Abstract
Estrogens inhibit stellate cell activation and fibrogenesis. Thus, gender and reproductive states may influence the degree of fibrosis in patients with nonalcoholic steatohepatitis (NASH). To investigate the association between gender, menopause, and the severity of liver fibrosis in patients with NASH, we analyzed 541 adult patients enrolled from our Duke Liver Clinics (n=338) and the Duke Metabolic and Weight Loss Surgery Program (n=203) who had a histologic diagnosis of NASH. Multiple ordinal logistic regression models were used to assess the association between gender, menopause and severity of liver fibrosis. Overall, men, pre-menopausal and post-menopausal women composed 35.1%, 28.4%, and 36.5% of the population, respectively. The mean age was 48 years and 22% had advanced fibrosis. After adjusting for covariates (enrolling site, grades of portal inflammation, and hepatocyte ballooning) and potential confounders (race, body mass index, diabetes/prediabetes, hypertension), adjusted cumulative odd ratio (ACOR) and 95% confidence interval (CI) for greater fibrosis severity was 1.4 [0.9, 2.1] (p=0.17) for post-menopausal women and 1.6 [1.0, 2.5] (p=0.03) for men, having pre-menopausal women as a reference. There was borderline interaction between gender and age group divided by age 50, the average age at menopause in the US (p=0.08): ACOR and 95% CI of having greater fibrosis severity in men compared to women was 1.8 [1.1, 2.9] for patients with age <50 years (p=0.02) and 1.2 [0.7, 2.1] for patients with age ≥ 50 years (p=0.59).
Conclusion
Men are at a higher risk of having more severe fibrosis compared to women before menopause, while post-menopausal women have a similar severity of liver fibrosis compared to men. These findings may be explained by the protective effects of estrogen against fibrogenesis.
Keywords: nonalcoholic fatty liver disease, histologic severity, sex difference, estrogen
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a spectrum of liver disorders ranging from simple hepatic steatosis to inflammation, fibrosis, and cirrhosis. The prevalence of diabetes, obesity and metabolic syndrome have been rapidly increasing in the US.1 The prevalence of NAFLD, the hepatic manifestation of insulin resistance, has been increasing accordingly in a parallel way.2 NAFLD is the most common liver disease in the US and the disease burden of NAFLD is growing rapidly. The prevalence of NAFLD has been estimated to be around 10–30%.3 However, recent large single center prospective study showed that the prevalence of NAFLD and NASH was 46%, and 12.2%, respectively.4 Moreover, NAFLD was reported to be the third most common cause of the liver cancer in the US.5, 6
The majority of patients with NAFLD have simple hepatic steatosis (fat >5% of liver weight) and these patients are asymptomatic and do not have increased mortality.7 However, some patients with NAFLD present with nonalcoholic steatohepatitis (NASH), which was shown to increase overall mortality8. Necroinflammation in the liver in patients with NASH can contribute to hepatic fibrogenesis, which can progress to cirrhosis. Age, diabetes, and obesity were reported to be associated with severe liver fibrosis.9 Among the histologic features of NAFLD, hepatocyte ballooning and portal inflammation have been shown to be strong predictors of advanced fibrosis10, 11 Knowledge on factors modifying the disease progression of NAFLD, however, remains insufficient.
Women after menopause have increased risk of insulin resistance, hyperlipidemia, and visceral fat accumulation, all of which are known risk factors for NAFLD.12 Previous epidemiological studies showed that gender and menopause impact the prevalence and incidence of NAFLD.13 During reproductive age, the prevalence of NAFLD is higher in men compared to women, while, after menopause, the protective effect in women appears to be eliminated.14,15 Hormone replacement therapy (HRT) was shown to be protective against having NAFLD after menopause.14, 16 In patients with chronic hepatitis C, post-menopausal women are associated with an increased risk of having advanced fibrosis compared to pre-menopausal women and hormone replacement therapy was protective against hepatic fibrosis.17 The protective effect of pre-menopausal women or HRT against fibrogenesis in hepatitis C virus (HCV) related liver disease appears to be mediated by estrogen. Estrogen was shown to prevent stellate cell activations in-vitro and regression of fibrogenesis in vivo.18 Currently, how gender and reproductive state impact the severity of fibrosis in patients with NAFLD remains largely unknown.
We theorize that pre-menopausal women are at a decreased risk of fibrosis progression compared to men, while post-menopausal women are at a similar risk for fibrosis progression as men due to the protective effect of estrogen among adult women patients with NAFLD during their reproductive age. We aimed to assess, in adult patients with NASH, whether gender and menopause are associated with the severity of fibrosis after taking into account other factors affecting the severity of liver fibrosis.
Methods
Study design and data source
This is a cross sectional study designed to investigate the effect of gender and menopause on the severity of liver fibrosis in patients with NASH. We used a data set from Duke University Health System (DUHS) NAFLD Clinical Database, which is a prospective open-enrolling database established in 2007 to collect clinical and research information from patients with NAFLD. The detailed design of this database project has been described in our previous publication.19 Briefly, patients with clinical suspicion of NAFLD and age of 18 or older who had clinical indications of liver biopsy were approached and enrolled through Duke Liver Clinic and Duke Metabolic and Weight Loss Surgery Program. All the liver biopsy slides were reviewed and scored by hepatopathologists at Duke University using the NASH clinical research network (CRN) scoring system.20 The DUHS NAFLD clinical database project was approved by the Duke University Institutional Review Board, and provided de-identified data sets to conduct this analysis.
