Abstract
Melatonin is a well-documented time-keeping hormone that can entrain an individual's physiology and behavior to the day–night cycle, though surprisingly little is known about its influence on the neural basis of social behavior, including vocalization. Male midshipman fish (Porichthys notatus) produce several call types distinguishable by duration and by daily and seasonal cycles in their production. We investigated melatonin's influence on the known nocturnal- and breeding season-dependent increase in excitability of the midshipman's vocal network (VN) that directly patterns natural calls. VN output is readily recorded from the vocal nerve as a ‘fictive call’. Five days of constant light significantly increased stimulus threshold levels for calls electrically evoked from vocally active sites in the medial midbrain, supporting previous findings that light suppresses VN excitability, while 2-iodomelatonin (2-IMel; a melatonin analog) implantation decreased threshold. 2-IMel also increased fictive call duration evoked from medial sites as well as lateral midbrain sites that produced several-fold longer calls irrespective of photoregime or drug treatment. When stimulus intensity was incrementally increased, 2-IMel increased duration only at lateral sites, suggesting that melatonin action is stronger in the lateral midbrain. For animals receiving 5 days of constant darkness, known to increase VN excitability, systemic injections of either of two mammalian melatonin receptor antagonists increased threshold and decreased duration for calls evoked from medial sites. Our results demonstrate melatonin modulation of VN excitability and suggest that social context-dependent call types differing in duration may be determined by neuro-hormonal action within specific regions of a midbrain vocal-acoustic network.
KEY WORDS: Melatonin, Vocalization, Periaqueductal gray, Midshipman fish
INTRODUCTION
Conserved features of vertebrate vocal-acoustic communication include the production of context-dependent vocal call types, occurrence over predictable daily and seasonal cycles, and the ability of neuro-hormones to modulate vocal motor output by acting upon dedicated neural networks (Bass and Remage-Healey, 2008; Goodson and Bass, 2001; Tramontin and Brenowitz, 2000). Most studies to date on fish circadian rhythms have investigated the effects of photoperiod and the time-keeping pineal hormone, melatonin, on locomotor or feeding activity, while little attention has been paid to melatonin's action on courtship behaviors such as vocalization, or more generally on underlying neural circuitry (Azpeleta et al., 2010; López-Olmeda et al., 2006; Piccinetti et al., 2010; Pinillos et al., 2001; Zhdanova et al., 2001). In songbirds, a melatonin-sensitive circadian rhythm in song and call behaviors has only recently been shown (Wang et al., 2012), even though melatonin influence on song nuclei volume and the expression of melatonin receptors in song nuclei has been well documented (Bentley, 2003; Bentley and Ball, 2000; Bentley et al., 1999; Bentley et al., 2013; Cassone et al., 1995; Cassone et al., 2008; Gahr and Kosar, 1996; Jansen et al., 2005; Whitfield-Rucker and Cassone, 1996). Additionally, melatonin inhibited the spontaneous firing rate of a vocal premotor nucleus in the zebra finch, suggesting that it can act directly on vocal circuits to influence vocal patterning (Jansen et al., 2005). Here, we use a fish model to investigate melatonin influence on the temporal patterning of a brainstem neural circuit dedicated to sound production.
Male plainfin midshipman (Porichthys notatus) (Girard, 1854) contract sonic swim bladder muscles at ~100 Hz to produce several call types distinguishable mainly by their duration and the social context under which they are produced (Brantley and Bass, 1994; Bass et al., 1999). Nest-guarding males produce very long-duration (minutes to hours) advertisement/courtship ‘hums’ or short-duration agonistic ‘grunts’ (~50–200 ms) (e.g. Fig. 1A) (Bass et al., 1999; Bass and McKibben, 2003; Brantley and Bass, 1994). Well-defined forebrain, midbrain and hindbrain nuclei form a vocal-acoustic network (Bass et al., 1994; Goodson and Bass, 2002), the output of which is readily recorded in vivo from paired vocal occipital nerves that innervate the sonic muscles and are considered homologs of hypoglossal nerve roots (Fig. 1B) (Bass et al., 2008). The spike-like vocal nerve motor volley is referred to as a fictive call in the absence of muscle activation because each nerve spike directly translates into a single muscle contraction and, in turn, one sound pulse (Fig. 1A) (Bass and Baker, 1990; Cohen and Winn, 1967). Each spike reflects the synchronous firing of vocal motor neurons whose activity is patterned by hindbrain premotor nuclei (Bass and Baker, 1990; Chagnaud et al., 2011; Chagnaud et al., 2012). Hence, fictive calls are a reliable proxy for assessing hormonal influences mediated by specific receptors on a discrete vocal network that directly determines natural call properties (Forlano et al., 2005; Forlano et al., 2010; Goodson and Bass, 2000a; Goodson and Bass, 2000b; Goodson et al., 2003; Fergus and Bass, 2013; Remage-Healey and Bass, 2004; Remage-Healey and Bass, 2007). Given the importance of day length on regulating reproductive physiology, melatonin's role as the main time-keeping hormone among vertebrates, and evidence of its interaction with the hypothalamo-pituitary-gonadal axis (Bhattacharya et al., 2007; Falcón et al., 2007; Falcón et al., 2010), the midshipman presents a tractable model for investigating potential melatonin action on the excitability of neural networks regulating courtship behaviors.
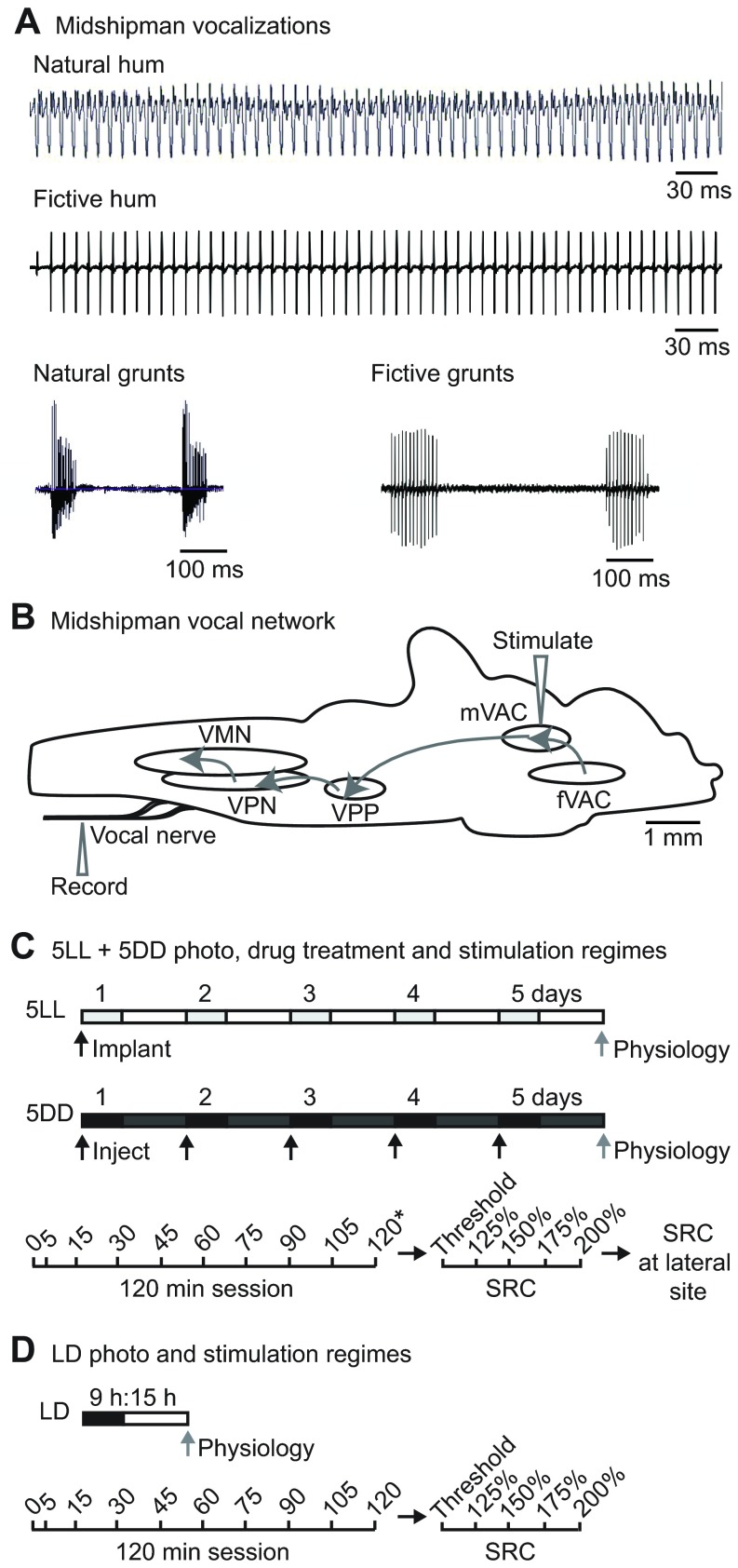
Fig. 1.
Midshipman vocalizations and experimental setup. (A) Natural (adapted from Rubow and Bass, 2009) and fictive calls of male midshipman fish. Long-duration advertisement hums are produced in the summer at nighttime. Short-duration agonistic grunt trains can be produced at any time of day or year. (B) A schematic sagittal view of the midshipman brain showing the vocal control network (adapted from Chagnaud et al., 2011). Stimulation in the midbrain vocal-acoustic complex (mVAC) evokes readily recorded fictive calls from the vocal nerve. The mVAC receives input from the forebrain vocal-acoustic complex (fVAC), from which fictive calls can also be evoked. The mVAC drives the hindbrain vocal pattern generator, which provides a precise and synchronous code for sonic muscle contraction and consists of the vocal pre-pacemaker nucleus (VPP), vocal pacemaker nucleus (VPN) and vocal motor nucleus (VMN). (C,D) Schematics of photoperiod, drug treatment and neurophysiology stimulation regimes used in this study. (C) 5LL fish were implanted with 2-indolmelatonin (2-IMel) or vehicle before subjective lights-off and moved to constant light (LL) for 5 days. Light gray boxes represent subjective night. 5DD fish were held in 5 days of constant darkness (DD) and injected daily around subjective lights-off with vehicle, luzindole or 4P-PDOT. Dark gray boxes represent subjective day. Black arrows indicate time of treatment, and the gray arrow indicates time of neurophysiology for both 5LL and 5DD fish. Sessions (120 min) consisted of stimulation at indicated times after fish were acclimated on the rig for 1 h; 40 stimuli were delivered at each time point except for 120 min, when an additional 60 stimuli for a total of 100 were delivered (highlighted by *). Ten minutes later, a stimulus–response curve (SRC) was collected without moving the electrode, where stimulus intensity was increased to the indicated % of baseline threshold, recording 10 fictive calls every 5 min. The stimulus electrode was then immediately moved to a lateral site in the midbrain to collect another SRC. (D) LD fish were tested for medial and lateral midbrain stimulation comparisons and were housed in 15 h:9 h light:dark. The neurophysiology stimulation regime followed that of 5LL and 5DD animals except only 40 stimuli were delivered at the 120 min trial, and only one SRC was collected after 120 min sessions without moving the electrode.
