It was also supported by the Interreg IV a France (Channel) – England Marinexus project n°4073 funded by the FEDER programme.The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Pechenik JA (1999) On the advantages and disadvantages of larval stages in benthic marine invertebrate life cycles. Mar Ecol-Prog Ser 177: 269–297. [Google Scholar]
- 2. Peck LS, Souster T, Clark MS (2013) Juveniles are more resistant to warming than adults in 4 species of Antarctic marine invertebrates. PLoS One 8: e66033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.IPCC (2013) Working Group I Contribution to the IPCC Fifth Assessment Report - Climate Change 2013: The Physical Science Basis - Summary for Policymakers.
- 4. Feely RA, Doney SC, Cooley SR (2009) Ocean acidification: present conditions and future changes in a high-CO2 world. Oceanography 22: 36–47. [Google Scholar]
- 5. Pörtner H-O (2008) Ecosystem effects of ocean acidification in times of ocean warming: a physiologist's view. Mar Ecol-Prog Ser 373: 203–217. [Google Scholar]
- 6. Widdicombe S, Spicer JI (2008) Predicting the impact of ocean acidification on benthic biodiversity: what can animal physiology tell us? J Exp Mar Biol Ecol 366: 187–197. [Google Scholar]
- 7. Doney SC, Fabry VJ, Feely RA, Kleypas JA (2009) Ocean acidification: the other CO2 problem. Ann Rev Mar Sc 1: 169–192. [DOI] [PubMed] [Google Scholar]
- 8. Hoegh-Guldberg O (2009) Climate change and coral reefs: Trojan horse or false prophecy? Coral Reefs 28: 569–575. [Google Scholar]
- 9.Byrne M (2011) Impact of ocean warming and ocean acidification on marine invertebrate life history stages: vulnerabilities and potential for persistence in a changing ocean. In: Gibson R, Atkinson R, Gordon J, Smith I, Hughes D, editors. Oceanography and Marine Biology: An Annual Review: Taylor & Francis. pp. 1–42.
- 10. Feely RA, Sabine CL, Lee K, Berelson W, Kleypas J, et al. (2004) Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science 305: 362–366. [DOI] [PubMed] [Google Scholar]
- 11. Kroeker KJ, Kordas RL, Crim RN, Singh GG (2010) Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecol Lett 13: 1419–1434. [DOI] [PubMed] [Google Scholar]
- 12. Dupont S, Thorndyke M (2009) Impact of CO2-driven ocean acidification on invertebrates early life-history - What we know, what we need to know and what we can do. . Biogeosciences Discuss. 6: 3109–3131. [Google Scholar]
- 13. Kurihara H (2008) Effects of CO2-driven ocean acidification on the early developmental stages of invertebrates. Mar Ecol-Prog Ser 373: 275–284. [Google Scholar]
- 14. Nguyen HD, Doo SS, Soars NA, Byrne M (2012) Noncalcifying larvae in a changing ocean: warming, not acidification/hypercapnia, is the dominant stressor on development of the sea star Meridiastra calcar . Glob Change Biol18: 2466–2476. [Google Scholar]
- 15. Pansch C, Schlegel P, Havenhand J (2013) Larval development of the barnacle Amphibalanus improvisus responds variably but robustly to near-future ocean acidification. ICES J Mar Sci 70: 805–811. [Google Scholar]
- 16. Bechmann RK, Taban IC, Westerlund S, Godal BF, Arnberg M, et al. (2011) Effects of ocean acidification on early life stages of shrimp (Pandalus borealis) and mussel (Mytilus edulis). J Toxic Envir Health-Part A 74: 424–438. [DOI] [PubMed] [Google Scholar]
- 17. Range P, Pilo D, Ben-Hamadou R, Chicharo M, Matias D, et al. (2012) Seawater acidification by CO2 in a coastal lagoon environment: effects on life history traits of juvenile mussels Mytilus galloprovincialis . J Exp Mar Biol Ecol 424: 89–98. [Google Scholar]
- 18. Dupont S, Dorey N, Thorndyke M (2010) What meta-analysis can tell us about vulnerability of marine biodiversity to ocean acidification? Estuar Coast Shelf Sci 89: 182–185. [Google Scholar]
