Abstract
Background
It is not clear whether subgroups of patients with peripheral artery disease (PAD) and claudication respond more favorably to exercise rehabilitation than others. We determined whether sex and diabetes were factors associated with the response to exercise rehabilitation in patients with claudication.
Methods
Eighty patients were randomized to home-based and supervised exercise programs, and 60 finished with complete exercise intervention data. Exercise consisted of intermittent walking to near maximal claudication pain for three months. Primary outcome measures included claudication onset time (COT) and peak walking time (PWT). Patients were partitioned into diabetic and non-diabetic groups, and then further partitioned by sex to form four groups.
Results
Overall, exercise adherence was high (84%), and there was no significant difference (p > 0.05) in the amount of exercise completed among the four groups. All groups had significant improvements (p < 0.05) in COT and PWT following exercise rehabilitation, except for diabetic women (p > 0.05). Only 37% of women with diabetes had an increase in COT compared to 100% of men with diabetes (p < 0.01), and their risk ratio for non-response was 9.2 (p < 0.0001).
Conclusions
Women with PAD and claudication, particularly those with diabetes, represent a vulnerable subgroup of patients who respond poorly to a program of exercise rehabilitation. Diabetic women with PAD and claudication may either need a greater dose of exercise, or another intervention separate from or in combination with exercise to elicit improvements in claudication measures that are similar to non-diabetic women and to diabetic and non-diabetic men.
INTRODUCTION
Peripheral artery disease (PAD) is prevalent in eight million Americans,1 and is associated with high health economic costs, and high rates of morbidity and mortality similar to coronary heart disease and ischemic stroke.2, 3 Although the prevalence of PAD in women is similar to men at all ages, the burden of PAD, defined as the total number of individuals who have PAD, is greater in women.4 These numbers are especially impressive given that women are less likely to report symptoms than men,5 particularly those characteristic of classic intermittent claudication.6 Once becoming symptomatic, women have a two-fold higher mortality rate,7 a more functionally dependent lifestyle,5, 8 shorter distances to the onset of claudication pain and to maximal pain,9 and more impaired oxygen saturation of the calf muscle during ambulation than men.10 More severe impairments in claudication and in calf muscle oxygen saturation in women with PAD may make them particularly vulnerable for progressively worse lower extremity function when these limitations are combined with co-morbid conditions that impair microcirculation, such as diabetes.11, 12
Supervised exercise programs are efficacious for clinical management of claudication,13–16 and have been given a Class IA recommendation by the American College of Cardiology (ACC) and the American Heart Association (AHA).17 Although treatment of claudication with exercise rehabilitation is well documented, it is not clear whether subgroups of PAD patients respond more favorably than others. For example, there is a paucity of data on the efficacy of exercise rehabilitation in women who have PAD and claudication, as only a small percentage (27%) of eligible patients in 32 previous randomized exercise trials have been women.4 Furthermore, comparing sex-specific responses to exercise has not been addressed. Diabetes is another example in which there is conflicting, and surprisingly little data on the efficacy of exercise rehabilitation. One recent study found that claudication distances did not improve in PAD patients with diabetes following six months of exercise,18 whereas another recent report found that diabetic patients improved to a similar extent compared to non-diabetic patients.19 To address the dearth of information on the potential influences of sex and diabetes on responses to an exercise program, we conducted a follow-up analysis to our recently published20 prospective, randomized controlled exercise trial in PAD patients with intermittent claudication. The primary aim of the current study was to determine whether sex and diabetes were factors associated with the response to exercise rehabilitation in patients with claudication. Secondary aims were to determine whether the amount of exercise completed during intervention was different according to sex and diabetes status, whether the amount of exercise completed was associated with the change scores of claudication onset time (COT) and peak walking time (PWT), and to determine the characteristics of patients who do not respond to exercise intervention. Our primary hypothesis was that women will experience less improvement in the primary outcome measures of COT and PWT, that the presence of diabetes will blunt the improvement in the primary outcomes, and that the effect of diabetes will be greater in women than in men.
METHODS
Patients
Recruitment
Patients participated at the General Clinical Research Center (GCRC), University of Oklahoma Health Sciences Center (HSC) from September, 2004 to April, 2007. Patients were recruited by HSC vascular clinic referrals, as well as by newspaper advertisements. Procedures used were approved by the Institutional Review Board at the University of Oklahoma HSC. Written informed consent was obtained from each patient prior to investigation.
Screening
Patients who had claudication secondary to vascular insufficiency were included if they met the following criteria: (a) a history of any type of exertional leg pain, (b) ambulation during a graded treadmill test limited by leg pain consistent with claudication,21 and (c) an ankle-brachial index (ABI) ≤ 0.90 at rest17 or an ABI ≤ 0.73 after exercise.22 Patients were excluded for the following conditions: (a) absence of PAD (ABI > 0.90 at rest and ABI > 0.73 after exercise), (b) inability to obtain an ABI measure due to non-compressible vessels, (c) asymptomatic PAD determined from the medical history and verified during the graded treadmill test, (d) use of cilostazol or pentoxifylline initiated within three months prior to investigation, (e) exercise tolerance limited by any disease process other than PAD, (f) active cancer, (g) end stage renal disease defined as stage 5 chronic kidney disease, (h) abnormal liver function, and (g) randomization into the usual care control group. As shown in our previous report,20 a total of 80 patients were randomized to either the home-based exercise program (n = 40) or to the supervised exercise program (n = 40), and 60 patients completed both the exercise training programs and the post-tests (n = 29 and n = 31, respectively). The remaining 20 patients were not included in our primary analyses because 14 made personal decisions to discontinue (n = 14), 4 were medically excluded because of experiencing a stroke, myocardial infarction, leg revascularization, and hernia surgery during the study, and 2 were excluded because they had incomplete exercise training data.
