Abstract
Significant progress has been made in the field of cancer immunotherapy, where the goal is to activate or modulate the body’s immune response against cancer. However, current immunotherapy approaches exhibit limitations of safety and efficacy due to systemic delivery. In this context, the use of nanotechnology for the delivery of cancer vaccines and immune adjuvants presents a number of advantages such as targeted delivery to immune cells, enhanced therapeutic effect, and reduced adverse outcomes. Recently, gold nanoparticles (AuNP) have been explored as immunotherapy carriers, creating new AuNP applications that merit a critical overview. This review highlights recent advances in the development of AuNP mediated immunotherapies that harness AuNP biodistribution, optical properties and their ability to deliver macromolecules such as peptides and oligonucleotides. It has been demonstrated that the use of AuNP carriers can improve the delivery and safety of immunotherapy agents, and that AuNP immunotherapies are well suited for synergistic combination therapy with existing cancer therapies like photothermal ablation.
Keywords: Gold nanoparticles, Immunotherapy, Biodistribution, Immune system, Cancer
Introduction
Cancer immunotherapy is a promising treatment modality that is a subject of ongoing and extensive study. The goal of this treatment approach is to stimulate the host immune system to detect and eradicate cancer cells, which have developed numerous mechanisms of evading immune recognition. For example, tumor cells down-regulate expression of surface antigens and of co-stimulatory molecules, thus reducing T cell recognition and stimulation.1,2 Tumor cells also secrete immunosuppressive cytokines such as IL-10 and TGFβ, creating an environment that is not conducive to dendritic cell (DC) maturation.1,2 In addition, they are capable of producing factors such as TRAIL and FasL, which induce apoptosis in T cells.1,2 Finally, cancerous tissue can also attract a number of immune suppressive cell types to the tumor microenvironment. These cells include tumor associated macrophages (TAMs), regulatory T cells (Treg), and myeloid derived suppressor cells (MDSCs). TAMs have been shown to promote cancer progression through the release of cytokines that induce angiogenesis, metastasis, and cell growth and can produce anti-inflammatory signals that suppress immune effectors such as natural killer cells and T cells.3–5 Tregs can suppress various immune cells, including cytotoxic T cells and DCs; this suppression occurs through cell to cell contact and Treg expression of inhibitory molecules such as cytotoxic lymphocyte antigen 4 (CTLA-4) and programmed cell death ligand 1 (PD-L1).6 MDSCs originate from the bone marrow and are composed of immature myeloid cells and precursors of cells such as macrophages, granulocytes, and dendritic cells. This population is expanded in a number of tissues in tumor bearing mice, including the liver, lungs, spleen, peripheral blood, and the tumor microenvironment, and it can suppress T cell activity as well as promote the development of Tregs.7–9
Targeting these immune suppressive populations as well as stimulating immune effector cells against tumors is a major goal of cancer immunotherapy.10 As reviewed by Vanneman et al., combining immunotherapies with targeted molecular treatments is a particularly promising approach.10 Agents such as sunitinib (Sutent®), a small molecule receptor tyrosine kinsase inhibitor, and cetuximab (Erbitux®), a chimeric (mouse/human) monoclonal antibody that inhibits EGFR, induce immune anti-tumor responses that could be complemented with immune therapies such as cancer vaccines. For instance, cetuximab has been shown to promote dendritic cell maturation and NK cell mediated tumor killing and is currently being tested in combination with a pancreatic cancer cell vaccine.10
In turn, the delivery and efficacy of immunotherapeutic agents and molecular therapies can be enhanced through the use of nanotechnology. Nanoparticles are well suited for delivery of immune therapies such as vaccines or adjuvants because they preferentially accumulate within tissues and cells of the immune system.11–14 Moon et al. discuss a number of nanoparticle mediated immunotherapies that have been explored recently, demonstrating how nanoparticle delivery can improve therapeutic effect and reduce systemic toxicities.15 Various designs have been explored, including polymeric poly(lactic-co-glycolic acid) (PLGA), liposomes, gelatin based nanoparticles, and AuNPs. For example, Kwong and colleagues showed that liposomes could deliver the immune stimulatory CpG oligonucleotide and anti-CD40 antibody, inducing an enhanced anti-tumor response without causing a systemic increase in inflammatory cytokines typically associated with these treatments.16 Gelatin nanoparticles have also been used to simultaneously deliver the ovalbumin antigen and the CpG adjuvant, demonstrating enhanced effect and reduced toxicity.17
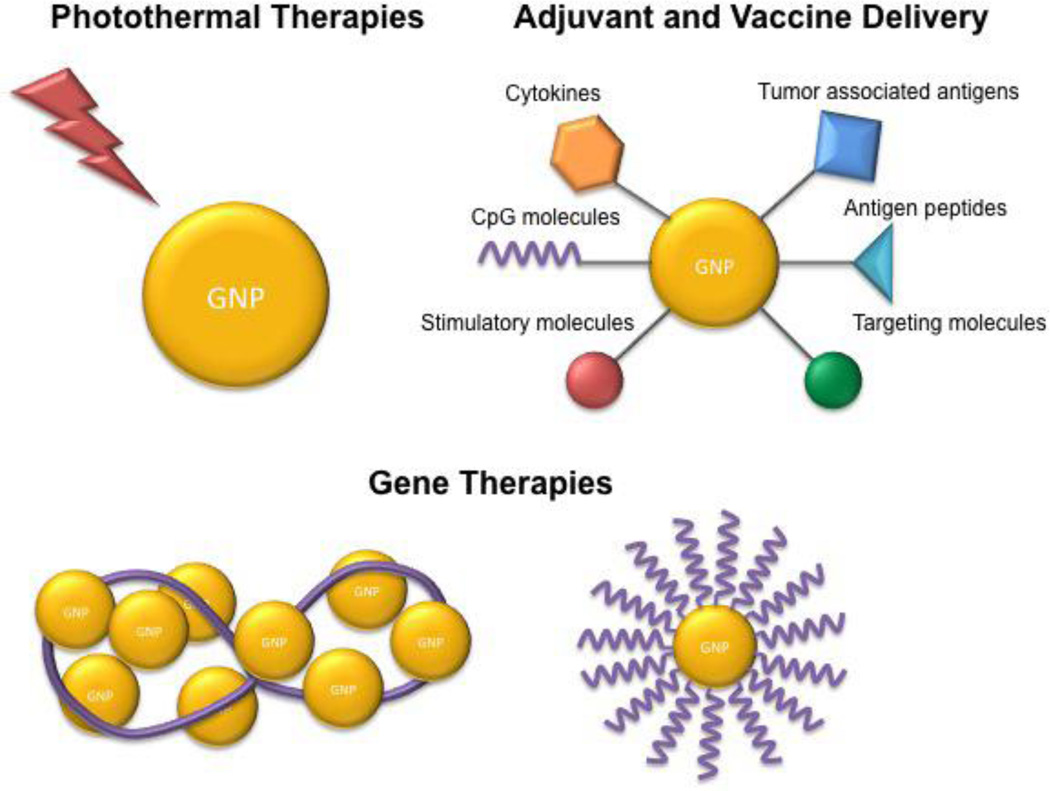
Recently, AuNPs have been applied in immunotherapies, including cancer antigen and immune adjuvant delivery.18–23 AuNPs are a promising carrier for immune therapies because, like other nanoparticles, they easily accumulate in the immune system.12, 13 In addition, AuNPs are bioinert, can be functionalized with drugs and other ligands, and can also be easily tuned to a desired size or shape. Importantly, however, AuNPs, have unique optical properties that can be exploited for immune therapies, particularly their applications in photothermal ablation and light triggered drug delivery.24–26 In this review, we discuss the most recent understanding of the biodistribution and immune interactions of AuNPs. In addition, we discuss their unique optical properties and how photothermal therapies can be used for immune applications. Finally, we review their recent use in immunotherapy, including drug and gene delivery studies. Overall, the multiple functionalities of AuNPs make them promising vehicles for immune therapies, particularly for combinatorial treatment approaches that target multiple immune pathways (Figure 1).
Figure 1.
Applications of gold nanoparticles in cancer immunotherapy.