- 19. Ries JB, Cohen AL, McCorkle DC (2009) Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification. Geology 37: 1131–1134. [Google Scholar]
- 20. Chua CM, Leggat W, Moya A, Baird AH (2013) Temperature affects the early life history stages of corals more than near future ocean acidification. Mar Ecol-Prog Ser 475: 85–92. [Google Scholar]
- 21. Putnam HM, Mayfield AB, Fan TY, Chen CS, Gates RD (2013) The physiological and molecular responses of larvae from the reef-building coral Pocillopora damicornis exposed to near-future increases in temperature and pCO2 . Mar Biol 160: 2157–2173. [Google Scholar]
- 22. Dupont S, Havenhand J, Thorndyke W, Peck L, Thorndyke M (2008) Near-future level of CO2-driven ocean acidification radically affects larval survival and development in the brittlestar Ophiothrix fragilis . Mar Ecol-Prog Ser 373: 285–294. [Google Scholar]
- 23. Martin S, Richier S, Pedrotti ML, Dupont S, Castejon C, et al. (2011) Early development and molecular plasticity in the Mediterranean sea urchin Paracentrotus lividus exposed to CO2-driven acidification. J Exp Biol 214: 1357–1368. [DOI] [PubMed] [Google Scholar]
- 24. Padilla-Gamino JL, Kelly MW, Evans TG, Hofmann GE (2013) Temperature and CO2 additively regulate physiology, morphology and genomic responses of larval sea urchins, Strongylocentrotus purpuratus . P Roy Soc Lond, B Biol 280: 20130155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Egilsdottir H, Spicer JI, Rundle SD (2009) The effect of CO2 acidified sea water and reduced salinity on aspects of the embryonic development of the amphipod Echinogammarus marinus (Leach). Mar Poll Bull 58: 1187–1191. [DOI] [PubMed] [Google Scholar]
- 26. Gazeau F, Parker L, Comeau S, Gattuso J-P, O'Connor W, et al. (2013) Impacts of ocean acidification on marine shelled molluscs. Mar Biol 160: 2207–2245. [Google Scholar]
- 27. Gutowska MA, Melzner F (2009) Abiotic conditions in cephalopod (Sepia officinalis) eggs: embryonic development at low pH and high pCO2 . Mar Biol 156: 515–519. [Google Scholar]
- 28. Moran D, Stottrup JG (2011) The effect of carbon dioxide on growth of juvenile Atlantic cod Gadus morhua L. Aquat Toxicol. 102: 24–30. [DOI] [PubMed] [Google Scholar]
- 29. Munday PL, Gagliano M, Donelson JM, Dixson DL, Thorrold SR (2011) Ocean acidification does not affect the early life history development of a tropical marine fish. Mar Ecol-Prog Ser 423: 211–221. [Google Scholar]
- 30. Parker LM, Ross PM, O'Connor WA, Pörtner H-O, Scanes E, et al. (2013) Predicting the response of molluscs to the impact of ocean acidification. Biology 2: 651–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Parker LM, Ross PM, O'Connor WA (2009) The effect of ocean acidification and temperature on the fertilization and embryonic development of the Sydney rock oyster Saccostrea glomerata (Gould 1850). Glob Change Biol 15: 2123–2136. [Google Scholar]
- 32. Barros P, Sobral P, Range P, Chicharo L, Matias D (2013) Effects of seawater acidification on fertilization and larval development of the oyster Crassostrea gigas . J Exp Mar Biol Ecol 440: 200–206. [Google Scholar]
- 33. Kimura R, Takami H, Ono T, Onitsuka T, Nojiri Y (2011) Effects of elevated pCO2 on the early development of the commercially important gastropod, Ezo abalone Haliotis discus hannai . Fish Oceanogr 20: 357–366. [Google Scholar]
- 34. Li J, Jiang Z, Zhang J, Qiu J-W, Du M, et al. (2013) Detrimental effects of reduced seawater pH on the early development of the Pacific abalone. Mar Poll Bull 74: 320–324. [DOI] [PubMed] [Google Scholar]
- 35. Davis AR, Coleman D, Broad A, Byrne M, Dworjanyn SA, et al. (2013) Complex responses of intertidal molluscan embryos to a warming and acidifying ocean in the presence of UV radiation. PLoS One 8: e55939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Crim RN, Sunday JM, Harley CDG (2011) Elevated seawater CO2 concentrations impair larval development and reduce larval survival in endangered northern abalone (Haliotis kamtschatkana). J Exp Mar Biol Ecol 400: 272–277. [Google Scholar]
