Abstract
Objective
To evaluate the efficacy and safety of intratympanic AM-101 in patients with persistent acute inner ear tinnitus after acute acoustic trauma, idiopathic sudden sensorineural hearing loss (ISSNHL), or acute otitis media.
Study Design
Prospective, double-blind, randomized, placebo-controlled study with follow-up visits on Days 7, 30, and 90.
Setting
Twenty-eight European sites (academic tertiary referral centers and private ENT practices).
Patients
248 patients aged 16 to 65 years.
Interventions
Three intratympanic injections of AM-101 (0.27 or 0.81 mg/ml) or placebo over 3 consecutive days.
Main Outcome Measures
Efficacy was assessed by changes in minimum masking level (MML; primary end point), loudness match, tinnitus loudness, tinnitus annoyance, and sleep difficulties on a 0 to 100 numerical rating scale, THI-12 questionnaire, and patient global impression of change. Safety was evaluated using the frequency of clinically relevant hearing deterioration and adverse events.
Results
The study overall failed to demonstrate a treatment benefit based on the change in MML. However, AM-101 0.81 mg/ml showed statistically significantly better improvement for tinnitus loudness, annoyance, sleep difficulties, and tinnitus impact in patients with tinnitus after noise trauma or otitis media. The subgroup of ISSNHL-related tinnitus patients did not show conclusive results. The study drug and I.T. injections were well tolerated.
Conclusion
The study established proof of concept for AM-101 in the treatment of tinnitus arising from cochlear glutamate excitotoxicity. Patient-reported outcomes seem to be more relevant and reliable efficacy measures for assessing treatment-related changes in tinnitus than psychoacoustic tests.
Key Words: Acute acoustic trauma, Cochlea, Idiopathic sudden sensorineural hearing loss, Intratympanic, Otitis Media, Therapy, Tinnitus
Tinnitus, the perception of sound without external acoustic stimulation, is a very common disorder. According to some recent estimates, approximately 25% of American adults have experienced tinnitus, with nearly 8% having frequent occurrences (1). European population studies estimate that 7% to 14% of the population has spoken to their physician about tinnitus, whereas potentially disabling tinnitus occurs in 1% to 2.4% of people (2). Tinnitus may seriously impact the ability to sleep or relax or lead to tiredness, irritation, nervousness, despair, frustration, or depression (3,4).
Many tinnitus patients are prepared to try a wide variety of treatments in the search for effective relief (5). Although approaches, such as tinnitus retraining therapy (6) or cognitive behavioral therapy (7), may provide relief for certain patients, there exists no universal standard of care for tinnitus or approved tinnitus drug (8), provoking substantial frustration among patients and physicians (9).
AM-101 (Esketamine hydrochloride gel; Auris Medical AG, Basel, Switzerland) is a small molecule noncompetitive NMDA receptor antagonist that is being developed for treatment of acute inner ear tinnitus. Cochlear NMDA receptors are located at the inner hair cell postsynapse (10) and upregulated during neosynaptogenesis after glutamate excitotoxicity (11), which can be triggered, for example, by acoustic trauma or hypoxia (12). In cochlear neosynaptogenesis, glutamate has a neurotrophic role via the activation of NMDA receptors (13). It has been proposed that during this critical process of regrowth and synaptic repair of auditory dendrites, the auditory nerve may be particularly susceptible to (aberrant) excitation via NMDA receptors (14), thus generating “phantom noise.”
It has been suggested that the initiation phase of cochlear tinnitus is dependent on NMDA receptor activity in primary auditory neurons similar to memory processes during a consolidation window (15,16). AM-101 aims to treat tinnitus in the acute stage before it becomes centralized or memorized at higher structures of the auditory system and/or brain. It is delivered in a hyaluronic acid gel formulation by intratympanic (I.T.) injection into the middle ear from where the active substance diffuses across the round window membrane into the cochlea. This approach provides for a highly targeted cochlear therapy with only minimal systemic exposure (17,18).
Clinical development with AM-101 was initiated with a randomized, double-blind, placebo-controlled phase I/II study, which enrolled 24 patients with acute tinnitus after acute acoustic trauma (AAT) or ISSNHL (19). Single doses of AM-101 were well tolerated up to the maximum tested concentration of 0.81 mg/ml, and only small traces of the active substance and its primary metabolite (<0.3 ng/ml) could be detected in blood plasma, confirming minimal systemic exposure. In addition, gradual improvement in tinnitus was observed over the 60-day observation period.
Based on these outcomes, a larger study was designed to evaluate AM-101’s efficacy and safety. The eligible patient population was broadened to include also patients with tinnitus after acute otitis media (OM). Inner ear hearing loss and tinnitus are sometimes sequelae of middle ear inflammation as presumably pathogens migrate from the middle into the inner ear; the proinflammatory cytokine IL-1β has been suggested as a trigger of glutamate excitotoxicity in various neurologic disorders (20). The dose regimen was extended from single to triple injections to address potential variability in drug exposure at the round window membrane, and the observation period was extended to 90 days to capture any continued effects.
MATERIALS AND METHODS
Study Design and Participants
This was a multicenter, double-blind, randomized, placebo-controlled, parallel-group phase II study involving 28 study sites in Germany, Belgium, Poland, and the Netherlands (ENT clinical departments as well as private practices). It was registered on ClinicalTrials.gov (NCT00860808) and conducted in compliance with the Declaration of Helsinki and the International Conference on Harmonisation and Good Clinical Practice guidelines. The study was approved by appropriate independent ethics committees and by the competent national health authorities.
Eligible participants were aged 18 to 65 years, had persistent tinnitus after AAT, ISSNHL, or acute otitis media, with onset less than 3 months ago, and an MML of 5 dB or greater sensation level (SL). The tinnitus-inducing incident had to be documented by a detailed medical report or a referral letter and was further checked by the investigator based on audiometry, otoscopy, and when taking the subject’s medical history. In those rare cases where the subject had preexisting tinnitus from another origin, the subject was not included as specific assessment of the acute tinnitus was not feasible. Exclusion criteria included tinnitus that was not completely maskable, fluctuating, or intermittent; Menière’s disease; ongoing acute or chronic otitis media or externa; any ongoing therapy known as potentially tinnitus inducing or for inner ear hearing loss or otitis media; concomitant use of any other NMDA receptor antagonist; and other tinnitus treatments for the study duration. Although depression was not expected to be a frequent comorbidity in the acute tinnitus population, it was to be excluded if judged clinically relevant by the investigator. Women who were breast feeding, pregnant, or who planned a pregnancy during the study, or women of childbearing potential who declared being unwilling or unable to practice an effective method of contraception were excluded. Written informed consent was obtained from each patient before the performance of any study-specific procedures.
