Abstract
Protein phosphorylation is an important mechanism for regulating ionotropic glutamate receptors (iGluRs). Early studies have established that major iGluR subtypes, including α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptors and N-methyl-D-aspartate (NMDA) receptors, are subject to phosphorylation. Multiple serine, threonine, and tyrosine residues predominantly within the C-terminal regions of AMPA receptor and NMDA receptor subunits have been identified as sensitive phosphorylation sites. These distinct sites undergo either constitutive phosphorylation or activity-dependent phosphorylation induced by changing cellular and synaptic inputs as reversible events. An increasing number of synapse-enriched protein kinases have been found to phosphorylate iGluR. The common kinases include protein kinase A, protein kinase C, Ca2+/calmodulin-dependent protein kinase II, Src/Fyn non-receptor tyrosine kinases, and cyclin dependent kinase-5. Regulated phosphorylation plays a well-documented role in modulating the biochemical, biophysical, and functional properties of the receptor. In the future, identifying the precise mechanisms how phosphorylation regulates iGluR activities and finding the link between iGluR phosphorylation and the pathogenesis of various brain diseases, including psychiatric and neurodegenerative diseases, chronic pain, stroke, Alzheimer’s disease and substance addiction, will be hot topics and could contribute to the development of novel pharmacotherapies, by targeting the defined phosphorylation process, for suppressing iGluR-related disorders.
Keywords: Excitatory amino acid, AMPA, NMDA, PKA, PKC, CaMKII, Cdk5, tyrosine kinase
1. Introduction
Ionotropic glutamate receptors (iGluRs) are ligand-gated ion channels and are classified into α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptors, N-methyl-D-aspartate (NMDA) receptors, and kainate receptors (Traynelis et al., 2010). These receptors become functional upon a homomeric and mainly heteromeric assembly of multiple subunits. AMPA receptors, for example, are assembled into a tetrameric structure composed of four subunits (GluA1-4, formerly known as GluR1-4), whereas NMDA receptor tetramers are composed of two obligatory GluN1 (or NR1) and two modulatory GluN2 (or NR2) subunits. All subunits share the similar conformation in the plasma membrane which includes three membrane-spanning domains (M1, M3, and M4), a hydrophobic hairpin domain (M2), an extracellular N-terminus, and an intracellular C-terminus (CT). Intracellular domains, including loop 1, loop 2 and mainly CT, are key zones for phosphorylation. Multiple serine, threonine, and tyrosine residues in the CT of AMPA receptor and NMDA receptor subunits have been identified as sensitive sites that are phosphorylated by a set of synapse-enriched protein kinases, including protein kinase A (PKA), protein kinase C (PKC), Ca2+/calmodulin-dependent protein kinase II (CaMKII), non-receptor tyrosine kinases (NRTK), and others (Mao et al., 2011; Lu and Roche, 2012; Sanz-Clemente et al., 2013b). Phosphorylation at a specific site is either largely constitutive or activity-dependent as a dynamic and reversible modification in nature. By regulating phosphorylation levels, protein kinases control the biochemistry, biophysics, and physiology of iGluRs, usually in a fashion associated with the concomitant modulation of synaptic plasticity. This perspective provides a brief overview on the role of phosphorylation in regulating iGluRs with a focus on recent progress, which is followed by a perspective on future studies linking phosphorylation biology of iGluRs to neurological disorders.
