Abstract
Cell-generated traction forces induce integrin activation, leading to focal adhesion growth and cell spreading. It remains unknown, however, whether integrin activation feeds back to impact the generation of cytoskeletal tension. Here, we used elastomeric micropost arrays to measure cellular traction forces in wildtype and integrin-null cells. We report that activation of β1 but not β3 integrin, by either increasing density of immobilized fibronectin or treating with manganese, elicited fibroblast spreading and cytoskeletal tension. Furthermore, this force generation required Rho kinase and myosin activity. These findings suggest that integrin activation and cell traction forces comprise a bi-directional signaling unit of cell adhesion.
Keywords: integrin, adhesion, cytoskeletal tension, cell traction force, micropost array, cell spreading
1. Introduction
The binding of integrins to extracellular matrix (ECM) initiates cell adhesion, which can be described as a series of processes including cell spreading against the underlying matrix, assembly of focal adhesions (FAs), and generation of actomyosin-mediated cytoskeletal tension against these adhesions [1]. Each of these processes appears to be linked through several pathways. For example, the degree of cell spreading against a micropatterned substrate regulates RhoA activity and cytoskeletal tension [2,3], and this cytoskeletal tension is important for adhesion assembly [4,5]. Conversely, it has been shown that the clustering of integrins required for adhesion assembly is critical to support cell spreading and tension generation [6,7]. Because cell spreading, adhesion assembly, and cytoskeletal tension each have been shown to regulate many cellular functions including proliferation, differentiation, and migration, understanding how these processes are regulated is an important question.
Integrin receptors undergo conformational activation from a low affinity to high affinity state [8,9], and these changes in integrin activity may contribute to the regulation of cell spreading and FA assembly. Indeed, direct activation of integrins via manganese (Mn2+) [10] or conformation-modulating antibodies [11] appears to enhance cell spreading and adhesion assembly [12,13]. Although numerous studies have linked integrin activation to FA growth and superior cell adhesion and spreading on ECM, it is unclear whether integrin activation can also directly regulate cytoskeletal tension generation.
In this study, we found that β1 integrin activation via increased fibronectin (FN) density or Mn2+ leads to enhanced generation of cellular traction forces. We measured these forces by culturing cells on FN-functionalized arrays of uniformly spaced elastomeric microposts, a system we developed previously to enable studies of traction force dynamics [5,14]. Our data indicate that the activation state of integrins is intimately connected to basic adherent cell behaviors like contractility, which has implications for improving our understanding of the regulation of cell shape, mechanics, and function.
2. Materials and Methods
2.1. Cell culture
Wildtype and β3 integrin-null MEFs were provided by Dr. Richard Assoian (University of Pennsylvania) and Dr. Richard Hynes (MIT), respectively. β1 integrin-null MEFs were maintained as previously described [15]. All cells were cultured in 10% FBS/DMEM (Atlanta Biologicals).
2.2. Reagents and antibodies
Reagents were obtained as follows: fibronectin (BD); vitronectin (Sigma); lysophosphatidic acid (Avanti Polar Lipids); Y27632 (Tocris Bioscience); blebbistatin (Calbiochem); FN blocking antibody 16G3 (20 μg/ml; gift of Dr. Martin Schwartz, University of Virginia); β1 integrin blocking antibody BMC5 and rat control IgG (10 μg/ml; Chemicon); anti-β1 integrin (BD); anti-GAPDH (Ambion); anti-active-β1 integrin (clone 9EG7, BD); anti-vinculin (hVin1, Sigma-Aldrich); adenoviral sh-α5 integrin and scrambled sequence (gift of Dr. Rebecca Wells, University of Pennsylvania).
2.3. Cell attachment assay
Plates were coated overnight at 4°C with FN in triplicate (BD Biosciences) and blocked with 50 μg/ml BSA/PBS. Cells were seeded, gently rinsed after 1 hour with warm PBS, and quantified using CyQuant (Invitrogen Molecular Probes).
2.4. Substrate preparation
Micropost array detectors (mPADs) were fabricated using PDMS-based replica-molding as previously described [5,16]. Microcontact printing FN on these or flat substrates, with either continuous or 625 μm2 islands, was performed as described previously [17]. FN concentrations of 0.0625 or 4.0 μg/ml FN in 50 μg/ml BSA are designated as low or high FN density, respectively.
2.5. Western blotting
Cells were lysed in Laemmli sample buffer (Bio-Rad), separated via SDS-PAGE, transferred to PVDF, immunoblotted, and detected using SuperSignal West Dura detection kit (Thermo Scientific).