Randomization and Masking
At baseline on Day 0, study participants were randomized to receive AM-101 0.27 mg/ml (“low dose”), AM-101 0.81 mg/ml (“high dose”), or placebo at a 1:1:1 ratio. The study drug formulation was identical in appearance for all AM-101 doses and placebo and revealed no differences during or after administration. The study medication was provided to study sites in identical kits and sequentially numbered with an identifier for the study site and one for each individual patient. A separate randomization sequence was generated for each study site. The randomization was blocked in groups of 3 to balance the number of patients in each treatment group without revealing the block size to study staff. Study patients and investigators remained blinded to the study drug allocation throughout the entire study.
Procedures
For each patient, the study consisted of a baseline assessment (Day 0) and 5 further study visits on Days 1, 2, 7, 30, and 90. Baseline assessments included a general physical examination, recording of medical history and demographics, vital signs, and a urine pregnancy test for women of childbearing age. Persistence of tinnitus was considered given when patients indicated that they could always hear their tinnitus when they were thinking of it in the past 2 weeks and that their tinnitus during that period occurred either all of the time, most of the time or a good bit of the time.
At each study visit, the following efficacy outcome variables were determined: MML, tinnitus loudness match, tinnitus loudness (TLQ), and tinnitus annoyance (TAQ) on a 0 to 100 numerical rating scale (NRS). In addition, the THI-12 questionnaire (21) and tinnitus-related sleep difficulties (falling asleep, lying awake, and getting back to sleep; items 5, 20, and 18 of the Iowa Tinnitus Activities Questionnaire (22)) on a 0 to 100 NRS were collected at baseline and at Day 90, and a patient global impression of change (PGIC) in tinnitus severity at Day 90. The primary efficacy end point was defined as the absolute improvement of the MML from baseline to Day 90; improvement in TLQ and TAQ were coprimary end points.
The MML and loudness match are part of the standard battery of psychoacoustic tinnitus assessments and has been proposed as useful and responsive outcome measure (23). It relates to daily experiences of quite many patients who seek to find sources of masking such as background noise, music, and so on to diminish the perception of their tinnitus (24). First, the threshold to pulsed standard speech audiometry broadband masking noise was determined using the descending method of limits with bracketing at 5 dB resolution. The measure was performed twice and results averaged. The MML was then determined with a descending method of limits, starting with presentation of the pulsed masking noise at 70-dB hearing level (HL). The midpoint of the last stimulus levels where the tinnitus was audible, respectively inaudible, was defined as the MML.
For the tinnitus loudness match a pulsed 1 kHz tone (24) was presented to patients 15 dB above hearing threshold. Patients were asked to indicate whether their tinnitus was louder or softer than the tone. The loudness was then adjusted upward or downward in 1-dB steps until the tinnitus loudness was bracketed and the second last value confirmed by a repeat measure. Both the MML and the loudness match procedures were performed 3 times, and the resulting values were averaged, provided they were all within a 5-dB range; if not, a fourth measure was performed to replace the most extreme value. Testing was conducted for the affected ear only, and separately for each ear in case of bilateral tinnitus.
For determining tinnitus loudness and annoyance, patients were presented the 2 questions: “Describe the loudness of your tinnitus right now using a scale from 0–100” and “Describe the annoyance of your tinnitus today using a scale from 0–100.” The scale was anchored at the extremes 0 = no tinnitus heard, respectively not annoying at all, and 100 = very loud, respectively very annoying. For the patient global impression of change (PGIC), patients were asked: “When comparing with the severity of your tinnitus at the beginning of the clinical trial, which was 3 months ago, how would you describe the severity of the tinnitus today?” Response categories were very much worse; much worse; minimally worse; unchanged; minimally improved; much improved; very much improved.
The hearing function was tested using pure tone audiometry (0.25, 0.5, 1, 2, 3, 4, 6, and 8 kHz) using the descending method of limits at baseline and at Days 7, 30, and 90. The primary safety end point was the frequency of clinically relevant hearing deterioration in the treated ear, defined as threshold shift of 15 dB or greater from baseline to Day 30 in any 2 contiguous test frequencies. Further safety assessments included nystagmoscopy/nystagmography, otoscopy, and tympanometry.
Study drug was administered on Days 0, 1, and 2 by I.T. injection under local anesthesia of the tympanic membrane. Approximately 0.25 ml of the study drug was gently injected into the middle ear through a small tympanotomy with the patient’s head placed in a position tilted 45 degrees toward the unaffected ear. Patients remained in their reclined or supine position for approximately 30 minutes to allow the active substance to diffuse into the cochlea. In case of bilateral tinnitus, only the worse affected ear was treated as a precautionary safety measure. The injection procedure was repeated on Days 1 and 2.
Statistical Methods
The sample size was determined based on an expected effect size of 0.5 regarding the difference in the primary end point between the high dose and the placebo group and assuming a 2-sided type I error rate of 5% and a statistical power of 80%. This resulted in a planned sample size of 79 patients per treatment group, rounded up to 240 in total.
Efficacy analyses were primarily performed on the analysis set “Valid for efficacy” (VfE), based on the “Intention to treat” principle, and secondarily on the “Per Protocol” (PP) analysis set. In general, safety analyses were performed for the analysis set “Valid for safety” (VfS), which included all patients who received at least 1 injection of the study medication. Missing values for MML, TLQ, or TAQ on Day 90 were replaced only for the VfE set by random imputation based on Day 30 values of the respective treatment group.
For continuous efficacy end points, analysis of covariance (ANCOVA) models were used including treatment group as fixed class effect, and the baseline values (Day 0) of the respective end point as covariate. For the discrete end point PGIC, the Cochran Mantel Haenszel test for trend was applied for each AM-101 group versus placebo. All secondary efficacy end points were tested using exploratory methods.
To assess whether unilateral and bilateral tinnitus cases could be analyzed together, an ANCOVA including treatment group and tinnitus laterality as main class effects, the interaction term of treatment and laterality and baseline MML as covariate was to be performed before the primary efficacy analysis. If the interaction term yielded a significant result (p < 0.10), all subsequent efficacy analyses would be restricted to the (larger) group of unilateral patients.
The frequency of patients meeting the primary safety end point was compared by treatment group with the Fisher exact test. The frequency of clinically relevant hearing loss was compared between treated and untreated contralateral ears applying McNemar’s test on symmetry.
RESULTS
The trial profile in accordance with the CONSORT statement (25) is shown in Figure 1. A total of 284 patients were screened, and 248 patients were randomized and treated. The majority of treated patients completed the study (89% to 97% across treatment groups). The most common reasons for patient discontinuation were lost to follow-up and withdrawal of consent.
FIG. 1.