2. Phosphorylation of AMPA receptors
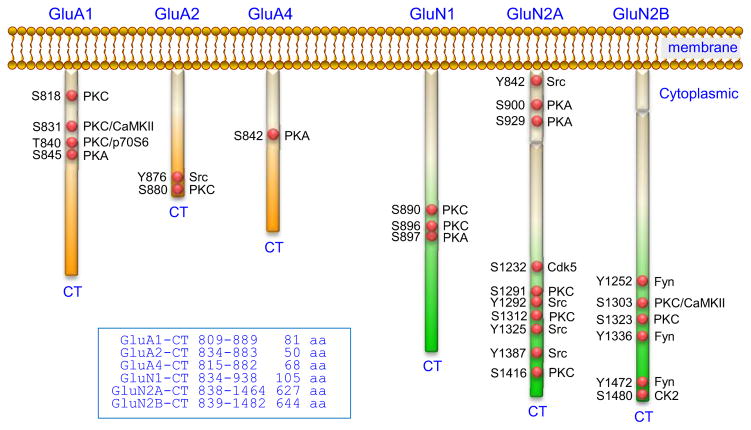
Reliable serine or threonine phosphorylation occurs in AMPA receptor subunit CT regions (Mao et al., 2011; Lu and Roche, 2012) (Figure 1). The first set of phosphorylation sites identified include serine 831 (S831) and S845 in GluA1 (Roche et al., 1996; Barria et al., 1997; Mammen et al., 1997). The former is phosphorylated by PKC and CaMKII, whereas the latter is phosphorylated by PKA. Additionally, GluA1 is phosphorylated at S818 by PKC (Boehm et al., 2006) and threonine 840 (T840) by PKC (Lee et al., 2007) and p70S6 (Delgado et al., 2007). Other subunits are also subject to phosphorylation. GluA2 contains a PKC site (S880) (Matsuda et al., 1999; Chung et al., 2000) and GluA4 has a primary site (S842) sensitive to PKA and possibly other kinases (Carvalho et al., 1999). In addition to serine and threonine, phosphorylation occurs at tyrosine 876 (Y876) in GluA2 in response to Src NRTKs (Hayashi and Huganir, 2004).
Figure 1. Phosphorylation sites in the CT regions of AMPA receptor and NMDA receptor subunits.
Multiple serine, threonine, and tyrosine phosphorylation sites have been identified in the CT regions of AMPA receptor subunits (GluA1, GluA2, and GluA4) and NMDA receptor subunits (GluN1, GluN2A, and GluN2B). The GluN2A CT and GluN2B CT are particularly large, containing 627 and 644 amino acids (aa), respectively. Most phosphorylation sites in the GluN2A/B CT are located in the distal segments.
Phosphorylation at these sites has a significant impact on AMPA receptors. Biochemically, phosphorylation regulates trafficking of modified subunits, resetting the number of the receptor among different subcellular/subsynaptic compartments. S845 is obviously a key site controlling GluA1 trafficking. Phosphorylation at this site consistently traffics receptors to extrasynaptic membranes and primes extrasynaptic receptors for synaptic insertion based on early and recent studies (Estaban et al., 2003; He et al., 2009; Incontro et al., 2013). Other phosphorylation sites, including GluA1 S818 and GluA4 S842, exert the same effect (Estaban et al., 2003; Boehm et al., 2006; Gomes et al., 2007). S818 phosphorylation was recently shown to achieve this effect by increasing the GluA1 interaction with a neuronal specific actin-binding protein 4.1N (Lin et al., 2009). In contrast to accelerated exocytosis with increased synaptic insertion of receptors, phosphorylation also enables endocytosis and reduces the abundance of synaptic receptors. In GluA2, the two major phosphorylation sites (Y876 and S880) are noticeably adjacent to the end of CT and overlap with the binding domain (880-SVKI) for PDZ domain-containing scaffold proteins, such as glutamate receptor interacting proteins 1 and 2 (GRIP1/2). Thus, enhanced phosphorylation at Y876 or S880 disrupted the association of GluA2 with GRIP1/2, thereby accelerating endocytosis of GluA2 and reducing the abundance of surface-expressed AMPA receptors (Matsuda et al., 1999; 2000; Chung et al., 2000; Seidenman et al., 2003; Hayashi and Huganir, 2004). However, complex of the role of GRIPs in regulating AMPA receptor trafficking is underscored by the finding that GRIP interactions with GluA2 were not required for surface expression of GluA2 in cultured hippocampal neurons (Braithwaite et al., 2002).
Phosphorylation also alters biophysical properties of AMPA receptor channels. An early study found that GluA1 S831 phosphorylation by CaMKII increased single channel conductance (Derkach et al., 1999). This effect was recently replicated in PKC-phosphorylated S831 (Jenkins and Travnelis, 2012). Moreover, the S831 regulation relies on coexpression of GluA1/A2 with transmembrane AMPA receptor regulatory proteins (TARPs) (Kristensen et al., 2011). S845 phosphorylation enhanced the channel open probability and the current peak (Roche et al., 1996; Banke et al., 2000). Recently, it was shown that adenosine A(2A) receptors seem to engage this PKA-S845 pathway to increase the availability of GluA1-containing AMPA receptors at extrasynaptic pools for synaptic insertion and augment AMPA currents in hippocampal neurons (Dias et al., 2012).