Biodistribution and immune response
The blood clearance and organ accumulation of AuNPs in vivo is affected by various factors such as particle size, shape, charge, and coating.12, 13, 27, 28 In general, smaller particles circulate in the blood longer and distribute more widely than larger particles, and surface coating with polyethylene glycol (PEG) can reduce opsonization and uptake by the reticuloendothelial system. For instance, Zhang and colleagues have shown that 20 nm PEGylated AuNPs accumulate in the liver and spleen to a lesser extent than 80 nm particles and that a higher percent dose of the 20 nm particles reaches the targeted tumor site.29 The group postulates that smaller particles permit a more dense coating on the particle surface. Similarly, Perrault et al. have reported that AuNP blood half-life increases with decreasing particle size and increasing PEG molecular weight.30 Recently, however, Larson et al. have shown that the PEG on the AuNP surface can be displaced with cysteine and cystine present in the blood, thereby causing protein absorption and macrophage uptake. The group improved upon the typical PEG design by adding an alkyl linker between the PEG and the thiol that binds to the gold surface, thereby reducing PEG displacement and macrophage uptake.31
A number of groups have investigated the mechanisms of AuNP uptake by various cell types as well as the particle characteristics affecting such uptake. Recently, Liu and colleagues characterized the effect of particle charge on AuNP uptake in both phagocytic and nonphagocytic cells.32 Positively charged particles were taken up to a much higher extent by nonphagocytic cells than negatively charged ones. On the other hand, particle charge had little effect on uptake by phagocytic cells. The particles in non-phagocytic cells were localized to secondary lysosomes and formed small aggregates while the ones in phagocytic cells were found in phagosomes, indicating that non-phagocytic cells take up particles through clathrin-mediated endocytosis while the phagocytic ones take them up through phagocytosis.32 Franca et al. further elucidated phagocytic cell uptake by characterizing the uptake of AuNPs 30 nm and 150 nm in diameter.33 Both particle sizes could be phagocytosed, but the group found that clathrin mediated pinocytosis could induce uptake of 30 nm particles but not 150 nm ones. Scavenger receptor mediated phagocytosis was a major factor in 150 nm AuNP uptake. Nevertheless, inhibition of clathrin and calveolin mediated pathways did not completely block AuNP uptake, indicating that there are other pathways involved, and elucidating such mechanisms merits further study.33
Upon uptake, studies have shown that particles can retain in the body for extended periods of time. For instance, Sadauskas et al. observed gold retention within macrophage clusters in the liver over a 6 month period.34 Balasubramanian et al., in turn, observed high gold content in the liver and spleen of rats after 2 months.35 In general, smaller particles excrete more readily from the body than larger particles because the smaller size facilitates renal and hepato-biliary clearance.27, 35, 36 Zhang et al. exploited this characteristic to develop glutathione (GSH) coated gold nanoclusters that demonstrated low retention in the liver and spleen and high renal excretion.37 As opposed to bovine serum albumin (BSA) coated nanoclusters that aggregated to a size between 40 and 80 nm and showed approximately 1% urine excretion, the GSH protected clusters remained between 5 and 30 nm in size and showed 36% urine excretion.37
Nevertheless, despite such design changes, AuNPs inevitably accumulate in high concentrations in the liver and spleen, and as such, it is important to understand how AuNPs interact with the immune system. Research on the immune effects of gold nanoparticles is still in its infancy, but a number of in vitro and in vivo reports are noteworthy. Yen et al. examined AuNP effects on macrophages in vitro and concluded that AuNPs can induce proinflammatory cytokine expression in a size dependent manner.38 Other groups, however, have found that AuNPs can inhibit IL-1β mediated inflammatory responses39 and toll like receptor 9 (TLR9) responses,40 also in a size dependent manner. These studies looked at particles in the 2 to 50 nm size range and found that the smallest particles (< 5 nm) had the highest impact on immune response. Kim and colleagues observed that oligonucleotide conjugated 13 nm AuNPs increased expression of innate immunity genes associated with inflammatory and defense responses in human peripheral blood mononuclear cells.41 Interestingly, they did not obtain the same results when testing the AuNPs on the 293T cell line, suggesting that assessing immunological and toxic impacts of AuNPs on immortalized cell lines as opposed to primary cells may yield differing results.41 Moyano et al., in turn, analyzed how the immune system would react to 2 nm AuNPs of varying hydrophobicity, and the group determined that the more hydrophobic nanoparticles would induce higher expression of inflammatory cytokines by mouse splenocytes.42
Tsai et al. found that 13 nm AuNPs could bind to the immune suppressive TGF-β1 protein secreted by murine bladder tumor 2 cells (MBT-2) in a time and dose dependent manner in vitro.43 The absorption of the cytokine to the nanoparticle surface also induced a conformational change that reduced the biological activity of TGF-β1, thereby reducing epithelial-mesenchymal transition of murine mammary gland cells in vitro. Finally, the group demonstrated that AuNPs could inhibit tumor growth when MBT-2 cells were implanted in mice in the presence of AuNPs, The particles were shown to reduce the systemic concentration of TGF-β1 as well as the concentration of the immune suppressive cytokine IL-10. In addition, tumors implanted in the presence of AuNPs showed higher infiltration of CD4+ and CD8+ T cells. Overall, their results demonstrate that AuNPs can attenuate TGF-β1 signaling and subsequently promote an anti-tumor immune response.43 Further studies on AuNP interactions with other immune modulating cytokines can potential yield new immune therapeutic applications for AuNPs.
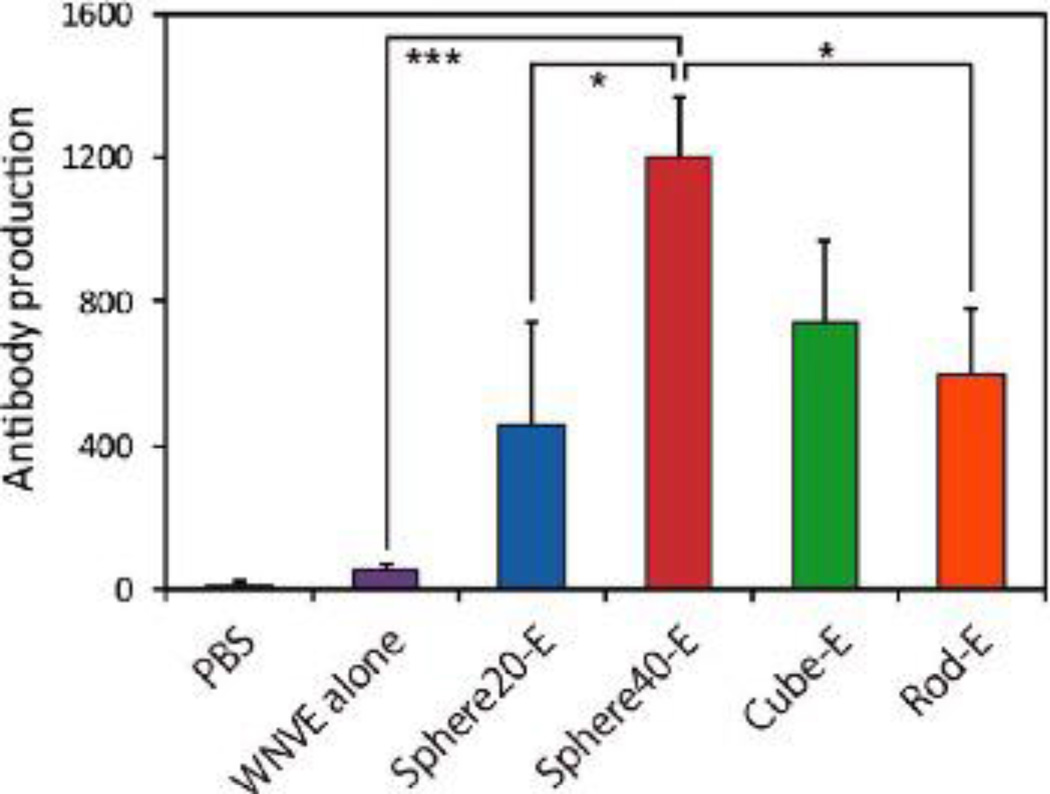
Similarly, Niikura et al. recently explored the size and shape effects of gold nanoparticles on inflammatory responses in vitro and in vivo.23 Their study showed that antigen coated 40 nm spherical and cubic gold nanoparticles induced secretion of cytokines such as TNFα, IL-12, and IL-6 in bone marrow derived dendritic cells (BMDCs) while 20 nm spherical particles and 36×10 nm gold nanorods did not.23 In addition, the 40 nm spherical AuNPs induced higher antigen specific antibody production after intraperitoneal injection in vivo than antigen alone or antigen coated particles of different designs (Figure 2). Overall, the group concluded that AuNPs can act as a vaccine adjuvant in a size and shape dependent manner.23 On the other hand, Chen et al. observed that 21 nm spherical gold nanoparticles inhibited TNFα expression in adipose tissue of mice after intraperitoneal injection, thereby indicating an anti-inflammatory effect of AuNPs.44 The immune response to AuNPs may therefore be tissue dependent, as well as particle size and shape dependent, and future studies should expand upon these findings.
Figure 2.
AuNPs enhance antibody production in vivo when compared to antigen delivery alone. Spherical, 40 nm AuNPs were the most effective design. Reproduced with permission from Niikura et al.23
Bartneck and colleagues assessed the immune modulatory characteristics of AuNPs by characterizing their effect in a hepatitis disease model.45 The group determined that, depending on nanorod surface coating, the particles could polarize liver macrophages to an inflammatory or anti-inflammatory state. For instance, nanorods coated with PEG and linked to the RGD tripeptide induced an anti-inflammatory M2 polarization characterized by IL4 expression in hepatic macrophages, while nanorods coated with the GLF tripeptide promoted the inflammatory M1 polarization characterized by TNFα expression.45 Both nanorod designs ultimately worsened liver injury in an acute hepatitis model, emphasizing the importance of understanding immune reactions to gold nanoparticles when applied in a disease context.
The differing results regarding the inflammatory or anti-inflammatory effects of AuNPs merit further consideration. The anti-inflammatory effects on TLR9 and IL-1β responsiveness appear to be mediated by AuNP interactions with proteins that modulate these pathways. Tsai et al., for instance, indicate that AuNPs can inhibit TLR9 signaling by binding to high mobility group box-1 (HMGB-1), an important regulator of that pathway.40 Sumbayev et al. suggest that AuNPs bind directly to IL-1β, thereby inhibiting its downstream signaling.39 The cause of AuNP inflammatory effects is less clear, and studies should ensure that endotoxin contamination does not influence the results.46 Vallhov et al. demonstrated that AuNPs with low endotoxin contamination showed only minor maturation effects on dendritic cells while contaminated AuNPs induced expression of co-stimulation markers and cytokine expression.46 In their study on macrophage uptake, Franca et al. demonstrated that the nanoparticles had low endotoxin content (<0.005 EU/ml) and found that these particles did not induce pro-inflammatory cytokine release in macrophages.33 Therefore, it is important that inflammatory findings account for the presence of endotoxin and that the potential inflammatory or anti-inflammatory effects of AuNPs be rigorously evaluated.
Overall, although the distribution and immune modulatory activity of gold nanoparticles in vivo can potentially induce inflammatory or anti-inflammatory side effects, these properties can also be manipulated for immune therapy applications, such as vaccine and immune adjuvant delivery. In the following sections we explore the unique characteristics of gold nanoparticles and how they have been used for immunotherapy and what future applications could be explored.
Optically responsive AuNPs for immune applications
AuNPs have unique optical properties that can be manipulated for diagnostic and therapeutic applications.24, 26 Importantly, light can excite free electrons on the surface of the nanoparticle and induce surface plasmon resonance, or a collective oscillation of the electrons. For photothermal therapy (PTT), the nanoparticles can be tuned by varying size and shell thickness to absorb incident light in the near infrared range. Within this range, light can penetrate healthy tissue and be absorbed by the nanoparticles, which then heat up and destroy the nearby cancer cells.26, 47 In addition to PTT, a number of thermal treatments and related therapies for cancer currently exist or are in development. These include radiofrequency ablation, cryoablation, photodynamic therapy and magnetic nanoparticle hyperthermia. Treatment with heat can induce death through different pathways, depending on temperature and duration. In general, heat treatments between 41 and 47 °C induce apoptosis in cancer cells while treatments above this range induce necrosis.48 Although heat treatments have generally focused on local tumors, studies have shown that these treatments can induce a tumor specific immune response.49–51 This response is mediated by the release of antigens and heat shock proteins from dying tumor cells, which are then captured by dendritic cells (DCs) and other antigen presenting cells (APCs). The immune system can subsequently mount a response against cancer cells in distal, untreated sites.