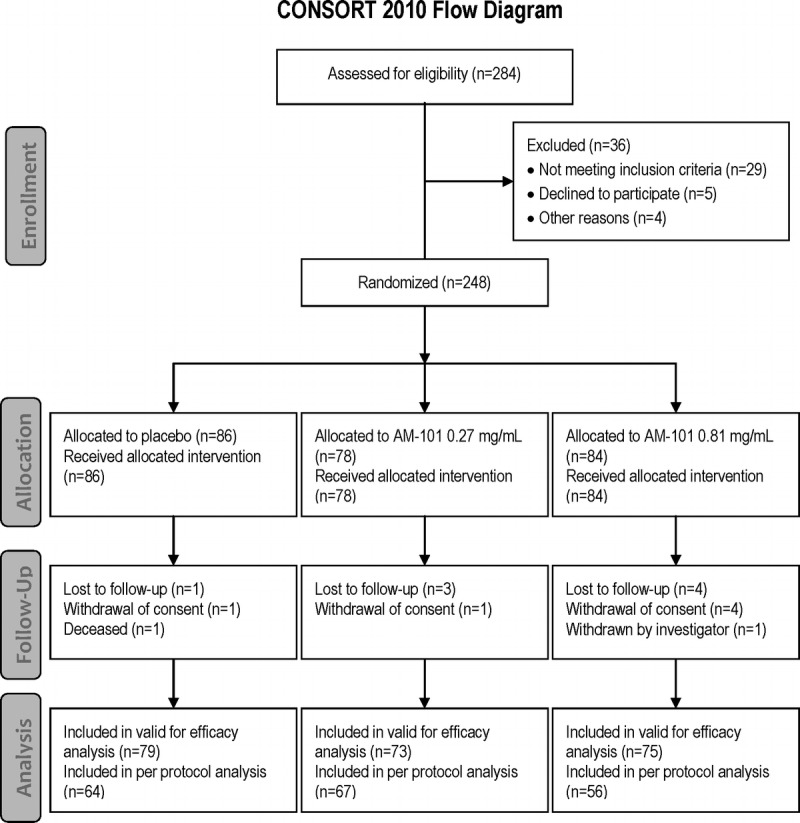
Patient flow diagram. Of the 284 patients, 248 were enrolled into the AM-101-CL-08-01 study. The most frequent reason for screening failure was by far the inability to mask the tinnitus at the stimulus limit of 70 dB and hence determine a baseline minimum masking level (MML) value. Eighty-six patients were enrolled into the placebo group, 78 into the AM-101 0.27 mg/ml group and 84 into the AM-101 0.81 mg/ml group. All of them received at least one i.t. injection of study drug and constituted the valid for safety analysis set (total of 248 patients). Seven patients (4 in the placebo group and 3 in the AM-101 0.81 mg/ml group) did not receive all injections as planned. Eight patients were lost to follow-up, 6 withdrew consent, 1 patient in the placebo group died because of severe cardiomyopathy (46 days after first dosing), and 1 patient in the AM-101 0.81 mg/ml group was withdrawn by the investigator because of ongoing tinnitus decompensation. A total of 227 patients were included in the valid for efficacy analysis set. The most common reasons for exclusion were last post baseline MML (SL) value not within allowed visit time window/no post baseline MML values available and inclusion criterion of MML greater than 5 dB sensation level not met. A total of 187 patients were included in the PP analysis set; the most frequent reasons for exclusion were lost to follow-up, lack of MML values, and use of prohibited concomitant medication.
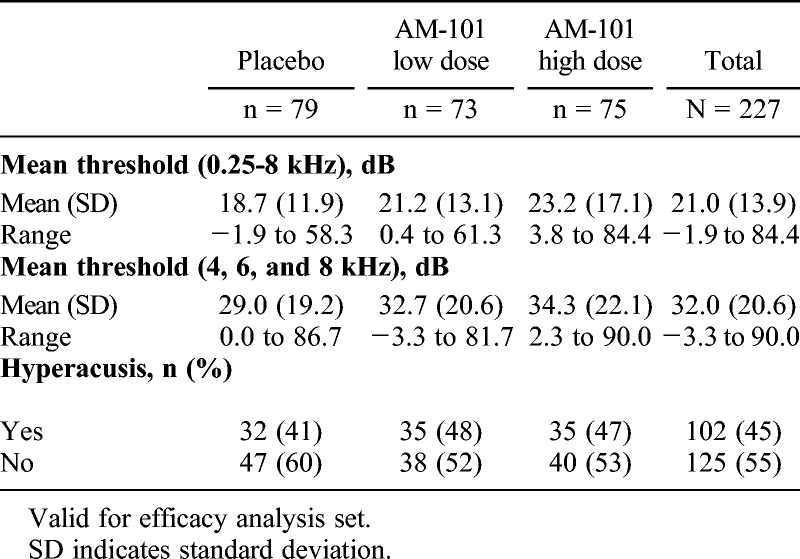
Baseline demographics for the study population (VfS set) are presented in Table 1 and tinnitus characteristics (VfE set) in Table 2. The majority of patients were male (70%), which is in line with previous data (26), and had unilateral tinnitus (79%). The most common tinnitus etiology was ISSNHL (48%), followed by AAT (39%) and OM (13%). Mean time since tinnitus onset was 61 days. As expected, patients showed hearing loss predominantly in the higher frequencies (Table 3).
TABLE 1.
Patient demographics at baseline
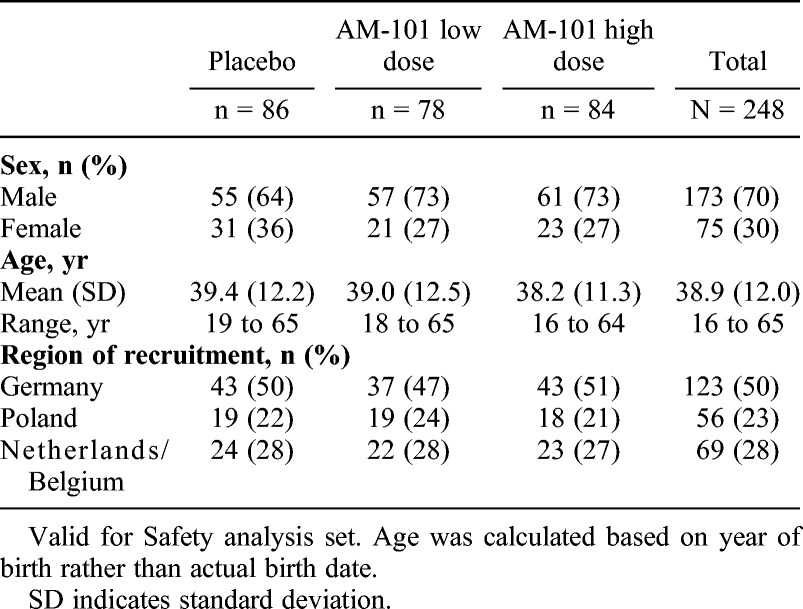
TABLE 2.
