Abstract
This study was designed to evaluate the safety and feasibility of high-dose interleukin-2 (HD IL-2) followed by sorafenib in patients with metastatic melanoma (MM) and renal cell carcinoma (RCC). Biomarkers relevant to the antitumor effects of IL-2 that may be altered by sorafenib including the percentages of natural T-regulatory cells (Tregs), myeloid-derived suppressor cells (MDSC), and STAT5 phosphorylation (pSTAT5) in T cells were evaluated. We hypothesized that the proposed treatment schedule is feasible and safe and may lead to enhanced tumor response. A phase I dose escalation trial was conducted in patients with either metastatic RCC or MM. HD IL-2 (600,000 IU/kg IV q8h×8–12 doses) was administered on days 1–5 and 15–19, followed by sorafenib on days 29–82. The sorafenib dose was escalated. The percentage of Tregs, MDSC, and pSTAT5 in T cells were evaluated in peripheral blood by flow cytometry. Twelve of the 18 patients were evaluable for dose-limiting toxicity. No dose-limiting toxicity was observed. The treatment-related toxicity was predictable and did not seem to be additive with this schedule of administration. Partial responses were seen in 3 patients. No significant changes in the percentage of circulating Treg and MDSC were observed, whereas sorafenib did not adversely affect the ability of IL-2 to induce pSTAT5 in T cells. HD IL-2 followed by sorafenib was safe and feasible in patients with MM and RCC and did not adversely affect T-cell signaling through STAT5 in response to IL-2.
Key Words: high-dose interleukin 2, sorafenib, renal cell carcinoma, melanoma
IL-2 is a T-cell growth factor that participates in the orchestration of T-cell-dependent immune responses, which are well documented in vitro and in rodent models. Yet, harnessing the full spectrum of anticancer activity from IL-2 in human cancer has been challenging. This is due in part to the complex heterogeneity of human immune responses. High-dose recombinant interleukin-2 (HD IL-2) has been utilized since 1984 in patients with good performance status for the treatment of metastatic renal cell carcinoma (RCC) and subsequently for metastatic melanoma (MM). Initial regulatory approval was based upon response rates of 15% with durable complete responses (CR) in 4%–6% of patients.1,2 Although RCC and melanoma are uniquely more sensitive to IL-2-based therapy, most evade active therapy. The molecular heterogeneity of these cancers coupled with genetic instability and continual evolution of the cancers to favor and perhaps promote immune tolerance likely contribute to the low response rate.3–5
Combining HD IL-2 with an agent with potential complementary anticancer features and few overlapping toxicities represents a seldom utilized strategy in part due to the significant toxicity associated with HD IL-2 monotherapy.6 Sorafenib is a multitargeted tyrosine kinase inhibitor (TKI) known to inhibit RAF, VEGFR2 (KDR), VEGFR3 (Flt4), RET, and c-kit. It has regulatory approval for the treatment of advanced RCC and hepatocellular carcinoma,7,8 and activity in other tumor types has been demonstrated.9,10
The rationales for combining HD IL-2 with sorafenib are numerous. First, the direct antitumor activity of sorafenib may combine in an additive manner with the immunomodulatory effects of HD IL-2. Second, there is evidence that IL-2 may alter the expression of proangiogenic factors within the tumor microenvironment that are modulated by sorafenib.11 Third, sorafenib has been shown to downregulate the level of immunosuppressive cells that might limit the antitumor activity of NK and T cells stimulated by IL-2.12,13 Indeed, neoadjuvant sorafenib was found to reduce the percentage of tumor-infiltrating regulatory T cells in RCC patients treated with nephrectomy.14 Despite these data, there is also concern that sorafenib might have an antagonistic effect on immune function.15 For example, preclinical studies have suggested that sorafenib may negatively affect dendritic cells and possibly inhibit IL-2-induced phosphorylation of STAT5 (pSTAT5) in T cells, a pathway responsible for proliferation and cytotoxicity.16,17
Sorafenib’s toxicity profile has been well documented18 and is rather distinct in comparison with HD IL-2 toxicity.6 We hypothesized that HD IL-2 could be safely administered as part of a regimen containing sorafenib. Although there are few recognized overlapping toxicities, our concern for unforeseen additive toxicity led us to pursue a phase I trial design to identify dose-limiting toxicity (DLT). There are several reversible grade 3 nonhematological toxicities commonly associated with HD IL-2 administration. For this trial we therefore defined DLT as toxicities that were unexpected, irreversible, or prolonged. Patients were considered evaluable for DLT only if they had received both agents. We chose to vary the sorafenib dose while maintaining the standard HD IL-2 dose.