Exercise Interventions
Home-Based Exercise Rehabilitation Program
Exercise sessions in our home-based exercise program were rigorously quantified with a step activity monitor (StepWatch3™, Orthoinnovations, Inc., Oklahoma City, OK) to accurately record the duration and cadence of ambulation. Home-based exercise program was designed to be as similar to the supervised exercise program as possible, and we were indeed successful in matching the exercise volume and exercise compliance of the two programs.20 The home-based exercise program consisted of three months of intermittent walking to near maximal claudication pain three days per week at a self-selected pace. Walking duration began at 20 minutes for the first two weeks, and progressively increased five minutes biweekly until a total of 45 minutes of walking was accomplished during the final two weeks of the program. Patients were given a step activity monitor and were instructed to wear it on the right ankle during each exercise session, and then to remove the monitor at the completion of each session. Additionally, they received an exercise logbook to record their walking sessions. Patients returned their step activity monitors and logbooks to the research staff at the end of week 1, 2, 4, 6, 8, 10, and 12 of the program, and data from the monitor was downloaded. During these brief 15-minute meetings, patients discussed their progress with an exercise physiologist, were given feedback about the data from the step activity monitor, and were given new instructions regarding changes in exercise duration. No exercise was performed by the patients in our facility during these meetings with the research staff.
Supervised Exercise Rehabilitation Program
The supervised program was designed to elicit increases in COT and PWT according to our previous studies.13, 20, 23, 24 This standardized program consisted of three months of supervised, intermittent treadmill walking, three days per week at a speed of approximately two mph. Walking duration began at 15 minutes for the first two weeks of the program, and progressively increased by 5 minutes biweekly until a total of 40 minutes of walking was accomplished during the final two weeks of the program. Patients exercised at a relatively low intensity by walking at a grade equal to 40% of the final work load from the baseline maximal treadmill test. Patients walked to the point of near maximal claudication pain, at which point they stopped to relieve their leg pain. Patients then repeated the intermittent walking and rest periods until the prescribed total number of minutes of exercise was attained for the training session. During each exercise session, patients wore a step activity monitor on the right ankle to quantify the cadence and time of ambulation. Patients in the supervised program were not given advice or instructions to perform additional exercise away from our research center.
Measurements
Medical History and Physical Examination
Patients arrived in the morning fasted, but were permitted to take their usual morning medication regimen. Demographic information, height, weight, cardiovascular risk factors, co-morbid conditions, claudication history, blood samples, a list of current medications, and ABI were obtained from a medical history and physical examination.
Gardner Maximal Treadmill Test
Patients performed a progressive, graded treadmill protocol (2 mph, 0% grade with 2% increase every two minutes) until maximal claudication pain on two separate days. The first test was to determine study eligibility by assessing whether exercise performance was limited by claudication, whereas the second test was done on another day to obtain the outcome measures of COT, and PWT. COT was defined as the walking time at which the patient first experienced pain, and PWT was defined as the time at which ambulation could not continue due to maximal pain. Using these procedures, the test-retest intraclass reliability coefficient in our laboratory is R = 0.89 for COT,21 and R = 0.93 for PWT.21
Statistical Analyses
Patients were partitioned into diabetic and non-diabetic groups, and then further partitioned by sex under each category to form four groups. Measurement variables were summarized within each group by reporting mean, median and standard deviation. Dichotomous variables are reported as percentage of subjects with each attribute. To avoid possible distortion of results due to departure from normal distribution, wherever parametric and non-parametric procedures were available, both were used to test hypotheses for measurement variables. Differences in pairs of groups were examined using independent t-test and corresponding Wilcoxon test. Differences among groups in the exercise rehabilitation variables were examined using One Way ANOVA and with Kruskal-Wallis non-parametric test.
The delta COT, defined as the change from baseline, was calculated as the 3-month value minus the baseline value. Patients with a positive delta COT were designated as “responders”. Delta COT values within each group were examined for difference from zero using one sample t-tests and corresponding Wilcoxon tests. Responder rates were tested using the two sided Sign test. Differences among delta COT means of the four groups were examined using a two factor (sex, and diabetes) ANOVA. Partial correlations controlled for diabetes, sex, and interaction of five exercise rehabilitation variables with delta COT were computed. The exercise rehabilitation variable with the largest correlation coefficient was used as a covariate in the ANCOVA examination of delta COT means. Delta PWT was similarly obtained and analyzed. Selected variables were examined as possible risk factors for failing to become a responder for COT. All analyses were performed using the NCSS statistical package. Statistical significance was set at p < 0.05.
RESULTS
Clinical Characteristics
Patients with PAD were classified into four groups based on their sex and diabetes status. The baseline clinical characteristics of these groups are displayed in Table I. In patients without diabetes, men were significantly younger (p<0.05) than women, and had greater body weight (p<0.05) and lower prevalence of smoking (p<0.05). In patients with diabetes, men had greater body weight (p<0.05) than women.