2.6. Immunofluorescence, cell imaging, and quantitative analysis of focal adhesions and strain energies
For immunofluorescence, cells were fixed with 3.7% paraformaldehyde (Electron Microscopy Sciences), permeabilized with 0.1% Triton X-100, and labeled using primary and then secondary antibodies. Quantitative analyses of adhesions and cell area were performed using a custom-developed MATLAB program [18]. For mPAD experiments, cells were labeled with CellTracker Green CMFDA (Invitrogen Molecular Probes). Quantitative analyses of cell area and total cell strain energies on mPADs were performed as previously described [5].
2.7. Knockdown of α5 integrin
MEFs were infected with adenovirus encoding either shRNA directed against α5 integrin or a scrambled sequence [19] at a MOI of 50. Cells were trypsinized at 48 hours post-infection and seeded on mPAD substrates.
2.8 Statistical Analysis
For each box-and-whisker plot, 15 or more cells per condition were imaged and analyzed across 3 or more experiments. Statistical comparisons between experimental conditions used either Mann-Whitney-U tests or Wilcoxon signed-rank tests, as indicated in individual figure legends. For all tests, statistical significance was assigned at p-value ≤ 0.05 (ns: non-significant, *: p≤0.05, **: p≤0.01, ***: p≤0.001).
3. Results
3.1. Integrin activation enhances cell spreading and traction force
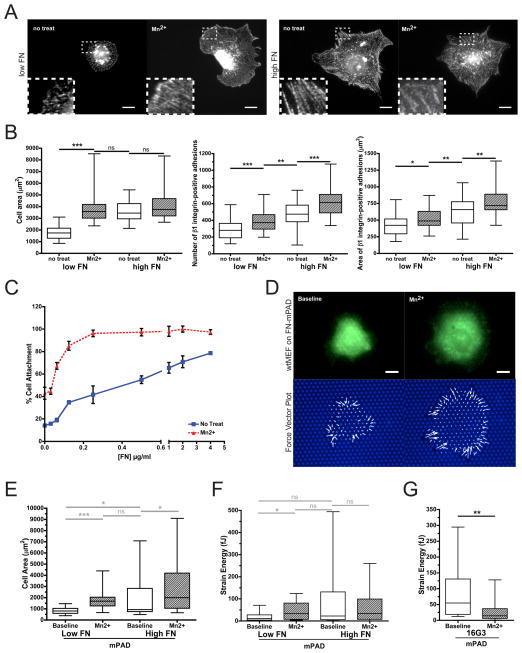
We first confirmed that increasing the density of immobilized FN and exposure to Mn2+ enhanced integrin activation [20,21] in our system. Wildtype mouse embryonic fibroblasts (wtMEFs) were plated on substrates coated with a range of FN densities, cultured in the presence or absence of 1 mM Mn2+ for one hour, and then immunostained for activated β1 integrin. In the absence of Mn2+, cells cultured on low FN density exhibited small peripheral β1 integrin-positive adhesions (Fig. 1A). In contrast, Mn2+ treatment of cells on low FN resulted in increases in spread cell area and the number and size of β1 integrin-positive adhesions (Fig. 1A,B). On high FN densities, cells displayed greater cell spreading and adhesion number and size relative to untreated cells on low FN, and Mn2+ treatment did not promote additional spreading (Fig. 1A,B). As a functional measure of integrin-mediated adhesion, we show that cell attachment was improved by increasing FN density and/or Mn2+ treatment (Fig. 1C). Together, these data confirm that shifting the equilibrium towards ECM-engaged integrin, by either increasing FN density or conformational activation of integrin by Mn2+, promotes cell attachment, spreading, and adhesion assembly.
Figure 1. Integrin activation enhances cell spreading and traction force.
(A) Immunofluorescent images and magnified insets of activated β1 integrin (9EG7) immunostaining in wtMEFs seeded on low or high FN density in the presence or absence of 1 mM Mn2+ for 1 hour (this Mn2+ concentration and timepoint is used throughout the text). Scale bar = 20 μm. (B) Quantification of spread cell area, number of β1 integrin-positive adhesions per cell, and area of β1 integrin-positive adhesions (n ≥ 35 cells/condition). (C) wtMEF attachment curves across varied FN densities in the presence or absence of Mn2+. Error bars indicate standard error of the mean for 3 independent experiments done in triplicate. (D) Representative images of a GFP-expressing wtMEF (top) and micropost tops with deflections rendered into force vectors (bottom), before and after Mn2+ treatment. Scale bar = 10 μm. (E,F) wtMEF spread cell area (E) and total strain energy (F) on low or high FN density, at baseline or after Mn2+ treatment (n ≥ 15 cells/condition for all mPAD measurements throughout the text). (G) wtMEF total strain energy on low FN, following 30 minutes of pretreatment with FN blocking antibody 16G3 (baseline) then 1 hour of Mn2+ (Mn2+). (B,E–G) Mann-Whitney-U test (ns: non-significant, *: p≤0.05, **: p≤0.01, ***: p≤0.001).