In addition to clinical endpoints, several correlative studies were integrated into the protocol to gain insight into how these agents affect tumor-associated immunosuppressive cells such as natural T-regulatory (Tregs) cells and myeloid-derived suppressor cells (MDSC) during the course of therapy. We also measured the effect of prior sorafenib on IL-2-induced pSTAT5 in T cells as this pathway may be antagonized by this TKI.19
MATERIALS AND METHODS
Patients
Eligible patients were 18 years of age and above with metastatic or unresectable RCC or MM with at least 1 measureable metastatic site. Patients were candidates for HD IL-2 treatment and therefore had ECOG PS 0 or 1. Prior sunitinib was allowed for patients with RCC. Normal organ/marrow function was required. Patients were required to have normal cardiac stress and pulmonary function testing. Patients with brain metastasis or a history of brain metastasis were excluded. Patients with RCC were required to be either in the good or intermediate prognostic groups20 and urged to undergo debulking nephrectomy. RCC patients were excluded if the histology was <50% clear cell. This trial closed to accrual before the FDA approval of ipilimumab and vemurafenib. This single institution phase I trial was approved by the Institutional Review Board of the Ohio State University. Each patient provided written informed consent.
Study Design
HD IL-2 (Proleukin; Prometheus Laboratories Inc.) was given in combination with sorafenib (Nexavar; Onyx Pharmaceuticals Inc. and Bayer Healthcare Pharmaceuticals Inc.) using dose escalation rules as part of a standard cohorts-of-3 phase I trial design. Sorafenib dose was increased in each dose level group, whereas the HD IL-2 dose remained constant (Table 1). The HD IL-2 was administered in a standard manner (600,000 IU/kg IV every 8 h days 1–5 up to 12 doses and repeated on days 15–19). Sorafenib was initiated on day 29 (week 5) if all HD IL-2-related toxicities had resolved to grade ≤2. Sorafenib was continued until week 12. Disease assessment took place at week 12. We defined a course of HD IL-2 as two 5-day cycles separated by 10 days. A series includes a course of HD IL-2+ and 7 weeks of sorafenib (Fig. 1). Patients were offered a second series (HD IL-2 and sorafenib) if tumor response or stable disease was documented. Sorafenib was discontinued at least 3 days before restarting HD IL-2. Treatment was continued for 1 series past best response.
TABLE 1.
Cohort Dose Levels
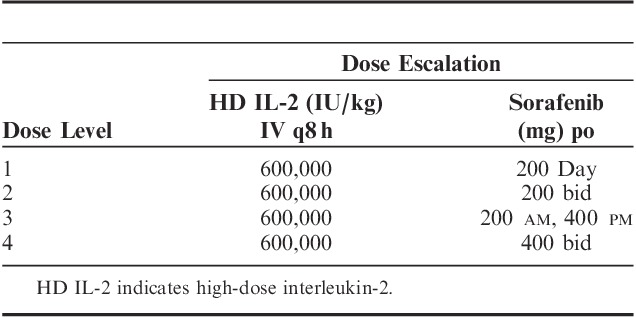
FIGURE 1.
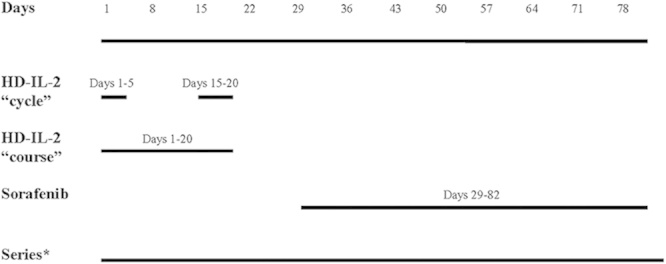
Schema. *Terminology: 1 course of aldesleukin is made up of two 5-day cycles separated by 1 week. One course of aldesleukin plus 8 weeks of sorafenib is a series.
Response Assessment and Toxicity Evaluation
Disease response was determined using the Response Evaluation Criteria in Solid Tumors guidelines.21 The duration of response was measured from the date when stable disease or partial response was documented until progressive disease was objectively measured. Toxicity assessments were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0.
Laboratory Correlative Studies
Procurement of Peripheral Blood
Approximately 8–10 mL of blood was drawn from patients into sodium heparin tubes on days 1 and 29 during each series. For each day 1 timepoint, blood was collected both at baseline and 1 hour after the infusion of IL-2. peripheral blood mononuclear cells (PBMCs) were separated using Ficoll-Paque and density gradient centrifugation and cryopreserved before batch analysis as previously described.22
Flow Cytometric Analysis of Immunosuppressive Cell Subsets
Cryopreserved PBMCs from each patient were assayed for phenotypic markers consistent with MDSCs and natural Treg cells as previously described.23 Briefly, PBMCs from each patient were suspended at a concentration of 1×107/mL in flow staining buffer (PBS plus 1% FBS). Cells were incubated with fluorochrome-labeled antibodies at 4°C. Specific antibodies include CD4-APC (Beckman Coulter), CD15 FITC (eBioscience), CD33 PE (BD Biosciences), HLA-DR PERCP-Cy5.5 (eBioscience), CD11b APC (BD Biosciences), and CD14 Pacific Blue (BD Biosciences). PBMCs were also labeled with the appropriate isotype control antibodies for each fluorochrome to use as negative controls. Cells were then washed with flow buffer, fixed with 1% formalin, and stored at 4°C until analysis. All samples were run on a BD LSR II flow cytometer, and were subsequently analyzed with FlowJo software (Tree Star Inc.). MDSCs were defined as cells positive for CD33, and lacking HLA-DR with subsets expressing CD15, CD14, and CD11b. Natural Treg cells were defined as CD4+CD25+FoxP3+ and assessed using the commercially available Human T-regulatory cell staining kit per manufacturer’s recommendations (BD Biosciences).