Table I.
Baseline clinical characteristics of 60 patients who completed three months of exercise rehabilitation. Values are means (standard deviation) or percentage of patients.
| Variables | Non- Diabetic Men (N = 19) |
Non- Diabetic Women (N = 16) |
Diabetic Men (N = 9) |
Diabetic Women (N = 16) |
Total Group (N = 60) |
|---|---|---|---|---|---|
| Age (years) | 64 (14)* | 74 (10) | 64 (9) | 60 (7) | 66 (12) |
| Weight (kg) | 82.7 (17.5)* | 70.7 (13.7) | 107.2 (22.0)† | 89.1 (13.1) | 84.9 (19.7) |
| Body Mass Index (kg/m2) | 27.0 (4.7) | 27.1 (5.3) | 34.5 (7.5) | 34.0 (5.2) | 30.0 (6.4) |
| Ankle/Brachial Index | 0.69 (0.22) | 0.73 (0.21) | 0.76 (0.20) | 0.65 (0.29) | 0.70 (0.23) |
| Claudication Onset Time (sec) | 235 (142) | 156 (109) | 257 (191) | 163 (120) | 198 (140) |
| Peak Walking Time (sec) | 464 (296) | 300 (188) | 409 (181) | 278 (173) | 362 (234) |
| Race (% Caucasian) | 63 | 75 | 44 | 25 | 47 |
| Current Smoking (% yes) | 0* | 25 | 11 | 0 | 8 |
| Hypertension (% yes) | 79 | 94 | 100 | 88 | 88 |
| Dyslipidemia (% yes) | 89 | 88 | 89 | 100 | 92 |
| Abdominal Obesity (% yes) | 21 | 44 | 78 | 88 | 53 |
| Metabolic Syndrome Components (n) | 2.8 (1.0) | 3.1 (1.2) | 4.4 (1.0) | 4.5 (0.6) | 3.6 (1.2) |
| Metabolic Syndrome (% yes) | 68 | 81 | 89 | 100 | 83 |
| Obesity (% yes) | 21 | 31 | 78 | 81 | 48 |
Significantly different than the non-diabetic women (p < 0.05).
Significantly different than the diabetic women (p < 0.05).
Exercise Intervention Measures
The exercise intervention measures of each group are shown in Table II. There was no significant difference among the four groups for each variable. Overall, the exercise adherence was high among the participants, as the mean percentage of exercise sessions completed was 84%. Of particular interest, women with diabetes were actually above average for adherence, as they completed 87% of the prescribed exercise sessions. During an average exercise session, the entire group of patients walked for 33 minutes at a cadence of 42 strides/min and took a total of 1344 strides. There was no significant difference among the four sex/diabetes groups for the number of patients who did not complete the study (p = 0.332), and there was no significant difference between the two exercise programs for non-completers (p = 0.606).
Table II.
Exercise intervention measures. Values are means (SD).
| Variables | Non- Diabetic Men (N = 19) |
Non- Diabetic Women (N = 16) |
Diabetic Men (N = 9) |
Diabetic Women (N = 16) |
Total Group (N = 60) |
|---|---|---|---|---|---|
| Percentage of Exercise Sessions Completed (%) | 92 (27) | 76 (25) | 74 (28) | 87 (15) | 84 (25) |
| Total Exercise Time (min) | 1135 (511) | 877 (390) | 1016 (606) | 956 (476) | 1001 (486) |
| Total Exercise Strides (strides) | 49702 (24619) | 35664 (15812) | 40632 (22872) | 37374 (15281) | 41310 (20356) |
| Average Exercise Time (min / session) | 33.3 (11.3) | 31.9 (13.1) | 39.3 (30.4) | 31.0 (17.1) | 33.2 (17.0) |
| Average Exercise Strides (strides / session) | 1430 (407) | 1285 (525) | 1535 (814) | 1195 (480) | 1344 (533) |
| Average Exercise Cadence (strides / min) | 43.8 (6.5) | 41.2 (7.7) | 43.0 (9.1) | 40.3 (11.9) | 42.1 (8.8) |