Midshipman courtship vocalization follows dramatic daily and seasonal rhythms, occurring at night during the summer breeding season (Brantley and Bass, 1994; Ibara et al., 1983). Field and captive studies of fish vocal behavior report robust daily periodicity, with activity peaking during nighttime in most species identified, including closely related toadfish species (Locascio and Mann, 2008; Rice and Bass, 2009). Directly complementing behavioral studies, in vivo neurophysiology in midshipman demonstrates a nocturnal increase in vocal network excitability, measured in increased duration and decreased stimulation threshold of midbrain-evoked fictive calls (Rubow and Bass, 2009). Constant light conditions abolish the nocturnal rise in excitability, while constant dark substantially increases excitability (Rubow and Bass, 2009). Although these studies support the existence of either daily or circadian rhythms in fish vocal behavior and neural circuit plasticity, potential control mechanisms remain unknown.
List of abbreviations
- 2-IMel
2-iodomelatonin
- 4P-PDOT
4-phenyl-2-propionamidotetralin
- 5DD
24 h of darkness for 5 days
- 5LL
24 h of light for 5 days
- AT
anterior tuberal hypothalamus
- AVT
arginine vasotocin
- BW
body weight
- CPG
central pattern generator
- DMSO
dimethyl sulfoxide
- IPI
interpulse interval
- LD
15 h:9 h light:dark cycle
- mVAC
midbrain vocal-acoustic complex
- PAG
periaqueductal gray
- PL
paralemniscal midbrain tegmentum
- POA
preoptic area
- PTT
paratoral midbrain tegmentum
- SIU
stimulus isolation unit
- SRC
stimulus–response curve
- TS
torus semicircularis
- TSd
deep layer of the torus semicircularis
- VN
vocal network
- vT
ventral tuberal hypothalamus
Given the ability of constant light to abolish pineal melatonin production and constant dark to increase baseline melatonin in many species of fish (Bayarri et al., 2002; Bhattacharya et al., 2007; Falcón et al., 2010), we tested the hypothesis that the stimulatory effects of constant darkness on vocal excitability were due, in part, to an increase in melatonin action in discrete vocal nuclei. Within the midshipman vocal network, the midbrain periaqueductal gray (PAG) and surrounding midbrain tegmentum play a crucial role in vocalization initiation, consistent with other vertebrates (Kittelberger and Bass, 2013). We report that, compared with medial stimulation sites, lateral midbrain sites produce longer duration calls comparable to natural advertisement/mate calls that are more sensitive to the stimulatory effects of melatonin than brief calls evoked from medial sites. We propose the existence of a neuroendocrine center located laterally within the previously described midbrain vocal-acoustic network that contributes to the generation of social context-dependent calls. To our knowledge, this study is the first demonstration of melatonin effects on the excitability of a neural network underlying vocal behavior in fishes, and one of few such studies in all of vertebrates (Jansen et al., 2005).
RESULTS
We used two electrical microstimulation regimes in the midbrain to probe vocal network excitability. The first regime evoked fictive responses at 10 time points over 120 min, following Rubow and Bass (Rubow and Bass, 2009). The second stimulation regime, referred to throughout as a stimulus–response curve (SRC), assessed fictive call responses to increasing stimulus current levels. Prior to each time point in 120 min sessions and each SRC, we measured the minimum stimulus current required to elicit responses (threshold). Fig. 1C,D provide schematics of photoperiod, drug treatment and neurophysiology stimulation regimes used in this study.
Although earlier studies were suggestive that more robust vocal stimulation sites could be found in lateral portions of the midbrain PAG and portions of the surrounding tegmentum in comparison to medial sites (Goodson and Bass, 2000b), no study systematically compared vocal output between midbrain regions or examined their neuroendocrine control. In addition to eliciting vocal output from medial sites in the medial PAG and nearby tegmentum (Kittelberger and Bass, 2013), we also stimulated sites in the lateral midbrain that could reliably elicit up to a magnitude longer duration output. We first provide evidence of photoregime effects and melatonin modulation in medial and lateral midbrain regions followed by detailed comparisons of their excitability.
Effects of photoperiod on vocal excitability inferred from control animals
Fish were held under three different photoregimes: one group was maintained on a 15 h:9 h light:dark cycle (LD), consistent with their breeding season photoperiod during late spring–summer. Another group was moved from LD to 24 h of light for 5 days (5LL). Constant light conditions have been equated to ‘functional pinealectomy’, which in fish, as in mammals, abolishes the nocturnal increase in melatonin secretion from the pineal gland (Bayarri et al., 2002; Bhattacharya et al., 2007; Carter et al., 1982; Porter et al., 1999). A third group was moved from LD to 24 h of darkness for 5 days (5DD). In constant darkness, the pineal gland of most teleosts exhibits endogenous cycling of melatonin production albeit at higher daytime levels than under normal light:dark cycles, with the exception of some that show constant upregulation of melatonin production within the duration of darkness (Ekström and Meissl, 1997; Oliveira et al., 2009). The 5LL and 5DD photoregimes were chosen because they exaggerate the observed decrease or increase in vocal excitability associated with daytime and nighttime, respectively (Rubow and Bass, 2009).
We first compared vocal output in control animals from each of the photoperiod groups (see above, and Results) to examine effects of photoperiod manipulation alone and to validate previous findings that 5LL decreased and 5DD increased vocal excitability (Rubow and Bass, 2009). For 120 min sessions and medial site SRC comparisons (Fig. 2A–C), data were from animals tested at medial stimulation sites, including 5LL implant controls for testing effects of a melatonin agonist on excitability (n=10; Fig. 3), 5DD vehicle-injected controls for testing effects of melatonin antagonists on excitability (n=6; Fig. 4), and LD animals for comparing vocal motor output from medial versus lateral midbrain sites (n=5; Fig. 5). For lateral SRCs (Fig. 2D), data were from the same 5LL and 5DD control animals as above, as well as LD animals that first received lateral stimulation over 120 min sessions (n=5; Fig. 5). We present the following results with the caveat that although all were control animals, differences in treatment methods (no treatment, implant, injection) could have contributed to variation in vocal excitability.
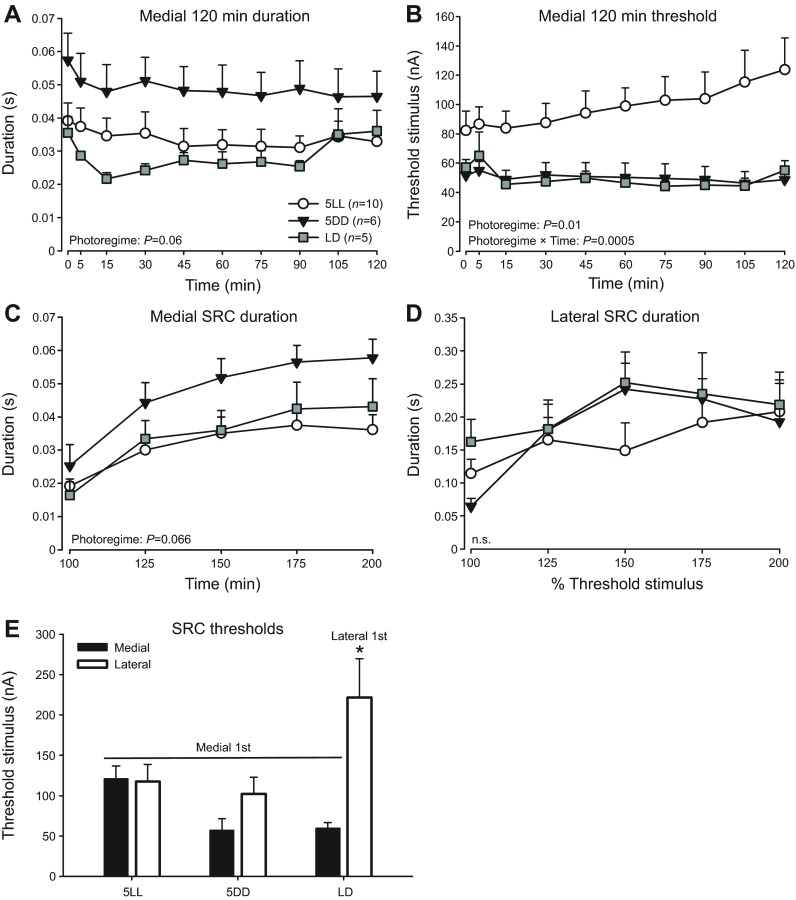
Fig. 2.
Effects of photoregime on vocal excitability inferred from control animals. Fictive call duration (A) and threshold (B) over 120 min sessions, as well as medial (C) and lateral (D) SRCs recorded from control fish of each photoregime/treatment group: LD, 5LL and 5DD. (E) Threshold levels at baseline trials of medial and lateral SRCs. For LD fish that received lateral midbrain stimulation only, lateral SRCs were recorded without prior medial SRCs (Lateral 1st). All other groups received medial stimulation first (Medial 1st). Data are presented as means ± s.e. n.s., non-significant; *P<0.042.
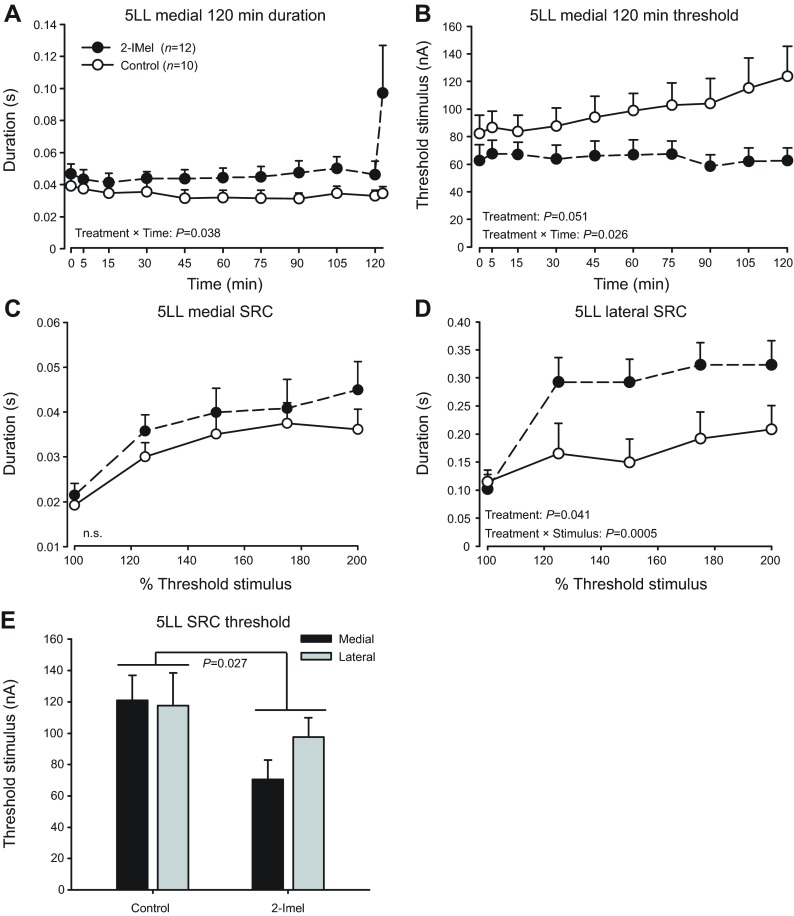
Fig. 3.