We next examined whether integrin activation impacts cytoskeletal tension, by using elastomeric micropost array detector substrates (mPADs) to measure cell traction forces [5]. wtMEFs attached to and spread on the posts (Fig. 1D – top left panel). Cell spreading correlated with FN density on mPADs similarly to flat substrates, and the deficiency in spreading on low FN was rescued by the addition of Mn2+ (Fig. 1D – top right panel, 1E). Importantly, we observed that Mn2+-induced integrin activation triggered enhanced cell traction forces on low FN, and increasing FN density also increased traction force generation (Fig. 1D – bottom panels, 1F). Moreover, this enhanced traction force production was blocked by the addition of a FN blocking antibody, 16G3 (Fig. 1G), demonstrating that Mn2+-triggered forces require the formation of new integrin-FN bonds. These data show that increasing the amount of ECM-engaged integrin leads to a net increase in traction forces.
3.2. β1KO MEFs have defects in Mn2+-induced spreading and traction force generation
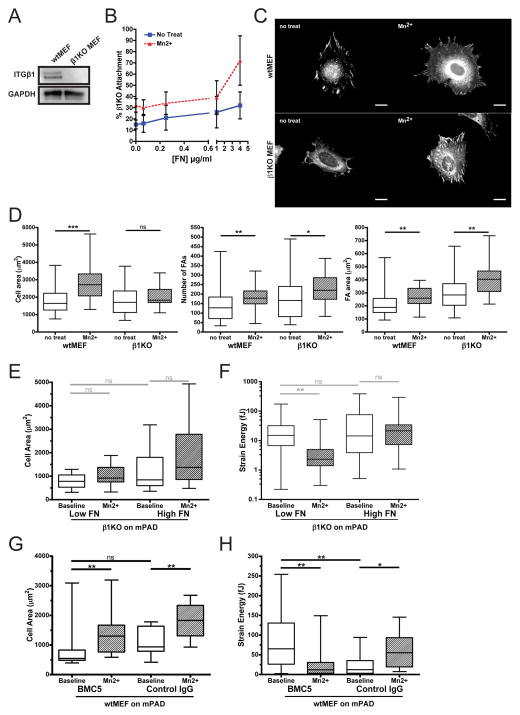
Although Mn2+ activates integrins indiscriminately, we hypothesized that specific integrin subtypes might be important for mediating the changes in cell spreading and traction force generation in our system. Therefore, we tested the responses of MEFs carrying a deletion of the β1 gene. As originally reported [15], expression of β1 integrin is undetectable in these cells, as illustrated here by the absence of reactivity in a anti-β1 western blot (Fig. 2A). β1KOs showed reduced attachment to FN (Fig. 2B), relative to the robust attachment curves seen for wtMEFs (Fig. 1C). To assess whether β1KO cells could respond to Mn2+, we assayed whether Mn2+ could induce cell spreading and FA assembly on low FN. While β1KOs failed to spread in the presence of Mn2+ (Fig. 2C,D,E), enhanced FA growth was still observed (Fig. 2D).
Figure 2. β1KO MEFs have defects in Mn2+-induced spreading and traction force generation.
(A) Western blotting for β1 integrin expression in wtMEFs and β1KO MEFs. The two bands correspond to a partially glycosylated precursor protein and the mature protein. (B) β1KO attachment curves across varied FN densities in the presence or absence of Mn2+. Error bars indicate standard error of the mean for 4 independent experiments done in triplicate. (C) Vinculin immunofluorescence for wtMEFs and β1KOs in the presence or absence of Mn2+ on low FN. Scale bar = 20 μm. (D) Quantification of spread cell area, average number of FAs per cell, and average FA area (n ≥ 25 cells/condition). (E,F) β1KO spread cell area (E) and total strain energy (F) on low or high FN density, at baseline or after Mn2+ treatment. (G,H) wtMEF spread cell area (G) and total strain energy (H) on low FN, following 30 minutes of pretreatment with either β1 blocking antibody BMC5 or control IgG antibody (baseline) then 1 hour of Mn2+ (Mn2+). (D–H) Mann-Whitney-U test (ns: non-significant, *: p≤0.05, **: p≤0.01, ***: p≤0.001).
We then investigated the effect of β1 knockout on Mn2+-induced cytoskeletal tension. Although β1KOs cells exhibited strong basal contractility compared to wildtype MEFS, the β1KOs failed to mount increased traction forces in response to either increased FN density or Mn2+ treatment (Fig. 2EF). In fact, Mn2+ treatment resulted in a statistically significant decrease in traction forces on low FN (Fig. 2F).