Synaptic plasticity, including long-term potentiation (LTP) and long-term depression (LTD), is evidently regulated by phosphorylation according to a large number of previous and recent studies (Lee, 2006; Lu and Roche, 2012). The regulation is largely based on a metaplastic basis that AMPA receptors undergo activity-dependent and phosphorylation-mediated recruitment to or removal from synapses during synaptic plasticity. S818, S831 or S845 phosphorylation alone or in combination seems to underlie or potentiate LTP expression (Estaban et al., 2003; Boehm et al., 2006; Oh et al., 2006; Lee et al., 2010; Makino et al., 2011). S845 is also critical for LTD expression since S845A but not S831A mutant mice lacked LTD (Lee et al., 2010). In contrast, GluA2 S880 phosphorylation that reduces GluA2 surface expression promoted LTD (Seidenman et al., 2003). Recent studies support the similar role of phosphorylation at these sites in synaptic plasticity (Dias et al., 2012; Fernandez-Monreal et al., 2012; Halt et al., 2012; Sanderson et al., 2012; Kohda et al., 2013; Ren et al., 2013). Interestingly, S845 phosphorylation serves as a prerequisite step for homeostatic synaptic plasticity (Goel et al., 2011) and a mechanism for sorting endocytically-removed AMPA receptors to endosomes for reinsertion or lysosomes for degradation (Fernandez-Monreal et al., 2012).
3. Phosphorylation of NMDA receptors
The CT domains of NMDA receptor subunits, especially GluN2A/B, are relatively large and accommodate most if not all phosphorylated amino acids identified so far (Mao et al., 2011; Sanz-Clemente et al., 2013b) (Figure 1). In the GluN1 CT, PKC phosphorylates S890 and S896 probably via different PKC isoforms, while PKA phosphorylates a neighboring site S897 (Tingley et al., 1997; Sanchez-Perez and Felipo, 2005). The GluN2A CT contains two PKA phosphorylation sites (S900 and S929) (Krupp et al., 2002), three PKC sites (S1291, S1312, and S1416) (Gardoni et al., 2001; Jones and Leonard, 2005), and a site (S1232) phosphorylated by cyclin dependent kinase-5 (Cdk5) (Li et al., 2001). GluN2B CT S1303 is a substrate site of CaMKII (Omkumar et al., 1996). PKC is another kinase for this site as well as S1323 (Liao et al., 2001). S1480 is a recently identified residue in GluN2B, which is phosphorylated by casein kinase (CK2) (Sanz-Clemente et al., 2010). Tyrosine phosphorylation is restricted to GluN2 (Lau and Huganir, 1995). Multiple tyrosine sites within the GluN2A CT (842, 1292, 1325, and 1387) and GluN2B CT (1252, 1336, and 1472) are phosphorylated by Src and/or Fyn NRTKs (Nakazawa et al., 2001; Vissel et al., 2001; Yang and Leonard, 2001; Taniguchi et al., 2009). Other phosphorylation sites may exist according to a recent study, although their functional relevance has not been determined (Ghafari et al., 2012).
As expected, phosphorylation significantly modulates trafficking and distribution of NMDA receptors. PKC-mediated S890 phosphorylation dispersed the surface clusters of GluN1 (Tingley et al., 1997). S896 and S897, when phosphorylated together, increased surface expression of NMDA receptors (Scott et al., 2001). CK2 phosphorylation of S1480 within the PDZ domain binding site disrupted the interaction between GluN2B and PSD-95, driving GluN2B endocytosis (Chung et al., 2004) and facilitating a well-known developmental switch from GluN2B to GluN2A at synapses (Sanz-Clemente et al., 2010). A recent study showed that activated CaMKII coupled GluN2B and CK2 to form a tri-molecular complex and increased CK2-mediated phosphorylation of GluN2B S1480 (Sanz-Clemente et al., 2013a). Tyrosine phosphorylation site-selectively impacts the receptor. Y1472 and Y1336 phosphorylation seems to enrich NMDA receptors at synaptic and extrasynaptic compartments, respectively (Goebel-Goody et al., 2009). Fyn phosphorylation of Y1336 site-dependently regulated GluN2B cleavage by calpain (Wu et al., 2007). As a major regulator, Fyn is believed to act as a point of convergence for many signaling pathways to modulate GluN2B/NMDA receptors (Trepanier et al., 2012).
