Abstract
Interleukin -22 (IL-22) is a member of IL-10 family cytokines that is produced by many different types of lymphocytes including both those of the innate and adaptive immune system. This includes activated T cells, most notably Th17 and Th22 cells, and NK cells, γδ T cells, LTi cells and LTi-like cells. IL-22 mediates its effects via the IL-22-IL-22R complex and subsequent Janus Kinase-signal transduces and activators transcription (JAK-STAT) signaling pathway. Recently accumulated evidence has indicated that IL-22 also plays an important role in the pathogenesis of many autoimmune diseases. In this review, we discuss the recent findings and advancement of the role for IL-22 in several autoimmune diseases, such as psoriasis, rheumatoid arthritis (RA), hepatitis, graft versus host disease (GHVD) and allergic diseases, implicating that target IL-22 may have a therapeutic potential in those autoimmune diseases.
Keywords: T-helper cell, Innate immunity, Interleukin-22, Cytokine receptor, Signal pathway, Antimicrobial immunity, Autoimmunity
1. Introduction
IL-22 was firstly identified in murine IL-9-stimulated BW5147 T-lymphoma cells (1), and followed by the identification of human IL-22 in two studies (1–2). IL-22 is a member of the IL-10 cytokine family. Its structure is similar to the well-known anti-inflammatory and immunosuppressive cytokine IL-10, for which IL-22 was initially named as IL-10-related-T-cell-derived inducible factor(IL-TIF). Human and mouse IL-TIF both consist of 179 amino acid residues including four aminothiopropionic acids, which show an overall sequence identity with IL-10 of 22% in the mouse and 25% in the human (3).
The human IL-22-encoding gene is located on chromosome 12q15, approximately 52-and 99-Kbp, at 90Kb from the IFN-γ gene, and 27Kb from the AK155 gene. The human IL-22 gene comprises five exons. The first 53 bp of exon 1 encodes the 5′-untranslated region. The other portions of exon 1 (186bp), the exon 2-4(66,144, and 66bp), and the first portion (79) of exon 5 contain the protein-coding part and the stop codon. The rest portions of exon 5 (554bp) encode the 3′-untranslated region, which includes six single and two overlapping copies of the ATTTA motif known to be involved in the regulation of mRNA degradation. The open reading frame is comprised of 537 bp (without the stop codon), predicting a length of 179 AA for the encoded protein. In the mouse, IL-22-encoding gene is located on chromosome 10, also near the IFN-γ gene. There are two copies in different mouse strains, which shows 98% nucleotide identity in the coding region, named IL-TIFα and IL-TIFβ (4). With the knowledge of IL-22, numerous studies regarding the role of IL-22 in autoimmune diseases are emerging.
2. The cellular sources of IL-22
IL-22 was originally thought to be a Th1-associated cytokine. With the discovery of new T helper cells, it has been determined that specific populations of T cells have the capacity to express IL-22, several of which accumulate at barrier surfaces. Th17 and Th22 cells were demonstrated to be important producers (5–7). In Th17 cells, IL-22 expression differs from IL-17 and other Th17-associated cytokines. The presence of transforming growth factor-β (TGF-β) and IL-6, which is mainly required for the generating IL-17A does not lead to optimal IL-22 expression; this is because TGF-β is inhibitory to IL-22 expression(8). Moreover, IL-17A is highly dependent on the nuclear hormone receptor transcription factors retinoic acid-related orphan receptor γt (RORγt) and RORα, whereas IL-22 expression requires the ligand-dependent transcription factor aryl hydrocarbon receptor (AHR)(9–10). In humans, a population of CD4+ T cells that localizes to the skin and can express IL-22, TNF-a, and IL-13, but not IL-17A, has been reported. Given the predominate expression of IL-22, these cells have been termed Th22 cells(7). If cultured in Th1-,Th2-, Th17- or Treg-polarizing conditions, Th22 clones continue to express IL-22 and not the other cytokines associated with these Th subsets(11). Th22 cells appear to be important for skin homeostasis and in inflammation.
In addition to CD4+ T cells, CD8+ T cells also express IL-22 when differentiated into Tc17 cells. Increased population of CD8+T cells expressing IL-22 has also been observed in the skin of patients with atopic dermatitis and correlated with increased disease severity(12). Additionally, similar to Th17 cells, the γδ T- cell has been showed to coexpress IL-22 and IL-17A, and has been implicated in pulmonary immune responses(13,14).