Loss of β1 integrin disrupts numerous integrin heterodimers, including the principal fibronectin receptor α5β1 integrin. To test whether α5β1 integrin was specifically required for Mn2+-induced cytoskeletal tension, we treated wtMEFs with a function-blocking α5β1 integrin antibody, BMC5. Inhibition of α5β1 integrin trended toward decreased cell spreading on low FN mPADs and did not prevent the spreading response to Mn2+ treatment (Fig. 2G). In spite of the trend toward decreased spreading, basal contractility showed an unexpected increase in response to BMC5 (Fig. 2H), whereas MEFs treated with an isotype-matched control IgG showed similar baseline contractility to untreated cells (Fig. 2H vs. 1F). Nonetheless, upon treatment with Mn2+, BMC5-treated cells showed a statistically significant decrease in strain energy that paralleled the response observed in β1KO cells (Fig. 2H vs. 2F). Moreover, we observed a similar loss of strain energy in response to Mn2+ treatment when α5 integrin was depleted by RNA interference (Supp. Fig. 1A,B). Taken together, these data suggest that the increased cell traction force upon stimulation of integrin activation requires β1 integrin and is likely mediated by α5β1.
3.3. β1 integrin-dependent tractions require spread cell shape, ROCK, and myosin activity
To better understand the requirement for β1 integrin in generating traction forces, we investigated whether these forces were mediated by non-muscle myosin II activity and whether β1KOs were competent to respond to other contractility agonists. We tested the role of myosin activity in Mn2+-induced traction by pretreating wtMEFs with Y27632 and blebbistatin, pharmacological inhibitors of Rho kinase (ROCK) and myosin, respectively. Both blebbistatin (Fig. 3A) and Y27632 (Fig. 3B) prevented Mn2+-induced traction forces. Prior work from our group demonstrated that cellular contractility can also be blocked by culturing cells on small micropatterned FN islands [5]. Here we observed that micropatterned islands (625 μm2) of high FN prevented cells from mounting traction forces in response to Mn2+ stimulation (Fig. 3C), consistent with a role for actomyosin contractile machinery in mediating Mn2+-dependent traction forces.
Figure 3. β1 integrin-dependent tractions require spread cell shape, ROCK, and myosin activity.
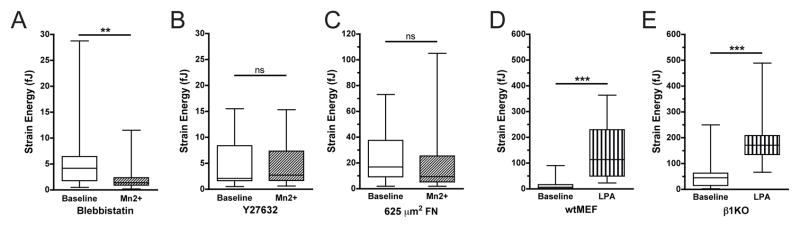
(A,B) Total strain energy for wtMEFs pretreated with 10 μM blebbistatin (A) or 10 μM Y27632 (B) for 30 minutes on low FN, at baseline or after Mn2+ treatment. (C) Total strain energy for wtMEFs restricted to 625 μm2 on high FN micropatterned islands, at baseline or after Mn2+ treatment. (D,E) Total strain energy for wtMEFs (D) and β1KOs (E) on low FN, at baseline or after 30 minutes of 10 μg/ml LPA treatment. (A–E) Wilcoxon signed-rank test (ns: non-significant, **: p≤0.01, ***: p≤0.001).
Restricting cell spreading by micropatterning results in a generalized defect in coupling extracellular stimuli with traction force production, a relationship we have reported in multiple systems [5,16]. We therefore tested whether the defect in traction force production in β1KOs could be attributed to a generalized defect in organizing or activating actomyosin contractility or was specific to Mn2+. Treatment of cells with lysophosphatidic acid (LPA), a strong agonist for Rho-mediated myosin activation, triggered a sustained increase in traction force in both wtMEFs and β1KOs within one minute (Fig. 3D,E), indicating that the coupling between soluble agonists and contractility remains intact in the β1KOs.