Study population
This study included a total of 541 patients with histological diagnosis of NASH, age of 18 or over, and being enrolled in the DUHS NAFLD clinical database between January 2007 and April 2010. Histologic diagnosis of NASH was defined as follows using the histologic scores recorded as described earlier: 1) steatosis (≥ grade 1) accompanied by hepatocyte ballooning (≥ grade 1) or lobular inflammation (≥ grade 2) or 2) steatosis (≥ grade 1) accompanied by fibrosis (≥ stage 1) with any lobular inflammation (≥ grade 1) or hepatocyte ballooning (≥ grade 1). Patients with a history of alcohol abuse or significant alcohol use (more than 14 serving per week for men and more than 7 serving for women), serologic or histologic evidence of co-existing other diseases (viral hepatitis, primary biliary cirrhosis, autoimmune hepatitis, hemochromatosis, Wilson´s disease, or alpha-1 antitrypsin deficiency) were excluded in our study.
Liver histology
All liver biopsy specimens were stained with hematoxylin-eosin and Masson trichrome stains and reviewed by the hepatopathologists using a standardized scoring form created based on the NASH CRN scoring system.20
The primary outcome of this study was the severity of hepatic fibrosis. Stage 3 and 4 were considered as advanced fibrosis and combined as a single category. Histologic degrees of ballooned hepatocytes and portal inflammation have been strongly associated with severity of fibrosis among patient with NASH21. The clinical associations have been endorsed by previous animal experiments which mechanistically linked ballooned hepatocyte/necroinflammation and portal inflammation to fibrogenesis22–24. Based on the current understanding, to investigate the potential impact of gender/menopause on fibrogenesis and/or fibrosis regression, we treated grades of hepatocyte ballooning and portal inflammation as covariates in the models and assessed potential associations between fibrosis severity and gender/menopause at a given degree of necroinflammation/portal inflammation.
Gender and reproductive variable
The primary predictor in this study was the gender/menopause classification: men, pre-menopausal women, and post-menopausal women. Menopausal state was classified based on self-reported reproductive information and history of oophorectomy, which were available via the DUHS NAFLD clinical database. When self-reported reproductive information or history of oophorectomy was not available (N=49 of the 541 subjects), an age of 50 years, the average age at menopause in US, was used to define menopausal state.25
Other study variables
Clinical information was collected at the time of liver biopsy using standardized questionnaires and medical chart review as a part of the DUHS NAFLD Clinical Database project. Other study variables analyzed in this study include enrollment site (Duke Liver Clinic vs. Duke Metabolic and Weight Loss Surgery Program), age, race, body mass index (BMI), smoking history, alcohol consumption, and the presence of diabetes mellitus, prediabetes, and hypertension. Information on alcohol consumption was collected using a self-reported questionnaire (i.e., averaged quantity and frequency) and expressed as average servings per week. Self-reported smoking history was classified into current smokers vs. others (including past smokers). Medication lists including estrogen were collected using standardized questionnaires and medical chart review. The presence of co-morbid conditions was defined by combining the physician’s diagnosis, medication history, both of which were obtained via chart review and the standardized questionnaires, and relevant laboratory data obtained within 30 days from the date of liver biopsy. Diabetes mellitus was defined by fasting blood glucose of ≥126mg/dl, blood glucose at 2 hours (75g glucose tolerant test) of ≥ 200mg/dl, hemoglobin A1c of ≥ 6.5%, physician’s diagnosis and/or the presence of any diabetic treatments. Prediabetes was defined by fasting blood glucose of ≥ 100 mg/dl and < 126mg/dl, blood glucose at 2 hours (75g glucose tolerant test) of ≥140mg/dl and < 200mg/dl, and/or the physician’s diagnosis. Diabetes mellitus and prediabetes were analyzed as a combined variable. Hypertension was defined by physician’s diagnosis and/or usage of anti-hypertensive.
Statistical analyses
Data were reported as the mean ± standard deviation for continuous variables and as a percentage for categorical variables. Clinical characteristics of the study population were compared among the gender/menopause categories (i.e., men, pre-menopausal women, and post-menopausal women) by using analysis of variance (ANOVA) for continuous variables or chi-square test for categorical variables. As our study population contains two populations enrolled at different sites, the variable of enrolled site was first assessed for potential interaction in the analyses. When no interaction was noted, the variable of enrolled site was included in the models as a covariate. Otherwise, separate models were developed for enrollment sites.
To assess the associations between gender/menopause categories and fibrosis severity, two different modeling approaches were used, taking into consideration enrollment site, grades of hepatocyte ballooning and portal inflammation, age, race, BMI, diabetes mellitus/prediabetes, and hypertension: recursive partitioning conditional inference tree model and multiple ordinal logistic regression model (MOLR).
The former, a non-parametric decision tree model, was used to identify factors that best classify fibrosis severity in a hierarchal manner among all the above-listed variables26. Conditional inference trees allow for complex interaction among variables and work as follows: 1) tests the global null hypothesis of independence between predictors and outcome, and implements a binary split for the selected variable with strongest association with response, 2) stop if hypothesis cannot be rejected; otherwise repeat step 1).
In the MOLR analysis, fibrosis stage was set as an outcome, the gender and menopause classification as the primary predictor, and grades of hepatocyte ballooning and portal inflammation and the enrolled sites as covariates. The variables of race, BMI, diabetes mellitus/prediabetes, and hypertension were also included in the models as potential confounders. Significance of the associations was determined using likelihood ratio tests. Magnitude of association was expressed as adjusted cumulative odds ratio (ACOR) and 95% confidence interval (CI), computed using pre-menopausal women as a reference group.
Further, in order to assess whether gender differently affects the severity of fibrosis before and after menopause, we developed a model including an interaction term (gender * age of <50 or ≥50) using an age cut-off of 50 years, the average age at menopause in the US25. Separate models for age < and ≥50 were also developed using the above-mentioned variables. In order to investigate the potential benefit of HRT against liver fibrosis, association between HRT and severity of liver fibrosis was assessed in post-menopause women.