Apart from expression of IL-22 by T cells, innate immune cells also have the capacity to express IL-22. IL-22 was reported to be expressed by blood-derived NK cells.9 There is also described mucosa-associated lymphoid tissue-residing NK cell population in humans, which expresses IL-22 in response to IL-23 (the so-called NK-22). Despite the absence of the classical NK cell effector functions, They rather provide the protection and regulate the mucosal homeostasis. They express NKp44, CCR6, and the transcription factors RORγt, RORα, AHR and IRF4, and produce IL-22, IL-26 and LIF(15,16).
Furthermore, IL-22 expression has been described in several populations of innate lymphoid cells (ILCs) with the capacity to produce IL-22 and coexpress NK cell and myeloid cell markers(17).IL-22-expressing ILCs constitute a heterogeneous population composed of CD4+ lymphoid tissue inducer (LTi) cells characterized by the repression of IL-17, lymphotoxin α1β2 and RORγt, LTi-like cells expressing RORγt, AHR and IL-17, and NKp46+ ILCs expressing RORγt mouse(18–20). Sharing a number of similar phenotypic and transcriptional profiles, these ILCs populations are present at barrier surfaces, and can express IL-22 following stimulation with IL-23 alone. Recent studies have showed that these populations have been implicated in promoting innate immunity and intestinal inflammation, and may represent a more primitive form of IL-22-producing adaptive immune cells(21).
3. IL-22 acts by the IL-22-IL-22R pathway
3a. IL-22R expressed only by non-hematopoietic cell lineages
IL-10 and IL-22 receptors are composed of heterodimeric chains. IL-10 is made up of IL-10R1 and IL-10R2. IL-22 receptor complex consists of IL-22R1 and IL-10R2. The unique signaling and the functional outcome of these two cytokines are maintained by the exclusive use of independent receptor subunits. the IL-22R1 subunit is restricted to cell lineages of a non-hematopoietic origin. In particular, non-hematopoietic cells that have been found to constitutively express a functional IL-22R1 are resident in the pancreas, kidney, and liver, as well as at barrier surfaces such as the skin, intestine, and lung(22–23). In contrast, bone marrow, peripheral blood mononuclear (PBMC), spleen or thymus, all of which contain a high proportion of immune cells, do not express IL-22R1(23). Furthermore, immune cells in general lack IL-22R1 expression and therefore are not targets of IL-22, which is different from its conventional designation as an interleukin(23).The restricted distribution of the IL-22R governs the functions of IL-22 as it restricts the biological effects of IL-22 to non-hematopoietic tissue-resident cells. Interestingly, IL-22R expression is up-regulated following stimulation of human skin cells with INF-γ and TNF-α, or following Con-A or LPS challenge of hepatocytes (23–24), suggesting that the IL-22 action may be affected by the dynamic expression of IL-22R1.
In addition to the surface-bound receptor, a soluble secreted receptor for IL-22 exists, termed IL-22BP or IL-22RA2. IL-22BP is expressed in various tissues, including the breast, lungs, and colon(25). However, the cellular sources of IL-22BP in these tissues remains unclear. IL-22BP binds IL-22 with a sufficient affinity to block IL-22R binding, therefore acting as a natural cytokine antagonist. IL-22BP expression in tissues can be regulated. During acute inflammation, while IL-22 was up-regulated in murine models of infection and colitis, IL-22BP was down-regulated, suggesting that IL-22BP may be important in regulating the in vivo biological consequences of IL-22 expression(26–27). However, further investigations are required to advance our understanding of the regulation and functions of IL-22BP in the context of infection and inflammation, as it may be an important pathway to consider when targeting IL-22.