3.4. β3 integrin is dispensable for Mn2+-induced traction forces
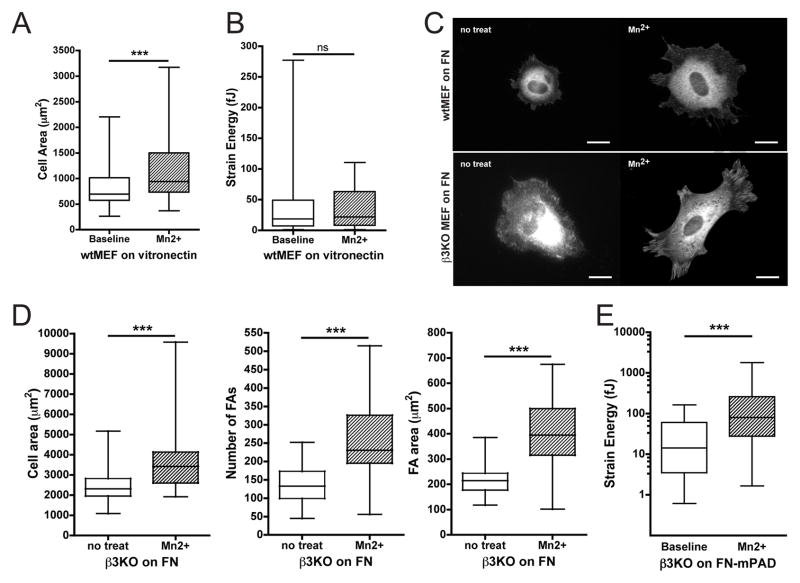
Our results suggest that, in the absence of β1 integrin, activation of integrins induces FA assembly, but fails to induce cell spreading or traction forces on low FN. Because fibroblasts also use β3 integrin to bind FN, we next evaluated whether β3 integrin activation could contribute to either enhanced cell spreading or traction forces following Mn2+ treatment. To do so, we first plated wtMEFs on vitronectin, a preferential ligand for β3 integrin [22]. Upon treatment with Mn2+, wtMEFs on vitronectin increased spread cell area (Fig. 4A) but generated no additional net traction force (Fig. 4B).
Figure 4. β3 integrin is dispensable for Mn2+-induced traction forces.
(A,B) wtMEF spread cell area (A) and total strain energy (B) on 20 μg/ml vitronectin, at baseline or after Mn2+ treatment. (C) Vinculin immunofluorescence for wtMEFs and β3KO MEFs in the presence or absence of Mn2+ on low FN. Scale bar = 20 μm. (D) Quantification of spread cell area, average number of FAs per cell, and average FA area (n ≥ 25 cells/condition). (E) β3KO total strain energy on low FN density, at baseline or after Mn2+ treatment. (A,B,E) Wilcoxon signed-rank test; (D) Mann-Whitney-U test (ns: non-significant, ***: p≤0.001).
These data suggest that β3 integrin engagement with ECM ligand is not sufficient to mediate Mn2+-induced traction forces but could support cell spreading. To test the role of β3 integrin in the response to Mn2+ more directly, we examined the effect of Mn2+ on β3-null MEFs (β3KOs) [23]. Similar to wtMEFs, β3KOs treated with Mn2+ demonstrated an increase in spread cell area and FA growth and number (Fig. 4C,D). Likewise, the β3KOs increased cell traction forces after Mn2+ treatment (Fig. 4E). Thus, β3 integrin activation can promote cell spreading; however, it is dispensable for Mn2+-induced traction force generation.
4. Discussion
Cellular traction forces play an integral role in cell adhesion to matrices. These forces regulate FA assembly, presumably by acting directly on integrins to activate them through “inside-out” signaling [4,5]. Additionally, myosin-mediated contractility regulates adhesion through recruitment of signaling proteins to FAs [24,25]. Signaling downstream from these FA proteins impacts proliferation [26], differentiation [27], migration [28,29], and other higher-level cellular functions. Here, we show “outside-in” signals that promote integrin activation (increased FN density, Mn2+) also trigger traction force generation. This finding clarifies how the cell might sense matrix density through ECM-modulated integrin affinity that directly adjusts cytoskeletal tension to befit the microenvironment.
Integrin activation could modulate cell traction forces through several possible mechanisms. Here, we reveal that traction forces induced by integrin activation require Rho kinase and myosin activity, suggesting that Rho GTPases could be involved. Alternatively, integrin adhesion complexes can nucleate actin polymerization via Arp2/3 in a manner dependent on the density of ligated integrins [30,31] and independently of Rho GTPases [32]. Actin polymerization creates protrusive force [33] that can drive cell motility [34] and may contribute to the integrin-mediated forces reported in this work.
Different integrin subtypes often have overlapping functions, with some instances where distinct integrins produce unique effects on cells. For example, both α5β1 and αVβ3 integrins bind FN, but drive divergent migratory behavior; α5β1-FN adhesion promotes thin cell protrusions and random cell migration whereas αVβ3-FN adhesion supports persistent migration with broad lamellipodia [35]. In fact, it has been proposed that these effects are mediated through a change in the balance of Rho/Rac signaling [35]. Here we show that in normal cells, Mn2+ stimulates cell traction forces in a β1 integrin-dependent manner, whereas in cells deficient in α5 or β1, Mn2+ stimulation leads to a decrease in traction forces. While the mechanism of decreased traction forces in α5 or β1 deficient cells is as yet unknown, it is interesting to speculate that αvβ3-specific signaling, such as to Rac1 GTPase, may play a role.