2-IMel increases vocal excitability in animals kept under 5LL. (A) 2-IMel treatment increased fictive call duration in a time-dependent manner during the 120 min stimulus trial and decreased stimulus threshold (B). (C,D) 2-IMel increased fictive call duration in stimulus response curves (SRC) only when lateral midbrain was stimulated. (E) 2-IMel decreased stimulus threshold measured at the onset of SRCs. All fish were stimulated at a medial site first for the 120 min session and SRC, followed by recording a second SRC at a lateral site. Data are presented as means ± s.e. n.s., non-significant. Note for the 120 min session duration (A), the means from the first 40 responses were separated from the last 60 fictive call responses (displaced to the right), hence the two data points at 120 min.
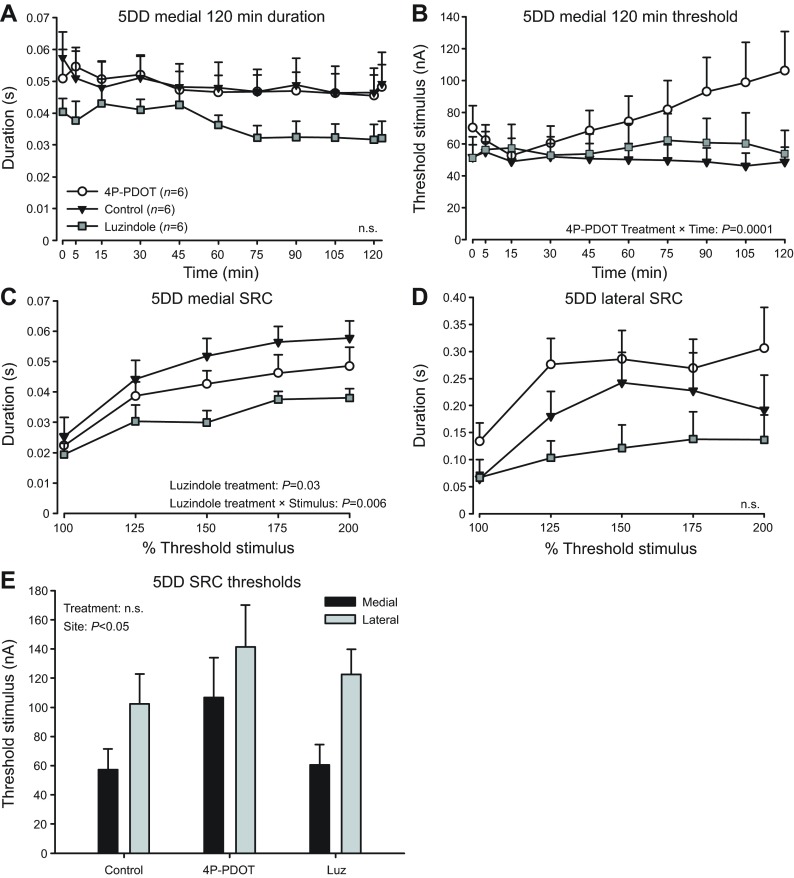
Fig. 4.
Effects of melatonin receptor antagonists on vocal excitability in animals kept under 5DD. Melatonin receptor antagonists luzindole and 4P-PDOT have mixed effects on fictive call 120 min session duration (A), 120 min session threshold (B), medial SRC duration (C) and lateral SRC duration (D). (E) Treatments had no effect on SRC threshold levels, which were higher at lateral sites. Data are presented as means ± s.e. n.s., non-significant. Note for 120 min session duration measurements (A), the means from the first 40 responses were separated from the last 60 fictive call responses (displaced to the right), hence the two data points at 120 min.
Fig. 5.
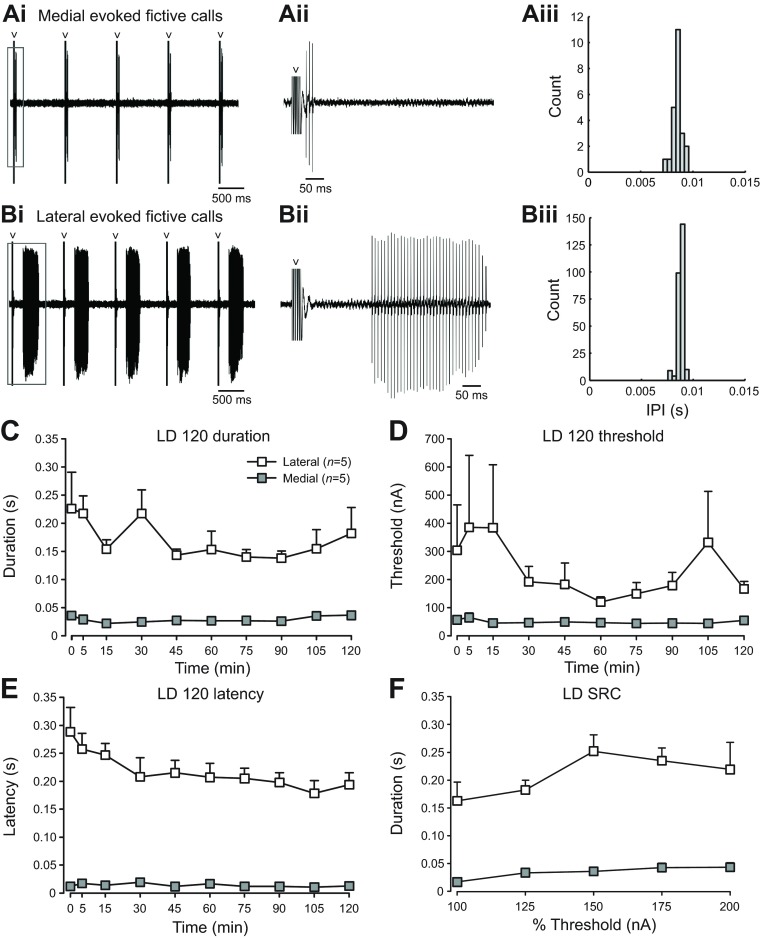
Vocal attributes depend on site of midbrain stimulation. (A,B) Five representative fictive vocal responses evoked by medial (Ai) and lateral (Bi) midbrain stimulation at 200% SRC, taken from a fish that was treated with 2-IMel. Fictive vocal traces outlined in gray boxes were enlarged in Aii and Bii. Gray arrowheads point to stimulus artifacts. From the same fish and stimulation trial, fictive call firing frequency, measured in inter-pulse intervals (IPI), taken from 200% threshold medial SRC trial (Aiii) and lateral SRC trial (Biii) showed no significant site-dependent differences. (C–F) Quantification of fictive call duration (C), stimulus threshold (D), latency (E) and stimulus response curves (F) resulting from either medial or lateral midbrain stimulation in non-treated fish held in normal LD cycles (n=5 per group). Data are presented as means ± s.e. All stimulus-site comparisons shown in C–F are statistically significant (P<0.006).
For the medial midbrain, we observed a strong trend of photoregime effect on call duration recorded over 120 min, with 5DD animals having the longest fictive calls (P=0.06; Fig. 2A). There was a significant effect of photoregime on stimulus threshold over the 120 min session (F2,18.0=6.02, P=0.01; Fig. 2B) and a significant photoregime × time interaction (F18,161=2.68, P=0.0005), with 5LL animals having the highest thresholds (Tukey's HSD: 5LL versus 5DD: P=0.018; 5LL versus LD: P=0.043). These results for medial sites suggested that 5DD increased and 5LL suppressed vocal excitability, as best revealed by threshold levels.
There was a similar strong trend for a photoregime effect on medial site SRC burst duration (P=0.066), with 5DD animals having the highest duration (Fig. 2C). For lateral site SRC (Fig. 2D), we detected no significant photoregime effect (P=0.73). There was no photoregime × stimulus intensity interaction for either medial (P=0.12) or lateral (P=0.17) SRCs.
Finally, we compared medial versus lateral site excitability by examining SRC thresholds in control animals (Fig. 2E). When we examined the effects of photoregime, stimulus site and their interaction on SRC thresholds, we found a near-significant photoregime effect (P=0.061), and a significant stimulus site effect (F1,1=12.48, P=0.001) and photoregime × stimulus site interaction (F2,2=6.41, P=0.004). Post hoc Tukey's HSD showed that the lateral SRC threshold of LD animals who did not receive prior medial stimulation was significantly higher than all others who received medial stimulation first (P<0.042; Fig. 2E). These results imply that stimulation at medial sites first with a 120 min session disinhibits lateral sites by decreasing threshold, but stimulation at lateral sites first does not.
We concluded that photoregime had a significant influence on vocal excitability. This was most strikingly revealed by threshold comparisons (Fig. 2B), consistent with the results of Rubow and Bass (Rubow and Bass, 2009) and our hypothesis that constant light inhibits vocal excitability.
2-Iodomelatonin treatment in 5LL animals
To test the prediction that melatonin replacement can rescue the decreased vocal motor excitability seen in 5LL animals (Rubow and Bass, 2009), fish held in 5LL received an implant for 5 days of either a vehicle or 2-iodomelatonin (2-IMel), a high-affinity melatonin analog that acts on both MT1/Mel1a and MT2/Mel1b receptor subtypes in mammals (Dubocovich et al., 2010; Boutin et al., 2005; Stankov et al., 1993).