- 37. Gaylord B, Hill TM, Sanford E, Lenz EA, Jacobs LA, et al. (2011) Functional impacts of ocean acidification in an ecologically critical foundation species. J Exp Biol 214: 2586–2594. [DOI] [PubMed] [Google Scholar]
- 38. Talmage SC, Gobler CJ (2009) The effects of elevated carbon dioxide concentrations on the metamorphosis, size, and survival of larval hard clams (Mercenaria mercenaria), bay scallops (Argopecten irradians), and Eastern oysters (Crassostrea virginica). Limnol Oceanogr 54: 2072–2080. [Google Scholar]
- 39. Gazeau F, Gattuso J-P, Dawber C, Pronker AE, Peene F, et al. (2010) Effect of ocean acidification on the early life stages of the blue mussel Mytilus edulis . Biogeosciences 7: 2051–2060. [Google Scholar]
- 40. Kurihara H, Kato S, Ishimatsu A (2007) Effects of increased seawater pCO2 on early development of the oyster Crassostrea gigas . Aquat Biol 1: 91–98. [Google Scholar]
- 41. Ellis RP, Bersey J, Rundle SD, Hall-Spencer JM, Spicer JI (2009) Subtle but significant effects of CO2 acidified seawater on embryos of the intertidal snail, Littorina obtusata . Aquat Biol 5: 41–48. [Google Scholar]
- 42. Van Colen C, Debusschere E, Braeckman U, Van Gansbeke D, Vincx M (2012) The early life history of the clam Macoma balthica in a high CO2 world. PLoS One 7: e44655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Talmage SC, Gobler CJ (2010) Effects of past, present, and future ocean carbon dioxide concentrations on the growth and survival of larval shellfish. P N atl Acad Sci107: 17246–17251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Byrne M, Ho M, Wong E, Soars NA, Selvakumaraswamy P, et al. (2010) Unshelled abalone and corrupted urchins: development of marine calcifiers in a changing ocean. P Roy Soc Lond, B Biol 278: 2376–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ross PM, Parker LM, O'Connor WA, Bailey EA (2011) The impact of ocean acidification on reproduction, early development and settlement of marine organisms. Water 3: 1005–1030. [Google Scholar]
- 46. Przeslawski R (2004) A review of the effects of environmental stress on embryonic development within intertidal gastropod egg masses. Molluscan Res 24: 43–63. [Google Scholar]
- 47. Chaparro OR, Segura CJ, Montory JA, Navarro JM, Pechenik JA (2009) Brood chamber isolation during salinity stress in two estuarine mollusk species: from a protective nursery to a dangerous prison. Mar Ecol-Prog Ser 374: 145–155. [Google Scholar]
- 48. Pechenik JA (1982) Ability of some gastropod egg capsules to protect against low-salinity stress. J Exp Mar Biol Ecol 63: 195–208. [Google Scholar]
- 49. Pechenik JA (1983) Egg capsules of Nucella lapillus (l.) protect against low-salinity stress. J Exp Mar Biol Ecol 71: 165–179. [Google Scholar]
- 50. Chaparro OR, Montory JA, Segura CJ, Pechenik JA (2009) Effect of reduced pH on shells of brooded veligers in the estuarine bivalve Ostrea chilensis Philippi 1845. J Exp Mar Biol Ecol 377: 107–112. [Google Scholar]
- 51. Montory JA, Chaparro OR, Cubillos VM, Pechenik JA (2009) Isolation of incubation chambers during brooding: effect of reduced pH on protoconch development in the estuarine gastropod Crepipatella dilatata (Calyptraeidae). Mar Ecol-Prog Ser 374: 157–166. [Google Scholar]
- 52.Allen TR (2012) The effects of ocean acidification and sea surface warming on the embryonic development of the opistobranch gastropod Stylocheilus stiatus. Student Research Papers, Fall 2012, UCB Moorea Class: Biology and Geomorphology of Tropical Islands, Berkeley Natural History Museum, UC Berkeley, 18 p.