Our finding that activation of β1 integrin contributes to enhanced traction forces is consistent with previous studies in which force production on fibronectin substrates is disrupted by α5β1 blocking antibody in fibroblasts [36] or myocytes [37]. It is interesting that the same β1 integrin subtype that induces intracellular forces also undergoes conformational activation in response to extracellular forces and uniquely displays catch-bond and adhesion strength-reinforcing behavior upon force transmission [38–41]. In view of these previous studies, our results suggest that feedback between force sensing and traction force generation may be a necessary component to the cell mechanotransduction system. Further studies on the regulation of basic cell functions by integrins will help shed light on how cells manage complex behavior in response to mechanical and adhesive cues.
Supplementary Material
(A,B) wtMEF spread cell area (A) and total strain energy (B) on low FN density, following adenoviral delivery of shRNA against either α5 integrin or scrambled control sequence (baseline) then 1 hour of Mn2+ (Mn2+). (A,B) Mann-Whitney-U test (ns: non-significant, *: p≤0.05).
Highlights.
Integrin activation induces cell spreading and cytoskeletal tension.
Elastomeric micropost arrays report integrin activation-mediated traction forces.
Activation of β1 integrin but not β3 integrin drives cell traction forces.
Acknowledgments
We thank R. Hynes for his kind gift of β3 integrin-null MEFs, R. Assoian for the wildtype MEFs, M. Schwartz for the FN blocking antibody, R. Wells for the adenoviral sh-α5, M. Yang and M. Lynch for assistance with mPADs, and J. Eyckmans and C. Choi for helpful discussions. This work was supported in part by grants from the National Institutes of Health (EB00262 and GM74048), the RESBIO Technology Resource for Polymeric Biomaterials, and Center for Engineering Cells and Regeneration of the University of Pennsylvania. D.M.C. acknowledges financial support from the Ruth L. Kirschstein National Research Service Award, and R.A.D. was supported by the National Science Foundation.
Abbreviations
- β1KO
β1-integrin null mouse embryonic fibroblast
- β3KO
β3-integrin null mouse embryonic fibroblast
- BSA
bovine serum albumin
- ECM
extracellular matrix
- FA
focal adhesion
- FN
fibronectin
- LPA
lysophosphatidic acid
- mPAD
micropost array detector
- PDMS
polydimethylsiloxane
- ROCK
Rho kinase
- wtMEF
wildtype mouse embryonic fibroblast
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Grace L. Lin, Email: grlin@mail.med.upenn.edu.
Daniel M. Cohen, Email: cohendm@seas.upenn.edu.
Ravi A. Desai, Email: rdesai@seas.upenn.edu.
Mark T. Breckenridge, Email: markbrec@seas.upenn.edu.
Lin Gao, Email: lingao@seas.upenn.edu.
Martin J. Humphries, Email: martin.humphries@manchester.ac.uk.
Christopher S. Chen, Email: chrischen@seas.upenn.edu.
References
- 1.Galbraith CG, Sheetz MP. Forces on adhesive contacts affect cell function. Curr Opin Cell Biol. 1998;10:566–71. doi: 10.1016/s0955-0674(98)80030-6. [DOI] [PubMed] [Google Scholar]
- 2.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–95. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 3.Bhadriraju K, Yang M, Alom Ruiz S, Pirone D, Tan J, Chen CS. Activation of ROCK by RhoA is regulated by cell adhesion, shape, and cytoskeletal tension. Exp Cell Res. 2007;313:3616–23. doi: 10.1016/j.yexcr.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–15. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan JL, Tien J, Pirone DM, Gray DS, Bhadriraju K, Chen CS. Cells lying on a bed of microneedles: an approach to isolate mechanical force. Proc Natl Acad Sci USA. 2003;100:1484–9. doi: 10.1073/pnas.0235407100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cavalcanti-Adam EA, Volberg T, Micoulet A, Kessler H, Geiger B, Spatz JP. Cell spreading and focal adhesion dynamics are regulated by spacing of integrin ligands. Biophys J. 2007;92:2964–74. doi: 10.1529/biophysj.106.089730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selhuber-Unkel C, Erdmann T, Lopez-Garcia M, Kessler H, Schwarz US, Spatz JP. Cell adhesion strength is controlled by intermolecular spacing of adhesion receptors. Biophys J. 2010;98:543–51. doi: 10.1016/j.bpj.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell ID, Humphries MJ. Integrin structure, activation, and interactions. Cold Spring Harb Perspect Biol. 2011;3:a004994. doi: 10.1101/cshperspect.a004994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim C, Ye F, Ginsberg MH. Regulation of integrin activation. Annu Rev Cell Dev Biol. 2011;27:321–45. doi: 10.1146/annurev-cellbio-100109-104104. [DOI] [PubMed] [Google Scholar]