Baseline tinnitus characteristics
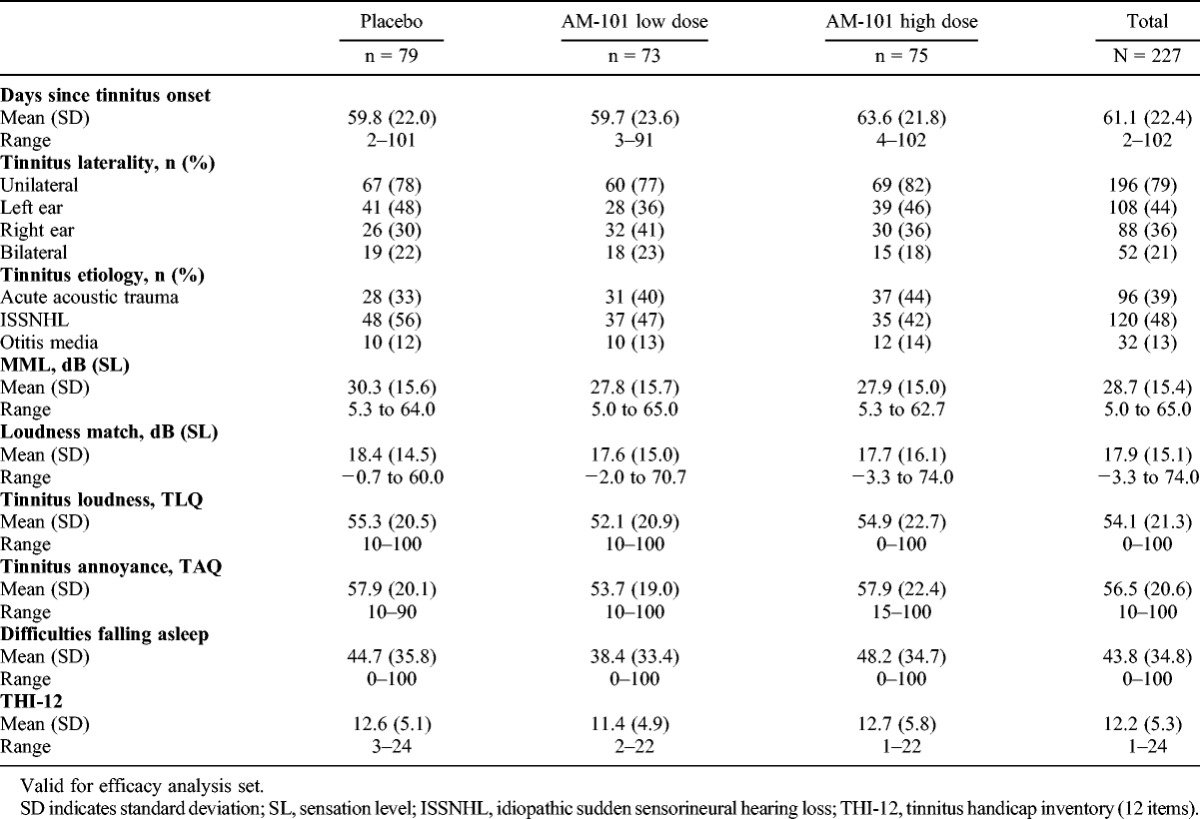
TABLE 3.
Baseline hearing characteristics (treated ear)
Because there was no statistical significance for the interaction term (p = 0.4775), unilateral and bilateral patients were analyzed together for the efficacy endpoints, with laterality considered as a subgroup. The global null hypothesis of no differences between treatment groups in the change of MML from baseline to Day 90 could not be rejected: least square means were 8.2, 7.9, and 7.9 dB (SL) for the placebo, low- and high-dose groups, respectively (p = 0.9919). The study thus failed to meet its primary efficacy end point.
However, further analysis of descriptive statistics and ANCOVA models of the primary and coprimary end points with the prespecified covariates and subgroups revealed consistent differences between changes in MML and changes in tinnitus loudness and tinnitus annoyance. In addition, tinnitus etiology and tinnitus laterality seemed to be important and interrelated influencing factors, but their baseline distribution was uneven: for example, in the placebo group ISSNHL-related tinnitus was overrepresented (56%) and AAT-related tinnitus underrepresented (32%; Table 2). ISSNHL-related cases showed no treatment effects or any influential variables in the ANCOVA models; in addition, complete tinnitus remission in placebo treated patients by Day 90 was more frequent in this etiology subgroup (22%) than in the AAT (12%) and OM (10%) subgroups. Further exploratory analysis of outcomes was therefore considered appropriate.
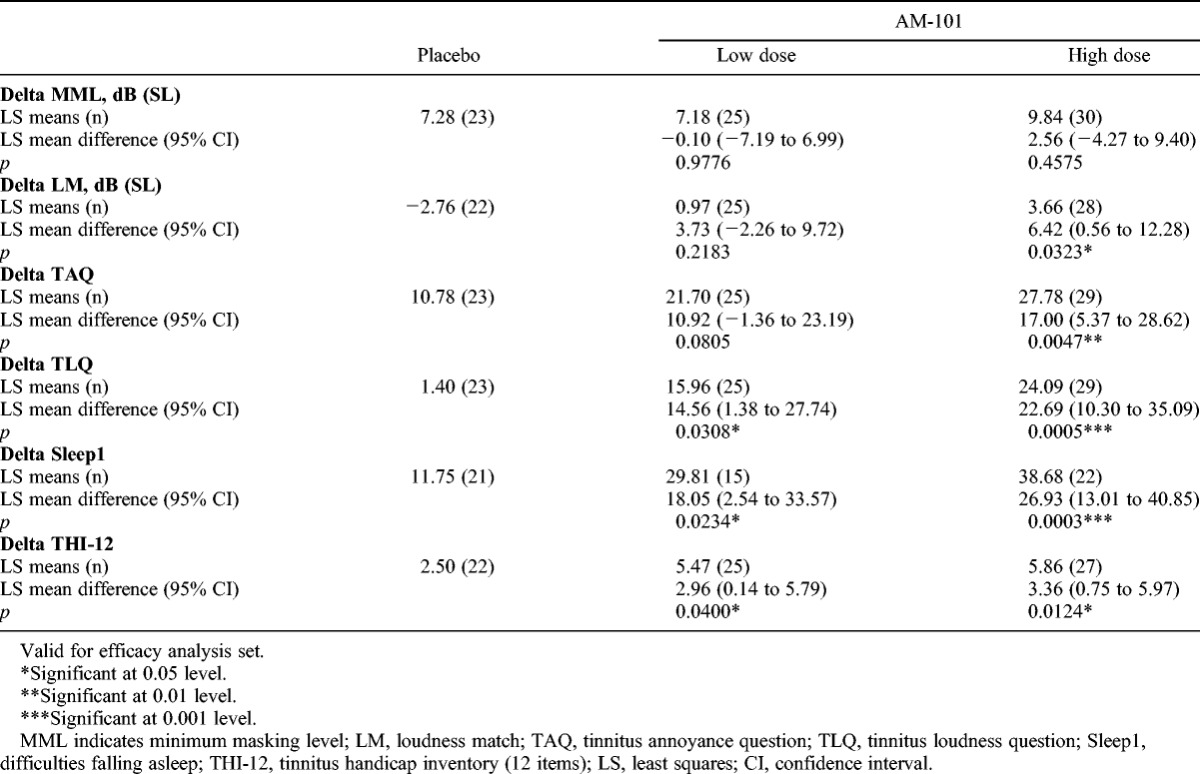
A subgroup “OM + AAT” was defined to comprise all respective patients with either OM or AAT as tinnitus onset factors. In this subgroup with known tinnitus etiology by involvement of cochlear glutamate excitotoxicity, the ANCOVA demonstrated superiority of the high dose with respect to placebo for the change in TLQ, sleep difficulties (e.g., falling asleep), and THI-12 from baseline to Day 90 (Table 4). When restricting the OM + AAT population to unilateral cases (71% of the subgroup), the treatment effects became more pronounced for the change in TLQ, sleep difficulties (e.g., falling asleep), and THI-12 (Table 5). In addition, the differences in mean changes in TAQ and loudness match also became statistically significant. The low-dose group overall showed improvement between the high-dose and the placebo groups.