Flow Cytometric Analysis of pSTAT5
Cryopreserved T-cell subsets from each patient were assessed for levels of pSTAT5 protein by multiparametric flow cytometry as previously described.19 Briefly, cells were resuspended in 100 μL RPMI-1640 supplemented with 10% FBS, fixed with Fix and Perm Reagent A (Invitrogen) for 2–3 minutes at room temperature, and then incubated for 10 minutes with 3 mL cold methanol. Cells were washed in flow buffer (PBS supplemented with 5% FBS) and incubated in 100 μL of Fix and Perm Reagent B (Invitrogen) containing rabbit anti-human pSTAT5 antibody conjugated to alexafluor488, and APC-conjugated extracellular antibodies against CD4 (Beckman Coulter) or CD8 (Beckman Coulter) for 1 hour at room temperature. Fluorochrome-conjugated isotype control antibodies were used to account for background staining. Cells were then washed in flow buffer, fixed in 1% formalin, and analyzed on a Becton Dickinson FACSCalibur flow cytometer (BD Biosciences) using at least 10,000 events gated in the region of the lymphocyte population, as determined by light scatter properties.
Statistical Analysis
Adverse event data were summarized for patients within dose levels and tabulated based on type, severity, and perceived attribution to study treatment regimen. In addition, presence of any DLTs that occurred in patients receiving both HD IL-2 and sorafenib were also assessed by dose level. Clinical outcomes such as best response to treatment as well as progression-free and overall survival were summarized in an exploratory manner given the inherent limitations to evaluate clinical efficacy in the phase I setting across multiple limited size dose levels. Immunologic markers such as Treg cells, MDSC, CD4, and CD8 levels were evaluated and descriptively summarized. Changes in these markers at different timepoints (eg, post-HD IL-2 vs. post-sorafenib, before and after cycle 1, cycle 1 day 0 to cycle 2 day 29) were assessed graphically across dose levels. These changes were also descriptively summarized, and the nonparametric test for paired samples, the Wilcoxon signed-rank test, was used to explore potential significant changes in these markers during the course of the treatment regimen.
RESULTS
Patient Disposition
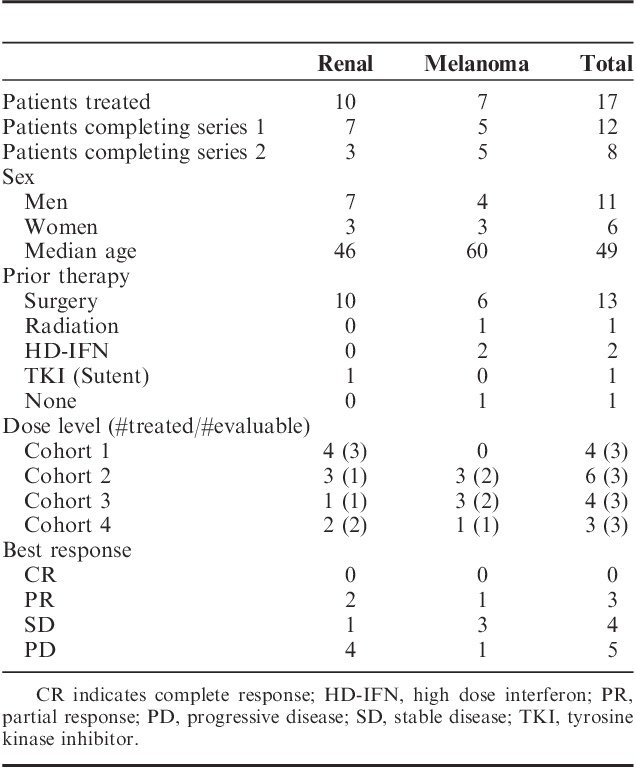
A total of 18 patients (median age, 49 y; range, 37–78) signed the consent. One patient with MM was taken off trial before receiving any protocol treatment due to rapid disease progression. The consent was withdrawn and the patient was replaced. The characteristics of the remaining 17 patients (10 RCC and 7 melanoma; 11 men and 6 women) are listed in (Table 2). All 10 of the RCC patients had undergone prior nephrectomy. Five patients received HD IL-2 but never received sorafenib for a variety of reasons including 1 patient with MM who experienced sudden death. The remaining 12 patients treated on 4 different dose levels completed at least 1 series (both HD IL-2 and sorafenib). No DLT was seen in these patients. Dose levels 1 through 3 had cohorts that included >3 patients because, as described previously, some were not considered evaluable and therefore replaced. Eight patients completed a second series. The median number of doses of HD IL-2 received in series 1 was 17.5. For series 2 the median number of doses received was 16.