- 10.Xiong JP, Stehle T, Zhang R, Joachimiak A, Frech M, Goodman SL, Arnaout MA. Crystal structure of the extracellular segment of integrin alphaVbeta3 in complex with an Arg-Gly-Asp ligand. Science. 2002;296:151–5. doi: 10.1126/science.1069040. [DOI] [PubMed] [Google Scholar]
- 11.Byron A, Humphries JD, Askari JA, Craig SE, Mould AP, Humphries MJ. Anti-integrin monoclonal antibodies. J Cell Sci. 2009;122:4009–11. doi: 10.1242/jcs.056770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards JG, Hameed H, Campbell G. Induction of fibroblast spreading by Mn2+: a possible role for unusual binding sites for divalent cations in receptors for proteins containing Arg-Gly-Asp. J Cell Sci. 1988;89 (Pt 4):507–13. doi: 10.1242/jcs.89.4.507. [DOI] [PubMed] [Google Scholar]
- 13.Cluzel C, Saltel F, Lussi J, Paulhe F, Imhof BA, Wehrle-Haller B. The mechanisms and dynamics of alphaVbeta3 integrin clustering in living cells. J Cell Biol. 2005;171:383–92. doi: 10.1083/jcb.200503017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang MT, Reich DH, Chen CS. Measurement and analysis of traction force dynamics in response to vasoactive agonists. Integr Biol (Camb) 2011;3:663–74. doi: 10.1039/c0ib00156b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parsons M, Messent AJ, Humphries JD, Deakin NO, Humphries MJ. Quantification of integrin receptor agonism by fluorescence lifetime imaging. J Cell Sci. 2008;121:265–71. doi: 10.1242/jcs.018440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu J, Wang YK, Yang MT, Desai RA, Yu X, Liu Z, Chen CS. Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nat Methods. 2010;7:733–6. doi: 10.1038/nmeth.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan JL, Liu W, Nelson CM, Raghavan S, Chen CS. Simple approach to micropattern cells on common culture substrates by tuning substrate wettability. Tissue Eng. 2004;10:865–72. doi: 10.1089/1076327041348365. [DOI] [PubMed] [Google Scholar]
- 18.Nelson CM, Pirone DM, Tan JL, Chen CS. Vascular endothelial-cadherin regulates cytoskeletal tension, cell spreading, and focal adhesions by stimulating RhoA. Mol Biol Cell. 2004;15:2943–53. doi: 10.1091/mbc.E03-10-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olsen AL, Sackey BK, Marcinkiewicz C, Boettiger D, Wells RG. Fibronectin extra domain-A promotes hepatic stellate cell motility but not differentiation into myofibroblasts. Gastroenterology. 142:928–937. e3. doi: 10.1053/j.gastro.2011.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lenter M, Uhlig H, Hamann A, Jeno P, Imhof B, Vestweber D. A monoclonal antibody against an activation epitope on mouse integrin chain beta 1 blocks adhesion of lymphocytes to the endothelial integrin alpha6beta1. Proc Natl Acad Sci USA. 1993;90:9051–5. doi: 10.1073/pnas.90.19.9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bazzoni G, Shih DT, Buck CA, Hemler ME. Monoclonal antibody 9EG7 defines a novel beta1 integrin epitope induced by soluble ligand and manganese, but inhibited by calcium. J Biol Chem. 1995;270:25570–7. doi: 10.1074/jbc.270.43.25570. [DOI] [PubMed] [Google Scholar]
- 22.Humphries JD, Byron A, Humphries MJ. Integrin ligands at a glance. J Cell Sci. 2006;119:3901–3. doi: 10.1242/jcs.03098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hodivala-Dilke KM, et al. Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Invest. 1999;103:229–38. doi: 10.1172/JCI5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schiller HB, Friedel CC, Boulegue C, Fässler R. Quantitative proteomics of the integrin adhesome show a myosin II-dependent recruitment of LIM domain proteins. EMBO Rep. 2011;12:259–66. doi: 10.1038/embor.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pasapera AM, Schneider IC, Rericha E, Schlaepfer DD, Waterman CM. Myosin II activity regulates vinculin recruitment to focal adhesions through FAK-mediated paxillin phosphorylation. J Cell Biol. 2010;188:877–90. doi: 10.1083/jcb.200906012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Assoian RK, Schwartz MA. Coordinate signaling by integrins and receptor tyrosine kinases in the regulation of G1 phase cell-cycle progression. Curr Opin Genet Dev. 2001;11:48–53. doi: 10.1016/s0959-437x(00)00155-6. [DOI] [PubMed] [Google Scholar]