- 53. Dorey N, Melzner F, Martin S, Oberhaensli F, Teyssie JL, et al. (2013) Ocean acidification and temperature rise: effects on calcification during early development of the cuttlefish Sepia officinalis . Mar Biol 160: 2007–2022. [Google Scholar]
- 54. Fernandes DAO, Podolsky RD (2012) Effects of ocean acidification on growth, development, and calcification of gastropod embryos: does encapsulation matter? Integrative and comparative biology 52: e244–e244. [Google Scholar]
- 55. Blanchard M (1995) Origine et état de la population de Crepidula fornicata (Gastropoda Prosobranchia) sur le littoral français. Haliotis 24: 75–86. [Google Scholar]
- 56. Blanchard M (1997) Spread of the slipper limpet Crepidula fornicata (L. 1758) in Europe. Current state and consequences. Sci Mar 61: 109–118. [Google Scholar]
- 57. Brante A, Fernandez M, Viard F (2009) Limiting factors to encapsulation: the combined effects of dissolved protein and oxygen availability on embryonic growth and survival of species with contrasting feeding strategies. J Exp Biol 212: 2287–2295. [DOI] [PubMed] [Google Scholar]
- 58. Orton J (1912) An account of the natural history of the slipper-limpet (Crepidula fornicata) with some remarks on its occurrence on the oyster grounds on the Essex coast. J Mar Biol Assoc UK 9: 437–443. [Google Scholar]
- 59.Rigal F (2009) Dynamique spatio-temporelle du nuage larvaire du gastéropode introduit Crepidula fornicata au sein d'une baie mégatidale, la baie de Morlaix (France). PhD thesis. Paris: Université Pierre et Marie Curie. 160p.
- 60. Pechenik JA (1984) The relationship between temperature, growth rate, and duration of planktonic life for larvae of the gastropod Crepidula fornicata (L.). J Exp Mar Biol Ecol 74: 241–257. [Google Scholar]
- 61. Richard J, Huet M, Thouzeau G, Paulet YM (2006) Reproduction of the invasive slipper limpet, Crepidula fornicata, in the Bay of Brest, France. Mar Biol 149: 789–801. [Google Scholar]
- 62. Noisette F, Duong G, Six C, Davoult D, Martin S (2013) Effects of elevated pCO2 on the metabolism of a temperate rhodolith Lithothamnion corallioides grown under different temperatures. J Phycol 49: 746–757. [DOI] [PubMed] [Google Scholar]
- 63.Barry JP, Tyrrell T, Hansson L, Plattner GK, Gattuso JP (2010) Atmospheric CO2 targets for ocean acidification perturbation experiments. In: Riebesell U FVJ, Hansson L. & Gattuso J.-P., editor. Guide to best practices for ocean acidification research and data reporting. Luxembourg: Publications Office of the European Union. pp. 260.
- 64.Dickson AG, Sabine CL, Christian JR, editors (2007) Guide to best practices for ocean CO2 measurements. Sidney, British Columbia: North Pacific Marine Science Organization. 176 p.
- 65.Lewis E, Wallace DWR (1998) Program developed for CO2 system calculations. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, U.S. Department of Energy
- 66. Mehrbach C, Culberso C, Hawley JE, Pytkowic RM (1973) Measurement of apparent dissociation-constants of carbonic-acid in seawater at atmospheric-pressure. Limnol Oceanogr 18: 897–907. [Google Scholar]
- 67. Dickson AG, Millero FJ (1987) A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep Sea Res 34: 1733–1743. [Google Scholar]
- 69.Rasband WS (2012) ImageJ version 1.46r. In: Health USNIo, editor. imagejnihgov/ij/, 1997–2012. Bethesda, Maryland, USA.