Statistical analyses were performed using JMP statistical software version 9.0 (SAS Institute Inc., Cary, NC) and R version 2.13.1 (http://www.r-project.org/), and considered differences statistically significant when the p-value(s) were less than 0.05. P-values have not been adjusted for multiple comparisons.
Results
Patients Characteristics
Between January 2007 and April 2010, there were 722 patients with NAFLD who were enrolled in the database study and whose NAFLD histologic scores were available. Three quarters of the patients (N= 541) were classified as NASH according to our study definition and served our study population. Among the 541 patients, 338 patients (62%) were enrolled at Duke Liver Clinic while 203 patients (38%) at Duke Metabolic and Weight Loss Surgery Program. Clinical characteristics of the study population are summarized in Table 1. Overall, men, pre-menopausal and post-menopausal women composed 35%, 28%, and 37% of the population.
Table 1.
Clinical Characteristics of the Study Population
| Pre-menopausal women (N=153) |
Post- menopausal women(N=199) |
Men (N=189) |
P value | |
|---|---|---|---|---|
| Age, years (SD) | 40.1[7.8] | 56.0 [7.4] | 45.9 [11.7] | <0.01 |
| Enrolling site, Metabolic and Weight Loss surgery % | 84 (54.9 %) | 68 (34.2 %) | 51 (27.0 %) | <0.01 |
| Race, Black | 28 (18.3 %) | 19 (9.6 %) | 11 (5.8 %) | <0.01 |
| BMI, kg/m2 | 44.6+10.6 | 39.2+9.4 | 37.6+8.4 | <0.01 |
| Diabetes/impaired glucose tolerance, % | 76 (49.7 %) | 115 (57.8 %) | 82 (43.4 %) | 0.02 |
| Hypertension, % | 94 (61.4 %) | 159 (79.9 %) | 133 (70.4 %) | <0.01 |
| Alcohol consumption*, servings per week | 1.11±0.13 | 0.41±0.12 | 0.23±0.15 | <0.0001 |
| Current smoking* | 7 (6.0%) | 5 (3.5%) | 3 (3.8%) | 0.59 |
| Hepatocyte ballooning | <0.01 | |||
| Grade 0 | 57(38.0 %) | 43(21.7 %) | 43(22.8 %) | |
| Grade 1 | 59(39.3 %) | 90(45.5 %) | 112(59.3 %) | |
| Grade 2 | 34 (22.7 %) | 65 (32.8 %) | 34 (18.0 %) | |
| Lobular inflammation | 0.93 | |||
| Grade 0 | 4 (2.7 %) | 3 (1.5 %) | 3 (1.6 %) | |
| Grade 1 | 103 (68.2 %) | 138 (70.4 %) | 132 (71.0 %) | |
| Grade 2–3 | 44 (29.1 %) | 55 (28.1 %) | 51 (27.4 %) | |
| Steatosis | <0.01 | |||
| Grade 1 | 62 (40.5%) | 99 (49.8 %) | 72 (38.1 %) | |
| Grade 2 | 50 (32.7 %) | 60 (30.2 %) | 88 (46.6 %) | |
| Grade 3 | 41 (26.8 %) | 40 (20.1 %) | 29 (15.3 %) | |
| Portal inflammation | 0.06 | |||
| Grade 0 | 86 (55.7%) | 105 (53.8%) | 121 (65.0%) | |
| Grade 1 | 66 (44.3%) | 90 (46.2%) | 66 (35.0%) | |
| Fibrosis | 0.02 | |||
| Stage 0 | 17 (11.1%) | 18 (9.1%) | 18 (9.5%) | |
| Stage 1 | 77 (50.3%) | 70 (35.2%) | 70 (37.0%) | |
| Stage 2 | 37 (24.2%) | 56 (28.1%) | 59 (31.2%) | |
| Stage 3–4 | 22 (14.4 %) | 55 (27.6 %) | 42 (22.2 %) |
Information on alcohol consumption was missing in 207 subjects while information on smoking status was missing in 201 subjects. The variables were not considered in further analyses due to the high frequency of the missing data.
Mean age of the group was the highest in post-menopausal women (56±7 years), followed by men (46±12 years) and pre-menopausal women (40±8 years) (p<0.01). Compared to the post-menopausal women and men, premenopausal women had a higher prevalence of Black race (p<0.01) and higher BMI (p<0.01) and were more frequently enrolled at Duke Metabolic and Weight Loss Surgery Program (p<0.01). On the other hand, the proportion of the patients with diabetes/prediabetes (p=0.02) and hypertension (p<0.01) was higher in post-menopausal women. Stage of hepatic fibrosis (p=0.02), grades of hepatocyte ballooning (p<0.01) and steatosis (p<0.01) were significantly different among the gender/menopause categories.
Relationship of fibrosis severity to grades of other histologic features and clinical variables
After adjusting for the site of enrollment, grade of hepatocyte ballooning, lobular inflammation, steatosis, and portal inflammation had positive associations with the severity of liver fibrosis.(Table 2) Among histological variables, the grade of portal inflammation and hepatocyte ballooning were independently associated with the severity of liver fibrosis (Supplementary table 1). In addition to histologic variables, age, diabetes/prediabetes, and hypertension had positive associations with the severity of liver fibrosis while black race had an inverse association with the severity of liver fibrosis, independent of the site of enrollment.
Table 2.