A number of nanoparticles have been designed for PTT, including hollow gold nanoshells, gold silica nanoshells, and gold-gold sulfide nanoparticles, the characteristics of which have been previously reviewed.47, 52 The use of AuNPs for PTT was pioneered by the Halas and West groups, demonstrating that PTT could eradicate mouse tumors and enhance survival.25, 53 Although gold nanoparticle PTT is effective, it is limited to accessible sites and is not currently applicable in metastatic disease. Yet, the immune response following PTT has not been thoroughly studied and can potentially be enhanced to treat distant sites not accessible by PTT. A recent study on immune modulating properties of this treatment was tested in vitro.54 Nguyen and colleagues found that tumor cell necrosis following gold nanoshell thermal treatment in vitro caused the release of damage associated molecular patterns (DAMPs) such as ATP and uric acid, but this release did not induce stimulation of macrophages.54 A related in vivo study used AuNPs as immunotherapy delivery vehicles but did not assess the effects of PTT. In this study Visaria and colleagues used TNF conjugated gold nanoparticles to promote the antitumor activity of hyperthermia.55 The group shows that TNF pre-treatment enhances the damage caused by hyperthermia, and they posit that TNF treatment induced inflammatory pathways that caused vascular damage at the tumor site.55 Although this method promoted tumor death at the local site, its efficacy in treating distant tumors was not demonstrated.
Bear et al. assessed the systemic effects of PTT in a melanoma model, and observed that PTT can promote a tumor specific immune response against a distant, subcutaneous B16-ovalbumin (B16-OVA) tumor.56 This response was mediated by the infiltration of CD4+ helper T cells and CD8+ cytotoxic T cells (CTLs). However, the response was also marked by a significant increase in myeloid derived suppressor cells (MDSCs) at the distant site and at the spleen compared to mice that did not receive treatment. In addition, PTT was followed by a systemic increase in inflammatory cytokines such as IL-6 and IL-1β, as well as an increase in factors such as GM-CSF and G-CSF. Such findings suggest that PTT can produce immune stimulatory and immune inhibitory effects that could be exploited in the treatment of metastatic disease. In this study, Bear and colleagues combined PTT with adoptive T cell therapy and found that the combination therapy could inhibit metastatic tumor growth to a greater extent than either treatment alone.56
Other potential combination treatments include the use of PTT in conjunction with other immune modulatory agents such as drugs, oligonucleotides, or proteins. In delivering such agents, the optical properties of AuNPs could again be exploited. For instance, Dreaden et al. developed macrolide-coated, NIR tuned gold nanorods to target macrophages and stimulate their response against breast carcinoma cells in vitro.57 This nanorod design could potentially be used to better target tumors in vivo and as a combination therapy of PTT and immunotherapy.57 Gold nanoparticles have also been used for thermally controlled release of DNA molecules and chemotherapy drugs.58–61 Yavuz and colleagues developed doxorubicin encapsulating gold nanocages coated with a thermally responsive polymer that would change conformation upon heating.61 The nanocages were tuned to absorb light in the NIR range and would heat up upon light exposure, thereby releasing the encapsulated drug.61 Similarly, You et al. employed doxorubicin loaded hollow gold nanoshells to apply a PTT and chemotherapy combination treatment in vivo, demonstrating that the combination was more effective than either treatment alone and that it reduced the systemic toxicity of doxoxrubicin.62 This combination therapy could potentially be applied in a metastatic disease model, as the effects of both the drug and the thermal treatment could induce a systemic immune response against distant, untreated sites. Doxorubicin has been shown to cause immunogenic cell death in cancer cells,63 an effect that could be exploited in favor of anti-tumor vaccines and other immunotherapies.64 Ultimately, the inflammatory response, antigen release, and immunogenic apoptosis caused by a combination of PTT and doxorubicin could prove to cause a potent immune response against metastatic cancer sites.
The ability to construct thermally responsive AuNPs that carry nucleic acids also opens a range of combination treatment possibilities. Lu et al. developed siRNA carrying hollow gold nanospheres that were tuned to absorb light in the NIR range.59 The group postulated that the siRNA was released due to the collapse of the particles upon heating and due to breaking of the thiol bond holding the siRNA to the particle (Figure 3). The siRNA was specific for the NF-κB p65 subunit, and Lu et al. showed that this molecule could be specifically silenced only in tumors that were irradiated with NIR light. As such, nonspecific delivery of the siRNA to non-irradiated organs such as the liver and spleen did not cause downregulation of the molecule.59 Still, the release of nucleic acids from AuNPs can be further refined, as demonstrated by Poon et al.58 In their work the group showed that double stranded DNA release from gold nanoshells could be tuned using laser power and conjugation strategies so as to release single stranded DNA by denaturation as opposed to double stranded DNA by breakage of the thiol bond.58
Figure 3.
Hollow gold nanoshells can be irradiated with NIR light to cause particle collapse and release of conjugated siRNA. Reproduced with permission from Lu et al.59
The ability to release nucleic acids from AuNPs may be promising for immune therapies, as thermal ablation or light triggered release could be combined with delivery of nucleic acid immune adjuvants such as toll like receptor (TLR) agonists. For example, the CpG oligonucleotide adjuvant has been conjugated to gold nanoparticles, and it has been demonstrated that AuNPs enhance the effect of CpG in vitro and in vivo.18, 19, 22 Of course, optically triggered nanoparticles are not limited to these applications. For instance, Yeheskely-Havon et al. recently utilized AuNPs targeted to both DCs and lymphoma cells in order to bring the cells together and then induce membrane fusion by irradiating the particles at their resonant frequency.65 A number of treatment modalities using AuNP-mediated delivery of immunotherapeutic agents have been devised, all of which could potentially be combined with the optical and thermal properties of these nanoparticles. The following sections will discuss recent advances in AuNP mediated delivery of immunotherapies and their potential implications for future studies.
Gold nanoparticle mediated adjuvant delivery
AuNPs are regarded as efficient nanocarriers as they are inert, chemically robust and able to protect molecules like antigens (Ag) and cytokines from degradation.66 AuNPs can be functionalized with molecules that modulate dendritic cell (DC) and T-cell activation, or induce humoral responses.67, 68 Furthermore, AuNPs can be scaled to be less than 100 nm in diameter, allowing for efficient passive delivery of conjugated molecules to DCs residing in the lymph nodes (LNs).67 AuNP mediated immunotherapy includes a wide variety of treatments that stimulate different cellular pathways.
In the normal biological environment, inflammatory cytokines or pathogens produce danger signals for immune cell activation. Vaccines, for example, mimic this behavior through the use of natural or synthetic adjuvants.66 The goal of adjuvants is to activate the immune system, often to prime it for a subsequent immunotherapy. As discussed above, AuNPs alone have been shown to stimulate the immune system and thus provide attractive candidates for adjuvant delivery. For example, Bastus et al. showed that 10 nm AuNPs conjugated with two peptides stimulated macrophage activation as evidenced by induction of TNF, IL-1β, IL-6 and NO, while the macrophages did not recognize the peptides or AuNP alone.69 Similarly, Fallarini and co-workers functionalized 2–5 nm AuNPs with disaccharides modeled after the Neisseria meningitides bacterium and found that these particles induced macrophage activation, T-cell proliferation and increased IL-2 production.68
As aforementioned, several groups have investigated AuNP mediated CpG delivery. CpG molecules are short DNA sequences that mimic bacterial DNA and thus stimulate immune cells via interaction with TLR9, found in the endolysosomal compartments of cells.70 AuNPs and CpG constructs highlight the benefits that nanoparticle delivery provides for immunotherapy since AuNPs are rapidly uptaken by endolysosomal compartments of phagocytic immune cells, where CpG is active. Wei et al. took 15 and 30 nm AuNPs and formed a self-assembled layer of thiolated CpG to investigate the immunostimulatory effects of AuCpG particles. The group found that AuCpG induced TNFalpha and IL-6 production, confirming that the bioactivity of CpG is not altered by the AuNP carrier. Furthermore, they found that the AuNP carrier makes the system much more efficient, with 15-fold more TNF secretion stimulated by 30-fold less CpG.22 Lin and colleagues recently published a study evaluating different AuCpG designs in vitro and in vivo. The group developed a triethylene glycol (TEG) modified CpG functionalized AuNP, resulting in inhibition of tumor growth compared to free CpG. Lin also found that the smaller 15 nm AuNP cores resulted in more immune stimulation compared to 30 and 80 nm cores.19 Of note, Tsai et al. showed that when bare AuNPs were co-delivered with CpG-ODNs, the AuNP competed for high-mobility group box-1 binding, resulting in inhibited CpG-mediated TLR9 function.40 Therefore, by binding CpG to the surface of AuNPs, such competitive binding can be avoided, and the AuNPs can in turn facilitate access of CpG to TLR9.