For 120 min recording sessions at medial sites, 2-IMel significantly increased fictive call duration in a time-dependent manner (Fig. 3A). There was a significant effect of time (F10,200=2.9, P=0.002) and time × treatment interaction (F10,200=2.0; P=0.038), but no treatment effect alone (P=0.09). Additionally, 2-IMel significantly decreased overall stimulus threshold in 5LL males across 120 min sessions (F1,20=4.3, P=0.0514; Fig. 3B) and there was a significant treatment × time interaction (F9,180=2.17, P=0.026). In sum, 120 min recording sessions revealed that 2-IMel increased vocal excitability at medial stimulation sites in 5LL fish.
For SRCs, 2-IMel exerted a site-dependent effect on duration with a significant stimulatory effect at lateral, but not medial, stimulation sites (Fig. 3C,D). At lateral sites, 2-IMel treatment significantly increased duration (F1,20=4.8, P=0.041), with a significant treatment × stimulus intensity interaction (F4,80=5.6, P=0.0005). At medial sites, we observed no treatment (P=0.59) or treatment × stimulus intensity interactions (P=0.91). We also compared the threshold stimulus levels for SRCs (Fig. 3E). There was a significant treatment effect of 2I-Mel for decreasing threshold (F1,40=5.28, P=0.027), but no stimulus-site effect (P=0.44) or stimulus site × treatment interaction (P=0.33).
Taken together, the results indicated that 2-IMel increased excitability at vocal midbrain sites, irrespective of stimulating electrode location. However, the SRC paradigm revealed a particularly robust stimulatory effect of melatonin on call duration specific to lateral midbrain sites.
Melatonin receptor antagonist treatment in 5DD animals
We set out to test the hypothesis that melatonin action on specific receptor subtypes could explain the increased vocal excitability observed in 5DD males. Because our comparisons of control groups showed that 5DD decreased threshold levels at medial sites (Fig. 2B), we predicted that treatment with melatonin receptor antagonists would reverse this effect. Fish held in 5DD received daily intramuscular injections for 5 days of a receptor antagonist or vehicle (Fig. 1C). The antagonists used were luzindole, a general Mel1a/b antagonist, or 4-phenyl-2-propionamidotetralin (4P-PDOT), a Mel1b-specific antagonist (Dubocovich et al., 2010).
For 120 min sessions, although luzindole values for duration were generally lower at all time points, there was no significant effect (P=0.16) or treatment × time interaction (P=0.58) when compared with animals injected with vehicle control. No treatment (P=1.00) or treatment × time interaction (P=0.76) on duration were observed between vehicle and 4P-PDOT (Fig. 4A). For vehicle to luzindole stimulus threshold comparisons, there was no effect of treatment (P=0.84) or treatment × time interaction (P=0.74). For vehicle to 4P-PDOT threshold comparisons, we found no effect of treatment (P=0.18) alone, but there was a significant treatment × time interaction (F9,90=4.34, P=0.0001; Fig. 4B). In sum, 4P-PDOT increased threshold in a time-dependent manner, suggesting that disrupting Mel1b-mediated melatonin action impacts vocal excitability at medial sites in 5DD animals.
For SRCs evoked at medial sites, luzindole significantly decreased fictive call duration (F1,10=6.42, P=0.03) with a significant treatment × stimulus intensity interaction (F4,40=4.23, P=0.006); 4P-PDOT had no effect (P=0.32; Fig. 4C). In lateral SRCs, treatment with either luzindole (P=0.21) or 4P-PDOT (P=0.25) had no effect on duration (Fig. 4D). Thus, luzindole, like 4P-PDOT, inhibited vocal excitability at medial sites in 5DD animals. Finally, there was no significant treatment effect on SRC threshold levels (luzindole: P=0.56; 4P-PDOT: P=0.11) or treatment × stimulus site interaction (luzindole: P=0.75; 4P-PDOT: P=0.59; Fig. 4E), but there was a stimulus-site effect of lateral sites having significantly higher thresholds (luzindole: F1,9.4=14.5; P=0.004; 4P-PDOT: F1,10=5.0; P=0.048; Fig. 4E).
In summary, for calls evoked from medial sites, antagonizing Mel1b receptors (4P-PDOT) increased threshold in 120 min sessions, while antagonizing Mel1a/b receptors (luzindole) decreased duration in SRCs. This was consistent with our predication that the increased vocal excitability seen in 5DD control animals was due to melatonin action on specific melatonin receptors.
Medial versus lateral midbrain vocal sites
We wanted to ensure that the several-fold longer duration calls consistently elicited from stimulation sites in the lateral compared with the medial midbrain (Fig. 2C,D, Fig. 3C,D, Fig. 4C,D) were not due to a priming effect of stimulating for 120 min at a medial site first. We tested non-treated LD males at either (1) only a medial or only a lateral site for a 120 min session delivering only 40 stimuli at the 120 min trial, followed by one SRC at the same site (Fig. 1D); or (2) a lateral site for a 120 min session and SRC followed by a second SRC at a medial site (supplementary material Fig. S1).
Stimulation at lateral midbrain sites often led to several-fold longer duration fictive calls than those elicited from medial sites (e.g. Fig. 5Ai,ii,Bi,ii). Examination of fictive call frequency, measured in inter-pulse intervals (IPIs), of all fictive calls taken from one pair of representative medial and lateral SRCs revealed no significant site-dependent differences (P=0.14; e.g. Fig. 5Aiii,Biii). For 120 min trials, lateral-evoked calls had a significantly longer duration (F1,7.9=217.6, P<0.0001; Fig. 5C), higher threshold (F1,7.9=14.0, P=0.006; Fig. 5D) and longer latency (F1,8=316.8, P<0.0001; Fig. 5E). Medial site stimulation successfully evoked fictive calls for 98.8±1.0% (mean ± s.e., 120 min trial means) of the 40 stimuli delivered in each trial, whereas lateral stimulation was less reliable at 65.0±2.2% (data not shown).
Similar to the results for 120 min trials (Fig. 5C), lateral site SRCs had significantly longer calls (F1,8=68.4, P<0.0001; Fig. 5F). In a pilot study (n=3), we found the same response patterns when first stimulating in a lateral site for a 120 min session and SRC and then moving to a medial site for a second SRC (F1,18=138.9, P<0.0001; supplementary material Fig. S1).
Taken together, although calls elicited from lateral sites were longer duration than those from medial sites, they required more stimulus current to initiate and exhibited longer latencies.
Electrolytic lesions in a subset of animals, including those from drug treatment experiments, localized medial (n=11) and lateral (n=10) midbrain stimulation sites. Fictive call duration was highly dependent upon the site of stimulation segregated along the medial–lateral midbrain axis (supplementary material Fig. S2). Medial sites were located in the midbrain tegmentum and the medial PAG. Lateral sites were in the paratoral tegmentum (PTT), deep layer of the torus semicircularis (TSd), and just below and within the ventral aspect of the paralemniscal tegmentum (PL).
DISCUSSION
Our results support the hypothesis that nocturnal melatonin action contributes to increased vocal excitability during the midshipman breeding season. The SRC stimulation paradigm highlighted the lateral midbrain vocal-acoustic network as a potential neuroendocrine node that contributes to the production of distinct social context-dependent vocal outputs. We propose that these findings apply to other lineages of vocal vertebrates given the wide occurrence of daily and seasonal cyclicity in vocal behaviors and conserved vocal network organization.
Melatonin regulation of vocal excitability
We found that in control animals, constant light (5LL) inhibited vocal excitability by increasing stimulus threshold at the medial site compared with fish held in constant dark (5DD) and normal light:dark cycles, consistent with previous findings (Rubow and Bass, 2009). This inhibitory effect was rescued by melatonin agonist (2-IMel) implants in fish held under 5LL (Fig. 3B). The 2-IMel implant also led to increased call duration across 120 min sessions in a time-dependent manner. Interestingly, 2IMel increased duration in SRCs only at lateral, but not medial, midbrain stimulation sites (Fig. 3C,D). For 5DD fish, daily injections of 4P-PDOT, a Mel1b-specific receptor antagonist, increased thresholds across 120 min sessions in a time-dependent manner; luzindole, a general Mel1a/b antagonist, resulted in consistently low call durations in both 120 min sessions and SRCs, but these effects were only significant in SRCs (Fig. 4). These mammalian-specific antagonists (Dubocovich et al., 2010) may be less effective in fish, though some fish studies have effectively antagonized melatonin effects on locomotor and feeding activity via peripheral delivery of luzindole (Pinillos et al., 2001; Zhdanova et al., 2001). Together, our results support the hypothesis that a nocturnal melatonin action increases vocal network excitability in male midshipman fish during the breeding season.
Our experimental design was inspired by a previous study where 5DD and 5LL manipulations significantly increased or decreased vocal network excitability, respectively (Rubow and Bass, 2009). Surprisingly at first, we found that medial midbrain stimulation in 5DD fish did not readily evoke the grunt-hums observed in the prior study that are structurally comparable to natural amplitude-modulated growls (see Rubow and Bass, 2009). Rather, we consistently elicited long-duration fictive calls reminiscent of non-amplitude-modulated hum advertisement calls (see Fig. 1A), but only from the lateral midbrain. Our comparisons of control groups showed that although a 5DD effect on duration was apparent, it was only near significant (Fig. 2A). Two prominent methodological differences could explain this inconsistency. First, Rubow and Bass (Rubow and Bass, 2009) noted that an increase in stimulus intensity in conjunction with the 100 stimulus presentation at 120 min was needed to evoke the grunt-hums, whereas in the present study stimulus intensity was kept low and consistent across the 120 min sessions. Second, the long-duration fictive calls reported by Rubow and Bass (Rubow and Bass, 2009) were only readily evoked in males that were held in captivity for less than 1 month (Rubow, 2010) (A.H.B., unpublished observations). Most fish used in the present study were first tested after being held captive for at least 1 month and did not produce long calls either at baseline or with repeated stimulation at the medial site, consistent with our earlier studies.
Results from control animal comparisons (Fig. 2) may shed light on the apparent conundrum that midshipman fish are both long-day and nocturnal breeders. The short duration of nocturnal melatonin experienced under long days would lead to the prediction that melatonin is inhibitory to courtship behaviors, while being nocturnally active during peak diel levels of melatonin would lead to the inverse prediction. In support of an inhibitory role of melatonin in vocalization of diurnally active species, melatonin treatment in songbirds mimics the effect of short days by decreasing song nuclei volumes (Bentley et al., 1999; Cassone et al., 2008). Furthermore, daily melatonin treatment in pinealectomized zebra finches kept in constant dim light entrained song and call activity to occur during periods without melatonin (Wang et al., 2012). Our results in nocturnally active midshipman fish showed that fictive call duration was increased in 5LL+2-IMel animals, especially in the lateral midbrain (Fig. 3D), but not for vehicle-injected 5DD animals (Fig. 2D). One feasible explanation is that the combination of melatonin treatment and long durations of light experienced by 5LL+2-IMel animals increases vocal excitability by increasing melatonin sensitivity in the lateral midbrain, perhaps in the form of a higher density of melatonin receptors. In contrast, in fish that have experienced long durations of darkness (5DD), sensitivity to melatonin could have been diminished at the lateral midbrain, leading to no change in vocal excitability in response to constant darkness when melatonin levels are putatively high. However, we did observe significantly lower threshold levels at medial midbrain sites in 5DD animals (Fig. 2B), suggesting that 5DD does increase aspects of vocal excitability. These are testable hypotheses to be investigated in future studies on melatonin levels in 5DD animals, as well as photoperiod regulation of receptor density and localization.