Relationship of fibrosis stage to other histologic features and clinical variables -adjusted for enrolled sites
| COR | 95% CI | P value | |
|---|---|---|---|
| Hepatocyte ballooning | <0.01 | ||
| Grade 0 | - | - | |
| Grade 1 | 1.54 | 1.03–2.31 | |
| Grade 2 | 5.14 | 3.16–8.44 | |
| Lobular inflammation** | <0.01 | ||
| Grade 0 | - | - | |
| Grade 1 | 4.65 | 1.18– 18.75 | |
| Grade 2–3 | 7.43 | 1.85–30.69 | |
| Steatosis | 0.05 | ||
| Grade 1 | - | - | |
| Grade 2 | 1.13 | 0.79–1.60 | |
| Grade 3 | 1.66 | 1.10–2.50 | |
| Portal inflammation** | <0.01 | ||
| Grade 0 | - | - | |
| Grade 1 | 2.90 | 2.09–4.03 | |
| Age (per 5 year) | 1.16 | 1.05–1.22 | <0.01 |
| Race, Black | <0.01 | ||
| No | - | - | |
| Yes | 0.44 | 0.26–0.72 | |
| BMI, kg/m2 | 1.01 | 1.01–1.03 | 0.21 |
| Diabetes/Prediabetes | <0.01 | ||
| No | - | - | |
| Yes | 2.10 | 1.53–2.87 | |
| Hypertension | <0.01 | ||
| No | - | - | |
| Yes | 1.73 | 1.22–2.44 |
All the above analyses were performed with the adjustment for enrolled site.
: There was significant site-interaction (P<0.01). For portal inflammation, COR (95%CI) of grade 1 vs. grade 0 in having more advanced fibrosis was 1.47 (0.87–2.51, P=0.15) in the Metabolic and Weight Loss Surgery Program and 4.52 (2.94–7.03, P<0.01) in Duke Liver Clinic. For lobular inflammation, COR (95%CI) of grade 1 vs. grade 0 in having more advanced fibrosis was 12.00 (1.75–100.18, P<0.01) in the Metabolic and Weight Loss Surgery Program and 2.16 (0.32–15.56, P=0.43) in Duke Liver Clinic while COR of grade 2–3 vs. grade 0 was 6.55 (0.88–58.32, P=0.07) in the Metabolic and Weight Loss Surgery Program and 4.78 (0.69–35.13, P= 0.11) in Duke Liver Clinic.
Relationship of fibrosis severity to gender, menopause, and estrogen replacement therapy
After adjusting for the site of enrollment, grade of portal inflammation, and hepatocyte ballooning, the gender/menopause categories were significantly associated with fibrosis stage (p<0.04) and post-menopausal women and men had 1.6 [95%CI, 1.0–2.4], 1.7 [95%CI, 1.1–2.6] -fold increased risk of having greater severity of liver fibrosis than pre-menopausal women, respectively. This suggests that at any given degree of hepatocyte ballooning and portal inflammation, post-menopausal women and men have 60% to 70% increased risk of having a more advanced fibrosis stage when compared to pre-menopausal women (Table 3). When black race, BMI, diabetes/prediabetes, and hypertension were added to the model, there was a trend toward the association between the gender/menopause categories and fibrosis stages (p=0.10) and post-menopausal women and men had 1.4 [95%CI, 0.9–2.1], 1.6 [95%CI, 1.0–2.5], -fold increased risk of having greater severity of liver fibrosis than pre-menopausal women. (Table 3) After excluding the 49 women whose reproductive information was not available, we ran the full model. The association between the gender/menopause categories and fibrosis stages was consistent and even slightly stronger (p=0.05); post-menopausal women and men had 1.5 [95%CI, 1.0–2.4], 1.8 [95%CI, 1.1–2.9], -fold increased risk of having greater severity of liver fibrosis than pre-menopausal women. (Supplementation table 2)
Table 3.
Association between gender/menopause and fibrosis severity with and without adjusting for other variables.
| Model 1 | P value |
Model 2 | P value |
Model 3 | P value |
|
|---|---|---|---|---|---|---|
| COR [95% CI] | COR [95% CI] | COR 95% CI | ||||
| Gender and menopause | 0.03 | 0.04 | 0.10 | |||
| Pre-menopausal women | - | - | - | - | ||
| Men | 1.36 [0.91–2.03] | 1.66 [1.09–2.54] | 1.62 [1.04–2.53] | |||
| Post-menopausal women | 1.70 [1.15–2.52] | 1.56 [1.03–2.35] | 1.35 [0.88–2.07] | |||
| Hepatocyte ballooning | <0.01 | <0.01 | ||||
| Grade 0 | - | - | - | |||
| Grade 1 | - | 1.65 [1.08–2.51] | 1.48 [0.97–2.26] | |||
| Grade 2 | - | 4.73 [2.87–7.85] | 4.15 [2.50–6.94] | |||
| Portal inflammation | <0.01 | <0.01 | ||||
| Grade 0 | - | - | - | |||
| Grade 1 | - | 2.68 [1.91–2.68] | 2.85 [2.02–4.05] | |||
| Enrolling site Metabolic and Weight Loss Surgery | <0.01 | <0.01 | 0.02 | |||
| No | - | - | - | |||
| Yes | 0.51 [0.37–0.71] | 0.61 [0.42–0.89] | 0.56 [0.35–0.91] | |||
| Race, Black | <0.01 | |||||
| No | - | - | ||||
| Yes | - | - | 0.38 [0.22–0.65] | |||
| BMI, kg/m2 | - | - | 1.01 [0.99–1.03] | 0.33 | ||
| Diabetes/Prediabetes | <0.01 | |||||
| No | - | - | ||||
| Yes | - | - | 2.17 [1.54–3.08] | |||
| Hypertension | 0.30 | |||||
| No | - | - | ||||
| Yes | - | - | 1.23 [0.84–1.80] |
Model 1: adjusted for the site of enrollment
Model 2: adjusted for the site, grade of portal inflammation, hepatocyte ballooning
Model 3: adjusted for the site, grade of portal inflammation, hepatocyte ballooning, black race, BMI, diabetes/prediabetes, and hypertension.