TABLE 4.
Analysis of covariance results for changes in continuous efficacy variables to Day 90 in OM + AAT subgroup
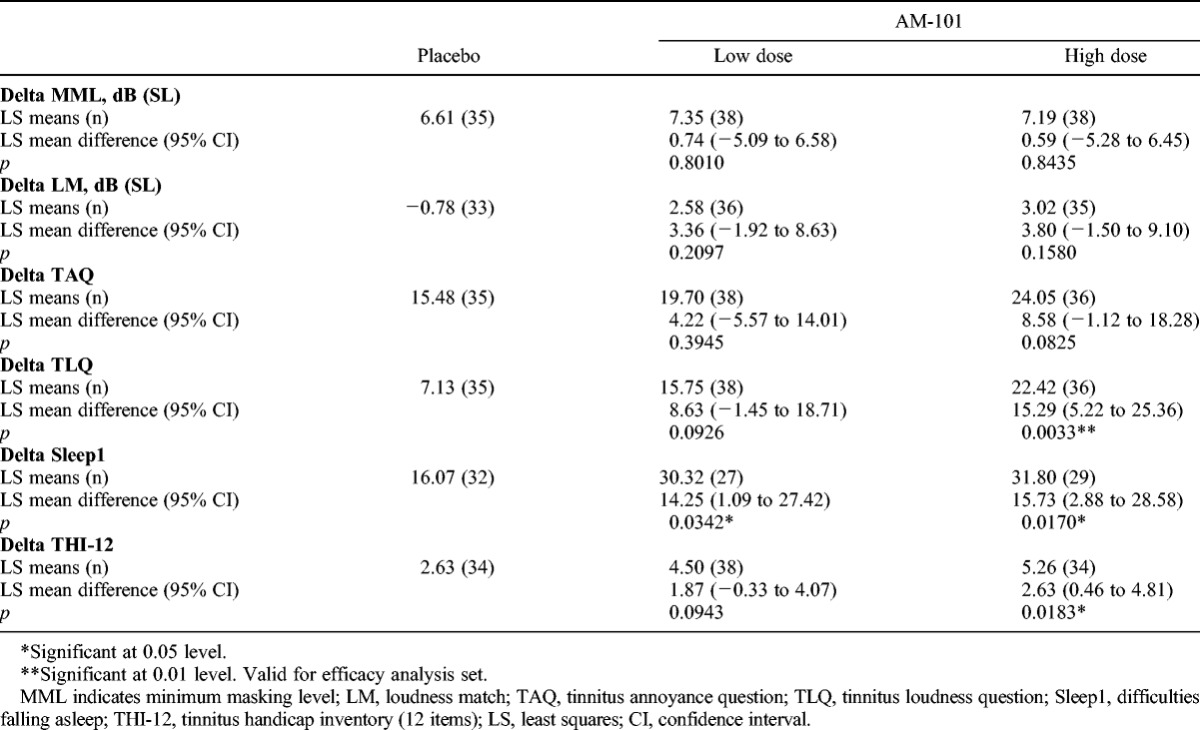
TABLE 5.
Analysis of covariance results for changes in continuous efficacy variables to day 90 in OM + AAT subgroup, unilateral cases only
Improvement in TLQ from baseline to Day 90 of at least 50% was seen for 14% of patients in the placebo group compared with 42% of patients in the high-dose group. Approximately 57% of patients in the high-dose group rated their tinnitus severity at Day 90 compared with baseline as “much improved” or “very much improved,” compared with 39% and 34% of patients in the low-dose and placebo groups. Again, differences were more pronounced among unilateral cases (64% in the high-dose group versus 44% and 35% in the low-dose and placebo group) (Fig. 2).
FIG. 2.
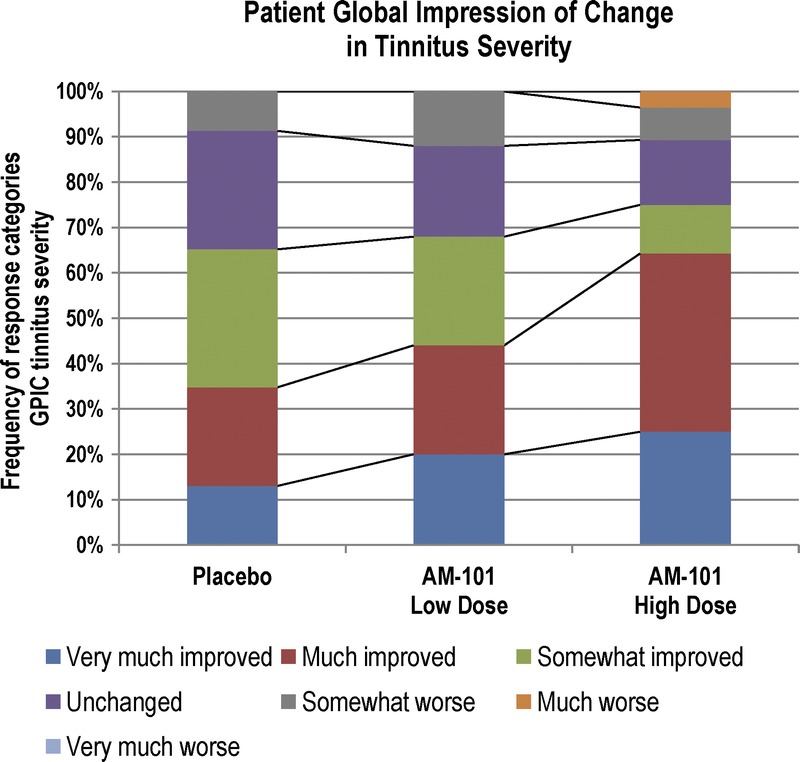
Global patient impression of change in tinnitus severity in OM + AAT subgroup. Responses by patients on Day 90 to the question “When comparing with the severity of your tinnitus at the beginning of the clinical trial, which was 3 months ago, how would you describe the severity of the tinnitus today?” Unilateral cases, n = 76.