Alternatively, increases in midshipman vocal excitability at night may rely on an increase in peak nocturnal melatonin levels during the summer breeding season. In some fish species, it has been found that although the duration of a nocturnal rise in circulating melatonin levels is shortened in response to long day lengths, the amplitude of the rise is higher during the spring/summer breeding season and is positively correlated with water temperature (García-Allegue et al., 2001; Iigo and Aida, 1995; Vera et al., 2007). If this is also true for the midshipman, as males migrate from wintering in deeper, colder waters to shallow, warmer intertidal zones for breeding (see Bass, 1996), a higher nocturnal peak in melatonin levels could lead to increased vocal excitability at night.
Melatonin has been shown to interact with neuropeptide and steroid pathways in many vertebrates, including teleost fishes (Falcón et al., 2010; Maitra and Chattoraj, 2006), frogs (Lutterschmidt and Wilczynski, 2012) and birds (Chowdhury et al., 2010; Ubuka et al., 2005). Unlike nonapeptides and steroid hormones (Goodson and Bass, 2000a; Remage-Healey and Bass, 2004), pilot studies showed that acute melatonin injections did not rapidly (5–120 min) change the output of the midshipman vocal system under our testing conditions (N.Y.F., personal observations). Hence, the effects of melatonin on vocal excitability documented here were likely via slower, transcriptionally dependent events. Melatonin may interact with multiple neurotransmitter and neuromodulatory systems synergistically to stimulate the full expression of nocturnal behavior. In other words, melatonin could increase baseline vocal network excitability at night so that in the presence of other activating factors such as neuro-hormones and social cues, the vocal system is capable of responding by producing long-duration calls.
A potential mechanism for melatonin to influence network excitability is through the inhibitory neurotransmitter gamma-aminobutyric acid (GABA). Acute melatonin application to mammalian brain slices increases or decreases GABAergic currents via Mel1a or Mel1b receptors, respectively (Wan et al., 1999). Supporting a stimulatory role for Mel1b-mediated action, antagonizing Mel1b receptors in zebra finches (which are specifically expressed in song control nuclei) at night decreased song duration the following day (Jansen et al., 2005). Song nucleus-specific Mel1b mRNA expression is also positively correlated with immediate early gene expression (Bentley et al., 2013), suggesting that melatonin action contributes to increased neural activity in vocal centers. Our result of 4P-PDOT (mammalian Mel1b antagonist) increasing threshold during the 120 min session is consistent with Mel1b-mediated events as stimulatory on the vocal system. Given GABA's essential role in midshipman vocal network function (Chagnaud et al., 2011; Chagnaud et al., 2012), melatonin might alter vocal network excitability via GABA.
Medial versus lateral midbrain vocal excitability
How the brain generates social context-dependent calls, distinguishable by vocal attributes such as duration, is still largely unresolved. Together with evidence from some mammalian species (Bandler and Carrive, 1988; Fenzl and Schuller, 2007), our study supports the possibility that vocal-acoustic centers in the midbrain contribute to modulation of different call types. We showed that activation of medial and lateral midbrain regions led to short- or long-duration calls, respectively. The vocally active sites identified here are inclusive of those anatomically mapped in prior studies of midshipman fish (Goodson and Bass, 2002; Kittelberger et al., 2006; Kittelberger and Bass, 2013). Whether medial and lateral sites represent two parallel subdivisions of the descending vocal system or the lateral midbrain feeds into the medial to activate the hindbrain vocal central pattern generator (CPG) is beyond the scope of this study and requires further investigation of midbrain microcircuitry. We propose, for vertebrates in general, that the neural control of vocal attributes is sculpted by differential activation of midbrain populations given that (1) call duration is a salient trait distinguishing natural call types in the midshipman (Bass and McKibben, 2003; McKibben and Bass, 1998; MicKibben and Bass, 2001), and vertebrates in general (Bradbury and Vehrencamp, 2011), and (2) midbrain vocal sites are present in tetrapods (see Kittelberger et al., 2006).
Specifically, we showed that the duration of fictive calls elicited from lateral midbrain sites was up to an order of magnitude longer than those elicited from medial sites, regardless of stimulus order. In some experiments, medial stimulation may have directly activated the medial longitudinal fasciculus that also evokes brief calls (Kittelberger et al., 2006). Calls elicited from lateral sites also exhibited higher stimulus thresholds and longer latencies than medial-elicited calls, suggesting that the lateral site is overall less excitable and/or is part of a multi-synaptic pathway that eventually leads to activation of the hindbrain vocal CPG (see below). Strikingly, the latency of lateral midbrain-evoked fictive calls are comparable to the latency of the hum component in medial-midbrain-evoked ‘grunt-hums’/growls (Rubow and Bass, 2009), suggesting that this component could have been produced by a disinhibited lateral site. Importantly, glutamate injection into the lateral midbrain elicits long-duration responses like those we report here (Weeg et al., 2005), suggesting stimulation of local neuronal populations. A more complete investigation using, for example, focal glutamate injection can more precisely map vocally active sites in the midbrain. We propose that divergent medial versus lateral midbrain responses is reliant on differential patterns of vocal-acoustic connectivity and/or neuroendocrine modulation.
In midshipman fish, the midbrain PAG is a crucial node in vocalization initiation and is highly interconnected with auditory centers, consistent with its role in sensorimotor integration and vocal initiation in birds and mammals (Jürgens, 2009; Holstege, 1989; Kingsbury et al., 2011; Kittelberger and Bass, 2013). A recent study revealed that the midshipman lateral PAG is more extensively connected than the medial PAG to auditory-recipient nuclei including the TSd (Kittelberger and Bass, 2013), consistent with our finding that TSd stimulation can elicit longer calls (supplementary material Fig. S2). The lateral PAG also shows greater connectivity to other brainstem vocal sites, as well as the anterior, ventral tuberal hypothalamus (vT), which is directly connected to and activates the PAG (Goodson and Bass, 2000b; Goodson and Bass, 2002; Kittelberger et al., 2006; Kittelberger and Bass, 2013). The latency (Fig. 5E) and percentage of successfully evoked fictive calls from lateral midbrain sites recorded here (~65%, see Results) closely resemble values for calls evoked from the vT, providing a neurophysiological complement to anatomical evidence showing stronger vT input to lateral than medial PAG (Kittelberger et al., 2006; Kittelberger and Bass, 2013). However, our lateral sites evoked notably longer calls than those reported for vT stimulation (Kittelberger et al., 2006), suggesting that while we stimulated the vT–PAG pathway, we were also activating a more extensive network. Furthermore, lateral PAG has stronger connections with the anterior tuberal hypothalamus (AT), a stimulation site that elicits calls similar to those from the lateral midbrain and PL [see fig. 4 in Goodson and Bass (Goodson and Bass, 2000b)], so both AT and vT inputs into the lateral midbrain could be important for generating long calls. Rostromedial PAG, by contrast, has stronger connections to downstream hindbrain auditory-recipient nuclei that are absent from lateral PAG output (Kittelberger and Bass, 2013). Together, these results support a functional separation of the medial and lateral PAG/midbrain tegmentum in eliciting context-dependent call types, e.g. brief agonistic grunts versus longer advertisement calls, dependent on sensory inputs and local neuroendocrine influences.
Vocal and acoustic structures in the midbrain such as the PAG, torus semicircularis, PTT and PL are highly interconnected and have been described together as the midbrain vocal-acoustic complex (mVAC) (Bass et al., 2000; Goodson and Bass, 2002). Anatomical studies of neuropeptide input and steroid receptor distribution in the midshipman midbrain support potential medial versus lateral nodes of neuroendocrine action. Isotocin-expressing fibers and terminals originating from the preoptic area (POA) show dense expression in the mVAC, including the PTT, which receives dense input from the lateral PAG and is a stimulation site resulting in long calls (supplementary material Fig. S2D,E) (Goodson et al., 2003; Weeg et al., 2005; Kittelberger and Bass, 2013). Sparse labeling of isotocin was also found in the TSd, another site for eliciting long fictive calls (supplementary material Fig. S2D). A dorsal bundle of arginine vasotocin (AVT)-expressing fiber and terminals, also originating from the POA, can be found in the PTT. Unlike the diffuse expression of isotocin throughout the caudal midbrain, AVT immunoreactivity was concentrated in a dense band within the midbrain PL (Goodson and Bass, 2000b; Goodson et al., 2003). Although Goodson and Bass (Goodson and Bass, 2000b) report no effects of AVT on fictive call duration following AVT injections in the PL [unlike in the POA (Goodson and Bass, 2000a)], the one illustration of a stimulation site (their fig. 5A, comparable to our supplementary material Fig. S2F) suggests that injections were at sites caudal and medial to the majority identified in our study (supplementary material Fig. S2E).
Previous in vivo physiology experiments are suggestive of steroid action at midbrain levels, where sustained (>30 min) stimulatory effects of steroids on fictive call duration are dependent on descending midbrain input into the hindbrain CPG (Remage-Healey and Bass, 2004). Subsequent studies show androgen and estrogen receptor subtypes in the PAG (Forlano et al., 2010; Fergus and Bass, 2013), and high concentrations of the estrogen-synthesizing enzyme aromatase in the medial PAG (see Forlano et al., 2005). Presumably, endogenous steroids could act upon midbrain-specific receptors to increase vocal network excitability. However, because these studies used systemic steroid injections, medial versus lateral midbrain specific effects cannot be parsed out.
Taken together, the available evidence strongly suggests that further investigation is warranted to assess differential peptidergic and steroid control of specific midbrain vocal-acoustic regions.