TABLE 2.
Patient Summary
Toxicity
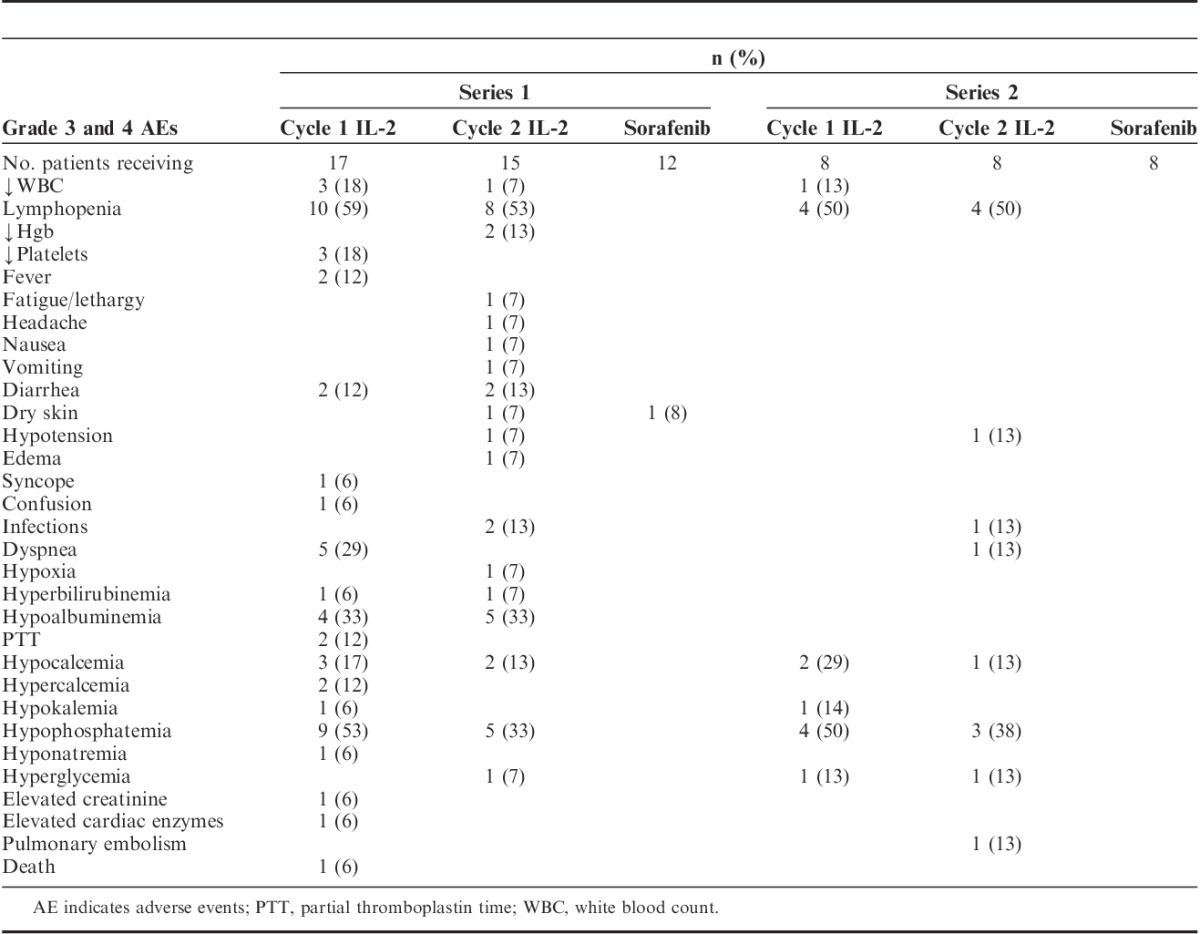
All grade 3 and 4 toxicities are listed in Table 3. Many of the toxicities associated with HD IL-2 did not reach grade 3 or 4 levels. There was no DLT. Patients who received a second series of treatment experienced less toxicity when compared with their first series with the exception of higher rates of hypophosphatemia during HD IL-2 administration. There were no trends appreciated between the frequency or severity of toxicity and the dose of sorafenib.
TABLE 3.
Grade 3 and 4 Toxicities
Efficacy
Because of the phase I setting for this trial and the focus on identification of a tolerable dose level for this combination, the small number of patients in each group, and lack of a control group, only descriptive and preliminary data can be obtained in relation to clinical efficacy. No CRs were observed. Partial responses were seen in 3 patients (dose levels 2 and 4). One additional patient with stable disease by imaging (dose level 3) experienced a significant clinical improvement associated with the reduction of a large esophageal mass (70% decline) by endoscopic evaluation. The partial responses were maintained from 3 to 14+ months. Stable disease was observed in 4 patients and maintained from 4 to 13 months. Progressive disease was recorded for 5 patients. One of these 5 with low-volume disease was initially considered to have stable disease and received a second series of treatment. Extended survival has been observed in patients that have progressed and received subsequent treatment for 14+ to 48+ months.