In another adjuvant delivery approach, cytokines like tumor necrosis factor (TNF) can be functionalized on AuNPs. Paciotti et al. conjugated thiolated polyethylene glycol (PEG-SH) and recombinant human TNF onto 33 nm AuNPs, and delivered the particles intravenously in human prostate tumor-bearing mice, where rapid uptake was shown within 4 hours in the MC-38 colon carcinoma tumors with minimal reticuloendothelial system (RES) uptake.71, 72 Paciotti and Myer showed that conjugating TNF onto the AuNP resulted in maximal antitumor responses with lower doses of TNF and decreased off-site toxicity compared to TNF alone. The biocompatibility of the particles was proposed to be due to PEG-SH, which allowed the particle to avoid detection and clearance by the RES, thus allowing accumulation in the solid tumor.71 Goel and co-workers continued this work, reaching phase 1 clinical trials in 2005 under the name CYT-6091 (Aurimune™) for 29 enrolled patients with solid tumors that were not responsive to traditional therapies. The therapy showed the highest reported systemically administered TNF dose in humans without adverse effects beyond controllable fever, more than 3 times the maximum tolerated dose previously reported. Phase 2 clinical trials will evaluate combination treatment with CYT-6091 and conventional chemotherapy for non-resectable tumors.73 CYT-6091 has also been used as a vascular disrupting agent in conjunction with cryosurgery or hyperthermia, and a synergistic antitumor response was shown that was greater than the sum of either treatment alone.72,74
Gold nanoparticle cancer vaccines
In addition to molecular adjuvants, vaccines are one of the major cancer immunotherapy approaches pursued due to their specific, strong and persistent immune response.23 However, one of the large drawbacks for cancer vaccines is the weak immune-stimulating capacity of tumor antigen to overcome tumor load and to induce potent humoral and cellular immunity.18 Several groups have investigated AuNP vaccines for their ability to deliver a large payload and for their size tunability.23, 75–77 Arnaiz et al. have used this approach recently in an HIV model. The group used 1.8 nm AuNPs functionalized with clusters of gp120, an HIV Ag. The clustering of various gp120 structural motifs on the AuNP surface served to mimic the presentation of oligomannosides by HIV to DCs, resulting in endocytosis by DCs and inhibition of HIV infection in T cells. Such a biomimicry approach could be implemented in a cancer immunotherapy application to improve DC targeting and uptake of AuNP vaccines by utilizing multivalent presentation of Ag.78 Lin and colleagues used 30 nm AuNPs functionalized with selfassembling modified PEGs and tumor-associated antigen peptides as a cancer vaccine platform. The AuNP construct, termed gold nanovaccine (AuNV), was fabricated by a facile one-pot synthesis, with a final particle that was less than 80 nm in diameter and able to carry large doses of peptides. The AuNV particles were tested in vitro for their ability to stimulate splenocytes as measured by an IFN-γ enzyme-linked immunosorbent spot assay (ELISPOT). The ELISPOT assay evaluated Ag-specific CD8+ CTL IFN-γ secretion, which highly correlates to anti-tumor immunogenicity. Lin et al. found that the AuNV particles were able to stimulate T cells at approximately four-fold efficiency compared to free antigen peptide. The design was also shown to be highly antigen specific and biocompatible.20
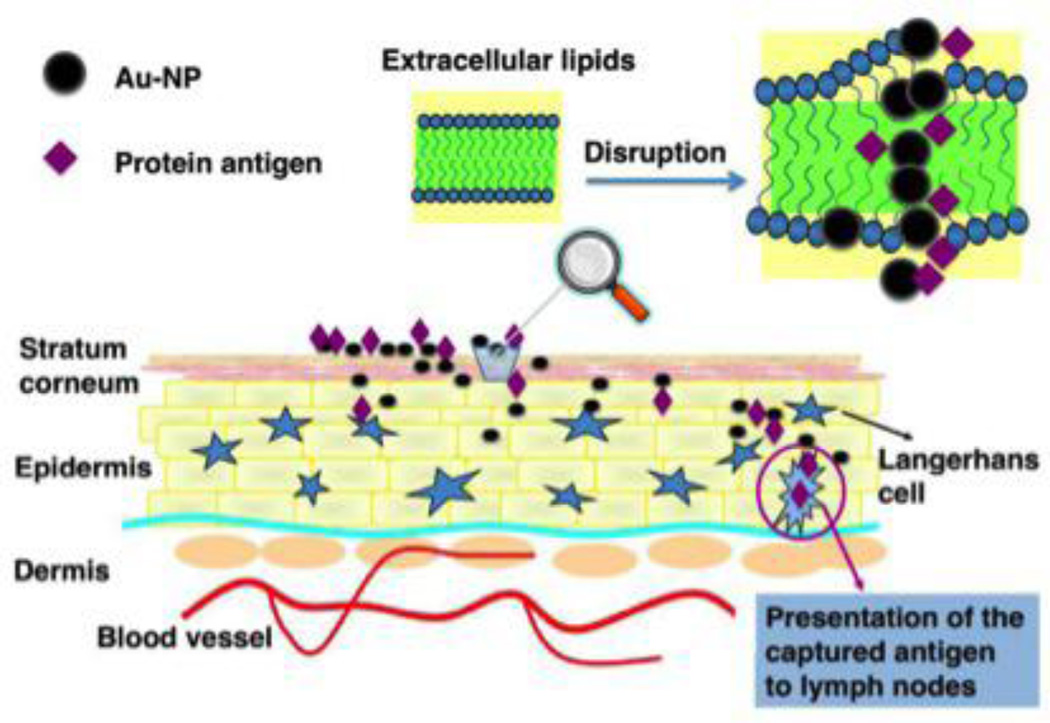
Recent work in the delivery of cancer vaccines using AuNPs has investigated the use of sub-10 nm AuNPs for needle-less vaccination. Small AuNPs have been shown to be skin permeable with the ability to migrate into the deep layers. Huang and colleagues posit that this penetration of the skin barrier is facilitated by interaction with extracellular skin lipids that result in transient openings in the stratum corneum (Figure 4). 79 Huang utilized 5 nm AuNPs coadministered with a protein Ag, OVA, and showed a robust immune response in mice as measured by anti-OVA IgG levels and compared to controls with free OVA protein administered topically and injected intramuscularly. Thus, future studies using small AuNPs functionalized or even simply co-delivered with tumor antigens or peptides may facilitate the use of topically administered vaccines.79
Figure 4.
AuNPs facilitate transcutaneous delivery of protein antigen. Reproduced with permission from Huang et al.79
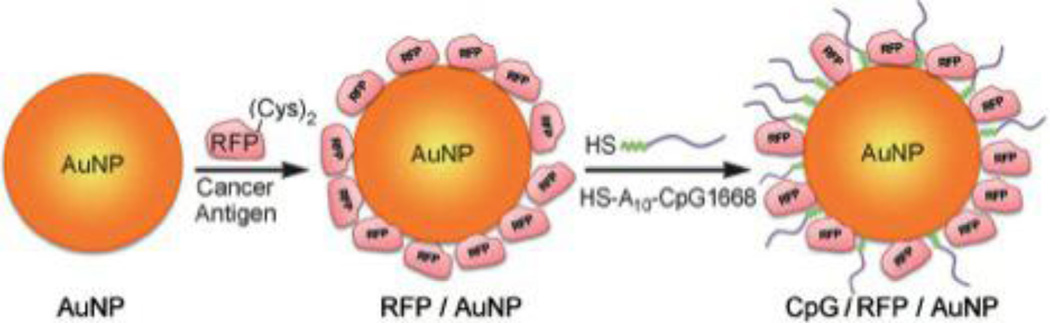
Co-delivery of adjuvants with vaccination has proven to be a highly effective and synergistic approach.80–83 The goal of dual vaccine-adjuvant therapy is to elicit a strong, antigen specific CTL response as well as induction of antibody responses to existing tumors. New approaches have explored the co-delivery of both adjuvant and vaccine molecules on one gold nanoparticle delivery vector. Lee and colleagues investigated a dual vaccine-adjuvant delivery system by conjugating 7 nm AuNPs with red fluorescent protein (RFP) as a model Ag and CpG 1668 as the adjuvant (Figure 5). The particles were delivered three times at one-week intervals via footpad injection in B16F10 melanoma tumor-bearing mice. The particles accumulated in proximal lymph nodes where they interacted with DCs, resulting in significant antitumor activity and potent induction of Ab production through a Th1-mediated pathway. Of note, the vaccine was also shown to prevent lung metastasis due to the potent T cell response.18 The group concluded, similar to Arnaiz et al.,78 that AuNP systems that mimic viral properties with respect to size, geometry and antigen presentation are attractive vaccine candidates due to their improved LN accumulation and interaction with APCs. Furthermore, the addition of an adjuvant can activate DCs and induce T-cell responses.18
Figure 5.
AuNPs coated to deliver protein antigen and CpG oligonucleotide adjuvant. Reproduced with permission from Lee et al.18
Cruz et al. developed a targeted construct where a synthetic peptide-based vaccine for androgen-responsive prostate cancer was grafted onto the surface of 10–25 nm AuNPs and targeted specifically to the Fc receptor (FcR) of human DCs in vitro.66, 67 The peptide consisted of luteinizing hormone-releasing hormone (LHRH) in tandem with a T-helper epitope tetanus toxoid fragment (TT) and a C-terminal Cys residue to facilitate dative binding on the AuNP. The goal was to induce an “anti-self” immunity to LHRH by modifying the target molecule on a synthetic peptide immunogen, LHRH-TT. Their results showed a strong immune response compared to non-targeted AuNP-LHRH-TT constructs and naked antigen.66 The TT fragment was shown to be an important contributor to inducing a T-cell response,66 and DC targeting was shown to prevent antigen dispersion in the bloodstream, thus increasing DC antigen uptake and nearly doubling CTL proliferation in vitro.67 In another dual vaccine-adjuvant approach, Brinas et al. conjugated 3–5 nm AuNP cores with tumor associated glycopeptide antigens, thiolated spacers and a B cell activating protein adjuvant at different sites. The group found that mice immunized with these particles produced both IgM and IgG isotypes against each glycopeptide Ag, and that the vaccines with a carbohydrate Ag were more effective than those with 2 contiguous disaccharides.84 This again highlights the utility of designing multivalent AuNP particles to accomplish multiple immune stimulating goals.
In addition to the aforementioned studies, there exist many other AuNP mediated delivery approaches. One emerging example is the use of immune cells as a targeted delivery system, where cells like macrophages, T cells, or DCs are loaded with AuNPs. For instance, Choi et al. fed macrophages gold nanoshells composed of a 60 nm gold core and 27 nm silica shell, and found that the macrophages were able to deliver the nanoshells to the hypoxic center of tumor spheroids, resulting in more significant photoinduced tumor reduction.85 Kennedy and colleagues demonstrated that T cells can be expanded ex vivo and efficiently loaded with 45 nm AuNPs without affecting T cell function or viability, as measured by migration and cytokine production. The group showed the AuNP-loaded T cells were able to migrate to tumor sites in a human tumor xenograft mouse model and that the targeting improved 4-fold with the T cell delivery system. This approach of AuNP loaded immune cells has numerous applications in cancer immunotherapy such as PTT.86
Gene therapy
Gene therapy presents another method that can be tailored to cancer immunotherapies wherein genes are introduced to initiate tumor death or immune activation. There exist a wide range of genes and vectors that have been implemented in clinical trials with successful outcomes.87 AuNPs have been used successfully in many gene therapy applications, and are still being actively investigated.88–97 For example, DNA vaccines can be designed to deliver genes encoding tumor-associated antigens, cytokines, or other molecules in order achieve adoptive immunity.98
Many early DNA-based cancer vaccines were motivated by ballistic DNA delivery methods. Mahvi et al. used a plasmid encoding for a cytokine, granulocyte-macrophage colonystimulating factor (GM-CSF), to transfect resting tumor cells. They accomplished this by coating gold particles with DNA and using a gene gun to transfect tumor cells, followed by exposure to irradiated cancer cells. This method of vaccination resulted in 58% of mice being protected from tumor challenge compared to 2% of the controls.99 Cassaday et al. continued this work to create a complete DNA vaccine with one gene to induce expression of an immunogenic tumor associated Ag (TAA), and a second gene to stimulate APCs. They chose gp100 and GM-CSF, respectively. The results of this approach only yielded a modest immune response, and further studies will be taken to induce stronger anti-tumoral T cell immunity.100 DeLong et al. used a novel approach where nucleic acids were complexed with protamine and attached to gold particles for delivery by gene gun. The particles were tested by delivering plasmid DNA encoding Hepatitis B core (cAg) and surface Ag (sAg). They demonstrated that Au-protamine-DNA conjugates resisted physical and chemical degradation and retained DNA vaccine structure and function in mice, as determined by enzyme-linked immunosorbent assay (ELISA) analysis of several Ags, IFN-gamma, and IL-4.101 Furthermore, these particles illustrated chemotherapy functionality.