Concluding remarks
Vertebrates occupy divergent temporal niches (Challet, 2007; Helfman, 1993; Reebs, 2002; Steiger et al., 2013) despite sharing a highly conserved and predictable pattern of melatonin secretion from the pineal gland at night (but see Wikelski et al., 2006). Thus, species-typical patterns of melatonin-dependent circadian and diel behaviors must rely on divergent downstream molecular and physiological events to interpret the melatonin signal accordingly at a particular time of day and year for a given species. Evidence for such plasticity is found in the varying distribution and abundance of melatonin receptors within neural pathways of songbirds across species, sexes, developmental stages and seasons (Bentley et al., 1999; Bentley et al., 2013; Cassone et al., 1995; Gahr and Kosar, 1996; Whitfield-Rucker and Cassone, 1996), the pro- or anti-gonadal effects of exogenous melatonin treatment in fishes (Maitra and Chattoraj, 2006), and the differential effects of melatonin on locomotor activity in nocturnal versus diurnal fishes (López-Olmeda et al., 2006). Results presented here suggest that differential melatonin sensitivity exists within subregions of a central vocal motor network to regulate the production of seasonal and nocturnal-dependent advertisement calling. Comparative approaches studying behaviors with different daily and seasonal expression patterns at the level of specific neural pathways, such as the vocal network controlling midshipman nocturnal vocalization, will contribute to a more predictive mechanistic model for melatonin regulation of behavior.
MATERIALS AND METHODS
Animals
Midshipman fish have two male reproductive morphs: type I males that acoustically court females and are the focus of this study, and type II males that sneak spawn (Brantley and Bass, 1994). Nesting type I males (standard length 15.1±0.24 cm) were hand collected from rocky intertidal zones in northern California between May 2011 and August 2012. Fish were temporarily (1–7 days) held in large outdoor tanks with running seawater at the University of California Bodega Marine Laboratory before being shipped overnight to Cornell University, where they were housed in individual tanks under 15 h:9 h light:dark (LD) for at least 2 weeks before experimentation. To better simulate sunrise and sunset, a small lamp turned on at 02:00 h EST, half an hour before room lights turned on, which turned off half an hour before the small lamp at 17:00 h EST. Visual inspection of the gonads and swim bladder muscles at the time of killing confirmed type I male status (Bass, 1996). All methods were approved by the Institutional Animal Care and Use Committee at Cornell.
Drug treatment
Fish held in 5LL received either a control implant (coconut oil vehicle) or a 50 mmol l−1 2-IMel implant (Santa Cruz Biotechnology Inc., CA, USA) for 5 days (Fig. 1C). 2-IMel was dissolved to 2 μg ml−1 in heat-liquefied coconut oil, sonicated to homogeneity, pipetted into 10 mm plastic tubing with 0.5 mm inner diameter (BD Infusion Therapy Systems Inc., Sandy, UT, USA) and stored at −20°C in parafilm-sealed Eppendorf tubes until use. After normalization by body weight (BW) of implanted males (n=12), implant dosages were 0.36±0.05 μg g−1 BW, well within reported doses used in fishes and tetrapods that range from 0.1 to 300 μg g−1 BW (Aarseth et al., 2010; Alvariño et al., 2001; Amano et al., 2000; Dubocovich et al., 1998; Handeland et al., 2013; López-Olmeda et al., 2006; Pinillos et al., 2001; Porter et al., 1998; Rubio et al., 2004; Stankov et al., 1993). Following general anesthesia in 0.025% benzocaine (Sigma-Aldrich, St Louis, MO, USA), implants were inserted into the abdominal cavity of males through a small incision, which was closed by suture and Vetbond Tissue Adhesive (3M Animal Care Products, St Paul, MN, USA). Fish were observed to fully recover within ~15 min of the surgical procedure.
Fish held in 5DD received daily intramuscular injections for 5 days via 27 gauge butterfly needles (BD Infusion Therapy Systems Inc.) of 2 μg g−1 BW of a receptor antagonist or only the vehicle (DMSO; J. T. Baker, Phillipsburg, NJ, USA) (Fig. 1C). Stocks of 50 mmol l−1 luzindole and 4P-PDOT were dissolved in DMSO and stored at −20°C in parafilm-sealed Eppendorf tubes until use. On the first day of injection, fish were anesthetized under 0.025% benzocaine and weighed in order to calculate the appropriate volume of luzindole, 4P-PDOT or DMSO to reach 2 μg g−1 BW, to be added to 5 μl g−1 BW teleost saline solution or water as an injection carrier. Subsequently, daily injections were carried out within 1 h of subjective lights off, between 16:00 and 17:00 h EST except for the day of neurophysiology (Fig. 1C). We chose to use 2 μg g−1 BW for luzindole and 4P-PDOT because this dose was within the range used in studies looking at locomotion or feeding (Dubocovich et al., 1998; Pinillos et al., 2001). Treatment groups were staggered to ensure that fish were tested under similar conditions, such as total time spent in captivity.
In vivo neurophysiology
The in vivo fictive call (defined in Introduction) preparation used here follows Rubow and Bass (Rubow and Bass, 2009). Briefly, a dorsal craniotomy exposed the brain, rostral spinal cord and vocal/occipital nerves after general anesthesia with 0.025% benzocaine and local injection of 0.25% Bupivacaine (Hospira, Inc., Lake Forest, IL, USA), a long-lasting local anesthetic. After surgery, fish were immobilized with an intramuscular injection of pancuronium bromide (0.5 mg kg−1; MP Biomedicals, LLC, Solon, OH, USA) and stabilized on a platform within a Plexiglas tank with chilled saltwater (15–17°C) perfused through the mouth. After 1 h of acclimation, an insulated tungsten electrode (125 μm diameter, 8 deg tip angle, 5 MΩ impedance, 20 μm exposed tips; A-M Systems, Sequim, WA, USA) was used to evoke fictive calls from midbrain sites using well-documented surface landmarks and depth measurements as guidance for electrode placement (Goodson and Bass, 2002; Kittelberger et al., 2006). Fictive calls were recorded from vocal nerve roots unilaterally [reflects synchronous bilateral activity (Bass and Baker, 1990)] with an extracellular Teflon-coated silver electrode with an exposed ball tip (50–100 μm diameter) and digitized using MATLAB software designed by Dr Bruce R. Land (School of Electrical and Computer Engineering, Cornell University).
In 120 min sessions, each stimulation trial consisted of 40 brief stimulus trains delivered to midbrain sites. Baseline (0 min) and subsequent recording trials were performed at 5, 15, 30, 45, 60, 75, 90, 105 and 120 min (Fig. 1C,D). For 5LL and 5DD animals (Fig. 1C), we delivered 100 stimulus trains instead of 40 at the time of the 120 min trial because Rubow and Bass (Rubow and Bass, 2009) found that the additional 60 stimuli evoked longer duration responses resembling natural calls in 5DD and 14 h:10 h light:dark males. We generated a SRC at the end of the 120 min recording session to measure the relationship between stimulus intensity and fictive call duration (Fig. 1C,D). At 10 min after the 120 min recording session and without moving the stimulating electrode, we recorded 10 fictive calls evoked by 100, 125, 150, 175 and 200% of threshold stimulus, with each recording separated by 5 min. For some animals (see Fig. 1C,D) the stimulating electrode was immediately moved to a lateral midbrain site and another SRC was recorded. Each stimulus train consisted of five square pulses (0.1 ms pulse width, 200 Hz), with inter-train intervals of 1 s delivered via a stimulus isolation unit (SIU; Model A350D-A, World Precision Instruments, Sarasota, FL, USA). SIU current output (nA) was interpolated from a linear relationship between SIU dial level and current output derived using Ohm's law by measuring voltage across a resistor.
All animals were tested 1 h post surgery starting between 17:00 and 18:00 h EST under darkness with the experimenter wearing a red headlamp (except for 5LL animals, which were tested under light). 5DD fish experienced ~15 min of exposure to low-level scope light during craniotomy.
Medial versus lateral midbrain lesions
At the end of the experiments in a subset of animals including fish from drug treatment experiments that received stimulation at medial and/or lateral sites, electrolytic lesions were made by passing 10 μA of current with 50% duty cycle for 4–15 s through the stimulus electrode to mark the stimulation site. Fish were then deeply anesthetized under 0.025% benzocaine and perfused transcardially with teleost Ringer's solution followed by 4% paraformaldehyde in 0.1 mol l−1 phosphate buffer (pH 7.2). Prior to sectioning, brains were cryoprotected in 30% sucrose solution overnight, frozen in Cryo-M-Bed (Hacker Instruments, Winnsboro, SC, USA), sectioned at 30 μm in the transverse plane on a crysostat, and mounted onto Superfrost Plus slides (Erie Scientific, Portsmouth, NH, USA). Photomicrographs were taken of selected sections using the Spot FLEX imaging system (Diagnostics Instruments, Inc., Sterling Heights, MI, USA) on a Nikon Eclipse E800 compound microscope.
Neurophysiological and statistical analysis
Measurements of fictive call duration, latency (delay from end of stimulus to first fictive pulse) and IPI were performed by a customized MATLAB program designed by Dr Bruce Land, Cornell University. In some cases where more than one fictive burst was elicited from the lateral midbrain, we combined the total duration of all bursts for analysis.
All statistical analyses were performed in JMP 9 (SAS Institute Inc., Cary, NC, USA) using means obtained from individual recording trials and in consultation with the Cornell University Statistical Consulting Unit. Log or square root transformations were performed whenever assumptions of normality were violated. We used a linear mixed model with appropriate fixed effects (details below) and fish nested within treatment as a random effect.
In order to examine effects of photoperiod manipulation, we first compared data taken from 5LL and 5DD control animals (oil-implanted or vehicle-injected) and LD non-treated animals used in medial versus lateral comparisons. For 120 min trial and SRC measurements, our fixed effects were photoregime, time/stimulus intensity and photoregime × time/stimulus intensity interaction. Three-way ANOVA followed by Tukey's HSD post hoc comparison was performed on SRC stimulus thresholds.
For 5LL and 5DD 120 min sessions, we looked for effects of treatment, time and treatment × time interaction on fictive call duration and threshold. Because luzindole and 4P-PDOT act on melatonin receptors with different affinities (Dubocovich et al., 2010), we performed separate analyses for each. The 100 stimuli delivered on the 120 min trial in 5LL and 5DD animals were split into the first 40 and last 60 calls for analyses. We combined data from 2011 and 2012 5LL animals because year was not a significant factor (120 min session duration: P=0.34; 120 min session threshold: P=0.27; medial SRC duration: P=0.80; lateral SRC duration: P=0.76). For SRC duration comparisons, fixed effects were treatment, stimulus intensity and treatment × stimulus intensity. To compare SRC threshold levels across treatment groups, we performed a three-way ANOVA for each antagonist.
In LD groups used for medial versus lateral comparisons, we looked for effects of stimulus site, time and stimulus site × time interaction on duration, stimulus threshold and latency of fictive responses in 120 min sessions. The fixed effects for SRCs were stimulus site, stimulus intensity and their interaction. Additionally, we performed a Student's t-test on a total of 116 IPIs from medial SRC and 1149 IPIs from lateral SRC in one individual to assess potential site-dependent differences in call frequency.