Correlative Outcomes
Effects of IL-2 and Sorafenib on Immunosuppressive Cells
We were interested in determining whether the combination of IL-2 and sorafenib might lead to changes in the number of MDSC or Treg cells in peripheral blood of patients. For this purpose, the percentage of cells with phenotypic markers for MDSC was measured in the peripheral blood of patients at day 0 and day 29 of series 1 and 2 by flow cytometry. Eight patients completed >1 series and were therefore evaluable for this correlative analysis. At the start of the study (series 1, day 1), the percentage of cells with a MDSC phenotype (HLA-DRlowCD11b+CD33+) was variable among patients (range, 0.3%–16% of total PBMCs). There was no significant difference between the percentage of MDSC at baseline as compared with either series 2, day 0 (P=0.38) or series 2, day 29 (P=0.31) (Fig. 2). Analysis of cells with a natural Treg cell phenotype (CD4+CD25+FoxP3+) in these 8 patients revealed less variability (median=2.42; range, 1.91%–3.46% of total PBMCs); however, there was a slight trend toward increasing percentages of natural Treg cell after 1 series of therapy (median=3.05; P=0.078), although these results did not reach statistical significance (Fig. 3). Our study was not powered to formally assess whether or not changes in MDSC or Treg cells were related to clinical response.
FIGURE 2.
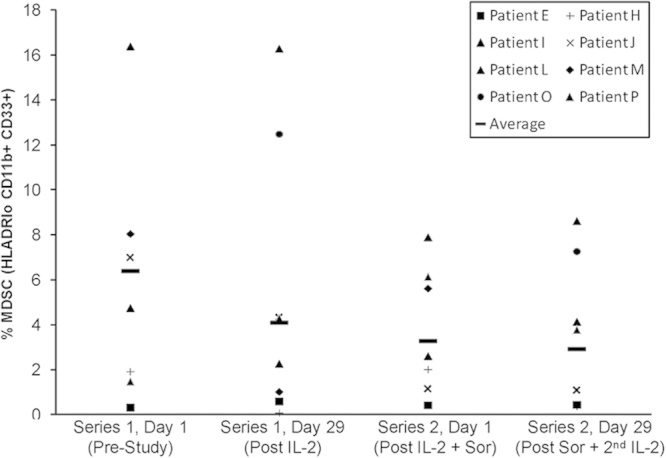
Phenotypic analysis of myeloid cells in patient PBMCs. PBMCs from patients obtained at various timepoints were analyzed by flow cytometry for cells expressing CD33+CD11b+HLA-DRlow markers, consistent with a myeloid-derived suppressor cell phenotype. Data are presented as the percentage of cells staining positive for these markers.
FIGURE 3.
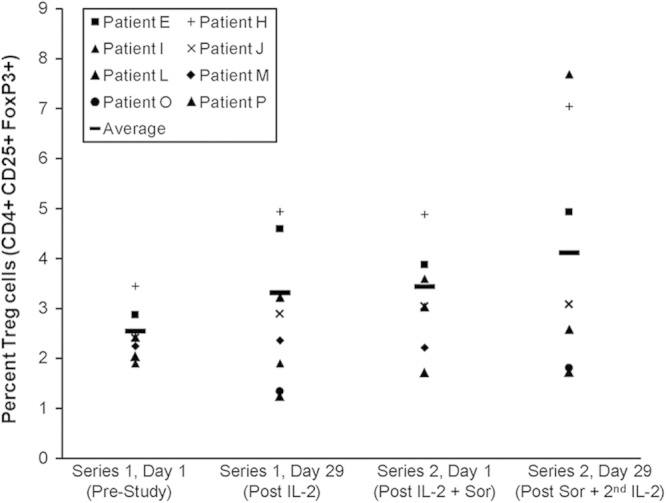
Phenotypic analysis of natural T-regulatory cells in patient PBMCs. PBMCs from patients obtained at various timepoints were analyzed by flow cytometry for cells expressing CD4+CD25+FoxP3+ markers, consistent with a T-regulatory cell phenotype. Data are presented as the percentage of cells staining positive for these markers.
Effects of Sorafenib on IL-2-induced pSTAT5 in T-Cell Subsets
This clinical trial design provided a unique opportunity to assess whether prior therapy with sorafenib might adversely affect IL-2-induced signal transduction within T cells. This correlative readout is of interest as prior in vitro studies from our group have indicated that a pretreatment with sorafenib may inhibit the subsequent cellular response to IL-2 stimulation ex vivo.17 For these studies, blood samples were available from 6 patients before and after sorafenib administration. The percentage of CD4+ and CD8+ T cells with pSTAT5 was assessed at baseline and 1 hour after IL-2 stimulation during series 1 and series 2. Consistent with prior studies by our group,19 the percentage of CD4+ and CD8+ T cells with pSTAT5 1 hour after IL-2 infusion was elevated as compared with baseline during both series 1 and 2. There was no significant difference in the percentage of pSTAT5-positive CD4+ or CD8+ T cells after IL-2 during series 1 versus series 2 (all P>0.4, Fig. 4). These data suggest that prior therapy with sorafenib may not adversely affect STAT5 signal transduction in T cells after subsequent IL-2 administration. We did not perform the same analysis in individuals who did not go on to receive a second series.