Age was not included in the multivariate model due to the strong correlation between menopause and age
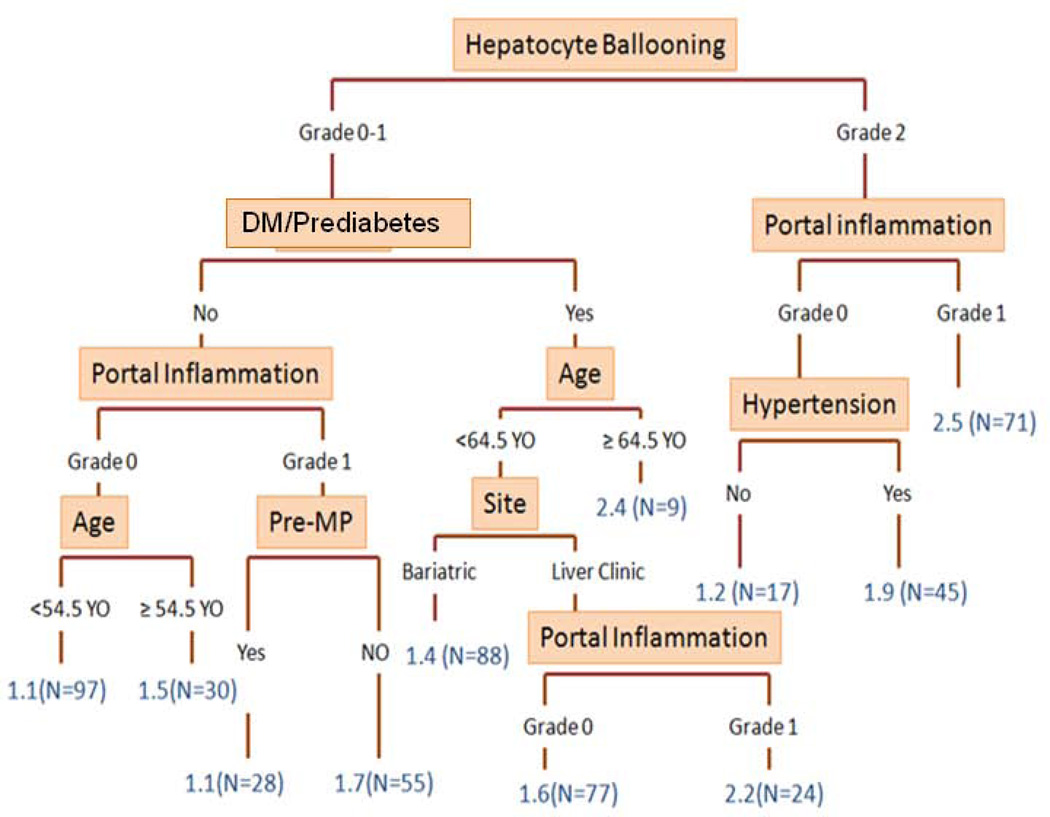
The result of the conditional inference tree model is summarized in Figure 1. The model identified the category of pre-menopausal women vs. others as a significant factor in classifying the severity of fibrosis in patients with NASH, along with hepatocyte ballooning, diabetes/prediabetes, portal inflammation, age, hypertension, and enrolling sites (Figure 1).
Figure 1. Conditional Inference Tree Model.
The figure demonstrates a decision tree developed with the same set of the variables used for ordinal logistic regression models. In the tree, variables with the strongest association fibrosis severity were split in a hierarchical manner. Detailed methodological explanation and relevant references were provided in the method section.
Numbers in blue: median fibrosis stage in the groups
DM: diabetes mellitus; Pre-MP: pre-menopausal women
Further, to assess whether gender would differentially affect the severity of fibrosis before and after the age 50 years (the average age of menopause in the US), we developed a model including age-gender interaction term. There was a borderline interaction between gender and the age groups in the relationship to fibrosis stage (p=0.08). To compare the different gender effect before and after age 50 years, we developed separate models as shown in Table 4. Before age 50, men had 1.8 [95%CI, 1.1–2.9; p=0.02]- fold increased risk of having greater severity of fibrosis than women after adjusting for the site of enrollment, grade of portal inflammation, and hepatocyte ballooning, black race, BMI, diabetes/prediabetes, hypertension, and age. In contrast, after age 50, the protective effect observed in women appeared to be eliminated (1.2 [95%CI, 0.7–2.0; p=0.59]. Of note, 16% (N=31) of postmenopausal women were <50 years old, while 11% (N=16) of pre-menopausal women were ≥ 50 years old in this population. After removing the 47 subjects, overall results were essentially the same with smaller p value of 0.05 for interaction term between gender and age groups. (Supplementary table 3)
Table 4.
Gender and severity of liver fibrosis: subgroup analysis according to the age above or below 50 years old
| <50 years | ≥ 50 years | |||
|---|---|---|---|---|
| Adjusted COR & 95%CI |
P value | Adjusted COR & 95%CI | P value | |
| Women | - | - | ||
| Men | 1.77 [1.08–2.90] | 0.02 | 1.17 [0.67–2.04] | 0.59 |
Models were adjusted for the site, grade of portal inflammation, hepatocyte ballooning, black race, DM/Prediabetes, and age.
Interaction of gender*age group (< and ≥ average age of menopause, 50 years old): p=0.08. P-values: likelihood ratio tests
In this cohort, 16% (N=31) of postmenopausal women were <50 years old, while 11% (N=16) of pre-menopausal women were ≥ 50 years old.