3b. Signal transduction pathways activated downstream of IL-22R ligation
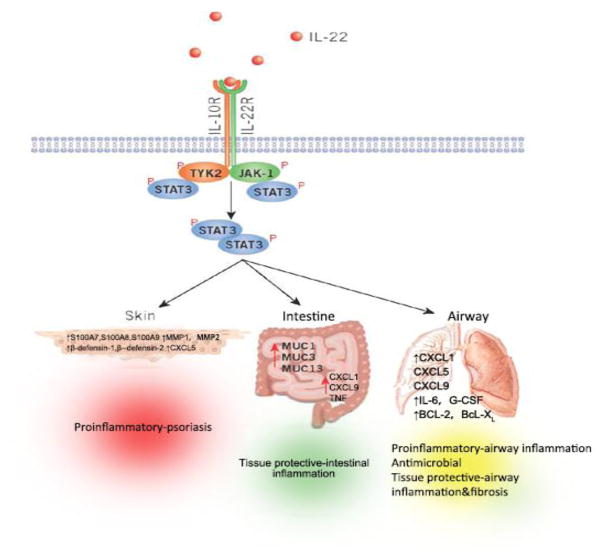
IL-22 binding to IL-22R complex leads to a cascade of downstream signaling pathways. Initial studies utilizing a murine kidney cell line revealed that IL-22R ligation induced phosphorylation of STAT3, and to a lesser extent, STAT5, while other studies observed phosphorylation of STAT1, STAT3, and STAT5 in a human kidney cell line(1). Further analysis also demonstrated that IL-22 signaling utilizes Jak1 and Tyk2 to propagate downstream phosphorylation signals, including several MAPK pathways (ERK1/2, MEK1/2, JNK, and p38 kinase), and STAT1, STAT3, and STAT5(28). IL-22 as well as other members of the IL-10 cytokine family utilizes the common pathway of STAT3-mediated signaling. However, IL-22 signaling exhibits a number of unique properties. For example, in comparison to IL-10 stimulation that induces phosphorylation of tyrosine residues on STAT3, IL-22 stimulation induces STAT3 phosphorylation on both tyrosine and serine residues, and also strongly activates the ERK1/2 pathway(28). The observed differences in signal transduction pathways can likely be attributed to differences between IL-10R1 and IL-22R1. STAT3 phosphorylation is an essential pathway in mediating the effects of IL-22 on epithelial cells at barrier surfaces, as phosphorylation of STAT3 in intestinal epithelial cells following chemical-induced colitis is IL-22-dependent, and furthermore, conditional deletion of epithelial-intrinsic STAT3 from intestinal epithelial cells phenocopied that of Il-22-definice-mice during chemical-induced colitis, implicating a requirement for STAT3 in in vivo IL-22-mediated signaling(29). Consistent with that, studies of mouse model systems have identified a critical role for signaling by IL-22 through its receptor (IL-22R) in the promotion of antimicrobial immunity, inflammation and tissue repair at barrier surfaces (Fig. 1)(30).
Figure 1.
Functional consequences of IL-22–IL-22R pathway. IL-22 receptor complex consists of IL-22R1 and IL-10R2. By binding to its receptor, IL-22 activates tyrosine kinase receptor-2(TYK2) and Janus kinase-1(JAk-1), ultimately leading to the activation of STATS3, which can activate many diverse processes involved in antimicrobial immunity, inflammation and tissue repair at barrier surfaces including the skin, intestine and lung. Depending on the cytokine milieu and tissue in which it is expressed, IL-22 can regulate the expression of genes encoding molecules associated with inflammation, repair or chemotaxis or the expression of antimicrobial peptides.
4. IL-22 knock out
To assess the role of IL-22 in autoimmune diseases, IL-22–deficient mice models have provided the best ideal tool. The IL-22-deficient mice were originally generated in 129 background and were subsequently backcrossed with BALB/c mice for 15 generations and or with C57BL/6 for 13 generations(31). Analysis of IL-22-deficient mice has indicated that IL-22 plays a pathogenic or protective role in chronic inflammatory diseases.