Primary Outcome Measures: Change Scores for COT and PWT
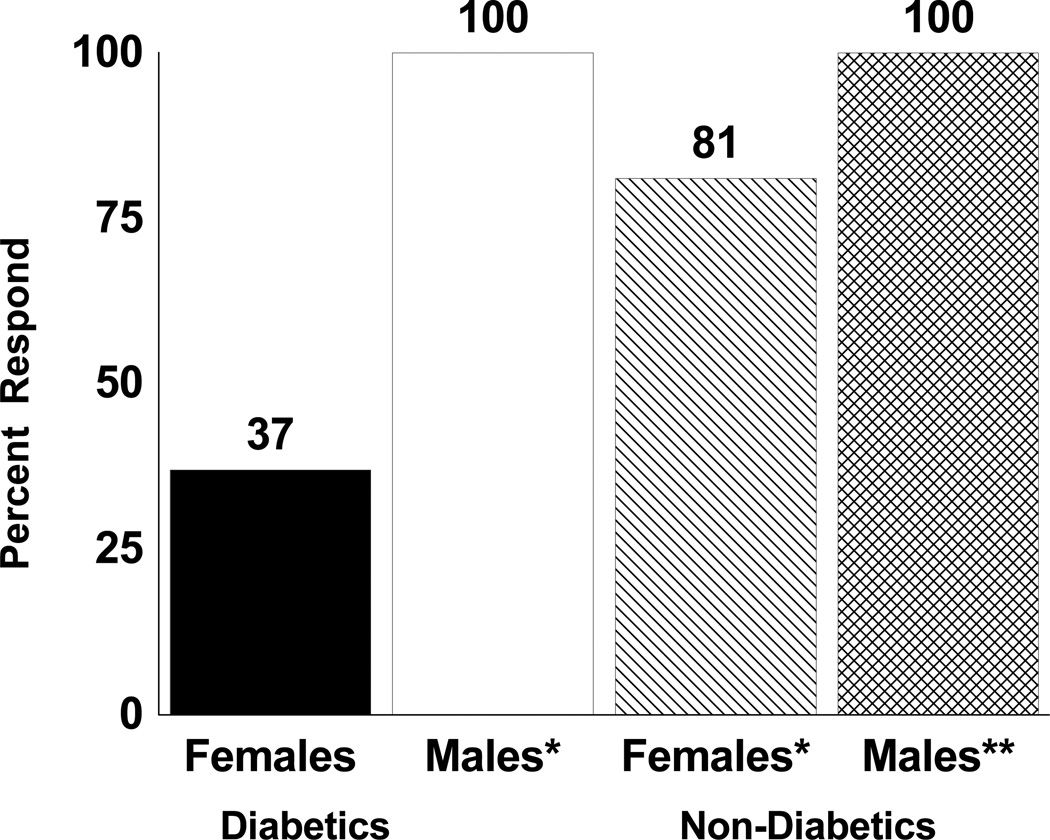
The change scores for COT in each group are shown in Table III. All groups had significant improvements (p<0.05) in mean and median COT following exercise rehabilitation, except for diabetic women (p>0.05). Overall, there was a significant sex effect for mean COT change score (p=0.005), as men had a 121 second greater increase than women. Furthermore, the sex by diabetes interaction for mean COT change score was significant after adjusting for total exercise strides taken during the exercise rehabilitation program (p=0.035). This is evident by noting that in patients with diabetes, the increase in mean COT change score was 221 seconds greater in men than in women (p<0.05), whereas in those without diabetes the increase in COT change score was only 50 seconds greater in men (p>0.05). This finding is further highlighted in Figure 1, as only 37% of women with diabetes had an increase in COT compared to 100% of men with diabetes (p<0.01).
Table III.
Change scores for claudication onset time (sec) in peripheral artery disease patients with and without diabetes.
| Diabetes Status | Men | Women | Difference |
|---|---|---|---|
| No Diabetes | |||
| Mean (SD) | 189 (146)** | 139 (191)* | 50 |
| Median | 165** | 49** | 116 |
| Diabetes | |||
| Mean (SD) | 273 (212)** | 52 (155) | 221* |
| Median | 294** | −12 | 306* |
| All Patients | |||
| Mean (SD) | 216 (171)** | 95 (177)** | 121** |
| Median | 169** | 15* | 154** |
ANOVA p values: Sex effect = 0.005, Diabetes effect = 0.982, Sex by Diabetes interaction = 0.069.
ANCOVA p values: Sex effect = 0.015, Diabetes effect = 0.870, Sex by Diabetes interaction = 0.035, Covariate (total exercise strides) = 0.044.
Significantly different than zero:
p < 0.05,
p < 0.01.
Figure 1.
Percentage of patients, stratified by sex and diabetes status, who had an increase in their change score for claudication onset time (i.e., responder) following exercise rehabilitation. Significantly different than zero: * p < 0.05, ** p < 0.01.
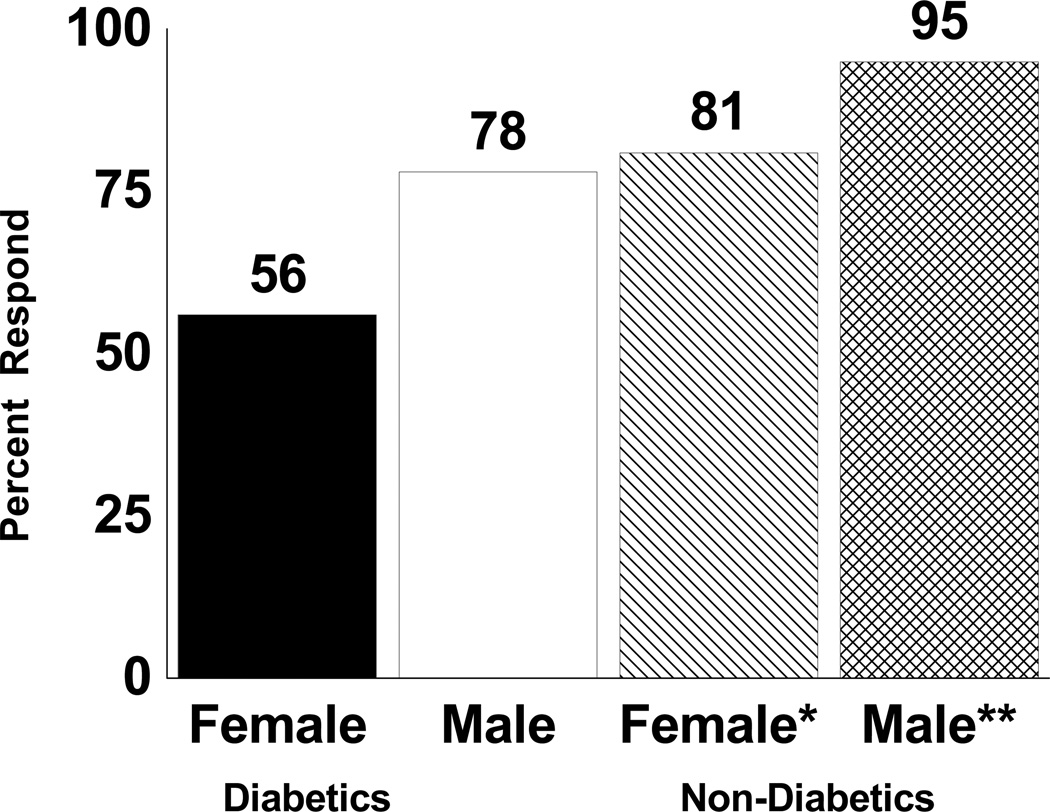
The change scores for PWT in each group are shown in Table IV. All groups had significant improvements (p<0.05) in mean and median PWT following exercise rehabilitation, except for diabetic women (p>0.05). Overall, there was a significant sex effect for mean PWT change score (p=0.003), as men had a 167 second greater increase than women. There was a trend for a diabetes effect on mean PWT change score (p=0.056), as non-diabetic men and women had larger increases than their diabetic counterparts. Similar to the COT results, the diabetic women had the lowest percentage of responders to exercise, as only 56% had an increase in PWT (Figure 2). The sex by diabetes interaction for mean PWT change score was not significant (p=0.917), as the difference between men and women was similar in diabetic and non-diabetic patients.
Table IV.
Change scores for peak walking time (sec) in peripheral artery disease patients with and without diabetes.
| Diabetes Status | Men | Women | Difference |
|---|---|---|---|
| No Diabetes | |||
| Mean (SD) | 290 (200)** | 144 (194)** | 146* |
| Median | 251** | 89** | 162* |