PKC potentiated GluN1/GluN2A-mediated currents by phosphorylating GluN2A S1291 and S1312 (Jones and Leonard, 2005). PKCζ seems to be the isoform carrying out this potentiation based on a recent study (Jones et al., 2012). Similarly, PKC augmented GluN1/GluN2B currents via GluN2B S1303 and S1323 analogous to GluN2A S1291 and S1312 (Liao et al., 2001). S1323 may also be a unique site regulating GluN1/GluN2B stretch sensitivity (Singh et al., 2012). GluN2A S900 and S929 phosphorylation modulates desensitization of GluN2A/NMDA receptors according to early and recent studies (Krupp et al., 2002; Maki et al., 2013). GluN2A S1232 phosphorylation may contribute to NMDA receptor currents and NMDA receptor-dependent LTP induction (Li et al., 2001). Recently, NRTKs (Src and Fyn) were found to differentially regulate GluN2A versus GluN2B receptors (Yang et al., 2012). While Src selectively links pituitary adenylate cyclase activating peptide 1 receptors (PAC1R) to tyrosine phosphorylation of GluN2A which in turn potentiates NMDA receptor currents and lowers the LTP threshold, Fyn connects dopamine D1 receptors to GluN2B phosphorylation, leading to augmented NMDA receptor currents and enhanced LTD.
4. General conclusions and future perspectives
The phosphorylation-dependent posttranslational modification of iGluRs has been intensively investigated since 1996. Multiple serine, threonine, and tyrosine amino acids have been identified primarily in the CT regions of AMPA receptor and NMDA receptor subunits. Early studies show that either constitutive or induced phosphorylation at a specific site exerts the distinct regulation of the biochemical, biophysical and functional properties of modified receptors. These previous observations have paved the solid way for future studies aimed to advance our understanding of glutamate receptor phosphorylation in many perspectives. First, mechanistic insights into the phosphorylation-mediated regulation are poorly understood at present. More studies are needed to elucidate how phosphorylation alters the biochemical features of phosphorylated molecules and how this biochemical alteration leads to stepwise changes in receptor expression and function. Second, structural biology could be included in the future multidisciplinary studies to map new phosphorylation sites in combination of discovering the structural state acquired for interactions between substrates and kinases or between iGluRs and submembranous proteins. The knowledge of structural biology is useful to predict and characterize potential phosphorylation sites and protein-protein interactions. Also, crystallographic analysis provides a powerful tool to reveal a structural basis for kinase-iGluR binding and to discover phosphorylation-triggered structural changes. Third, given the fact that the same kinase phosphorylates multiple iGluR subunits while different kinases can phosphorylate the same subunit and sometimes even at the same site, it is essential to investigate the distinct role of each individual kinase versus the role of multiple kinases when converged at the same site. It is expected that various kinases work in concert to phosphorylate and regulate iGluRs. To add an additional layer of the mechanism, protein phosphatases that have been less studied as compared to protein kinases need more attention as the phosphorylation level of iGluRs is most likely determined by the balance between kinases and phosphatases. Additionally, other types of posttranslational modifications, such as palmitoylation, ubiquitination and sumoylation, co-occur with phosphorylation to iGluRs. Like phosphorylation, these modifications are an enzymatic process catalyzed by discrete enzyme systems. They are inducible, regulatable, and reversible and are common mechanisms for regulating iGluRs and excitatory synapses (Mao et al., 2011). Thus, how these different types of modifications interact with each other will be an interesting topic for future studies. Finally and more importantly, future disease-based studies are in need of intensification to directly link iGluR phosphorylation to a state of disease. Altered phosphorylation levels of iGluRs in relevant brain regions have been associated with a variety of neurological disorders (Mao et al., 2011). Recently increasing lines of loss-of-function mutation mice (by replacing serine, threonine, or tyrosine alone or together by alanine or phenylalanine) provide a direct tool to evaluate the importance of a specific phosphorylation site or a set of defined phosphorylation sites in the pathogenesis or progression of various brain illnesses, including psychiatric and neurodegenerative diseases, chronic pain, stroke, Alzheimer’s disease and substance addiction. The increasing use of these mice will steadily advance the iGluR phosphorylation research to the desired functional level.