Supplementary Material
ACKNOWLEDGEMENTS
We sincerely thank Margaret Marchaterre for support with fish collection and neuroanatomy, Boris Chagnaud for help with neurophysiology and comments on the manuscript, Dan Fergus and Kevin Rohmann for statistical advice, and Bruce Land for programming support.
FOOTNOTES
Competing interests
The authors declare no competing financial interests.
Funding
This research was supported by the National Science Foundation [NSF IOS 1120925], a National Institutes of Health predoctoral training grant (5 T32 GM007469), Cornell Research in Animal Behavior grants and Cornell Sigma Xi research grants. Deposited in PMC for release after 12 months.
Supplementary material
Supplementary material available online at http://jeb.biologists.org/lookup/suppl/doi:10.1242/jeb.096669/-/DC1
References
- Aarseth J. J., Froiland E., Jorgensen E. H. (2010). Melatonin implantation during spring and summer does not affect the seasonal rhythm of feeding in anadromous arctic charr (Salvelinus alpinus). Polar Biol. 33, 379-388 [Google Scholar]
- Alvariño J. M. R., Rebollar P. G., Olemdo M., Alvarez-Blázquez B., Peleteiro J. B. (2001). Effects of melatonin implants on reproduction and growth of turbot broodstock. Aquac. Int. 9, 477-487 [Google Scholar]
- Amano M., Iigo M., Ikuta K., Kitamura S., Yamada H., Yamamori K. (2000). Roles of melatonin in gonadal maturation of underyearling precocious male masu salmon. Gen. Comp. Endocrinol. 120, 190-197 [DOI] [PubMed] [Google Scholar]
- Azpeleta C., Martínez-Alvarez R. M., Delgado M. J., Isorna E., De Pedro N. (2010). Melatonin reduces locomotor activity and circulating cortisol in goldfish. Horm. Behav. 57, 323-329 [DOI] [PubMed] [Google Scholar]
- Bandler R., Carrive P. (1988). Integrated defence reaction elicited by excitatory amino acid microinjection in the midbrain periaqueductal grey region of the unrestrained cat. Brain Res. 439, 95-106 [DOI] [PubMed] [Google Scholar]
- Bass A. H. (1996). Shaping brain sexuality. Am. Sci. 84, 352-363 [Google Scholar]
- Bass A. H., Baker R. (1990). Sexual dimorphisms in the vocal control system of a teleost fish: morphology of physiologically identified neurons. J. Neurobiol. 21, 1155-1168 [DOI] [PubMed] [Google Scholar]
- Bass A. H., McKibben J. R. (2003). Neural mechanisms and behaviors for acoustic communication in teleost fish. Prog. Neurobiol. 69, 1-26 [DOI] [PubMed] [Google Scholar]
- Bass A. H., Remage-Healey L. (2008). Central pattern generators for social vocalization: androgen-dependent neurophysiological mechanisms. Horm. Behav. 53, 659-672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass A. H., Marchaterre M. A., Baker R. (1994). Vocal-acoustic pathways in a teleost fish. J. Neurosci. 14, 4025-4039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass A. H., Bodnar D. A., Marchaterre M. A. (1999) Complementary explanations for existing phenotypes in an acoustic communication system. In Neural Mechanisms of Communication (ed. Hauser M., Konishi M.), pp. 493-514 Cambridge, MA: MIT; [Google Scholar]
- Bass A. H., Bodnar D. A., Marchaterre M. A. (2000). Midbrain acoustic circuitry in a vocalizing fish. J. Comp. Neurol. 419, 505-531 [DOI] [PubMed] [Google Scholar]
- Bass A. H., Gilland E. H., Baker R. (2008). Evolutionary origins for social vocalization in a vertebrate hindbrain-spinal compartment. Science 321, 417-421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayarri M. J., Madrid J. A., Sánchez-Vázquez F. J. (2002). Influence of light intensity, spectrum and orientation on sea bass plasma and ocular melatonin. J. Pineal Res. 32, 34-40 [DOI] [PubMed] [Google Scholar]
- Bentley G. E. (2003). Melatonin receptor density in Area X of European starlings is correlated with reproductive state and is unaffected by plasma melatonin concentration. Gen. Comp. Endocrinol. 134, 187-192 [DOI] [PubMed] [Google Scholar]
- Bentley G. E., Ball G. F. (2000). Photoperiod-dependent and -independent regulation of melatonin receptors in the forebrain of songbirds. J. Neuroendocrinol. 12, 745-752 [DOI] [PubMed] [Google Scholar]
- Bentley G. E., Van't Hof T. J., Ball G. F. (1999). Seasonal neuroplasticity in the songbird telencephalon: a role for melatonin. Proc. Natl. Acad. Sci. USA 96, 4674-4679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley G. E., Perfito N., Calisi R. M. (2013). Season- and context-dependent sex differences in melatonin receptor activity in a forebrain song control nucleus. Horm. Behav. 63, 829-835 [DOI] [PubMed] [Google Scholar]
- Bhattacharya S., Chattoraj A., Maitra S. K. (2007). Melatonin in the regulation of annual testicular events in carp Catla catla: evidence from the studies on the effects of exogenous melatonin, continuous light, and continuous darkness. Chronobiol. Int. 24, 629-650 [DOI] [PubMed] [Google Scholar]
- Boutin J. A., Audinot V., Ferry G., Delagrange P. (2005). Molecular tools to study melatonin pathways and actions. Trends Pharmacol. Sci. 26, 412-419 [DOI] [PubMed] [Google Scholar]
- Bradbury J. W., Vehrencamp S. L. (2011). Principles of Animal Communication. Sunderland, MA: Sinauer Associates; [Google Scholar]
- Brantley R. K., Bass A. H. (1994). Alternative male spawning tactics and acoustic signals in the plainfin midshipman fish Porichthys notatus Girard (Teleostei, Batrachoididae). Ethology 96, 213-232 [Google Scholar]
- Carter D. S., Hall V. D., Tamarkin L., Goldman B. D. (1982). Pineal is required for testicular maintenance in the Turkish hamster (Mesocricetus brandti). Endocrinology 111, 863-871 [DOI] [PubMed] [Google Scholar]
- Cassone V. M., Brooks D. S., Kelm T. A. (1995). Comparative distribution of 2[125I]iodomelatonin binding in the brains of diurnal birds: outgroup analysis with turtles. Brain Behav. Evol. 45, 241-256 [DOI] [PubMed] [Google Scholar]
- Cassone V. M., Bartell P. A., Earnest B. J., Kumar V. (2008). Duration of melatonin regulates seasonal changes in song control nuclei of the house sparrow, Passer domesticus: independence from gonads and circadian entrainment. J. Biol. Rhythms 23, 49-58 [DOI] [PubMed] [Google Scholar]
- Chagnaud B. P., Baker R., Bass A. H. (2011). Vocalization frequency and duration are coded in separate hindbrain nuclei. Nat. Commun. 2, 346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagnaud B. P., Zee M. C., Baker R., Bass A. H. (2012). Innovations in motoneuron synchrony drive rapid temporal modulations in vertebrate acoustic signaling. J. Neurophysiol. 107, 3528-3542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challet E. (2007). Minireview: Entrainment of the suprachiasmatic clockwork in diurnal and nocturnal mammals. Endocrinology 148, 5648-5655 [DOI] [PubMed] [Google Scholar]
- Chowdhury V. S., Yamamoto K., Ubuka T., Bentley G. E., Hattori A., Tsutsui K. (2010). Melatonin stimulates the release of gonadotropin-inhibitory hormone by the avian hypothalamus. Endocrinology 151, 271-280 [DOI] [PubMed] [Google Scholar]
- Cohen M. J., Winn H. E. (1967). Electrophysiological observations on hearing and sound production in the fish, Porichthys notatus. J. Exp. Zool. 165, 355-369 [DOI] [PubMed] [Google Scholar]
- Dubocovich M. L., Yun K., Al-Ghoul W. M., Benloucif S., Masana M. I. (1998). Selective MT2 melatonin receptor antagonists block melatonin-mediated phase advances of circadian rhythms. FASEB J. 12, 1211-1220 [DOI] [PubMed] [Google Scholar]
- Dubocovich M. L., Delagrange P., Krause D. N., Sugden D., Cardinali D. P., Olcese J. (2010). International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol. Rev. 62, 343-380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekström P., Meissl H. (1997). The pineal organ of teleost fishes. Rev. Fish Biol. Fish. 7, 199-284 [Google Scholar]
- Falcón J., Besseau L., Sauzet S., Boeuf G. (2007). Melatonin effects on the hypothalamo-pituitary axis in fish. Trends Endocrinol. Metab. 18, 81-88 [DOI] [PubMed] [Google Scholar]
- Falcón J., Migaud H., Muñoz-Cueto J. A., Carrillo M. (2010). Current knowledge on the melatonin system in teleost fish. Gen. Comp. Endocrinol. 165, 469-482 [DOI] [PubMed] [Google Scholar]
- Fenzl T., Schuller G. (2007). Dissimilarities in the vocal control over communication and echolocation calls in bats. Behav. Brain Res. 182, 173-179 [DOI] [PubMed] [Google Scholar]
- Fergus D. J., Bass A. H. (2013). Localization and divergent profiles of estrogen receptors and aromatase in the vocal and auditory networks of a fish with alternative mating tactics. J. Comp. Neurol. 521, 2850-2869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlano P. M., Deitcher D. L., Bass A. H. (2005). Distribution of estrogen receptor alpha mRNA in the brain and inner ear of a vocal fish with comparisons to sites of aromatase expression. J. Comp. Neurol. 483, 91-113 [DOI] [PubMed] [Google Scholar]
- Forlano P. M., Marchaterre M., Deitcher D. L., Bass A. H. (2010). Distribution of androgen receptor mRNA expression in vocal, auditory, and neuroendocrine circuits in a teleost fish. J. Comp. Neurol. 518, 493-512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahr M., Kosar E. (1996). Identification, distribution, and developmental changes of a melatonin binding site in the song control system of the zebra finch. J. Comp. Neurol. 367, 308-318 [DOI] [PubMed] [Google Scholar]
- García-Allegue R., Madrid J. A., Sánchez-Vázquez F. J. (2001). Melatonin rhythms in European sea bass plasma and eye: influence of seasonal photoperiod and water temperature. J. Pineal Res. 31, 68-75 [DOI] [PubMed] [Google Scholar]
- Girard C. F. (1854). Enumeration of the species of marine fishes, collected at San Francisco, California, by Dr. C.B.R. Kennerly, naturalist attached to the survey of the Pacific railroad route, under Lieut. A. W. Whipple. P. Acad. Nat. Sci. Phila. 7, 141-142 [Google Scholar]