Furthermore, the effect of age ≥50 was assessed in men and women separately. After adjusting for the covariates (enrolling site, degrees of portal inflammation and hepatocyte ballooning), age ≥50 was associated with a significantly increased risk of having more advanced fibrosis only among women (ACOR=1.8 [1.2, 2.7], p<0.01) but not men (ACOR=1.0[0.6, 1.8], p=0.89). After adding the other potential confounders in the model (age, black race, BMI, diabetes/prediabetes, and hypertension), results remained similar: there was a trend that age of age ≥50 was associated with an increased risk of having more advanced fibrosis only among women (ACOR=1.5 [1.0, 2.4], p=0.06) but not men (ACOR=0.9 [0.5, 1.7], p=0.80).
As an exploratory analysis, we examined the effect of estrogen replacement therapy on fibrosis severity among post-menopausal women. A total of 23 out of 199 post-menopausal women (12%) were under estrogen replacement therapy. After adjusting for the covariates (the site of enrollment, grade of portal inflammation, and hepatocyte ballooning), estrogen replacement was associated with 50% risk reduction although the association did not reach statistical significance (ACOR= 0.5; 95%CI:0.2–1.2; p=0.11). After adding the other potential confounders in the model (age, black race, BMI, diabetes/prediabetes, and hypertension), ACOR did not change significantly: 0.6; 95%CI, 0.3–1.4; p=0.23.
Discussion
We have performed a cross-sectional study investigating the effect of gender and menopause on the severity of liver fibrosis using a large single center prospective database that included patients with histologic diagnosis of NASH. To address the impact of gender/menopause on hepatic fibrosis, we applied a hypothesis-driven analytic approach to adjust for two strong histologic predictors of advanced fibrosis (i.e., hepatocyte ballooning and portal inflammation), both of which have been mechanistically linked to fibrogenesis.21–23 In this study, we showed that post-menopausal women and men were associated with an increased risk of having greater severity of liver fibrosis than pre-menopausal women, at any given degree of hepatocyte ballooning and portal inflammation. The conditional inference tree models also identified pre-menopausal women (vs. others) as a significant factor in classifying the severity of fibrosis in patients with NASH. There was a trend for an age-gender interaction; before age 50, men had an increased risk of having severe fibrosis compared to women, while after the age 50, the protective effect observed in women was markedly eliminated. Further, the age effect (age of ≥50) on fibrosis severity appeared to be observed among women, but not men. Lastly, although underpowered, estrogen replacement among post-menopausal women was associated with a 50% decreased risk of having more advanced fibrosis compared to no estrogen replacement (although statistical significance was not reached). Taken together, these findings suggest that, at any given degree of hepatocyte ballooning and portal inflammation, pre-menopausal women (or age younger than 50 years) are protected from having more advanced fibrosis, compared to men although this protective effect seems to be eliminated after the age of menopause. And estrogen replacement among post-menopausal women appeared to be protective against developing more advanced fibrosis. Collectively, our findings support the aforementioned study hypothesis and suggest protective estrogen effects on hepatic fibrosis among patients with NASH.
The protective effect of estrogen was demonstrated particularly in patients with HCV. A single center prospective study with a total of 251 women with HCV showed that the menopause and hormone replacement therapy affect the severity of liver fibrosis: post-menopausal women were more likely to have moderate-severe liver fibrosis (OR: 3.7, p<0.01) compared to pre-menopause women but the probability of moderate-severe fibrosis was lower for post-menopausal women receiving HRT (OR:0.35, p= 0.01) compared to women not receiving HRT after adjusting for duration of infection, severity of steatosis, and BMI.17 Another retrospective study examined the effect of menopause and hormone replacement therapy on liver fibrosis progression in 472 HCV-infected women.27 Post-menopausal women were more likely to have higher mean fibrosis score (AOR: 9.3, p=0.02) and rate of fibrosis progression (p<0.05) compared to premenopausal women after adjusting for age, BMI, and necroinflammatory activity. The mean fibrosis score (p<0.05) and estimated rate of fibrosis progression (p=0.02) was lower in women who received HRT compared to those who did not receive HRT among the post-menopausal women.
Several studies investigated effect of estrogen/anti-estrogen in the development of NAFLD. In a large prospective randomized controlled chemoprevention trial with tamoxifen in Italy (n=5408), taxomifen use was associated with increased risk of developing NAFLD (hazard ratio, 2.0; P = 0.04).28 A study using National Health and Nutrition Examination Survey (NHANES) showed that the risk of NAFLD is lower in post- menopausal women taking HRT compared to post-menopausal women not taking HRT.(OR:0.69, P<0.05).14 Similarly, a randomized controlled trials with 50 diabetic women showed that low dose HRT for 6 months resulted in significant reduction in liver enzymes.16 Protective effect of estrogen against liver fibrosis was also suggested in pediatric populations. Our previous study showed that patients at or beyond puberty were less likely to have high-grade steatosis, severe portal inflammation, or a high stage of fibrosis than patients who had not entered puberty and we speculated that increased estrogen release at or beyond puberty are primarily responsible for this observation.29
Several experimental studies have shown the protective effect of estrogen against liver fibrosis. One study showed the effect of oophorectomy and estrogen replacement on hepatic fibrosis using rats.18 Hepatic fibrosis induced by dimethylnitrosamine (DMN) was more prominent in male rats compared to female rats. Estrogen decreased collagen synthesis in male rats exposed to DMN. Female rats with oophorectomy had increased expression of procollagen. Estrogen replacement has reversed enhanced fibrogenesis observed in female rats with oophorectomy. Stellate cells incubated with estrogen resulted in decreased collagen synthesis, suggesting that estrogen suppress the activity of hepatic stellate cells, thereby decrease the collagen production and progression of liver fibrosis. Estrogen was shown to decrease the generation of reactive oxygen species (ROS), transforming growth factor (TGF) beta -1 expression, hepatic stellate cell activation and proliferation, which are integral process of hepatic fibrogenesis.30
The major strength of our study is a large number of patients with NASH in a prospectively designed database. The diagnosis of NASH was based on liver histology, and it was confirmed by experienced pathologists based on current guideline. Also, we applied multiple modeling approaches to address our research question, which provided complementary information to enrich our data interpretation. Our study, however, has several limitations. First, due to the cross sectional nature of our study, we were unable to prove causality between gender/menopause and severity of fibrosis. Second, referral bias is also a potential limitation as we included patients from single academic institution and patients enrolled in DUHS NAFLD database. Third, our study population did not contain enough subjects who were under estrogen replacement therapy, which precluded us from conclusive analysis on potential beneficial association between estrogen replacement and severity of fibrosis among post-menopausal women. Our database did not provide anthropometric measures which could potentially have confounded our findings. Lastly, 49 of 352 women had no data on reproductive information and their menopause category was classified based on their age relative to the average age at menopause in the US population. Based on our sensitivity analysis after excluding the 49 women, our estimates of ACOR might have been underestimated due to the misclassification, and true effect may be even larger than what was observed in the current study.