| Diabetes | |||
| Mean (SD) | 198 (214)* | 42 (128) | 156* |
| Median | 158* | 4 | 154* |
| All Patients | |||
| Mean (SD) | 260 (206)** | 93 (170)** | 167** |
| Median | 240** | 25** | 215** |
ANOVA p values: Sex effect = 0.003, Diabetes effect = 0.056, Sex by Diabetes interaction = 0.917.
ANCOVA p values: Sex effect = 0.005, Diabetes effect = 0.062, Sex by Diabetes interaction = 0.900, Covariate (total exercise strides) = 0.862.
Significantly different than zero:
p < 0.05,
p < 0.01.
Figure 2.
Percentage of patients, stratified by sex and diabetes status, who had an increase in their change score for peak walking time (i.e., responder) following exercise rehabilitation. Significantly different than zero: * p < 0.05, ** p < 0.01.
The association between the exercise intervention measures and the change scores for COT and PWT are shown in Table V. The total number of strides accumulated throughout the exercise program was associated with the change score for COT (r=0.268, p=0.044), as was the average number of strides taken per exercise session (r=0.267, p=0.045). None of the other exercise intervention measures were correlated with the changes scores for COT or PWT.
Table V.
Exercise intervention measures and their associations with the change scores for claudication onset time and peak walking time.
| Variables | Δ Claudication Onset Time (r) |
Δ Peak Walking Time (r) |
|---|---|---|
| Exercise Sessions Completed (%) | 0.178 | 0.062 |
| Total Exercise Time (min) | 0.227 | 0.044 |
| Total Exercise Strides (strides) | 0.268* | 0.023 |
| Average Exercise Time (min / exercise session) | 0.193 | 0.032 |
| Average Exercise Strides (strides / exercise session) | 0.267* | 0.047 |
| Average Exercise Cadence (strides/min) | 0.076 | 0.136 |
r = Pearson partial correlation coefficients controlled for diabetes, sex, and interaction.
p < 0.05.
Risk for Non-Response to Exercise Intervention
To examine whether subsets of patients were more likely to have a poor response to exercise rehabilitation, we calculated the risk ratio for non-response for each clinical characteristic of the patients (Table VI). Forty percent of diabetic patients did not respond to exercise rehabilitation, and their risk ratio for non-response was 4.7 (p<0.005) compared to non-diabetic patients. Similarly, 41% of women did not respond to exercise rehabilitation compared to 0% of men (p<0.0001). The presence of diabetes in women was an especially poor combination, as 63% did not respond to exercise and their risk ratio for non-response was 9.2 (p<0.0001). Twenty-six percent of patients with metabolic syndrome did not respond to exercise compared to 0% of those without metabolic syndrome (p=0.099), but this trend was not as strong as that found for diabetes. However, two of the components of metabolic syndrome were more strongly related to poor response to exercise rehabilitation, as the risk ratio for non-response was 4.1 for elevated glucose (p=0.012) and 2.9 for abdominal obesity (p=0.066). Finally, it is important to note that poor response to exercise rehabilitation was not related (p=0.355) to whether the intervention was performed in a supervised exercise or home-based exercise setting.
Table VI.