Unfortunately, DNA-based vaccines are limited in potency due to their inability to spread in vivo.102 Several groups have explored different transfection routes to overcome this limitation. Hung and co-workers sought to addressed the issue by delivering a gold particle DNA vaccine with a fusion of VP22, an HSV-1 protein that is known to increase intercellular transport, and a model Ag, HPV type 16 E7. DNA-coated AuNPs were injected to the abdominal region of mice once a week for 2 weeks for the vaccination. One week later, mice were challenged subcutaneously with tumor cells in the right leg and monitored.102 By incorporating the VP22 protein, Hung showed a 50-fold increase in Ag-specific CD8+ T cell precursors in vaccinated mice with E7-expressing tumors due to enhanced spreading and major histocompatibility complex (MHC) class I presentation of Ag and potent antitumor activity. Cheng and co-workers expanded upon this by evaluating a dual immunotherapy and antiangiogenesis gold particle DNA vaccine for the treatment of systemic tumors at multiple sites. In this approach, they developed a DNA vaccine encoding calreticulin (CRT) linked to HPV-16 E7 and determined that the N domain of CRT elicits the largest E7-specific antitumor immunity and antiangiogenic effects.103
Cytokine based immunotherapy approaches were used by groups such as Wang et al., who utilized gold particle mediated gene transfer to transfect tumors directly with a plasmid encoding IL-12.104 In this study, the cytokine encoding DNA was delivered in conjunction with an oral melanoma vaccine and the authors found that oral mucosa is among the most suitable tissues for gene gun mediated transfection. The production of IL-12 by the tumor resulted in suppressed tumor growth and improved survival.104
In contrast to the ballistic gene gun methods, Noh et al. showed that 1.4 nm cationic AuNPs resulted in enhanced intramuscular delivery and transfection with a plasmid encoding IL-2 compared to polyethylineimine (PEI), and with less cytotoxicity.105 Similarly, Zhou and colleagues showed that 1.9 nm AuNP conjugated with chitosan were able to deliver a plasmid encoding Hep B Ag via intramuscular injection and successfully immunized mice against challenge.106 Both of these examples highlight the ease with which existing AuNP vectors can be translated to cancer specific immunotherapy applications.
Future Outlook
AuNPs have numerous functionalities that can be harnessed for effective cancer immunotherapy. The photothermal properties are particularly important and can be combined to enhance the effect of other treatments such as cytokine, vaccine, or adjuvant delivery. The physiological changes that result from thermal treatment warrant further investigation so that they can be exploited for combination treatments, as has been recently accomplished with chemotherapy107 and adoptive T cell therapy. The inflammatory response generated by PTT itself can be manipulated to enhance cell therapies and vaccine treatments, but the ability to enhance delivery of immune modulating agents following thermal pre-treatment is particularly promising. For instance, von Maltzahn et al. used gold nanorods to thermally treat tumors and induce a coagulation cascade. Subsequently, doxorubicin-loaded liposomes targeted to the coagulation transglutaminase FXIII honed into the local coagulation of the tumor, delivering a 40 fold higher dose of doxorubicin than non-targeted controls.107 Similarly, Gormley et al. used heat to enhance the delivery of polymers to a tumor site by targeting them towards heat shock proteins which were overexpressed at the tumor following gold nanorod mediated thermal treatment. Untargeted polymers were eliminated from the tumor site while the targeted polymers were retained at the site for up to 12 hours.108 In a separate study, the group also determined that heat pre-treatment could increase the delivery of untargeted polymers by 1.5 fold, as the polymers could penetrate deeper into the tumor tissue.109
Given these findings, AuNP mediated thermal therapy could be used to promote the delivery of targeted nanoparticle vehicles carrying a number of immune modulating agents such as cytokines or oligonucleotides.110 The enhanced delivery in addition to the inflammatory response to PTT can promote systemic immune effects for the treatment of metastatic disease as well as vaccination from future tumor recurrence. In fact, Jackaman and colleagues have shown that treatment of a primary tumor with a combination of IL-2 cytokine and CD40 agonist can induce an immune response against distant, untreated tumor sites.111 Similarly, Fransen et al. demonstrated that local slow release of anti-CD40 antibody at a primary tumor site induced a tumor specific CTL response against distant tumors.112 Such therapies and other immune modulatory agents have been formulated in nanoparticles,15, 16 which could be targeted to thermally treated tumors as was accomplished in the von Maltzahn and Gromley studies.107–109 The physiological and inflammatory response to heat treatment could then potentially both promote delivery and enhance the immune response that results from the immune stimulatory agents.
No nanomaterial is without limitations, however, and AuNPs are no exception (Table 1). Unlike some nanomaterials, AuNPs are non-biodegradable and non-porous and thus are not ideal for time released delivery of drugs and small molecules. Another consideration with the use of AuNP therapeutics is that imaging and PTT is hindered by penetration depth. More importantly, the pharmacokinetics and biocompatibility of AuNPs is altered upon surface modification with ligands and thus each variant of AuNP agent must be characterized in a clinically relevant oncology model. For instance, their effect on normal, malignant and immune-modulating cells should be well studied during preclinical development.113 However, the benefits of gold make AuNPs particularly well suited for cancer immunotherapies and there is no sign of waning interest in this versatile nanomaterial114.
Table 1.
Advantages and limitations of AuNPs as a cancer therapy platform.
| Advantages |
|---|
| Biocompatible |
| Ease of synthesis (monodispersity, size and shape control) |
| Simple ligand conjugation chemistry (ionic, covalent and physical adsorption) |
| Shape and size-dependent optical properties (NIR tuning for PTT) |
| High photothermal conversion rate and photostability (PTT, laser cleavage of gold-thiol bonds) |
| Intense absorption and scattering (contrast agents, resistance to photobleaching) |
| Multifunctional vehicles (delivery, imaging, therapy) |
| Increased molecule loading per particle |
| Colloidal stability |
| Limitations |
|---|
| Limited penetration depth (imaging and PTT) |
| Non-biodegradable, non-porous (encapsulation, timed release) |
| Altered biodistribution, pharmacokinetics and toxicity profile upon surface modification |
| Lack of fundamental understanding of interaction with living cells |
Future work should focus on combination strategies and on new nanoparticle designs that can facilitate the combination of drug delivery and photothermal therapy. Recently, for example, Nam et al. developed 10 nm AuNPs that respond to the acidic tumor microenvironment and are able to both release doxorubicin from the surface and aggregate to form AuNP clusters in a pH-responsive manner. The AuNP clusters can then absorb light in the NIR range and be used for PTT. Such a nanoparticle, as well as the aforementioned polymer nanocage design developed by Yavuz et al.,61 could prove promising for AuNP mediated immunotherapies.
Conclusion
AuNPs are widely studied nanocarriers that have been tested for a number of cancer treatment applications, including drug and gene delivery and photothermal therapy. AuNP in vivo biodistribution has been thoroughly characterized, but the nanoparticles’ effect on the immune system has only recently been explored. Their applications in immunotherapy have great potential, and promising results in vaccine and adjuvant delivery have been reported in recent years. Furthermore, the optical properties of AuNPs create combination treatment possibilities that exploit the inflammatory and coagulation responses to thermal therapy. Future work should explore the development of AuNP combination treatments to induce a systemic, tumor specific immune response in the context of metastatic disease.
Acknowledgments
Funding: This work was supported by the National Institutes of Health R01 CA172836. J. Almeida was also funded by a training fellowship from the Keck Center of the Gulf Coast Consortia, on the Nanobiology Interdisciplinary Graduate Training Program, National Institute of Biomedical Imaging and Bioengineering (NIBIB) T32EB009379, the National Science Foundation Graduate Research Fellowship #0940902, and the Howard Hughes Medical Institute Med into Grad fellowship. E. Figueroa was funded by a pre-doctoral Ford Foundation fellowship as well as a training fellowship from the Keck Center of the Gulf Coast Consortia, on the Nanobiology Interdisciplinary Graduate Training Program, National Institute of Biomedical Imaging and Bioengineering (NIBIB) T32EB009379.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interest.
The authors certify that this manuscript, or any part of it, has not been published and will not be submitted elsewhere for publication while being considered by the journal Nanomedicine: Nanotechnology, Biology, and Medicine.