- 27.Salasznyk RM, Klees RF, Williams WA, Boskey A, Plopper GE. Focal adhesion kinase signaling pathways regulate the osteogenic differentiation of human mesenchymal stem cells. Exp Cell Res. 2007;313:22–37. doi: 10.1016/j.yexcr.2006.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sieg DJ, Hauck CR, Schlaepfer DD. Required role of focal adhesion kinase for integrin-stimulated cell migration. J Cell Sci. 1999;112 (Pt 16):2677–91. doi: 10.1242/jcs.112.16.2677. [DOI] [PubMed] [Google Scholar]
- 29.Xu W, Coll JL, Adamson ED. Rescue of the mutant phenotype by reexpression of full-length vinculin in null F9 cells; effects on cell locomotion by domain deleted vinculin. J Cell Sci. 1998;111 (Pt 11):1535–44. doi: 10.1242/jcs.111.11.1535. [DOI] [PubMed] [Google Scholar]
- 30.DeMali KA, Barlow CA, Burridge K. Recruitment of the Arp2/3 complex to vinculin: coupling membrane protrusion to matrix adhesion. J Cell Biol. 2002;159:881–91. doi: 10.1083/jcb.200206043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Butler B, Gao C, Mersich AT, Blystone SD. Purified integrin adhesion complexes exhibit actin-polymerization activity. Curr Biol. 2006;16:242–51. doi: 10.1016/j.cub.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 32.Rohatgi R, Nollau P, Ho HY, Kirschner MW, Mayer BJ. Nck and phosphatidylinositol 4,5-bisphosphate synergistically activate actin polymerization through the N-WASP-Arp2/3 pathway. J Biol Chem. 2001;276:26448–52. doi: 10.1074/jbc.M103856200. [DOI] [PubMed] [Google Scholar]
- 33.Miyata H, Nishiyama S, Akashi K, Kinosita K., Jr Protrusive growth from giant liposomes driven by actin polymerization. Proc Natl Acad Sci USA. 1999;96:2048–53. doi: 10.1073/pnas.96.5.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitchison TJ, Cramer LP. Actin-based cell motility and cell locomotion. Cell. 1996;84:371–9. doi: 10.1016/s0092-8674(00)81281-7. [DOI] [PubMed] [Google Scholar]
- 35.Danen EH, van Rheenen J, Franken W, Huveneers S, Sonneveld P, Jalink K, Sonnenberg A. Integrins control motile strategy through a Rho-cofilin pathway. J Cell Biol. 2005;169:515–26. doi: 10.1083/jcb.200412081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Legate KR, Takahashi S, Bonakdar N, Fabry B, Boettiger D, Zent R, Fassler R. Integrin adhesion and force coupling are independently regulated by localized PtdIns(4,5)2 synthesis. EMBO J. 2011;30:4539–53. doi: 10.1038/emboj.2011.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu X, Chakraborty S, Heaps CL, Davis MJ, Meininger GA, Muthuchamy M. Fibronectin increases the force production of mouse papillary muscles via alpha5beta1 integrin. J Mol Cell Cardiol. 2011;50:203–13. doi: 10.1016/j.yjmcc.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Friedland JC, Lee MH, Boettiger D. Mechanically activated integrin switch controls alpha5beta1 function. Science. 2009;323:642–4. doi: 10.1126/science.1168441. [DOI] [PubMed] [Google Scholar]
- 39.Garcia AJ, Huber F, Boettiger D. Force required to break alpha5beta1 integrin-fibronectin bonds in intact adherent cells is sensitive to integrin activation state. J Biol Chem. 1998;273:10988–93. doi: 10.1074/jbc.273.18.10988. [DOI] [PubMed] [Google Scholar]
- 40.Garcia AJ, Takagi J, Boettiger D. Two-stage activation for alpha5beta1 integrin binding to surface-adsorbed fibronectin. J Biol Chem. 1998;273:34710–5. doi: 10.1074/jbc.273.52.34710. [DOI] [PubMed] [Google Scholar]
- 41.Roca-Cusachs P, Gauthier NC, Del Rio A, Sheetz MP. Clustering of alpha5beta1 integrins determines adhesion strength whereas alphaVbeta3 and talin enable mechanotransduction. Proc Natl Acad Sci USA. 2009;106:16245–50. doi: 10.1073/pnas.0902818106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A,B) wtMEF spread cell area (A) and total strain energy (B) on low FN density, following adenoviral delivery of shRNA against either α5 integrin or scrambled control sequence (baseline) then 1 hour of Mn2+ (Mn2+). (A,B) Mann-Whitney-U test (ns: non-significant, *: p≤0.05).