FIGURE 4.
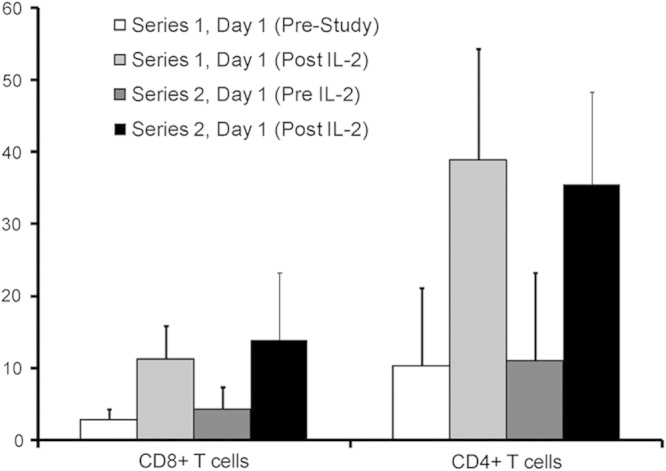
Analysis of STAT5 phosphorylation (pSTAT5) in T cells. PBMCs from patients obtained at various timepoints were analyzed by flow cytometry for pSTAT5 within either CD4+ or CD8+ T cells. Data are presented as the mean percentage of cells staining positive for pSTAT5 within each cell compartment across all patients analyzed. Error bars represent the SD for pSTAT5 staining from all patients.
DISCUSSION
We have shown that the combination of HD IL-2 and sorafenib as studied is feasible and safe. An abundance of caution led to the phase I design which was deemed necessary due to the potential for unforeseen overlapping or additive toxicities even though the agents were not administered simultaneously. Indeed, concern for serious cardiac toxicity in patients treated in nontrial settings with HD IL-2 following VEGF TKI’s was raised during the conduct of this trial.24 This was not observed in this trial. It is interesting to note that the toxicities during the second series (HD IL-2 after sorafenib) were numerically less than with the first series. This observation is certainly affected by selection bias. In retrospect, different schedules including a concurrent schedule may also have been safe. Early phase trials suggested clinical benefit with sorafenib in MM.25 This study was open and accruing participants before the completion of subsequent studies that showed a lack of clinical activity for sorafenib in MM. Concern for sub optimal dosing in these studies has been expressed.26,27
The biomarkers that account for clinical activity associated with HD IL-2 are likely multifactorial and involve changes in multiple immune effector cell compartments or the tumor microenvironment. The study design of the present trial allowed for a unique opportunity to evaluate the effect of prior sorafenib on IL-2-induced pSTAT5 in T cells at a number of prespecified timepoints. Prior preclinical studies from our group indicated that this canonical IL-2 signaling pathway might be inhibited by pretreatment of immune cells with sorafenib, and possibly compromise antitumor effects of exogenous IL-2.17 The data generated in this trial provided preliminary evidence that intracellular IL-2 signaling remained intact in patient T lymphocytes after a course of sorafenib treatment. The lack of an inhibitory effect of sorafenib on T-cell signaling is likely due to the schedule used, whereby the effects of sorafenib-mediated TK inhibition in T cells normalize before subsequent IL-2 therapy.
Because of its multitargeted nature at the level of TK inhibition, sorafenib may have inhibitory effects upon Treg cells or MDSC, which rely on cytokine signals to maintain homeostasis and function. We hypothesized that downregulating tumor-associated immunosuppression through sorafenib may enhance the immune-mediated antitumor effects of IL-2. However, sorafenib exposure followed by HD IL-2 had no significant effect on the percentage of circulating natural Treg cells (CD4+, CD25+, FoxP3+) or cells with phenotypic properties consistent with MDSC (HLADRIo, CD11b+, CD33+) using the schedule used in this trial. Clearly, analysis of immunosuppressive cell populations was somewhat limited in this trial, as more recently other relevant cell populations have received attention in advanced cancer patients including inducible Treg cells or MDSC expressing the CD14+ marker, among others. Because of limited availability of blood samples and ability to biopsy patients on this trial, the effects of this regimen on immune biomarkers in the tumor microenvironment were not evaluable.
CRs were not observed in our trial and we therefore do not know if sorafenib inhibited better responses. The partial responses suggest otherwise. Although not demonstrated in this small phase I trial, the potential remains that active single agents may affect the tumor’s ability to suppress the immune response and therefore lead to enhanced benefit from immunotherapy such as HD IL-2. We have demonstrated a schedule that is both feasible and safe. However, the small size of the trial and the highly selected group of relatively young and fit IL-2-eligible patients represent major limitations when attempting to generalize to larger populations with MM or RCC.