References
- 1.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 2.Bhutia SK, Mallick SK, Maiti TK. Tumour escape mechanisms and their therapeutic implications in combination tumour therapy. Cell Biology International. 2010;34:553–563. doi: 10.1042/CBI20090206. [DOI] [PubMed] [Google Scholar]
- 3.Weigert A, Sekar D, Brüne B. Tumor-associated macrophages as targets for tumor immunotherapy. Immunotherapy. 2009;1:83–95. doi: 10.2217/1750743X.1.1.83. [DOI] [PubMed] [Google Scholar]
- 4.Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. Journal of Leukocyte Biology. 2009;86:1065–1073. doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- 5.Wang HW, Joyce JA. Alternative activation of tumor-associated macrophages by IL-4: Priming for protumoral functions. Cell Cycle. 2010;9:4824–4835. doi: 10.4161/cc.9.24.14322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beyer M, Schultze JL. Regulatory T cells: Major players in the tumor microenvironment. Current Pharmaceutical Design. 2009;15:1879–1892. doi: 10.2174/138161209788453211. [DOI] [PubMed] [Google Scholar]
- 7.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nature Reviews Immunology. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: Recruitment, phenotype, properties, and mechanisms of immune suppression. Seminars in Cancer Biology. 2006;16:53–65. doi: 10.1016/j.semcancer.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Younos I, Donkor M, Hoke T, Dafferner A, Samson H, Westphal S, Talmadge J. Tumorand organ-dependent infiltration by myeloid-derived suppressor cells. International Immunopharmacology. 2011;11:814–824. doi: 10.1016/j.intimp.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 10.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nature Reviews Cancer. 2012;12:237–251. doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jewell CM, Bustamante Loṕez SC, Irvine DJ. In situ engineering of the lymph node microenvironment via intranodal injection of adjuvant-releasing polymer particles. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:15745–15750. doi: 10.1073/pnas.1105200108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khlebtsov N, Dykman L. Biodistribution and toxicity of engineered gold nanoparticles: a review of in vitro and in vivo studies. Chemical Society Reviews. 2011 doi: 10.1039/c0cs00018c. [DOI] [PubMed] [Google Scholar]
- 13.Almeida JPM, Chen AL, Foster A, Drezek R. In vivo biodistribution of nanoparticles. Nanomedicine. 2011;6:815–835. doi: 10.2217/nnm.11.79. [DOI] [PubMed] [Google Scholar]
- 14.Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Molecular Pharmaceutics. 2008;5:505–515. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moon JJ, Huang B, Irvine DJ. Engineering Nano- and microparticles to tune immunity. Advanced Materials. 2012;24:3724–3746. doi: 10.1002/adma.201200446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwong B, Liu H, Irvine DJ. Induction of potent anti-tumor responses while eliminating systemic side effects via liposome-anchored combinatorial immunotherapy. Biomaterials. 2011;32:5134–5147. doi: 10.1016/j.biomaterials.2011.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bourquin C, Anz D, Zwiorek K, Lanz AL, Fuchs S, Weigel S, Wurzenberger C, Von Der Borch P, Golic M, Moder S, Winter G, Coester C, Endres S. Targeting CpG oligonucleotides to the lymph node by nanoparticles elicits efficient antitumoral immunity. Journal of Immunology. 2008;181:2990–2998. doi: 10.4049/jimmunol.181.5.2990. [DOI] [PubMed] [Google Scholar]
- 18.Lee I-H, Kwon H-K, An S, Kim D, Kim S, Yu MK, Lee J-H, Lee T-S, Im S-H, Jon S. Imageable Antigen-Presenting Gold Nanoparticle Vaccines for Effective Cancer Immunotherapy In Vivo. Angewandte Chemie-International Edition. 2012;51:8800–8805. doi: 10.1002/anie.201203193. [DOI] [PubMed] [Google Scholar]
- 19.Lin AY, Mattos Almeida JP, Bear A, Liu N, Luo L, Foster AE, Drezek RA. Gold Nanoparticle Delivery of Modified CpG Stimulates Macrophages and Inhibits Tumor Growth for Enhanced Immunotherapy. PLoS ONE. 2013;8:e63550. doi: 10.1371/journal.pone.0063550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin AY, Lunsford J, Bear AS, Young JK, Eckels P, Luo L, Foster AE, Drezek RA. Highdensity sub-100-nm peptide-gold nanoparticle complexes improve vaccine presentation by dendritic cells in vitro. Nanoscale Research Letters. 2013:8. doi: 10.1186/1556-276X-8-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Massich MD, Giljohann DA, Schmucker AL, Patel PC, Mirkin CA. Cellular response of polyvalent oligonucleotide - Gold nanoparticle conjugates. ACS Nano. 2010;4:5641–5646. doi: 10.1021/nn102228s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei M, Chen N, Li J, Yin M, Liang L, He Y, Song H, Fan C, Huang Q. Polyvalent Immunostimulatory Nanoagents with Self-Assembled CpG Oligonucleotide-Conjugated Gold Nanoparticles. Angewandte Chemie-International Edition. 2012;51:1202–1206. doi: 10.1002/anie.201105187. [DOI] [PubMed] [Google Scholar]
- 23.Niikura K, Matsunaga T, Suzuki T, Kobayashi S, Yamaguchi H, Orba Y, Kawaguchi A, Hasegawa H, Kajino K, Ninomiya T, Ijiro K, Sawa H. Gold Nanoparticles as a Vaccine Platform: Influence of Size and Shape on Immunological Responses in Vitro and in Vivo. ACS Nano. 2013 doi: 10.1021/nn3057005. [DOI] [PubMed] [Google Scholar]
- 24.Arvizo R, Bhattacharya R, Mukherjee P. Gold nanoparticles: opportunities and challenges in nanomedicine. Expert Opinion on Drug Delivery. 2010;7:753–763. doi: 10.1517/17425241003777010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirsch LR, Stafford RJ, Bankson JA, Sershen SR, Rivera B, Price RE, Hazle JD, Halas NJ, West JL. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:13549–13554. doi: 10.1073/pnas.2232479100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang XH, Jain PK, El-Sayed IH, El-Sayed MA. Gold nanoparticles: interesting optical properties and recent applications in cancer diagnostic and therapy. Nanomedicine. 2007;2:681–693. doi: 10.2217/17435889.2.5.681. [DOI] [PubMed] [Google Scholar]
- 27.Hirn S, Semmler-Behnke M, Schleh C, Wenk A, Lipka J, Schäffler M, Takenaka S, Möller W, Schmid G, Simon U, Kreyling WG. Particle size-dependent and surface charge-dependent biodistribution of gold nanoparticles after intravenous administration. European Journal of Pharmaceutics and Biopharmaceutics. 2011;77:407–416. doi: 10.1016/j.ejpb.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sonavane G, Tomoda K, Makino K. Biodistribution of colloidal gold nanoparticles after intravenous administration: Effect of particle size. Colloids and Surfaces B-Biointerfaces. 2008;66:274–280. doi: 10.1016/j.colsurfb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Zhang GD, Yang Z, Lu W, Zhang R, Huang Q, Tian M, Li L, Liang D, Li C. Influence of anchoring ligands and particle size on the colloidal stability and in vivo biodistribution of polyethylene glycol-coated gold nanoparticles in tumor-xenografted mice. Biomaterials. 2009;30:1928–1936. doi: 10.1016/j.biomaterials.2008.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perrault SD, Walkey C, Jennings T, Fischer HC, Chan WCW. Mediating tumor targeting efficiency of nanoparticles through design. Nano Letters. 2009;9:1909–1915. doi: 10.1021/nl900031y. [DOI] [PubMed] [Google Scholar]
- 31.Larson TA, Joshi PP, Sokolov K. Preventing protein adsorption and macrophage uptake of gold nanoparticles via a hydrophobic shield. ACS Nano. 2012;6:9182–9190. doi: 10.1021/nn3035155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu X, Huang N, Li H, Jin Q, Ji J. Surface and Size Effects on Cell Interaction of Gold Nanoparticles with Both Phagocytic and Nonphagocytic Cells. Langmuir. 2013;29:9138–9148. doi: 10.1021/la401556k. [DOI] [PubMed] [Google Scholar]
- 33.França A, Aggarwal P, Barsov E, Kozlov S, Dobrovolskaia M, González-Fernández A. Macrophage scavenger receptor A mediates the uptake of gold colloids by macrophages in vitro. Nanomedicine (Lond) 2011 doi: 10.2217/nnm.11.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sadauskas E, Danscher G, Stoltenberg M, Vogel U, Larsen A, Wallin H. Protracted elimination of gold nanoparticles from mouse liver. Nanomedicine-Nanotechnology Biology and Medicine. 2009;5:162–169. doi: 10.1016/j.nano.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Balasubramanian SK, Jittiwat J, Manikandan J, Ong CN, Yu LE, Ong WY. Biodistribution of gold nanoparticles and gene expression changes in the liver and spleen after intravenous administration in rats. Biomaterials. 2010;31:2034–2042. doi: 10.1016/j.biomaterials.2009.11.079. [DOI] [PubMed] [Google Scholar]
- 36.Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Ipe BI, Bawendi MG, Frangioni JV. Renal clearance of quantum dots. Nature Biotechnology. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang X-D, Wu D, Shen X, Liu P-X, Fan F-Y, Fan S-J. In vivo renal clearance, biodistribution, toxicity of gold nanoclusters. Biomaterials. 2012;33:4628–4638. doi: 10.1016/j.biomaterials.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 38.Yen HJ, Hsu SH, Tsai CL. Cytotoxicity and immunological response of gold and silver nanoparticles of different sizes. Small. 2009;5:1553–1561. doi: 10.1002/smll.200900126. [DOI] [PubMed] [Google Scholar]
- 39.Sumbayev VV, Yasinska IM, Garcia CP, Gilliland D, Lall GS, Gibbs BF, Bonsall DR, Varani L, Rossi F, Calzolai L. Gold nanoparticles downregulate interleukin-lβ-induced proinflammatory responses. Small. 2013;9:472–477. doi: 10.1002/smll.201201528. [DOI] [PubMed] [Google Scholar]
- 40.Tsai C-Y, Lu S-L, Hu C-W, Yeh C-S, Lee G-B, Lei H-Y. Size-Dependent Attenuation of TLR9 Signaling by Gold Nanoparticles in Macrophages. Journal of Immunology. 2012;188:68–76. doi: 10.4049/jimmunol.1100344. [DOI] [PubMed] [Google Scholar]