Acknowledgments
The work by the authors discussed in this article was supported by NIH R01 DA010355 and R01 MH061469.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banke TG, Bowie D, Lee HK, Huganir RL, Schousboe A, Traynelis SF. Control of GluR1 AMPA receptor function by cAMP-dependent protein kinase. J Neurosci. 2000;20:89–102. doi: 10.1523/JNEUROSCI.20-01-00089.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria A, Derkach V, Soderling T. Identification of the Ca2+/calmodulin-dependent protein kinase II regulatory phosphorylation site in the α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate-type glutamate receptor. J Biol Chem. 1997;272:32727–32730. doi: 10.1074/jbc.272.52.32727. [DOI] [PubMed] [Google Scholar]
- Boehm J, Kang MG, Johnson RC, Esteban J, Huganir RL, Malinow R. Synaptic incorporation of AMPA receptors during LTP is controlled by a PKC phosphorylation site on GluR1. Neuron. 2006;51:213–225. doi: 10.1016/j.neuron.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Braithwaite SP, Xia H, Malenka RC. Differential roles for NSF and GRIP/ABP in AMPA receptor cycling. Proc Natl Acad Sci USA. 2002;99:7096–7101. doi: 10.1073/pnas.102156099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho AL, Kameyama K, Huganir RL. Characterization of phosphorylation sites on the glutamate receptor 4 subunit of the AMPA receptors. J Neurosci. 1999;19:4748–4754. doi: 10.1523/JNEUROSCI.19-12-04748.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HJ, Huang YH, Lau LF, Huganir RL. Regulation of the NMDA receptor complex and trafficking by activity-dependent phosphorylation of the NR2B subunit PDZ ligand. J Neurosci. 2004;24:10248–10259. doi: 10.1523/JNEUROSCI.0546-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HJ, Xia J, Scannevin RH, Zhang X, Huganir RL. Phosphorylation of the AMPA receptor subunit GluR2 differentially regulates its interaction with PDZ domain-containing proteins. J Neurosci. 2000;20:7258–7267. doi: 10.1523/JNEUROSCI.20-19-07258.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado JY, Coba M, Anderson CN, Thompson KR, Gray EE, Heusner CL, Martin KC, Grant SG, O’Dell TJ. NMDA receptor activation dephosphorylates AMPA receptor glutamate receptor 1 subunits at threonine 840. J Neurosci. 2007;27:13210–13221. doi: 10.1523/JNEUROSCI.3056-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkach V, Barria A, Soderling TR. Ca2+/calmodulin-kinase II enhances channel conductance of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate type glutamate receptors. Proc Natl Acad Sci USA. 1999;96:3269–3274. doi: 10.1073/pnas.96.6.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias RB, Ribeiro JA, Sebastiao AM. Enhancement of AMPA currents and GluR1 membrane expression through PKA-coupled adenosine A(2A) receptors. Hippocampus. 2012;22:276–291. doi: 10.1002/hipo.20894. [DOI] [PubMed] [Google Scholar]
- Estaban JA, Shi H, Wilson C, Nuriya M, Huganir RL, Malinow R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci. 2003;6:136–143. doi: 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- Fernandez-Monreal M, Brown TC, Royo M, Esteban JA. The balance between receptor recycling and trafficking toward lysosomes determines synaptic strength during long-term depression. J Neurosci. 2012;32:13200–13205. doi: 10.1523/JNEUROSCI.0061-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardoni F, Bellone C, Cattabeni F, Di Luca M. Protein kinase C activation modulates alpha-calmodulin kinase II binding to NR2A subunit of N-mehtyl-D-aspartate receptor complex. J Biol Chem. 2001;276:7609–7613. doi: 10.1074/jbc.M009922200. [DOI] [PubMed] [Google Scholar]
- Ghafari M, Hoger H, Keihan Falsafi S, Russo-Schlaff N, Pollak A, Lubec G. Mass spectrometrical identification of hippocampal NMDA receptor subunits NR1, NR2A-D and five phosphorylation sites on NR2A and NR2B. J Proteome Res. 2012;11:1891–1896. doi: 10.1021/pr201099u. [DOI] [PubMed] [Google Scholar]