- 70. Weiss IM, Tuross N, Addaddi l, Weiner S (2002) Mollusc larval shell formation: amorphous calcium carbonate is a precursor phase for aragonite. J Exp Zool 293: 478–491. [DOI] [PubMed] [Google Scholar]
- 71. Eyster LS (1986) Shell inorganic composition and onset of shell mineralization during bivalve and gastropod embryogenesis. Biol Bull 170: 211–231. [Google Scholar]
- 72. Schönitzer V, Weiss IM (2007) The structure of mollusc larval shells formed in the presence of the chitin synthase inhibitor Nikkomycin Z. . BMC structural biology 7: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.R Core Team (2013) R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
- 74.Zar JH, editor (1974) Biostatistical analysis: Englewood Cliffs, NJ: Prentice-Hall. 620 p.
- 75.Scherrer B, editor (1984) Biostatistiques: Gaetan Morin Editors. 850 p.
- 76. Chan KYK, Grunbaum D, O'Donnell MJ (2011) Effects of ocean-acidification-induced morphological changes on larval swimming and feeding. J Exp Biol 214: 3857–3867. [DOI] [PubMed] [Google Scholar]
- 77. Kim K-S, Shim J, Kim S (2013) Effects of ocean acidification on the larval growth of olive flounder (Paralichthys olivaceus). Biogeosciences Discuss 10: 7413–7431. [Google Scholar]
- 78. Parker LM, Ross PM, O'Connor WA (2010) Comparing the effect of elevated pCO2 and temperature on the fertilization and early development of two species of oysters. Mar Biol 157: 2435–2452. [Google Scholar]
- 79. Dupont S, Dorey N, Stumpp M, Melzner F, Thorndyke M (2013) Long-term and trans-life-cycle effects of exposure to ocean acidification in the green sea urchin Strongylocentrotus droebachiensis . Mar Biol 160: 1835–1843. [Google Scholar]
- 80. Parker LM, Ross PM, O'Connor WA, Borysko L, Raftos DA, et al. (2012) Adult exposure influences offspring response to ocean acidification in oysters. Glob Change Biol 18: 82–92. [Google Scholar]
- 81. Vehmaa A, Brutemark A, Engström-Öst J (2012) Maternal effects may act as an adaptation mechanism for copepods facing pH and temperature changes. PLoS One 7: e48538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gazeau F, Gattuso J-P, Greaves M, Elderfield H, Peene J, et al. (2011) Effect of carbonate chemistry alteration on the early embryonic development of the pacific oyster (Crassostrea gigas). PLoS One 6: e23010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Thiyagarajan V, Ko GWK (2012) Larval growth response of the Portuguese oyster (Crassostrea angulata) to multiple climate change stressors. Aquaculture 370–371: 90–95. [Google Scholar]
- 84. Watson S-A, Southgate PC, Tyler PA, Peck LS (2009) Early larval development of the Sydney rock oyster Saccostrea glomerata under near-future predictions of CO2-driven ocean acidification. J Shellfish Res 28: 431–437. [Google Scholar]
- 85. Andersen S, Grefsrud ES, Harboe T (2013) Effect of increased pCO2 level on early shell development in great scallop (Pecten maximus Lamarck) larvae. Biogeosciences 10: 6161–6184. [Google Scholar]
- 86. Kurihara H, Asai T, Kato S, Ishimatsu A (2009) Effects of elevated pCO2 on early development in the mussel Mytilus galloprovincialis . Aquat Biol 4: 225–233. [Google Scholar]
- 87. Marxen JC, Becker W, Finke D, Hasse B, Epple M (2003) Early mineralization in Biomphalaria glabrata: microscopic and structural results. J Molluscan Stud 69: 113–121. [Google Scholar]
- 88. Maeda-Martinez AN (2008) Osmotic and ionic concentration of the egg capsule fluid of Crepidula fornicata in relation to embryonic development. Mar Biol 154: 643–648. [Google Scholar]