- 41.Kim EY, Schulz R, Swantek P, Kunstman K, Malim MH, Wolinsky SM. Gold nanoparticle-mediated gene delivery induces widespread changes in the expression of innate immunity genes. Gene Therapy. 2012;19:347–353. doi: 10.1038/gt.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moyano DF, Goldsmith M, Solfiell DJ, Landesman-Milo D, Miranda OR, Peer D, Rotello VM. Nanoparticle hydrophobicity dictates immune response. Journal of the American Chemical Society. 2012;134:3965–3967. doi: 10.1021/ja2108905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsai YS, Chen YH, Cheng PC, Tsai HT, Shiau AL, Tzai TS, Wu CL. TGF-β1 conjugated to gold nanoparticles results in protein conformational changes and attenuates the biological function. Small. 2013;9:2119–2128. doi: 10.1002/smll.201202755. [DOI] [PubMed] [Google Scholar]
- 44.Chen H, Dorrigan A, Saad S, Hare DJ, Cortie MB, Valenzuela SM. In Vivo Study of Spherical Gold Nanoparticles: Inflammatory Effects and Distribution in Mice. PLoS ONE. 2013:8. doi: 10.1371/journal.pone.0058208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bartneck M, Ritz T, Keul HA, Wambach M, Bornemann J, Gbureck U, Ehling J, Lammers T, Heymann F, Gassler N, Lüdde T, Trautwein C, Groll J, Tacke F. Peptidefunctionalized gold nanorods increase liver injury in hepatitis. ACS Nano. 2012;6:8767–8777. doi: 10.1021/nn302502u. [DOI] [PubMed] [Google Scholar]
- 46.Vallhov H, Qin J, Johansson SM, Ahlborg N, Muhammed MA, Scheynius A, Gabrielsson S. The importance of an endotoxin-free environment during the production of nanoparticles used in medical applications. Nano Letters. 2006;6:1682–1686. doi: 10.1021/nl060860z. [DOI] [PubMed] [Google Scholar]
- 47.Kennedy LC, Bickford LR, Lewinski NA, Coughlin AJ, Hu Y, Day ES, West JL, Drezek RA. A new era for cancer treatment: Gold-nanoparticle-mediated thermal therapies. Small. 2011;7:169–183. doi: 10.1002/smll.201000134. [DOI] [PubMed] [Google Scholar]
- 48.Vauthier C, Tsapis N, Couvreur P. Nanoparticles: Heating tumors to death? Nanomedicine. 2011;6:99–109. doi: 10.2217/nnm.10.138. [DOI] [PubMed] [Google Scholar]
- 49.Greten TF, Korangy F. Radiofrequency ablation for the treatment of HCC - Maybe much more than simple tumor destruction? Journal of Hepatology. 2010;53:775–776. doi: 10.1016/j.jhep.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 50.Ito A, Honda H, Kobayashi T. Cancer immunotherapy based on intracellular hyperthermia using magnetite nanoparticles: A novel concept of "heat-controlled necrosis" with heat shock protein expression. Cancer Immunology, Immunotherapy. 2006;55:320–328. doi: 10.1007/s00262-005-0049-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Den Brok MHMGM, Sutmuller RPM, Van Der Voort R, Bennink EJ, Figdor CG, Ruers TJM, Adema GJ. In situ tumor ablation creates an antigen source for the generation of antitumor immunity. Cancer Research. 2004;64:4024–4029. doi: 10.1158/0008-5472.CAN-03-3949. [DOI] [PubMed] [Google Scholar]
- 52.Young JK, Figueroa ER, Drezek RA. Tunable nanostructures as photothermal theranostic agents. Annals of Biomedical Engineering. 2012;40:438–459. doi: 10.1007/s10439-011-0472-5. [DOI] [PubMed] [Google Scholar]
- 53.O'Neal DP, Hirsch LR, Halas NJ, Payne JD, West JL. Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Letters. 2004;209:171–176. doi: 10.1016/j.canlet.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 54.Nguyen HT, Tran KK, Sun B, Shen H. Activation of inflammasomes by tumor cell death mediated by gold nanoshells. Biomaterials. 2012;33:2197–2205. doi: 10.1016/j.biomaterials.2011.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Visaria RK, Griffin RJ, Williams BW, Ebbini ES, Paciotti GF, Song CW, Bischof JC. Enhancement of tumor thermal therapy using gold nanoparticle-assisted tumor necrosis factor-α delivery. Molecular Cancer Therapeutics. 2006;5:1014–1020. doi: 10.1158/1535-7163.MCT-05-0381. [DOI] [PubMed] [Google Scholar]
- 56.Bear AS, Kennedy LC, Young JK, Perna SK, Mattos Almeida JP, Lin AY, Eckels PC, Drezek RA, Foster AE. Elimination of Metastatic Melanoma Using Gold Nanoshell-Enabled Photothermal Therapy and Adoptive T Cell Transfer. PLoS ONE. 2013;8:e69073. doi: 10.1371/journal.pone.0069073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dreaden EC, Mwakwari SC, Austin LA, Kieffer MJ, Oyelere AK, El-Sayed MA. Small molecule-gold nanorod conjugates selectively target and induce macrophage cytotoxicity towards breast cancer cells. Small. 2012;8:2819–2822. doi: 10.1002/smll.201200333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Poon L, Zandberg W, Hsiao D, Erno Z, Sen D, Gates BD, Branda NR. Photothermal release of single-stranded DNA from the surface of gold nanoparticles through controlled denaturating and Au-S bond breaking. ACS Nano. 2010;4:6395–6403. doi: 10.1021/nn1016346. [DOI] [PubMed] [Google Scholar]
- 59.Lu W, Zhang G, Zhang R, Flores Ii LG, Huang Q, Gelovani JG, Li C. Tumor site-specific silencing of NF-κB p65 by targeted hollow gold nanosphere-mediated photothermal transfection. Cancer Research. 2010;70:3177–3188. doi: 10.1158/0008-5472.CAN-09-3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thibaudau F. Ultrafast photothermal release of DNA from gold nanoparticles. Journal of Physical Chemistry Letters. 2012;3:902–907. doi: 10.1021/jz3001213. [DOI] [PubMed] [Google Scholar]
- 61.Yavuz MS, Cheng Y, Chen J, Cobley CM, Zhang Q, Rycenga M, Xie J, Kim C, Song KH, Schwartz AG, Wang LV, Xia Y. Gold nanocages covered by smart polymers for controlled release with near-infrared light. Nature Materials. 2009;8:935–939. doi: 10.1038/nmat2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.You J, Zhang R, Zhang G, Zhong M, Liu Y, Van Pelt CS, Liang D, Wei W, Sood AK, Li C. Photothermal-chemotherapy with doxorubicin-loaded hollow gold nanospheres: A platform for nearinfrared light-trigged drug release. Journal of Controlled Release. 2012;158:319–328. doi: 10.1016/j.jconrel.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N, Schmitt E, Hamai A, Hervas-Stubbs S, Obeid M, Coutant F, Métivier D, Pichard E, Aucouturier P, Pierron G, Garrido C, Zitvogel L, Kroemer G. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. Journal of Experimental Medicine. 2005;202:1691–1701. doi: 10.1084/jem.20050915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garg AD, Nowis D, Golab J, Vandenabeele P, Krysko DV, Agostinis P. Immunogenic cell death, DAMPs and anticancer therapeutics: An emerging amalgamation. Biochimica et Biophysica Acta -Reviews on Cancer. 2010;1805:53–71. doi: 10.1016/j.bbcan.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 65.Yeheskely-Hayon D, Minai L, Golan L, Dann EJ, Yelin D. Optically Induced Cell Fusion Using Bispecific Nanoparticles. Small. 2013 doi: 10.1002/smll.201300696. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 66.Cruz LJ, Rueda F, Cordobilla B, Simon L, Hosta L, Albericio F, Caries Domingo J. Targeting Nanosystems to Human DCs via Fc Receptor as an Effective Strategy to Deliver Antigen for Immunotherapy. Molecular Pharmaceutics. 2011;8:104–116. doi: 10.1021/mp100178k. [DOI] [PubMed] [Google Scholar]
- 67.Cruz LJ, Tacken PJ, Rueda F, Carles Domingo J, Albericio F, Figdor CG. TARGETING NANOPARTICLES TO DENDRITIC CELLS FOR IMMUNOTHERAPY. Nanomedicine: Infectious Diseases, Immunotherapy, Diagnostics, Antifibrotics, Toxicology and Gene Medicine. 2012;509:143–163. doi: 10.1016/B978-0-12-391858-1.00008-3. [DOI] [PubMed] [Google Scholar]
- 68.Fallarini S, Paoletti T, Battaglini CO, Ronchi P, Lay L, Bonomi R, Jha S, Mancin F, Scrimin P, Lombardi G. Factors affecting T cell responses induced by fully synthetic glyco-goldnanoparticles. Nanoscale. 2013;5:390–400. doi: 10.1039/c2nr32338a. [DOI] [PubMed] [Google Scholar]
- 69.Bastus NG, Sanchez-Tillo E, Pujals S, Farrera C, Kogan MJ, Giralt E, Celada A, Lloberas J, Puntes V. Peptides conjugated to gold nanoparticles induce macrophage activation. Molecular Immunology. 2009;46:743–748. doi: 10.1016/j.molimm.2008.08.277. [DOI] [PubMed] [Google Scholar]
- 70.Badie B, Berlin JM. The future of CpG immunotherapy in cancer. Immunotherapy. 2013;5:1–3. doi: 10.2217/imt.12.148. [DOI] [PubMed] [Google Scholar]
- 71.Paciotti GF, Myer L, Weinreich D, Goia D, Pavel N, McLaughlin RE, Tamarkin L. Colloidal gold: A novel nanoparticle vector for tumor directed drug delivery. Drug Delivery. 2004;11:169–183. doi: 10.1080/10717540490433895. [DOI] [PubMed] [Google Scholar]
- 72.Goel R, Shah N, Visaria R, Paciotti GF, Bischof JC. Biodistribution of TNF-alpha-coated gold nanoparticles in an in vivo model system. Nanomedicine. 2009;4:401–410. doi: 10.2217/nnm.09.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Libutti SK, Paciotti GF, Byrnes AA, Alexander HR, Jr, Gannon WE, Walker M, Seidel GD, Yuldasheva N, Tamarkin L. Phase I and pharmacokinetic studies of CYT-6091, a novel PEGylated colloidal gold-rhTNF nanomedicine. Clinical Cancer Research. 2010;16:6139–6149. doi: 10.1158/1078-0432.CCR-10-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shenoi MM, Iltis I, Choi J, Koonce NA, Metzger GJ, Griffin RJ, Bischof JC. Nanoparticle Delivered Vascular Disrupting Agents (VDAs): Use of TNF-Alpha Conjugated Gold Nanoparticles for Multimodal Cancer Therapy. Molecular Pharmaceutics. 2013;10:1683–1694. doi: 10.1021/mp300505w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Safari D, Marradi M, Chiodo F, Dekker HAT, Shan Y, Adamo R, Oscarson S, Rijkers GT, Lahmann M, Kamerling JP, Penades S, Snippe H. Gold nanoparticles as carriers for a synthetic Streptococcus pneumoniae type 14 conjugate vaccine. Nanomedicine. 2012;7:651–662. doi: 10.2217/nnm.11.151. [DOI] [PubMed] [Google Scholar]
- 76.Pokharkar V, Bhumkar D, Suresh K, Shinde Y, Gairola S, Jadhav SS. Gold Nanoparticles as a Potential Carrier for Transmucosal Vaccine Delivery. Journal of Biomedical Nanotechnology. 2011;7:57–59. doi: 10.1166/jbn.2011.1200. [DOI] [PubMed] [Google Scholar]
- 77.Chen YS, Hung YC, Lin WH, Huang GS. Assessment of gold nanoparticles as a sizedependent vaccine carrier for enhancing the antibody response against synthetic foot-and-mouth disease virus peptide. Nanotechnology. 2010:21. doi: 10.1088/0957-4484/21/19/195101. [DOI] [PubMed] [Google Scholar]
- 78.Arnaiz B, Martinez-Avila O, Falcon-Perez JM, Penades S. Cellular Uptake of Gold Nanoparticles Bearing HIV gp120 Oligomannosides. Bioconjugate Chemistry. 2012;23:814–825. doi: 10.1021/bc200663r. [DOI] [PubMed] [Google Scholar]