The improvement in tinnitus was gradual over the 90-day observation period (Fig. 3). In the first days, tinnitus loudness increased slightly as the eardrum was still open and sound perception may have been temporarily altered. TLQ started to improve in the high-dose group on Day 2, before the third injection, breaking away from the 2 other groups. At Day 90, the mean tinnitus loudness in the high-dose group was 43.2% lower than at baseline (49.5% for unilateral cases). This compares with an improvement of 17.7% (10.0%) for the placebo group.
FIG. 3.
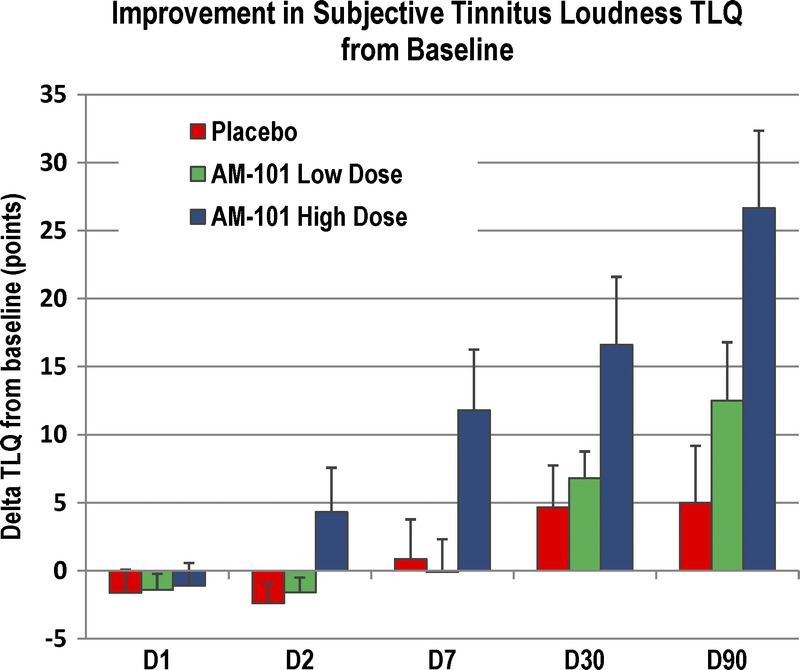
Improvement of subjective tinnitus loudness from baseline in OM + AAT subgroup. Mean absolute improvement of tinnitus loudness (TLQ) with standard error mean from the baseline D0 (before first injection) to D1 (before second injection), D2 (before third injection), and the follow-up visits at D7, D30, and D90. Unilateral cases, n = 84.
Mean hearing thresholds slightly improved for all treatment groups from baseline to Day 90 (1.4 dB in the placebo group, 0.3 dB in the low-dose group, and 2.4 dB in the high-dose group). A small transitory hearing deterioration was observed from baseline to Day 7 for the placebo and low-dose groups presumably related to the tympanotomy procedure; by Day 30, this small deterioration was fully reversed. Occurrence of clinically relevant hearing deterioration at Day 30 was observed in 1% (placebo), 4% (low dose), and 3% (high dose) of patients; overall visits, the frequency was 12%, 5%, and 8%. There was no statistically significant difference between treatment groups. The frequency of clinically relevant hearing deterioration in the treated ear differed from the untreated contralateral ear on Day 7 but not on Day 30 or 90.
Treatment-emergent adverse events were reported by similar proportions of patients across the treatment groups with no apparent clinically relevant differences in frequency, intensity, or relationship (Table 6). Local events accounted for greater than 50% of reported AEs and related mostly to anticipated transient changes in tinnitus perception and hearing after the tympanotomy. In 93% of cases, the eardrum was already fully cicatrized at Day 7. For 1 patient in the placebo group, the paracentesis was still open at Day 90; however, the patient was asymptomatic and declined a restorative intervention.
TABLE 6.
Incidence of treatment-emergent adverse events by treatment group
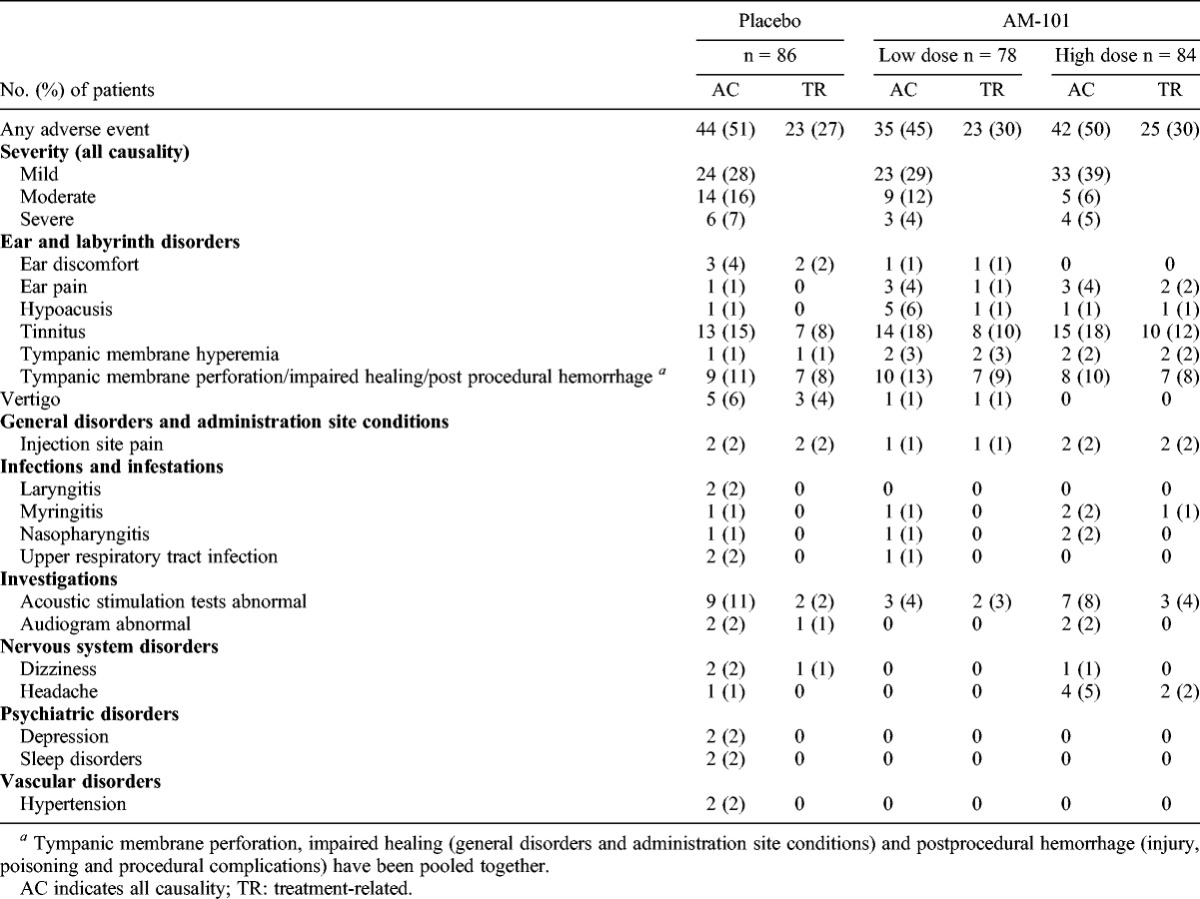
Seven patients experienced a total of 9 nonfatal serious AEs (SAEs), of which 4 occurred in the placebo group. All SAEs were considered either not related or unlikely related to treatment. In the placebo group, 1 patient died because of cardiomyopathy (considered unrelated). A total of 5 patients discontinued the study drug administration because of severe AEs: 3 in the placebo group (injection site discomfort, vertigo, and tinnitus) and 2 in the high-dose group (tinnitus and tympanic membrane hyperemia). All of these cases were resolved.