- Goebel-Goody SM, Davies KD, Alvestad Linger RM, Freund RK, Browning MD. Phospho-regulation of synaptic and extrasynaptic N-methyl-D-aspartate receptors in adult hippocampal slices. Neuroscience. 2009;158:1446–1459. doi: 10.1016/j.neuroscience.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Goel A, Xu LW, Snyder KP, Song L, Goenaga-Vazquez Y, Megill A, Takamiya K, Huganir RL, Lee HK. Phosphorylation of AMPA receptors is required for sensory deprivation-induced homeostatic synaptic plasticity. PLoS One. 2011;6:e18264. doi: 10.1371/journal.pone.0018264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes AR, Correia SS, Esteban JA, Duarte CB, Carvalho AL. PKC anchoring to GluR4 AMPA receptor subunit modulates PKC-driven receptor phosphorylation and surface expression. Traffic. 2007;8:259–269. doi: 10.1111/j.1600-0854.2006.00521.x. [DOI] [PubMed] [Google Scholar]
- Halt AR, Dallapiazza RF, Zhou Y, Stein IS, Qian H, Juntti S, Wojcik S, Borse N, Silva AJ, Hell JW. CaMKII binding to GluN2B is critical during memory consolidation. EMBO J. 2012;31:1203–1216. doi: 10.1038/emboj.2011.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Huganir RL. Tyrosine phosphorylation and regulation of the AMPA receptor by SRC family tyrosine kinases. J Neurosci. 2004;24:6152–6160. doi: 10.1523/JNEUROSCI.0799-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He K, Song L, Cummings LW, Goldman J, Huganir RL, Lee HK. Stabilization of Ca2+-permeable AMPA receptors at perisynaptic sites by GluR1-S845 phosphorylation. Proc Natl Acad Sci USA. 2009;106:20033–20038. doi: 10.1073/pnas.0910338106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incontro S, Ciruela F, Ziff E, Hormann F, Sanchez-Prieto J, Torres M. The type II cGMP dependent protein kinase regulates GluA1 levels at the plasma membrane of developing cerebellar granule cells. Biochim Biophys Acta. 2013 doi: 10.1016/j.bbamcr.2013.03.021. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins MA, Travnelis SF. PKC phosphorylates GluA1-Ser831 to enhance AMPA receptor conductance. Channels (Austin) 2012;6:60–64. doi: 10.4161/chan.18648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones ML, Leonard JP. PKC site mutations reveal differential modulation by insulin of NMDA receptors containing NR2A or NR2B subunits. J Neurochem. 2005;92:1431–1438. doi: 10.1111/j.1471-4159.2004.02985.x. [DOI] [PubMed] [Google Scholar]
- Jones ML, Liao GY, Malecki R, Li M, Salazar NM, Leonard JP. PI 3-kinase and PKCζ mediate insulin-induced potentiation of NMDA receptor currents in Xenopus oocytes. Brain Res. 2012;1432:7–14. doi: 10.1016/j.brainres.2011.11.020. [DOI] [PubMed] [Google Scholar]
- Kohda K, Kakegawa W, Matsuda S, Yamamoto T, Hirano H, Yuzaki M. The δ2 glutamate receptor gates long-term depression by coordinating interactions between two AMPA receptor phosphorylation sites. Proc Natl Acad Sci USA. 2013;110:E948–957. doi: 10.1073/pnas.1218380110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen AS, Jenkins MA, Banke TG, Schousboe A, Makino Y, Johnson RC, Huganir RL, Traynelis SF. Mechanism of Ca(2)/calmodulin-dependent kinase II regulation of AMPA receptor gating. Nat Neurosci. 2011;14:727–735. doi: 10.1038/nn.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupp JJ, Vissel B, Thomas CG, Heinemann SF, Westbrook GL. Calciueurin acts via the C-terminus of NR2A to modulate desensitization of NMDA receptors. Neuropharmacology. 2002;42:593–602. doi: 10.1016/s0028-3908(02)00031-x. [DOI] [PubMed] [Google Scholar]
- Lau LF, Huganir RL. Differential tyrosine phosphorylation of N-methyl-D-aspartate receptor subunits. J Biol Chem. 1995;270:20036–20041. doi: 10.1074/jbc.270.34.20036. [DOI] [PubMed] [Google Scholar]