Percentages and risk ratio for non-response to exercise intervention in patients with and without each attribute.
| Attribute | Attribute Absent (%) |
Attribute Present (%) |
Risk Ratio for Non-Response |
P Value |
|---|---|---|---|---|
| Diabetes | 8.6 | 40.0 | 4.7 | 0.005 |
| Female | 0 | 40.6 | --- | <0.0001 |
| Diabetic Female | 6.8 | 62.5 | 9.2 | <0.0001 |
| Caucasian | 25.0 | 18.8 | 0.8 | 0.558 |
| Current Smoking | 23.6 | 0.0 | 0 | 0.344 |
| Dyslipidemia | 0.0 | 23.6 | --- | 0.344 |
| Elevated Glucose | 9.1 | 37.0 | 4.1 | 0.012 |
| Hypertension | 28.6 | 20.8 | 0.7 | 0.639 |
| Abdominal Obesity | 10.7 | 31.3 | 2.9 | 0.066 |
| Metabolic Syndrome | 0 | 26 | --- | 0.099 |
| Obesity | 12.9 | 31.0 | 2.4 | 0.121 |
| Supervised Exercise Program | 27.6 | 16.1 | 0.6 | 0.355 |
DISCUSSION
Poor Response to Exercise in Diabetic Women
The primary finding was that the only subgroup of patients with PAD and claudication who did not significantly improve their COT and PWT following three months of exercise rehabilitation was diabetic women. Of the two factors, sex was more closely related to the exercise response than was diabetes, as the mean change scores for COT and PWT in women were less than half of those in men, and fewer women responded to exercise. This is the first study to directly compare the change scores of COT and PWT in men and women. The impaired response in women supports our hypothesis based on our previous baseline findings examining differences between men and women.9, 10, 25 Specifically, we have found that women with claudication have shorter walking distances to the onset of claudication pain9 and to maximal claudication pain9, 10 during standardized treadmill exercise than men, even though their ABI is similar.9, 10 Furthermore, compared to men, women with claudication have greater impairment in calf muscle hemoglobin oxygen saturation during and following exercise,10 ambulate slower in the community setting, particularly for short continuous durations of up to five minutes,25 and low daily ambulatory cadences correlate with poor calf muscle hemoglobin oxygen saturation during exercise in women.25 Collectively, these greater baseline limitations in claudication, calf muscle hemoglobin oxygen saturation, and daily ambulatory activity provided the basis of our hypothesis that women would respond less favorably to a program of exercise rehabilitation. However, it should be noted that in the current study the worse response to exercise rehabilitation by women was primarily found in those with diabetes, whereas non-diabetic women had significant improvements in COT and PWT.
The co-prevalence of diabetes in women with PAD and claudication appears to be a particularly difficult combination for treating claudication with an exercise program. In a recent study examining PAD patients with diabetes, of whom one-third were women, the walking distances to onset of claudication pain and to maximal pain did not change following six months of home-based exercise.18 These findings either suggest that diabetes interferes with the typical exercise-mediated improvements in claudication measures, or that the home-based program was not efficacious because it relied on self-report for most of the exercise sessions. Type 2 diabetes is associated with impaired microvascular function in patients with PAD and claudication, as reduced blood volume expansion12 and slower oxygen kinetics11 occur in the calf musculature during exercise than compared to non-diabetic PAD patients. Additionally, we have found that metabolic syndrome in patients with PAD, which is considered a precursor to diabetes, worsens claudication, physical function, health-related quality of life, and peripheral circulation than compared to patients without metabolic syndrome,26 and that abdominal obesity and elevated fasting glucose are the components of metabolic syndrome that are most predictive of these unfavorable outcomes.27 Collectively, these findings support the results of the current study which found that diabetic women do not respond well to an exercise program designed to treat claudication. However, some controversy exists regarding the impact that diabetes has on ambulation in patients with PAD, as a recent report found that six months of supervised exercise increased the walking distances to onset of pain and to maximal pain in diabetic patients with PAD, and their response was not different than patients without diabetes.19
Quantified Exercise Rehabilitation Stimulus and Characteristics of Non-Responders
A strength of the current investigation was that the exercise time, cadence, and ambulatory volume completed during the exercise programs were directly quantified with a step activity monitor worn around the ankle during each exercise session. Until our recent trial,20 no other randomized controlled exercise trial reported the exercise volume performed during the program. In the present study, none of the exercise rehabilitation measures were different among the four groups, and adherence to exercise was high in each group. Specifically, the diabetic women completed 87% of their exercise sessions, and during an average session they walked for 31 minutes at a cadence of 40 strides/min and took 1195 strides. Thus, performing less exercise than the other groups can be ruled out as a reason for why the diabetic women had smaller change scores for COT and PWT than the other groups. Another important finding was that the total number of strides taken during the program, and the average number of strides taken during each session were both positively correlated with the change score in COT, suggesting that more ambulatory strides completed during intervention was associated with delaying the onset of claudication. Collectively, these findings suggest that diabetic women with PAD may need to complete a higher volume of exercise to demonstrate efficacy than other groups of PAD patients.