- 89. Brante A, Fernandez M, Viard F (2008) Effect of oxygen conditions on intracapsular development in two calyptraeid species with different modes of larval development. Mar Ecol-Prog Ser 368: 197–207. [Google Scholar]
- 90. De Mahieu G, Penchaszadeh P, Casal A (1974) Algunos aspectos de las variaciones de proteinas y aminoacidos libres totales del liquido intracapsular en relacion al desarrollo embrionario en Adelomelon brasiliana (Lamarck, 1811)(Gastropoda, Prosobranchia, Volutidae). Cah Biol Mar 15: 215–227. [Google Scholar]
- 91. Maeda-Martinez AN (1987) The rates of calcium deposition in shells of molluscan larvae. Comp Biochem Physiol A-Mol Integr Physiol 86: 21–28. [Google Scholar]
- 92. Melzner F, Gutowska MA, Langenbuch M, Dupont S, Lucassen M, et al. (2009) Physiological basis for high CO2 tolerance in marine ectothermic animals: pre-adaptation through lifestyle and ontogeny? Biogeosciences 6: 2313–2331. [Google Scholar]
- 93. Thomsen J, Gutowska MA, Saphorster J, Heinemann A, Trubenbach K, et al. (2010) Calcifying invertebrates succeed in a naturally CO2-rich coastal habitat but are threatened by high levels of future acidification. Biogeosciences 7: 3879–3891. [Google Scholar]
- 94. Miller AW, Reynolds AC, Sobrino C, Riedel GF (2009) Shellfish face uncertain future in high CO2 world: influence of acidification on oyster larvae calcification and growth in estuaries. PLoS One 4: e5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hickman CS (2001) Evolution and development of gastropod larval shell morphology: experimental evidence for mechanical defense and repair. Evolution & Development 3: 18–23. [DOI] [PubMed] [Google Scholar]
- 96. Hickman CS (1999) Adaptive function of gastropod larval shell features. Invertebrate biology 118: 346–356. [Google Scholar]
- 97. Pechenik JA (2006) Larval experience and latent effects: metamorphosis is not a new beginning. Integrative and Comparative Biology 46: 323–333. [DOI] [PubMed] [Google Scholar]
- 98. Anil AC, Desai D, Khandeparker L (2001) Larval development and metamorphosis in Balanus amphitrite Darwin (Cirripedia; Thoracica): significance of food concentration, temperature and nucleic acids. J Exp Mar Biol Ecol 263: 125–141. [Google Scholar]
- 99. Occhipinti-Ambrogi A (2007) Global change and marine communities: Alien species and climate change. Mar Poll Bull 55: 342–352. [DOI] [PubMed] [Google Scholar]
- 100. Lenz M, da Gama BAP, Gerner NV, Gobin J, Groner F, et al. (2011) Non-native marine invertebrates are more tolerant towards environmental stress than taxonomically related native species: Results from a globally replicated study. Environ Res 111: 943–952. [DOI] [PubMed] [Google Scholar]
- 101. Ko GWK, Chan VBS, Dineshram R, Choi DKS, Li AJ, et al. (2013) Larval and post-larval stages of Pacific oyster Crassostrea gigas are resistant to elevated CO2 . PLoS One 8: e64147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. White MM, McCorkle DC, Mullineaux LS, Cohen AL (2013) Early exposure of bay scallops (Argopecten irradians) to high CO2 causes a decrease in larval shell growth. PLoS One 8: e61065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Jansson A, Norkko J, Norkko A (2013) Effects of reduced pH on Macoma balthica larvae from a system with naturally fluctuating pH-dynamics. PLoS One 8: e68198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hettinger A, Sanford E, Hill T, Hosfelt J, Russell A, et al. (2013) The influence of food supply on the response of Olympia oyster larvae to ocean acidification. Biogeosciences 10: 6629–6638. [Google Scholar]