- 79.Huang Y, Yu F, Park Y-S, Wang J, Shin M-C, Chung HS, Yang VC. Co-administration of protein drugs with gold nanoparticles to enable percutaneous delivery. Biomaterials. 2010;31:9086–9091. doi: 10.1016/j.biomaterials.2010.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jiang ZH, Koganty RR. Synthetic vaccines: The role of adjuvants in immune targeting. Current Medicinal Chemistry. 2003;10:1423–1439. doi: 10.2174/0929867033457340. [DOI] [PubMed] [Google Scholar]
- 81.Kortylewski M, Swiderski P, Herrmann A, Wang L, Kowolik C, Kujawski M, Lee H, Scuto A, Liu Y, Yang C, Deng J, Soifer HS, Raubitschek A, Forman S, Rossi JJ, Pardoll DM, Jove R, Yu H. In vivo delivery of siRNA to immune cells by conjugation to a TLR9 agonist enhances antitumor immune responses. Nature Biotechnology. 2009;27 doi: 10.1038/nbt.1564. 925-U88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Grossmann C, Tenbusch M, Nchinda G, Temchura V, Nabi G, Stone GW, Kornbluth RS, Ueberla K. Enhancement of the priming efficacy of DNA vaccines encoding dendritic cell-targeted antigens by synergistic toll-like receptor ligands. Bmc Immunology. 2009:10. doi: 10.1186/1471-2172-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Toubaji A, Hill S, Terabe M, Qian J, Floyd T, Simpson RM, Berzofsky JA, Khleif SN. The combination of GM-CSF and IL-2 as local adjuvant shows synergy in enhancing peptide vaccines and provides long term tumor protection. Vaccine. 2007;25:5882–5891. doi: 10.1016/j.vaccine.2007.05.040. [DOI] [PubMed] [Google Scholar]
- 84.Brinas RP, Sundgren A, Sahoo P, Morey S, Rittenhouse-Olson K, Wilding GE, Deng W, Barchi JJ., Jr Design and Synthesis of Multifunctional Gold Nanoparticles Bearing Tumor-Associated Glycopeptide Antigens as Potential Cancer Vaccines. Bioconjugate Chemistry. 2012;23:1513–1523. doi: 10.1021/bc200606s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Choi M-R, Stanton-Maxey KJ, Stanley JK, Levin CS, Bardhan R, Akin D, Badve S, Sturgis J, Robinson JP, Bashir R, Halas NJ, Clare SE. A cellular Trojan horse for delivery of therapeutic nanoparticles into tumors. Nano Letters. 2007;7:3759–3765. doi: 10.1021/nl072209h. [DOI] [PubMed] [Google Scholar]
- 86.Kennedy LC, Bear AS, Young JK, Lewinski NA, Kim J, Foster AE, Drezek RA. T cells enhance gold nanoparticle delivery to tumors in vivo. Nanoscale Research Letters. 2011:6. doi: 10.1186/1556-276X-6-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ginn SL, Alexander IE, Edelstein ML, Abedi MR, Wixon J. Gene therapy clinical trials worldwide to 2012 an update. Journal of Gene Medicine. 2013;15:65–77. doi: 10.1002/jgm.2698. [DOI] [PubMed] [Google Scholar]
- 88.Pissuwan D, Niidome T, Cortie MB. The forthcoming applications of gold nanoparticles in drug and gene delivery systems. Journal of Controlled Release. 2011;149:65–71. doi: 10.1016/j.jconrel.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 89.Rosi NL, Giljohann DA, Thaxton CS, Lytton-Jean AKR, Han MS, Mirkin CA. Oligonucleotide-modified gold nanoparticles for intracellular gene regulation. Science. 2006;312:1027–1030. doi: 10.1126/science.1125559. [DOI] [PubMed] [Google Scholar]
- 90.Ghosh PS, Kim C-K, Han G, Forbes NS, Rotello VM. Efficient Gene Delivery Vectors by Tuning the Surface Charge Density of Amino Acid-Functionalized Gold Nanoparticles. Acs Nano. 2008;2:2213–2218. doi: 10.1021/nn800507t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Massich MD, Giljohann DA, Seferos DS, Ludlow LE, Horvath CM, Mirkin CA. Regulating Immune Response Using Polyvalent Nucleic Acid-Gold Nanoparticle Conjugates. Molecular Pharmaceutics. 2009;6:1934–1940. doi: 10.1021/mp900172m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ryou S-M, Kim S, Jang HH, Kim J-H, Yeom J-H, Eom MS, Bae J, Han MS, Lee K. Delivery of shRNA using gold nanoparticle-DNA oligonucleotide conjugates as a universal carrier. Biochemical and Biophysical Research Communications. 2010;398:542–546. doi: 10.1016/j.bbrc.2010.06.115. [DOI] [PubMed] [Google Scholar]
- 93.Stobiecka M, Hepel M. Double-shell gold nanoparticle-based DNA-carriers with poly-L-lysine binding surface. Biomaterials. 2011;32:3312–3321. doi: 10.1016/j.biomaterials.2010.12.064. [DOI] [PubMed] [Google Scholar]
- 94.Sharma A, Tandon A, Tovey JCK, Gupta R, Robertson JD, Fortune JA, Klibanov AM, Cowden JW, Rieger FG, Mohan RR. Polyethylenimine-conjugated gold nanoparticles: Gene transfer potential and low toxicity in the cornea. Nanomedicine-Nanotechnology Biology and Medicine. 2011;7:505–513. doi: 10.1016/j.nano.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cebrian V, Martin-Saavedra F, Yaguee C, Arruebo M, Santamaria J, Vilaboa N. Sizedependent transfection efficiency of PEI-coated gold nanoparticles. Acta Biomaterialia. 2011;7:3645–3655. doi: 10.1016/j.actbio.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 96.Yan X, Blacklock J, Li J, Moehwald H. One-Pot Synthesis of Polypeptide-Gold Nanoconjugates for in Vitro Gene Transfection. Acs Nano. 2012;6:111–117. doi: 10.1021/nn202939s. [DOI] [PubMed] [Google Scholar]
- 97.Shan Y, Luo T, Peng C, Sheng R, Cao A, Cao X, Shen M, Guo R, Tomas H, Shi X. Gene delivery using dendrimer-entrapped gold nanoparticles as nonviral vectors. Biomaterials. 2012;33:3025–3035. doi: 10.1016/j.biomaterials.2011.12.045. [DOI] [PubMed] [Google Scholar]
- 98.Chlichlia K, Schirrmacher V, Sandaltzopoulos R. Cancer immunotherapy: Battling tumors with gene vaccines. Current Medicinal Chemistry: Anti-Inflammatory and Anti-Allergy Agents. 2005;4:353–365. [Google Scholar]
- 99.Mahvi DM, Burkholder JK, Turner J, Culp J, Malter JS, Sondel PM, Yang NS. Particle-mediated gene transfer of granulocyte-macrophage colony-stimulating factor cDNA to tumor cells: Implications for a clinically relevant tumor vaccine. Human Gene Therapy. 1996;7:1535–1543. doi: 10.1089/hum.1996.7.13-1535. [DOI] [PubMed] [Google Scholar]
- 100.Cassaday RD, Sondel PM, King DM, Macklin MD, Gan J, Warner TF, Zuleger CL, Bridges AJ, Schalch HG, Kim KM, Hank JA, Mahvi DM, Albertini MR. A phase I study of immunization using particle-mediated epidermal delivery of genes for gp100 and GM-CSF into uninvolved skin of melanoma patients. Clinical Cancer Research. 2007;13:540–549. doi: 10.1158/1078-0432.CCR-06-2039. [DOI] [PubMed] [Google Scholar]
- 101.DeLong RK, Akhtar U, Sallee M, Parker B, Barber S, Zhang J, Craig M, Garrad R, Hickey AJ, Engstrom E. Characterization and performance of nucleic acid nanoparticles combined with protamine and gold. Biomaterials. 2009;30:6451–6459. doi: 10.1016/j.biomaterials.2009.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hung CF, Cheng WF, Hsu KF, Chai CY, He L, Ling M, Wu TC. Improving vaccine potency through enhanced intercellular spreading and MHC class 1 presentation of antigen. Modern Pathology. 2001;14:185A–185A. doi: 10.4049/jimmunol.166.9.5733. [DOI] [PubMed] [Google Scholar]
- 103.Cheng WF, Hung CF, Chen CA, Lee CN, Su YN, Chai CY, Boyd DAK, Hsieh CY, Wu TC. Characterization of DNA vaccines encoding the domains of calreticulin for their ability to elicit tumor-specific immunity and antiangiogenesis. Vaccine. 2005;23:3864–3874. doi: 10.1016/j.vaccine.2004.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang J, Murakami T, Hakamata Y, Ajiki T, Jinbu Y, Akasaka Y, Ohtsuki M, Nakagawa H, Kobayashi E. Gene gun-mediated oral mucosal transfer of interleukin 12 cDNA coupled with an irradiated melanoma vaccine in a hamster model: Successful treatment of oral melanoma and distant skin lesion. Cancer Gene Therapy. 2001;8:705–712. doi: 10.1038/sj.cgt.7700363. [DOI] [PubMed] [Google Scholar]
- 105.Noh SM, Kim W-K, Kim SJ, Kim JM, Baek K-H, Oh Y-K. Enhanced cellular delivery and transfection efficiency of plasmid DNA using positively charged biocompatible colloidal gold nanoparticles. Biochimica Et Biophysica Acta-General Subjects. 2007;1770:747–752. doi: 10.1016/j.bbagen.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 106.Zhou X, Zhang X, Yu X, Zha X, Fu Q, Liu B, Wan X, Chen Y, Chen Y, Shan Y, Jin Y, Wu Y, Liu J, Kong W, Shen J. The effect of conjugation to gold nanoparticles on the ability of low molecular weight chitosan to transfer DNA vaccine. Biomaterials. 2008;29:111–117. doi: 10.1016/j.biomaterials.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 107.Von Maltzahn G, Park JH, Lin KY, Singh N, Schwöppe C, Mesters R, Berdel WE, Ruoslahti E, Sailor MJ, Bhatia SN. Nanoparticles that communicate in vivo to amplify tumour targeting. Nature Materials. 2011;10:545–552. doi: 10.1038/nmat3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gormley AJ, Larson N, Sadekar S, Robinson R, Ray A, Ghandehari H. Guided delivery of polymer therapeutics using plasmonic photothermal therapy. Nano Today. 2012;7:158–167. doi: 10.1016/j.nantod.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gormley AJ, Larson N, Banisadr A, Robinson R, Frazier N, Ray A, Ghandehari H. Plasmonic photothermal therapy increases the tumor mass penetration of HPMA copolymers. Journal of Controlled Release. 2013;166:130–138. doi: 10.1016/j.jconrel.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brinker CJ. Nanoparticle immunotherapy: Combo combat. Nature Materials. 2012;11:831–832. doi: 10.1038/nmat3434. [DOI] [PubMed] [Google Scholar]
- 111.Jackaman C, Lew AM, Zhan Y, Allan JE, Koloska B, Graham PT, Robinson BWS, Nelson DJ. Deliberately provoking local inflammation drives tumors to become their own protective vaccine site. International Immunology. 2008;20:1467–1479. doi: 10.1093/intimm/dxn104. [DOI] [PubMed] [Google Scholar]
- 112.Fransen MF, Sluijter M, Morreau H, Arens R, Melief CJM. Local activation of CD8 T cells and systemic tumor eradication without toxicity via slow release and local delivery of agonistic CD40 antibody. Clinical Cancer Research. 2011;17:2270–2280. doi: 10.1158/1078-0432.CCR-10-2888. [DOI] [PubMed] [Google Scholar]
- 113.Stern ST, Hall JB, Yu LL, Wood LJ, Paciotti GF, Tamarkin L, Long SE, McNeil SE. Translational considerations for cancer nanomedicine. Journal of Controlled Release. 2010;146:164–174. doi: 10.1016/j.jconrel.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Weintraub K. The new gold standard. Nature. 2013:S14–S16. doi: 10.1038/495S14a. [DOI] [PubMed] [Google Scholar]