Examination of whether clinical characteristics are predictive of non-response to exercise provides important information when considering an exercise intervention to treat claudication in PAD patients. As was found for the group analyses, female sex and diabetes were significantly associated with non-response to exercise, and having both characteristics was most significant by far. Interestingly, racial composition, current smoking status, and whether exercise was performed in a home-based program or a supervised program were not associated with non-response to exercise. Thus, many patients can benefit from an exercise program, regardless of whether the setting is home-based or an on-site supervised program. We have previously shown that smokers and non-smokers both improve COT and PWT following an exercise rehabilitation program,24 and that home-based and supervised exercise programs are efficacious,20 both of which support our current findings. Although metabolic syndrome and its’ individual components were not significantly associated with lack of response to exercise, there were trends in that direction. For example, those with abdominal obesity had a risk ratio of 2.9 for non-response, suggesting that they might also benefit from a program utilizing a higher volume of exercise than patients without abdominal obesity.
Limitations
Although the exercise rehabilitation programs were efficacious for increasing COT and PWT, several limitations exist. First, patients who participated in this trial were volunteers and therefore may represent those more interested in exercise, those who had better access to transportation to our research facilities, and those in relatively better health than patients who did not volunteer. Second, although 85% of the patients had a history of smoking as former smokers, our sample consisted of a relatively low percentage of current smokers. Third, to address whether sex, diabetes, and other clinical factors were related to the response to exercise, it was necessary to limit the analyses to only those who completed exercise rehabilitation. Previously, we found no difference in baseline characteristics between patients who completed this trial and patients who did not complete the study, and no differences were found between the observed data on those patients who completed the trial compared to intent-to-treat analyses using imputed data on all patients.20 It is possible that those who withdrew would have been poor responders to exercise if they had completed the study. However, we found that there was no significant difference among the 4 groups for the number of dropouts, indicating that dropouts did not bias our current results. Finally, because women were shorter than men on average, they may have needed to ambulate at slightly faster cadence during the treadmill test. However, since this would be true for both the pre-test and the post-test condition, we do not believe that sex difference in cadence during the treadmill test had an influence on the change scores for COT and PWT.
Conclusions and Clinical Implications
Our primary novel finding was that women had smaller change scores in COT and PWT following three months of exercise rehabilitation than men, primarily due to the negative influence of diabetes, as only 37% of diabetic women increased their COT. This poor response occurred despite diabetic women having comparable values to other patients in exercise compliance and in the amount of exercise accomplished during the rehabilitation program. We conclude that women with PAD and claudication, particularly those with diabetes, represent a vulnerable subgroup of patients who respond poorly to a program of exercise rehabilitation. The clinical implication is that diabetic women with PAD and claudication may either need a greater dose of exercise, or another intervention separate from or in combination with exercise to elicit improvements in claudication measures that are similar to non-diabetic women and to diabetic and non-diabetic men.
Acknowledgments
Supported by grants from the National Institute on Aging (R01-AG-24296; AWG), Oklahoma Center for the Advancement of Science and Technology grant (HR09-035; AWG), and OUHSC General Clinical Research Center grant (M01-RR-14467) sponsored by National Center for Research Resources (NCRR). The final peer-reviewed version of this manuscript is subject to the NIH Public Access Policy, and will be submitted to PubMed Central.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Executive summary: heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:188–197. doi: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- 2.Mahoney EM, Wang K, Cohen DJ, Hirsch AT, Alberts MJ, Eagle K, et al. One-year costs in patients with a history of or at risk for atherothrombosis in the United States. Circ Cardiovasc Qual Outcomes. 2008;1:38–45. doi: 10.1161/CIRCOUTCOMES.108.775247. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch AT, Hartman L, Town RJ, Virnig BA. National health care costs of peripheral arterial disease in the Medicare population. Vasc Med. 2008;13:209–215. doi: 10.1177/1358863X08089277. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch AT, Allison MA, Gomes AS, Corriere MA, Duval S, Ershow AG, et al. A call to action: women and peripheral artery disease: a scientific statement from the American Heart Association. Circulation. 2012;125:1449–1472. doi: 10.1161/CIR.0b013e31824c39ba. [DOI] [PubMed] [Google Scholar]
- 5.Vogt MT, Cauley JA, Kuller LH, Nevitt MC. Functional status and mobility among elderly women with lower extremity arterial disease: the Study of Osteoporotic Fractures. J.Am.Geriatr.Soc. 1994;42:923–929. doi: 10.1111/j.1532-5415.1994.tb06581.x. [DOI] [PubMed] [Google Scholar]