- Lee HK. Synaptic plasticity and phosphorylation. Pharmacol Ther. 2006;112:810–832. doi: 10.1016/j.pharmthera.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Takamiya K, Kameyama K, He K, Yu S, Rossetti L, Wilen D, Huganir RL. Identification and characterization of a novel phosphorylation site on the GluR1 subunit of AMPA receptors. Mol Cell Neurosci. 2007;36:86–94. doi: 10.1016/j.mcn.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Takamiya K, He K, Song L, Huganir RL. Specific roles of AMPA receptor subunit GluR1 (GluA1) phosphorylation sites in regulating synaptic plasticity in the CA1 region of hippocampus. J Neurophysiol. 2010;103:479–489. doi: 10.1152/jn.00835.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li BS, Sun MK, Zhang L, Takahashi S, Ma W, Vinade L, Kulkami AB, Brady RO, Pant HC. Regulation of NMDA receptors by cyclin-dependent kinase-5. Proc Natl Acad Sci USA. 2001;98:12742–12747. doi: 10.1073/pnas.211428098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao GY, Wagner DA, Hsu MH, Leonard JP. Evidence for direct protein kinase-C mediated modulation of N-methyl-D-aspartate receptor current. Mol Pharmacol. 2001;59:960–964. doi: 10.1124/mol.59.5.960. [DOI] [PubMed] [Google Scholar]
- Lin DT, Makino Y, Sharma K, Hayashi T, Neve R, Takamiya K, Huganir RL. Regulation of AMPA receptor extrasynaptic insertion by 4.1N, phosphorylation and palmitoylation. Nat Neurosci. 2009;12:879–887. doi: 10.1038/nn.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Roche KW. Posttranslational regulation of AMPA receptor trafficking and function. Curr Opin Neurobiol. 2012;22:470–479. doi: 10.1016/j.conb.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki BA, Cole R, Popescu GK. Two serine residues on GluN2A C-terminal tails control NMDA receptor current decay times. Channels (Austin) 2013;7:126–132. doi: 10.4161/chan.23968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino Y, Johnson RC, Yu Y, Takamiya K, Huganir RL. Enhanced synaptic plasticity in mice with phosphomimetic mutation of the GluA1 AMPA receptor. Proc Natl Acad Sci USA. 2011;108:8450–8455. doi: 10.1073/pnas.1105261108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammen AL, Kameyama K, Roche KW, Huganir RL. Phosphorylation of the alpha-amino-3-hydroxy-5-methylisoxazole4-propionic acid receptor GluR1 subunit by calcium/calmodulin-dependent kinase II. J Biol Chem. 1997;272:32528–32533. doi: 10.1074/jbc.272.51.32528. [DOI] [PubMed] [Google Scholar]
- Mao LM, Guo ML, Jin DZ, Fibuch EE, Choe ES, Wang JQ. Post-translational modification biology of glutamate receptors and drug addiction. Front Neuroanat. 2011;5:19. doi: 10.3389/fnana.2011.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda S, Mikawa S, Hirai H. Phosphorylation of serine-880 in GluR2 by protein kinase C prevents its C terminus from binding with glutamate receptor-interacting protein. J Neurochem. 1999;73:1765–1768. doi: 10.1046/j.1471-4159.1999.731765.x. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Launey T, Mikawa S, Hirai H. Disruption of AMPA receptor GluR2 clusters following long-term depression induction in cerebellar Purkinje neurons. EMBO J. 2000;19:2765–2774. doi: 10.1093/emboj/19.12.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa T, Komai S, Tezuka T, Hisatsune C, Umemori H, Semba K, Mishina M, Manabe T, Yamamoto T. Characterization of Fyn-mediated tyrosine phosphorylation sites on GluR epsilon 2 (NR2B) subunit of the N-methyl-D-aspartate receptor. J Biol Chem. 2001;276:693–699. doi: 10.1074/jbc.M008085200. [DOI] [PubMed] [Google Scholar]
- Oh MC, Derkach VA, Guire ES, Soderling TR. Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J Biol Chem. 2006;281:752–758. doi: 10.1074/jbc.M509677200. [DOI] [PubMed] [Google Scholar]
- Omkumar RV, Melinda O, Kiely MJ, Rosenstein AJ, Min KT, Kennedy MB. Identification of a phosphorylation site for calcium/calmodulin-dependent protein kinase II in the NR2B subunit of the N-methyl-D-aspartate receptor. J Biol Chem. 1996;271:31670–31678. doi: 10.1074/jbc.271.49.31670. [DOI] [PubMed] [Google Scholar]