DISCUSSION
This was the first large scale randomized controlled study with a medication specifically developed for the local treatment of acute inner ear tinnitus. It demonstrated that repeated I.T. injections as well as AM-101 are well tolerated and safe. Importantly, the medication had no impact on hearing; unlike AMPA receptors, cochlear NMDA receptors are not involved in fast excitatory neurotransmission (27). Although the study failed to demonstrate confirmatory efficacy of AM-101 in the whole study population and with the MML as primary efficacy outcome variable, exploratory analyses showed dose-dependent and significant improvements in patient-reported outcomes in tinnitus of established cochlear origin. Given the gradual amelioration, which very likely reflects plasticity changes at central levels, it may well be that treatment effects continued beyond the end of study visit at Day 90 or were at least maintained.
The striking discrepancy in efficacy outcomes between the psychoacoustic measures MML and LM, and the PROs seem to arise rather from reliability and technical issues with the former than from the latter, which showed plausible, consistent, and robust outcomes. Tinnitus loudness as a direct and intuitive measure of the tinnitus symptom started to improve shortly after treatment; the amelioration was corroborated by likewise and consistent improvements in sleep difficulty, tinnitus annoyance and tinnitus impact as measured by the THI-12 questionnaire. Unilateral cases improved more than bilateral cases, where treatment effects seemed to be diluted somewhat by the untreated contralateral ear.
Both the MML and the LM showed high variability with coefficients of variation between baseline and Day 90 in the placebo group of 1.6, respectively 3.7. This may reflect measurement heterogeneity, which is difficult to achieve in multisite trials, especially in the absence of specialized instrumentation (28) but also random fluctuations in patient responses to the presented acoustic stimuli. Last, but not least, the use of speech-shaped masking noise may also have influenced MML outcomes: some cases of high-pitched bilateral tinnitus required high-intensity stimuli for masking, which may have resulted in unintended contralateral masking by virtue of bone conduction.
The lack of conclusive results in ISSNHL-related tinnitus seems to reflect not only an unexpectedly high rate of spontaneous remission but also its idiopathic nature with uncertainty over involvement of cochlear glutamate excitotoxicity. Although noncochlear origins of ISSNHL have been reported as rare (29), and circulatory disturbance, one of the etiologies most commonly proposed as onset factor, is known to trigger excitotoxicity (12), there exists no reliable diagnostic to determine the precise pathophysiology. Hence, the possibility of noncochlear origins being more prominent than expected cannot be ruled out.
In contrast, OM-related tinnitus was found to be responsive to treatment with AM-101, although the involvement of glutamate excitotoxicity had only been hypothesized. This result is in line with recent animal data, which confirmed the migration of proinflammatory cytokines such as TNF-α and IL-1β from the inflamed middle ear into the inner ear (30), respectively showed their increased expression in a model of salicylate-induced tinnitus and correlation with elevated NMDA receptor expression (31). This points to cochlear neuroinflammation as tinnitus trigger after OM.
Although AAT and OM represent just two of many different etiologies of tinnitus, and some of them may not be amenable to treatment by AM-101 because of pathomechanisms other than glutamate excitotoxicity, the results from this trial represent major progress in tinnitus research. First of all, they show that clearly defined types of tinnitus can—at least during the acute stage—be pharmacologically modulated with I.T. AM-101 and that this effect, contrary to lidocaine, is not reversible and not fraught with inacceptable side effects. Second, acute noise trauma is one of the most important triggering incidents for tinnitus (26), and it seems possible that tinnitus arising from other types of traumatic insults to the cochlea may be treatable as well. Third, as the example of OM shows, the range of treatable tinnitus etiologies seems to extend beyond traumatic insults.
Future clinical studies with AM-101 should focus on tinnitus of established cochlear origin and rely on PROs for efficacy testing. Given the favorable safety profile, bilateral treatment may be considered, and the potential for efficacy explored beyond the 3-month time window since the cutoff point for discriminating between acute and chronic tinnitus must be considered arbitrary anyway (8). The determination of the most appropriate dosing regimen may also warrant further clinical evaluation.
Acknowledgments
The authors thank Emma Coombes for proofreading the manuscript, Prof. Uwe Baumann and Dr. Richard S. Tyler for the help and support in defining and specifying audiologic assessments, and Drs. Manfred Wargenau and Frauke Friedrichs for the biostatistical contributions. The authors also thank all participating tinnitus patients who made this study possible and to all investigators and their staff: Germany: Eberhard Biesinger, Traunstein, Robert Bodlaj, Lichtenfels, Detlef Brehmer, Göttingen, Marianne Grohé, Köln, Ralf Katzbach, Kiel, Thorsten Klimek, Ravensburg, Elisabeth Kühne, Halle, Heinz Maier, Ulm, Guido Mühlmeier, Ulm, Elmar Oestreicher, Meppen, Christian Otterstedde, Frankfurt, Frank Reintjes, Braunschweig, Elfi Seeger Schellerhoff, Porta Westfalica, Gerd Schultze Seemann, Berlin, Hasselt, Norbert Staab, Schlüchtern; Belgium and the Netherlands: Tony Cox, Christian Desloovere, Leuven, Ingeborg Dhooge, Gent, Frans Gordts, Brussel, Rudolf Kuhweide, Brugge, Philippe Lefebvre, Liège, Robert Stokroos, Maastricht, Paul van de Heyning and Sarah Rabaud, Antwerp; Poland: Wojciech Domka, Rzeszów, Anna Kabacinska, Szczecin, Ireneusz Kantor, Warszawa, Andrzej Krzyzaniak, Bydgoszcz, Grazyna Lisowska, Tarnowskie Góry, Krzysztof Morawski, Warszawa.