- 6.McDermott MM, Mehta S, Greenland P. Exertional leg symptoms other than intermittent claudication are common in peripheral arterial disease. Arch.Intern.Med. 1999;159:387–392. doi: 10.1001/archinte.159.4.387. [DOI] [PubMed] [Google Scholar]
- 7.Kannel WB, McGee DL. Update on some epidemiologic features of intermittent claudication: the Framingham Study. J Am Geriatr.Soc. 1985;33:13–18. doi: 10.1111/j.1532-5415.1985.tb02853.x. [DOI] [PubMed] [Google Scholar]
- 8.McDermott MM, Ferrucci L, Simonsick EM, Balfour J, Fried L, Ling S, et al. The ankle brachial index and change in lower extremity functioning over time: the Women's Health and Aging Study. J Am Geriatr.Soc. 2002;50:238–246. doi: 10.1046/j.1532-5415.2002.50054.x. [DOI] [PubMed] [Google Scholar]
- 9.Gardner AW. Sex differences in claudication pain in subjects with peripheral arterial disease. Med.Sci.Sports Exerc. 2002;34:1695–1698. doi: 10.1097/00005768-200211000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Gardner AW, Parker DE, Montgomery PS, Blevins SM, Nael R, Afaq A. Sex differences in calf muscle hemoglobin oxygen saturation in patients with intermittent claudication. J Vasc.Surg. 2009;50:77–82. doi: 10.1016/j.jvs.2008.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bauer TA, Reusch JE, Levi M, Regensteiner JG. Skeletal muscle deoxygenation after the onset of moderate exercise suggests slowed microvascular blood flow kinetics in type 2 diabetes. Diabetes Care. 2007;30:2880–2885. doi: 10.2337/dc07-0843. [DOI] [PubMed] [Google Scholar]
- 12.Mohler ER, III, Lech G, Supple GE, Wang H, Chance B. Impaired exercise-induced blood volume in type 2 diabetes with or without peripheral arterial disease measured by continuous-wave near-infrared spectroscopy. Diabetes Care. 2006;29:1856–1859. doi: 10.2337/dc06-0182. [DOI] [PubMed] [Google Scholar]
- 13.Gardner AW, Poehlman ET. Exercise rehabilitation programs for the treatment of claudication pain. A meta-analysis. JAMA. 1995;274:975–980. [PubMed] [Google Scholar]
- 14.Stewart KJ, Hiatt WR, Regensteiner JG, Hirsch AT. Exercise training for claudication. N.Engl.J.Med. 2002;347:1941–1951. doi: 10.1056/NEJMra021135. [DOI] [PubMed] [Google Scholar]
- 15.Stewart AH, Lamont PM. Exercise training for claudication. Surgeon. 2007;5:291–299. doi: 10.1016/s1479-666x(07)80028-x. [DOI] [PubMed] [Google Scholar]
- 16.Wind J, Koelemay MJ. Exercise therapy and the additional effect of supervision on exercise therapy in patients with intermittent claudication. Systematic review of randomised controlled trials. Eur J Vasc Endovasc Surg. 2007;34:1–9. doi: 10.1016/j.ejvs.2006.12.030. [DOI] [PubMed] [Google Scholar]
- 17.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; Vascular Disease Foundation. Circulation. 2006;113:e463–e654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 18.Collins TC, Lunos S, Carlson T, Henderson K, Lightbourne M, Nelson B, et al. Effects of a home-based walking intervention on mobility and quality of life in people with diabetes and peripheral arterial disease: a randomized controlled trial. Diabetes Care. 2011;34:2174–2179. doi: 10.2337/dc10-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Pul KM, Kruidenier LM, Nicolai SP, de Bie RA, Nieman FH, Prins MH, et al. Effect of supervised exercise therapy for intermittent claudication in patients with diabetes mellitus. Ann Vasc Surg. 2012;26:957–963. doi: 10.1016/j.avsg.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Gardner AW, Parker DE, Montgomery PS, Scott KJ, Blevins SM. Efficacy of quantified home-based exercise and supervised exercise in patients with intermittent claudication: a randomized controlled trial. Circulation. 2011;123:491–498. doi: 10.1161/CIRCULATIONAHA.110.963066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gardner AW, Skinner JS, Cantwell BW, Smith LK. Progressive vs single-stage treadmill tests for evaluation of claudication. Med.Sci.Sports Exerc. 1991;23:402–408. [PubMed] [Google Scholar]
- 22.Hiatt WR, Marshall JA, Baxter J, Sandoval R, Hildebrandt W, Kahn LR, et al. Diagnostic methods for peripheral arterial disease in the San Luis Valley Diabetes Study. J Clin Epidemiol. 1990;43:597–606. doi: 10.1016/0895-4356(90)90164-k. [DOI] [PubMed] [Google Scholar]
- 23.Gardner AW, Katzel LI, Sorkin JD, Bradham DD, Hochberg MC, Flinn WR, et al. Exercise rehabilitation improves functional outcomes and peripheral circulation in patients with intermittent claudication: a randomized controlled trial. J.Am.Geriatr.Soc. 2001;49:755–762. doi: 10.1046/j.1532-5415.2001.49152.x. [DOI] [PubMed] [Google Scholar]
- 24.Gardner AW, Killewich LA, Montgomery PS, Katzel LI. Response to exercise rehabilitation in smoking and nonsmoking patients with intermittent claudication. J.Vasc.Surg. 2004;39:531–538. doi: 10.1016/j.jvs.2003.08.037. [DOI] [PubMed] [Google Scholar]
- 25.Gardner AW, Parker DE, Montgomery PS, Khurana A, Ritti-Dias RM, Blevins SM. Gender differences in daily ambulatory activity patterns in patients with intermittent claudication. J Vasc Surg. 2010;52:1204–1210. doi: 10.1016/j.jvs.2010.05.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gardner AW, Montgomery PS, Parker DE. Metabolic syndrome impairs physical function, health-related quality of life, and peripheral circulation in patients with intermittent claudication. J.Vasc.Surg. 2006;43:1191–1196. doi: 10.1016/j.jvs.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 27.Gardner AW, Montgomery PS. The effect of metabolic syndrome components on exercise performance in patients with intermittent claudication. J Vasc.Surg. 2008;47:1251–1258. doi: 10.1016/j.jvs.2008.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]