- Goodson J. L., Bass A. H. (2000a). Forebrain peptides modulate sexually polymorphic vocal circuitry. Nature 403, 769-772 [DOI] [PubMed] [Google Scholar]
- Goodson J. L., Bass A. H. (2000b). Vasotocin innervation and modulation of vocal-acoustic circuitry in the teleost Porichthys notatus. J. Comp. Neurol. 422, 363-379 [DOI] [PubMed] [Google Scholar]
- Goodson J. L., Bass A. H. (2001). Social behavior functions and related anatomical characteristics of vasotocin/vasopressin systems in vertebrates. Brain Res. Brain Res. Rev. 35, 246-265 [DOI] [PubMed] [Google Scholar]
- Goodson J. L., Bass A. H. (2002). Vocal-acoustic circuitry and descending vocal pathways in teleost fish: convergence with terrestrial vertebrates reveals conserved traits. J. Comp. Neurol. 448, 298-322 [DOI] [PubMed] [Google Scholar]
- Goodson J. L., Evans A. K., Bass A. H. (2003). Putative isotocin distributions in sonic fish: relation to vasotocin and vocal-acoustic circuitry. J. Comp. Neurol. 462, 1-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handeland S. O., Imsland A. K., Björnsson B. T., Stefansson S. O., Porter M. (2013). Physiology during smoltification in Atlantic salmon: effect of melatonin implants. Fish Physiol. Biochem. 39, 1079-1088 [DOI] [PubMed] [Google Scholar]
- Helfman G. S. (1993). Fish behaviour by day, night and twilight. In Behaviour of Teleost Fishes, 2nd edn (ed. Pitcher T. J.), pp. 479-512 London: Chapman and Hall; [Google Scholar]
- Holstege G. (1989). Anatomical study of the final common pathway for vocalization in the cat. J. Comp. Neurol. 284, 242-252 [DOI] [PubMed] [Google Scholar]
- Ibara R. M., Penny L. T., Ebeling A. W., van Dykhuizen G., Cailliet G. (1983). The mating call of the plainfin midshipman fish, Porichthys notatus. In Predators and Prey in Fishes (ed. Noakes D. L. G., Lindquist D. G., Helfman G. S., Ward J. A.), pp. 205-212 The Hague, Netherlands: Dr W. Junk Publications; [Google Scholar]
- Iigo M., Aida K. (1995). Effects of season, temperature, and photoperiod on plasma melatonin rhythms in the goldfish, Carassius auratus. J. Pineal Res. 18, 62-68 [DOI] [PubMed] [Google Scholar]
- Jansen R., Metzdorf R., van der Roest M., Fusani L., ter Maat A., Gahr M. (2005). Melatonin affects the temporal organization of the song of the zebra finch. FASEB J. 19, 848-850 [DOI] [PubMed] [Google Scholar]
- Jürgens U. (2009). The neural control of vocalization in mammals: a review. J. Voice 23, 1-10 [DOI] [PubMed] [Google Scholar]
- Kingsbury M. A., Kelly A. M., Schrock S. E., Goodson J. L. (2011). Mammal-like organization of the avian midbrain central gray and a reappraisal of the intercollicular nucleus. PLoS ONE 6, e20720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittelberger J. M., Bass A. H. (2013). Vocal-motor and auditory connectivity of the midbrain periaqueductal gray in a teleost fish. J. Comp. Neurol. 521, 791-812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittelberger J. M., Land B. R., Bass A. H. (2006). Midbrain periaqueductal gray and vocal patterning in a teleost fish. J. Neurophysiol. 96, 71-85 [DOI] [PubMed] [Google Scholar]
- Locascio J. V., Mann D. A. (2008). Diel periodicity of fish sound production in Charlotte Harbor, Florida. Trans. Am. Fish. Soc. 137, 606-615 [Google Scholar]
- López-Olmeda J. F., Madrid J. A., Sánchez-Vázquez F. J. (2006). Melatonin effects on food intake and activity rhythms in two fish species with different activity patterns: diurnal (goldfish) and nocturnal (tench). Comp. Biochem. Physiol. 144A, 180-187 [DOI] [PubMed] [Google Scholar]
- Lutterschmidt D. I., Wilczynski W. (2012). Sexually dimorphic effects of melatonin on brain arginine vasotocin immunoreactivity in green treefrogs (Hyla cinerea). Brain Behav. Evol. 80, 222-232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra S. K., Chattoraj A. (2006). Photoperiod, pineal photoreceptors and melatonin as the signal of photoperiod in the regulation of reproduction in fish. J. Endocrinol. Reprod. 10, 73-87 [Google Scholar]
- McKibben J. R., Bass A. H. (1998). Behavioral assessment of acoustic parameters relevant to signal recognition and preference in a vocal fish. J. Acoust. Soc. Am. 104, 3520-3533 [DOI] [PubMed] [Google Scholar]
- McKibben J. R., Bass A. H. (2001). Effects of temporal envelope modulation on acoustic signal recognition in a vocal fish, the plainfin midshipman. J. Acoust. Soc. Am. 109, 2934-2943 [DOI] [PubMed] [Google Scholar]
- Oliveira C., Garcia E. M., López-Olmeda J. F., Sánchez-Vázquez F. J. (2009). Daily and circadian melatonin release in vitro by the pineal organ of two nocturnal teleost species: Senegal sole (Solea senegalensis) and tench (Tinca tinca). Comp. Biochem. Physiol. 153A, 297-302 [DOI] [PubMed] [Google Scholar]
- Piccinetti C. C., Migliarini B., Olivotto I., Coletti G., Amici A., Carnevali O. (2010). Appetite regulation: the central role of melatonin in Danio rerio. Horm. Behav. 58, 780-785 [DOI] [PubMed] [Google Scholar]
- Pinillos M. L., De Pedro N., Alonso-Gómez A. L., Alonso-Bedate M., Delgado M. J. (2001). Food intake inhibition by melatonin in goldfish (Carassius auratus). Physiol. Behav. 72, 629-634 [DOI] [PubMed] [Google Scholar]
- Porter M. J. R., Randall C. F., Bromage N. R., Thorpe J. E. (1998). The role of melatonin and the pineal gland on development and smoltification of Atlantic salmon (Salmo salar) parr. Aquaculture 168, 139-155 [Google Scholar]
- Porter M. J. R., Duncan N. J., Mitchell D., Bromagea N. R. (1999). The use of cage lighting to reduce plasma melatonin in Atlantic salmon (Salmo salar) and its effects on the inhibition of grilsing. Aquaculture 176, 237-244 [Google Scholar]
- Reebs S. G. (2002). Plasticity of diel and circadian activity rhythms in fishes. Rev. Fish Biol. Fish. 12, 349-371 [Google Scholar]
- Remage-Healey L., Bass A. H. (2004). Rapid, hierarchical modulation of vocal patterning by steroid hormones. J. Neurosci. 24, 5892-5900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L., Bass A. H. (2007). Plasticity in brain sexuality is revealed by the rapid actions of steroid hormones. J. Neurosci. 27, 1114-1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice A. N., Bass A. H. (2009). Novel vocal repertoire and paired swimbladders of the three-spined toadfish, Batrachomoeus trispinosus: insights into the diversity of the Batrachoididae. J. Exp. Biol. 212, 1377-1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio V. C., Sánchez-Vázquez F. J., Madrid J. A. (2004). Oral administration of melatonin reduces food intake and modifies macronutrient selection in European sea bass (Dicentrarchus labrax, L.). J. Pineal Res. 37, 42-47 [DOI] [PubMed] [Google Scholar]
- Rubow T. K. (2010). Seasonal and Diel Rhythms Regulate Multistability in a Teleost Vocal Pattern Generator. PhD dissertation, Cornell University, NY, USA: [Google Scholar]
- Rubow T. K., Bass A. H. (2009). Reproductive and diurnal rhythms regulate vocal motor plasticity in a teleost fish. J. Exp. Biol. 212, 3252-3262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankov B., Gervasoni M., Scaglione F., Perego R., Cova D., Marabini L., Fraschini F. (1993). Primary pharmaco-toxicological evaluation of 2-iodomelatonin, a potent melatonin agonist. Life Sci. 53, 1357-1365 [DOI] [PubMed] [Google Scholar]
- Steiger S. S., Valcu M., Spoelstra K., Helm B., Wikelski M., Kempenaers B. (2013). When the sun never sets: diverse activity rhythms under continuous daylight in free-living arctic-breeding birds. Proc. R. Soc. B 280, 20131016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramontin A. D., Brenowitz E. A. (2000). Seasonal plasticity in the adult brain. Trends Neurosci. 23, 251-258 [DOI] [PubMed] [Google Scholar]
- Ubuka T., Bentley G. E., Ukena K., Wingfield J. C., Tsutsui K. (2005). Melatonin induces the expression of gonadotropin-inhibitory hormone in the avian brain. Proc. Natl. Acad. Sci. USA 102, 3052-3057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera L. M., De Oliveira C., López-Olmeda J. F., Ramos J., Mañanós E., Madrid J. A., Sánchez-Vázquez F. J. (2007). Seasonal and daily plasma melatonin rhythms and reproduction in Senegal sole kept under natural photoperiod and natural or controlled water temperature. J. Pineal Res. 43, 50-55 [DOI] [PubMed] [Google Scholar]
- Wan Q., Man H.-Y., Liu F., Braunton J., Niznik H. B., Pang S. F., Brown G. M., Wang Y. T. (1999). Differential modulation of GABAA receptor function by Mel1a and Mel1b receptors. Nat. Neurosci. 2, 401-403 [DOI] [PubMed] [Google Scholar]
- Wang G., Harpole C. E., Trivedi A. K., Cassone V. M. (2012). Circadian regulation of bird song, call, and locomotor behavior by pineal melatonin in the zebra finch. J. Biol. Rhythms 27, 145-155 [DOI] [PubMed] [Google Scholar]
- Weeg M. S., Land B. R., Bass A. H. (2005). Vocal pathways modulate efferent neurons to the inner ear and lateral line. J. Neurosci. 25, 5967-5974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Rucker M. G., Cassone V. M. (1996). Melatonin binding in the house sparrow song control system: sexual dimorphism and the effect of photoperiod. Horm. Behav. 30, 528-537 [DOI] [PubMed] [Google Scholar]
- Wikelski M., Tarlow E. M., Eising C. M., Groothuis T. G. G., Gwinner E. (2006). Do night-active birds lack daily melatonin rhythms? A case study comparing a diurnal and a nocturnal-foraging gull species. J. Ornithol. 147, 107-111 [Google Scholar]
- Zhdanova I. V., Wang S. Y., Leclair O. U., Danilova N. P. (2001). Melatonin promotes sleep-like state in zebrafish. Brain Res. 903, 263-268 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.