Footnotes
Conflicts of interest and source of funding.
The study was supported in full by Auris Medical AG. T.M. is the Managing Director and a major shareholder of Auris Medical AG. The other authors declared no conflicts of interest.
P. V. D. H. and G. M. contributed equally and are joint first authors on this work.
REFERENCES
- 1. Shargorodsky J, Curhan GC, Farwell WR. Prevalence and characteristics of tinnitus among US adults. Am J Med 2010; 123: 711– 18 [DOI] [PubMed] [Google Scholar]
- 2. Vesterager V. Tinnitus—investigation and management. BMJ 1997; 7082: 728– 31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chan Y. Tinnitus: etiology, classification, characteristics, and treatment. Discov Med 2009; 42: 133– 36 [PubMed] [Google Scholar]
- 4. Stouffer JL, Tyler RS. Characterization of tinnitus by tinnitus patients. J Speech Hear Disord 1990; 55: 439– 53 [DOI] [PubMed] [Google Scholar]
- 5. Tyler RS. Patient preferences and willingness to pay for tinnitus treatments. J Am Acad Audiol 2012; 23: 115– 25 [DOI] [PubMed] [Google Scholar]
- 6. Jastreboff PJ. Tinnitus retraining therapy. Progr Brain Res 2007; 166: 415– 23 [DOI] [PubMed] [Google Scholar]
- 7. Cima RFF, Maes IH, Joore MA, et al. Specialised treatment based on cognitive behaviour therapy versus usual care for tinnitus: a randomised controlled trial. Lancet 2012; 379: 1951– 59 [DOI] [PubMed] [Google Scholar]
- 8. Langguth B, Elgoyhen AB. Current pharmacological treatments for tinnitus. Expert Opin Pharmacother 2012; 13: 2495– 509 [DOI] [PubMed] [Google Scholar]
- 9. Hall DA, Láinez MJ, Newman CW, et al. Treatment options for subjective tinnitus: self reports from a sample of general practitioners and ENT physicians within Europe and the USA. BMC Health Serv Res 2011; 11: 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ruel J, Chabbert C, Nouvian R, et al. Salicylate enables cochlear arachidonic-acid-sensitive NMDA receptor responses. J Neurosci 2008; 28: 7313– 23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Puel JL. Chemical synaptic transmission in the cochlea. Prog Neurobiol 1995; 47: 449– 76 [DOI] [PubMed] [Google Scholar]
- 12. Pujol R, Puel JL. Excitotoxicity, synaptic repair, and functional recovery in the mammalian cochlea: a review of recent findings. Ann N Y Acad Sci 1999; 884: 249– 54 [DOI] [PubMed] [Google Scholar]
- 13. D’Aldin CG, Ruel J, Assié R, et al. Implication of NMDA type glutamate receptors in neural regeneration and neoformation of synapses after excitotoxic injury in the guinea pig cochlea. Int J Dev Neurosci 1997; 15: 619– 29 [DOI] [PubMed] [Google Scholar]
- 14. Puel JL, Ruel J, Guitton M, et al. The inner hair cell synaptic complex: physiology, pharmacology and new therapeutic strategies. Audiol Neurotol 2002; 7: 49– 54 [DOI] [PubMed] [Google Scholar]
- 15. Guitton MJ. Tinnitus: pathology of synaptic plasticity at the cellular and system levels. Front Syst Neurosci 2012; 6: 1– 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guitton MJ, Dudai Y. Blockade of cochlear NMDA receptors prevents long-term tinnitus during a brief consolidation window after acoustic trauma. Neural Plast 2007; 2007: 80904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lustig LR. The history of intratympanic drug therapy in otology. Otolaryngol Clin North Am 2004; 37: 1001– 17 [DOI] [PubMed] [Google Scholar]
- 18. Meyer T. Intratympanic treatment for tinnitus: a review. Noise Health 2013; 15: 83– 90 [DOI] [PubMed] [Google Scholar]
- 19. Muehlmeier G, Biesinger E, Maier H. Safety of intratympanic injection of AM-101 in patients with acute inner ear tinnitus. Audiol & Neurotol 2011; 16: 388– 97 [DOI] [PubMed] [Google Scholar]
- 20. Fogal B, Hewett SJ. lnterleukin-1ß: a bridge between inflammation and excitotoxicity? J Neurochem 2008; 106: 1– 23 [DOI] [PubMed] [Google Scholar]
- 21. Bankstahl US, Elkin EP, Gebauer A, Görtelmeyer R. Validation of the THI-12 questionnaire for international use in assessing tinnitus: a multi-centre, prospective, observational study. Int J Audiol 2012; 51: 671– 77 [DOI] [PubMed] [Google Scholar]
- 22. Tyler RS, Gehringer AK, Noble W, et al. Tinnitus activities treatment. In: Tyler RS, ed. Tinnitus treatment: clinical protocols. New York, NY: Thieme, 2006; 116– 31 [Google Scholar]
- 23. Meikle MB, Stewart BJ, Griest SE, Henry JA. Tinnitus outcomes assessment. Trends Amplif 2008; 12: 223– 35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vernon JA, Meikle MB. Tinnitus: clinical measurement. Otolaryngol Clin of North Am 2002; 36: 293– 305 [DOI] [PubMed] [Google Scholar]
- 25. Moher D, Schulz KF, Altman D. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA 2001; 285: 1987– 91 [DOI] [PubMed] [Google Scholar]
- 26. Nicolas-Puel C, Akbaraly T, Lloyd R, et al. Characteristics of tinnitus in a population of 555 patients: specificities of tinnitus induced by noise trauma. Int Tinnitus J 2006; 12: 64– 70 [PubMed] [Google Scholar]
- 27. Ruel J, Wang J, Rebillard G, et al. Physiology, pharmacology and plasticity at the inner hair cell synaptic complex. Hear Res 2007; 227: 19– 27 [DOI] [PubMed] [Google Scholar]
- 28. Langguth B, Goodey R, Azevedo A, et al. Consensus for tinnitus patient assessment and treatment outcome measurement – Tinnitus Research Initiative meeting, Regensburg, July 2006, Progr Brain Res 2007; 166: 525– 36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rauch SD. Idiopathic sudden sensorineural hearing loss. New Engl J Med 2008; 359: 833– 40 [DOI] [PubMed] [Google Scholar]
- 30. MacArthur CJ, Pillers DA, Pang J, et al. Altered expression of middle and inner ear cytokines in mouse otitis media. Laryngoscope 2011; 121: 365– 71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hwang JH, Chen JC, Yang SY, et al. Expression of tumor necrosis factor-α and interleukin-1β genes in the cochlea and inferior colliculus in salicylate-induced tinnitus. J Neuroinflammation 2011; 8: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]