HD IL-2 remains relevant due to the remarkably durable CRs seen in a select few. In the nearly 20 years since HD IL-2 was approved by the FDA for the treatment of MM and RCC, several investigators have attempted clinical trials combining HD IL-2 with other agents.28–33 These studies demonstrated that the combination was feasible; however, the agents chosen to combine with HD IL-2 had very little single-agent activity. Sorafenib is one of the first in an expanding category of distinct but related agents. Its single-agent activity and nonoverlapping toxicity profile led us to perform this combination trial with HD IL-2. To the best of our knowledge, this is the first report of sorafenib or any other VEGF-TKI being combined with HD-IL-2. Sorafenib combined with lower dose subcutaneous IL-2 has been reported to be safe with no increase in efficacy compared with sorafenib alone.34 Given the safety of our treatment schedule, a phase II trial of this combination seems reasonable. We acknowledge that since this trial was initiated, there exists renewed enthusiasm for immune-based therapy based on new understanding and favorable clinical trial outcomes.35,36
CONCLUSIONS
Sorafenib when combined with HD IL-2 in the schedule evaluated was not associated with unexpected toxicity and no DLT was seen. Further study of sorafenib or other promising agents using a similar schedule combined with HD IL-2 seem warranted.
CONFLICTS OF INTEREST/FINANCIAL DISCLOSURES
Supported by Prometheus (P.M. and G.B.L.).
K.K is the member of Advisory Board (Prometheus). All other authors have declared there are no financial conflicts of interest with regard to this work.
REFERENCES
- 1.Fisher RI, Rosenberg SA, Fyfe G.Long-term survival update for high-dose recombinant interleukin-2 in patients with renal cell carcinoma.Cancer J Sci Am. 2000;6suppl 1S55–S57 [PubMed] [Google Scholar]
- 2.Fyfe G, Fisher RI, Rosenberg SA, et al. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy.J Clin Oncol. 1995;13:688–696 [DOI] [PubMed] [Google Scholar]
- 3.Zou W.Immunosuppressive networks in the tumour environment and their therapeutic relevance.Nat Rev Cancer. 2005;5:263–274 [DOI] [PubMed] [Google Scholar]
- 4.Curiel TJ.Tregs and rethinking cancer immunotherapy.J Clin Invest. 2007;117:1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakaguchi S, Sakaguchi N, Shimizu J, et al. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance.Immunol Rev. 2001;182:18–32 [DOI] [PubMed] [Google Scholar]
- 6.Schwartzentruber DJ.Guidelines for the safe administration of high-dose interleukin-2.J Immunother. 2001;24:287–293 [DOI] [PubMed] [Google Scholar]
- 7.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma.N Engl J Med. 2007;356:125–134 [DOI] [PubMed] [Google Scholar]
- 8.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma.N Engl J Med. 2008;359:378–390 [DOI] [PubMed] [Google Scholar]
- 9.Gian V, Rubin M, Hainsworth JD, et al. Sorafenib and continued erlotinib or sorafenib alone in patients with advanced non-small cell lung cancer progressing on erlotinib. A randomized phase II study of the Sarah Cannon Research Institute.Proc Am Soc Clin Oncol. 2012;30suppl30.abstr 7587 [DOI] [PubMed] [Google Scholar]
- 10.Lam ET, Ringel MD, Kloos RT, et al. Phase II clinical trial of sorafenib in metastatic medullary thyroid cancer.J Clin Oncol. 2010;28:2323–2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sabatino M, Kim-Schulze S, Panelli MC, et al. Serum vascular endothelial growth factor and fibronectin predict clinical response to high-dose interleukin-2 therapy.J Clin Oncol. 2009;27:2645–2652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Busse A, Asemissen A, Nonnenmacher A, et al. Immunomodulatory effects of sorafenib on peripheral immune effector cells in metastatic renal cell carcinoma.Eur J Cancer. 2011;47:690–696 [DOI] [PubMed] [Google Scholar]
- 13.Nagai H, Mukozu T, Matsui D, et al. Sorafenib prevents escape from host immunity in liver cirrhosis patients with advanced hepatocellular carcinoma.Clin Dev Immunol. 2012;2012:607851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desar IM, Jacobs JF, Hulsbergen-vandekaa CA, et al. Sorafenib reduces the percentage of tumour infiltrating regulatory T cells in renal cell carcinoma patients.Int J Cancer. 2011;129:507–512 [DOI] [PubMed] [Google Scholar]
- 15.Florcken A, Takvorian A, Van Lessen A, et al. Sorafenib, but not sunitinib, induces regulatory T cells in the peripheral blood of patients with metastatic renal cell carcinoma.Anticancer Drugs. 2012;23:298–302 [DOI] [PubMed] [Google Scholar]