- Ren SQ, Yan JZ, Zhang XY, Bu YF, Pan WW, Yao W, Tian T, Lu W. PKCλ is critical in AMPA receptor phosphorylation and synaptic incorporation during LTP. EMBO J. 2013 doi: 10.1038/emboj.2013.60. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche KW, O’Brien RJ, Mammen AL, Bernhardt J, Huganir RL. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 1996;16:1179–1188. doi: 10.1016/s0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- Sanchez-Perez AM, Felipo V. Serines 890 and 896 of the NMDA receptor subunit NR1 are differentially phosphorylated by protein kinase C isoforms. Neurochem Int. 2005;47:84–91. doi: 10.1016/j.neuint.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Sanderson JL, Gorski JA, Gibson ES, Lam P, Freund RK, Chick WS, Dell’Acqua ML. AKAP150-anchored calcineurin regulates synaptic plasticity by limiting synaptic incorporation of Ca2+-permeable AMPA receptors. J Neurosci. 2012;32:15036–15052. doi: 10.1523/JNEUROSCI.3326-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz-Clemente A, Gray JA, Ogilvie KA, Nicoll RA, Roche KW. Activated CaMKII couples GluN2B and casein kinase 2 to control synaptic NMDA receptors. Cell Rep. 2013a;3:607–614. doi: 10.1016/j.celrep.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz-Clemente A, Matta JA, Isaac JT, Roche KW. Casein kinase 2 regulates the NR2 subunit composition of synaptic NMDA receptors. Neuron. 2010;67:984–996. doi: 10.1016/j.neuron.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz-Clemente A, Nicoll RA, Roche KW. Diversity in NMDA receptor composition: many regulators, many consequences. Neuroscientist. 2013b;19:62–75. doi: 10.1177/1073858411435129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DB, Blanpied TA, Swanson GT, Zhang C, Ehlers MD. An NMDA receptor ER retention signal regulated by phosphorylation and alternative splicing. J Neurosci. 2001;21:3063–3072. doi: 10.1523/JNEUROSCI.21-09-03063.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenman KJ, Steinberg JP, Huganir RL, Malinow R. Glutamate receptor subunit 2 Serine 880 phosphorylation modulates synaptic transmission and mediates plasticity in CA1 pyramidal cells. J Neurosci. 2003;23:9220–9228. doi: 10.1523/JNEUROSCI.23-27-09220.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Doshi S, Spaethling JM, Hockenberry AJ, Patel TP, Geddes-Klein DM, Lynch DR, Meaney DF. N-methyl-D-aspartate receptor mechanosensitivity is governed by C terminus of NR2B subunit. J Biol Chem. 2012;287:4348–4359. doi: 10.1074/jbc.M111.253740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi S, Nakazawa T, Tanimura A, Kiyama Y, Tezuka T, Watabe AM, Katayama N, Yokoyama K, Inoue T, Izumi-Nakaseko H, Kakuta S, Sudo K, Iwakura Y, Umemori H, Inoue T, Murphy NP, Hashimoto K, Kano M, Manabe T, Yamamoto T. Involvement of NMDAR2A tyrosine phosphorylation in depression-related behaviour. EMBO J. 2009;28:3717–3729. doi: 10.1038/emboj.2009.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingley WG, Ehlers MD, Kameyama K, Doherty C, Ptak JB, Riley CT, Huganir RL. Characterization of protein kinase A and protein kinase C phosphorylation of the N-methyl-D-aspartate receptor NR1 subunit using phosphorylation site-specific antibodies. J Biol Chem. 1997;272:5157–5166. doi: 10.1074/jbc.272.8.5157. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepanier CH, Jackson MF, MacDonald JF. Regulation of NMDA receptors by the tyrosine kinase Fyn. FEBS J. 2012;279:12–19. doi: 10.1111/j.1742-4658.2011.08391.x. [DOI] [PubMed] [Google Scholar]
- Vissel B, Krupp JJ, Heinemann SF, Westbrook GL. A use-dependent tyrosine dephosphorylation of NMDA receptors is independent of ion flux. Nat Neurosci. 2001;4:587–596. doi: 10.1038/88404. [DOI] [PubMed] [Google Scholar]
- Wu HY, Hsu FC, Gleichman AJ, Baconguis I, Coulter DA, Lynch DR. Fyn-mediated phosphorylation of NR2B Tyr-1336 controls calpain-mediated NR2B cleavage in neurons and heterologous systems. J Biol Chem. 2007;282:20075–20087. doi: 10.1074/jbc.M700624200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Trepanier C, Sidhu B, Xie YF, Li H, Lei G, Salter MW, Orser BA, Nakazawa T, Yamamoto T, Jackson MF, MacDonald JF. Metaplasticity gated through differential regulation of GluN2A versus GluN2B receptors by Src family kinase. EMBO J. 2012;31:805–816. doi: 10.1038/emboj.2011.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Leonard JP. Identification of mouse NMDA receptor subunit NR2A C-terminal tyrosine sites phosphorylated by coexpression with v-Src. J Neurochem. 2001;77:580–588. doi: 10.1046/j.1471-4159.2001.00255.x. [DOI] [PubMed] [Google Scholar]