In summary, our study revealed that post-menopausal women and men are associated with an increased risk of having more advanced fibrosis compared to pre-menopausal women at a given degree of hepatocyte ballooning and portal inflammation. Considering the findings from previous animal experiments, the observed association between pre-menopausal women and a decreased risk of hepatic fibrosis may be explained by protective effects of estrogens in fibrogenesis. Further studies are warranted to investigate impact of estrogen on fibrosis progression in patients with NASH and potential preventive and/or therapeutic effects of estrogen among post-menopausal women with NASH.
Supplementary Material
Acknowledgement
The authors thank our colleagues in Duke Division of Gastroenterology, Duke Metabolic and Weight Loss Surgery Program and Center of Human Genetics for assistance with patient recruitment, acquisition and/or management of DUHS NAFLD Clinical Database. The authors also thank the NAFLD clinical research manager, study coordinators, clinical support staff, research and data management personnel, and the patients and their families without whom this study would not have been possible.
Grant support:
Drs. Diehl, Abdelmalek, Guy, Pang and Suzuki received support through an American Recovery and Reinvestment Act (ARRA) grant from the NIAAA: 5RC2 AA019399 (Anna Mae Diehl, Principal Investigator). Drs. Diehl and Abdelmalek received funding support from NIH/NIDDK grant U01-DK57149. Dr. Abdelmalek was supported by a NIH/NIDDK K23 Career Development Award (K23-DK062116).
Abbreviations
- ACOR
adjusted cumulative odds ratio
- BMI
body mass index
- COR
cumulative odds ratio
- CI
confidence interval
- DUHS
Duke University Health System
- HCV
hepatitis C virus
- HRT
hormone replacement therapy
- MOLR
multiple ordinal logistic regression model
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- NASH CRN
NASH clinical research network
- NHANES
National Health and Nutrition Examination Survey
Footnotes
Disclosure: The authors do not have conflict of interest related to this work.
References
- 1.Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132:2087–2102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 2.Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H, Srishord M. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9:524–530. e1. doi: 10.1016/j.cgh.2011.03.020. quiz e60. [DOI] [PubMed] [Google Scholar]
- 3.Ryan CK, Johnson LA, Germin BI, Marcos A. One hundred consecutive hepatic biopsies in the workup of living donors for right lobe liver transplantation. Liver Transpl. 2002;8:1114–1122. doi: 10.1053/jlts.2002.36740. [DOI] [PubMed] [Google Scholar]
- 4.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–131. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 5.Yang JD, Harmsen WS, Slettedahl SW, Chaiteerakij R, Enders FT, Therneau TM, Orsini L, Kim WR, Roberts LR. Factors that affect risk for hepatocellular carcinoma and effects of surveillance. Clin Gastroenterol Hepatol. 2011;9:617–23. e1. doi: 10.1016/j.cgh.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 6.Yang JD, Kim B, Sanderson SO, St Sauver JL, Yawn BP, Pedersen RA, Larson JJ, Therneau TM, Roberts LR, Kim WR. Hepatocellular carcinoma in olmsted county, Minnesota, 1976–2008. Mayo Clin Proc. 2012;87:9–16. doi: 10.1016/j.mayocp.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim D, Kim WR, Kim HJ, Therneau TM. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology. 2013;57:1357–1365. doi: 10.1002/hep.26156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ekstedt M, Franzen LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 9.Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30:1356–1362. doi: 10.1002/hep.510300604. [DOI] [PubMed] [Google Scholar]
- 10.Rakha EA, Adamson L, Bell E, Neal K, Ryder SD, Kaye PV, Aithal GP. Portal inflammation is associated with advanced histological changes in alcoholic and non-alcoholic fatty liver disease. J Clin Pathol. 2010;63:790–795. doi: 10.1136/jcp.2010.079145. [DOI] [PubMed] [Google Scholar]
- 11.Richardson MM, Jonsson JR, Powell EE, Brunt EM, Neuschwander-Tetri BA, Bhathal PS, Dixon JB, Weltman MD, Tilg H, Moschen AR, Purdie DM, Demetris AJ, Clouston AD. Progressive fibrosis in nonalcoholic steatohepatitis: association with altered regeneration and a ductular reaction. Gastroenterology. 2007;133:80–90. doi: 10.1053/j.gastro.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 12.Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes (Lond) 2008;32:949–958. doi: 10.1038/ijo.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki A, Abdelmalek MF. Nonalcoholic fatty liver disease in women. Womens Health (Lond Engl) 2009;5:191–203. doi: 10.2217/17455057.5.2.191. [DOI] [PubMed] [Google Scholar]