- 16.Hipp MM, Hif N, Walter S, et al. Sorafenib, but not sunitinib, affects function of dendritic cells and induction of primary immune responses.Blood. 2008;111:5610–5620 [DOI] [PubMed] [Google Scholar]
- 17.Raig ET, Kondadasula SV, Olencki T, et al. The Raf Kinase Inhibitor Sorafenib Inhibits Jak-STAT Signal Transduction in Human Immune Cells. 2008San Diego, CA:American Association for Cancer Research Annual Meeting; Abstract # 6214 [Google Scholar]
- 18.Escudier B, Eisen T, Stadler WM, et al. Sorafenib for treatment of renal cell carcinoma: final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial.J Clin Oncol. 2009;27:3312–3318 [DOI] [PubMed] [Google Scholar]
- 19.Varker KA, Kondadasula SV, Go MR, et al. Multiparametric flow cytometric analysis of signal transducer and activator of transcription 5 phosphorylation in immune cell subsets in vitro and following interleukin-2 immunotherapy.Clin Cancer Res. 2006;12:5850–5858 [DOI] [PubMed] [Google Scholar]
- 20.Motzer RJ, Mazumdar M, Bacik J, et al. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma.J Clin Oncol. 1999;17:2530–2540 [DOI] [PubMed] [Google Scholar]
- 21.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada.J Natl Cancer Inst. 2000;92:205–216 [DOI] [PubMed] [Google Scholar]
- 22.Lesinski GB, Kondadasula SV, Crespin T, et al. Multiparametric flow cytometric analysis of inter-patient variation in STAT1 phosphorylation following interferon Alfa immunotherapy.J Natl Cancer Inst. 2004;96:1331–1342 [DOI] [PubMed] [Google Scholar]
- 23.Ko JS, Zea AH, Rini BI, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients.Clin Cancer Res. 2009;15:2148–2157 [DOI] [PubMed] [Google Scholar]
- 24.Cho DC, Puzanov I, Regan MM, et al. Retrospective analysis of the safety and efficacy of interleukin-2 after prior VEGF-targeted therapy in patients with advanced renal cell carcinoma.J Immunother. 2009;32:181–185 [DOI] [PubMed] [Google Scholar]
- 25.Flaherty KT, Schiller J, Schuchter LM, et al. A phase I trial of the oral, multikinase inhibitor sorafenib in combination with carboplatin and paclitaxel.Clin Cancer Res. 2008;14:4836–4842 [DOI] [PubMed] [Google Scholar]
- 26.Flaherty KT, Lee SJ, Zhao F, et al. Phase III trial of carboplatin and paclitaxel with or without sorafenib in metastatic melanoma.J Clin Oncol. 2013;31:373–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pecuchet N, Lebbe C, Mir O, et al. Sorafenib in advanced melanoma: a critical role for pharmacokinetics?Br J Cancer. 2012;107:455–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Demchak PA, Mier JW, Robert NJ, et al. Interleukin-2 and high-dose cisplatin in patients with metastatic melanoma: a pilot study.J Clin Oncol. 1991;9:1821–1830 [DOI] [PubMed] [Google Scholar]
- 29.Trehu EG, Mier JW, Dubois JS, et al. Phase I trial of interleukin 2 in combination with the soluble tumor necrosis factor receptor p75 IgG chimera.Clin Cancer Res. 1996;2:1341–1351 [PubMed] [Google Scholar]
- 30.Atkins MB, Redman B, Mier J, et al. A phase I study of CNI-1493, an inhibitor of cytokine release, in combination with high-dose interleukin-2 in patients with renal cancer and melanoma.Clin Cancer Res. 2001;7:486–492 [PubMed] [Google Scholar]
- 31.Gollob JA, Sciambi CJ, Peterson BL, et al. Phase I trial of sequential low-dose 5-aza-2′-deoxycytidine plus high-dose intravenous bolus interleukin-2 in patients with melanoma or renal cell carcinoma.Clin Cancer Res. 2006;12:4619–4627 [DOI] [PubMed] [Google Scholar]
- 32.Schwartzentruber DJ, Lawson DH, Richards JM, et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma.N Engl J Med. 2011;364:2119–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Atchison E, Eklund J, Martone B, et al. A pilot study of denileukin diftitox (DD) in combination with high-dose interleukin-2 (IL-2) for patients with metastatic renal cell carcinoma (RCC).J Immunother. 2010;33:716–722 [DOI] [PubMed] [Google Scholar]
- 34.Procopio G, Verzoni E, Bracarda S, et al. Sorafenib with interleukin-2 vs. sorafenib alone in metastatic renal cell carcinoma: the ROSORC trial.Br J Cancer. 2011;104:1256–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer.N Engl J Med. 2012;366:2455–2465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mellman I, Coukos G, Dranoff G.Cancer immunotherapy comes of age.Nature. 2011;480:480–489 [DOI] [PMC free article] [PubMed] [Google Scholar]