- 14.Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology. 2002;122:1649–1657. doi: 10.1053/gast.2002.33573. [DOI] [PubMed] [Google Scholar]
- 15.Kojima S, Watanabe N, Numata M, Ogawa T, Matsuzaki S. Increase in the prevalence of fatty liver in Japan over the past 12 years: analysis of clinical background. J Gastroenterol. 2003;38:954–961. doi: 10.1007/s00535-003-1178-8. [DOI] [PubMed] [Google Scholar]
- 16.McKenzie J, Fisher BM, Jaap AJ, Stanley A, Paterson K, Sattar N. Effects of HRT on liver enzyme levels in women with type 2 diabetes: a randomized placebo-controlled trial. Clin Endocrinol (Oxf) 2006;65:40–44. doi: 10.1111/j.1365-2265.2006.02543.x. [DOI] [PubMed] [Google Scholar]
- 17.Codes L, Asselah T, Cazals-Hatem D, Tubach F, Vidaud D, Parana R, Bedossa P, Valla D, Marcellin P. Liver fibrosis in women with chronic hepatitis C: evidence for the negative role of the menopause and steatosis and the potential benefit of hormone replacement therapy. Gut. 2007;56:390–395. doi: 10.1136/gut.2006.101931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yasuda M, Shimizu I, Shiba M, Ito S. Suppressive effects of estradiol on dimethylnitrosamine-induced fibrosis of the liver in rats. Hepatology. 1999;29:719–727. doi: 10.1002/hep.510290307. [DOI] [PubMed] [Google Scholar]
- 19.Youssef NA, Abdelmalek MF, Binks M, Guy CD, Omenetti A, Smith AD, Diehl AM, Suzuki A. Associations of depression, anxiety and antidepressants with histological severity of nonalcoholic fatty liver disease. Liver Int. 2013 doi: 10.1111/liv.12165. [DOI] [PubMed] [Google Scholar]
- 20.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 21.Guy CD, Suzuki A, Zdanowicz M, Abdelmalek MF, Burchette J, Unalp A, Diehl AM. Hedgehog pathway activation parallels histologic severity of injury and fibrosis in human nonalcoholic fatty liver disease. Hepatology. 2012;55:1711–1721. doi: 10.1002/hep.25559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Omenetti A, Syn WK, Jung Y, Francis H, Porrello A, Witek RP, Choi SS, Yang L, Mayo MJ, Gershwin ME, Alpini G, Diehl AM. Repair-related activation of hedgehog signaling promotes cholangiocyte chemokine production. Hepatology. 2009;50:518–527. doi: 10.1002/hep.23019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rangwala F, Guy CD, Lu J, Suzuki A, Burchette JL, Abdelmalek MF, Chen W, Diehl AM. Increased production of sonic hedgehog by ballooned hepatocytes. J Pathol. 2011;224:401–410. doi: 10.1002/path.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Syn WK, Oo YH, Pereira TA, Karaca GF, Jung Y, Omenetti A, Witek RP, Choi SS, Guy CD, Fearing CM, Teaberry V, Pereira FE, Adams DH, Diehl AM. Accumulation of natural killer T cells in progressive nonalcoholic fatty liver disease. Hepatology. 2010;51:1998–2007. doi: 10.1002/hep.23599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nichols HB, Trentham-Dietz A, Hampton JM, Titus-Ernstoff L, Egan KM, Willett WC, Newcomb PA. From menarche to menopause: trends among US Women born from 1912 to 1969. Am J Epidemiol. 2006;164:1003–1011. doi: 10.1093/aje/kwj282. [DOI] [PubMed] [Google Scholar]
- 26.Torsten Hothorn KH, van de Wiel Mark A. Achim Zeileis A Lego System for Conditional Inference. AMER STATIST. 2006;60:257–263. [Google Scholar]
- 27.Di Martino V, Lebray P, Myers RP, Pannier E, Paradis V, Charlotte F, Moussalli J, Thabut D, Buffet C, Poynard T. Progression of liver fibrosis in women infected with hepatitis C: long-term benefit of estrogen exposure. Hepatology. 2004;40:1426–1433. doi: 10.1002/hep.20463. [DOI] [PubMed] [Google Scholar]
- 28.Bruno S, Maisonneuve P, Castellana P, Rotmensz N, Rossi S, Maggioni M, Persico M, Colombo A, Monasterolo F, Casadei-Giunchi D, Desiderio F, Stroffolini T, Sacchini V, Decensi A, Veronesi U. Incidence and risk factors for non-alcoholic steatohepatitis: prospective study of 5408 women enrolled in Italian tamoxifen chemoprevention trial. BMJ. 2005;330:932. doi: 10.1136/bmj.38391.663287.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki A, Abdelmalek MF, Schwimmer JB, Lavine JE, Scheimann AO, Unalp-Arida A, Yates KP, Sanyal AJ, Guy CD, Diehl AM. Association between puberty and features of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2012;10:786–794. doi: 10.1016/j.cgh.2012.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Itagaki T, Shimizu I, Cheng X, Yuan Y, Oshio A, Tamaki K, Fukuno H, Honda H, Okamura Y, Ito S. Opposing effects of oestradiol and progesterone on intracellular pathways and activation processes in the oxidative stress induced activation of cultured rat hepatic stellate cells. Gut. 2005;54:1782–1789. doi: 10.1136/gut.2005.053278. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.