The protective role of IL-22 in ConA-mediated liver injury was confirmed by use of IL-22-deficient mice, which were highly susceptible in this hepatitis model, as evidence by hepatic injury, necrosis and apoptosis(32). Similarly, in a DSS-induced innate mediated murine colitis, the Flavell group showed that IL-22-deficient mice developed severe morphological changes and higher mortality(33) The authors have reached the similar results when using a model of Th1-mediated colitis induced by adoptive transfer of CD4+CD45RB++CD25−T cells into Rag1/IL-22 double-deficient mice. They showed that these recipients lost more weight, developed a more severe phenotype and a high mortality when the transferred IL-22 deficient T cells. Recently, in the mouse graft versus host disease (GVHD) induced by an aggressively lethal MHC-mismatched murine bone marrow transplant (BMT) model of C57BL/6 (B6, H-2b) donor marrow and T cells transplanted into lethally irradiated BALB/C (H-2d) recipients, the Hanash group showed that transplantation with IL-22-deficient (IL22−/−) donor marrow or T cells had no impact on GVHD survival, but IL22−/− BMT recipients demonstrated significantly increased GVHD mortality and GVHD-associated organ pathology in the small and large intestines and liver, suggesting a critical role for host cells in the production of protective IL-22 post-BMT(34). However, in a mouse model of allogenic hematopoietic cell transplantation (allo-HCT) using IL22−/− mice, the Couturier group recently showed that donor-derived IL-22 has a key role in exacerbating the inflammation in the gastrointestinal tract and contributes to the severity of acute GVHD (aGHVD) but does not significantly interfere with the graft-versus-leukemia (GVL) effect. Moreover, the results are associated with the increased Foxp3+ regulatory T cells (Treg cells) in recipient mice that received IL22−/− T cells(35). Although the mechanism through which IL-22 deficiency results in Treg cells expansion is not clear, the protective effect of Treg on GHVD was already demonstrated in several models(36–37). Based on the controversy, the role of IL-22 needs to be further explored to delineate its pathogenic versus protective effect in GVHD.
The pathogenic role of IL-22 has been reported. For instance, IL-22-deficient mice were less susceptible to collagen-induced arthritis (CIA) with their decreased incidence of arthritis and decreased pannus formation, and lower numbers of mRNA copies of IL-1β, IL-6, TNFα and MMP-9 were found in their pooled synovium samples(38). Psoriasis is the first example of an organ-specific autoimmune disorder for which the role of IL-22 has been comprehensively investigated. The Renauld group recently showed that in the mouse imiquimod model, IL-22-deficient mice demonstrated almost totally little scaly skin lesions and a dramatic decrease in the development of pustules and a partial decrease in acanthosis, and the absence of IL-22 evidently decreased the expression of chemotactic factors such as CCL3 and CXCL3 and of biomarkers such as S100A8, S100A7, and keratin 14, which reflects the antimicrobial and hyper-proliferative responses of keratinocytes, suggesting IL-22 plays a major pathogenic role in psoriasis –like lesions(39).
5. Effect of IL-22 blockade in autoimmune diseases
5a. Psoriasis
In last few years, the discovery of IL-23/Th17 axis in pathophysiology of psoriatic disease shifts the cytokine paradigm from Th1 to Th17 cytokine mainly related to IL-17 and IL-22. IL-22 has been found to be a key mediator in the psoriasis pathogenesis. Psoriatic patients showed highly elevated IL-22 plasma levels, which correlated with the disease severity(40). Furthermore, IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation and mobility in keratinocytes: a potential role in psoriasis(40). The Sabat group showed that IL-22 regulates keratinocyte function in several ways: (a) facilitating to form a biological barrier of the skin by producing antimicrobial proteins (AMPs). (b) interfering with physiological desquamation process of skin by inhibiting the terminal differentiation of keratinocytes. (c) recruiting neutrophilic granulocytes in skin by inducing the production of chemokines (d) inducing production of matrix metalloproteinases 1 and 3 to help in extracellular tissue degradation(41). In addition, IL-22 synergized with other cytokines, such as TNF-α, IL-17 and IL-20, to form a cytokine network that orchestrates the progression of many different pathogenic features of psoriasis(42). Using an autoimmune psoriasis model, these mice treated with IL-22 neutralizing antibody demonstrated either no development or extremely mild development of the diseases. There was also a significant reduction of antimicrobial peptides in the IL-22 neutralized group, which suggests that IL-22 antagonism may lead to a therapeutic approach for th17 cell-medicated skin disease. Another recent study on the role of IL-22 in a mouse model with psoriasis skin inflammation showed similarly that blocking IL-22 can both affect keratinocyte dysregulation and neutrophil infiltration(39). These data further support the potential clinical effectiveness of IL-22 inhibitors in psoriasis patients.
5b. Rheumatoid arthritis (RA)
The RA is characterized by synovial inflammation and destruction of bone and joint cartilage. Cytokines play a key role in driving T cell activation and migration that lead to join destruction. In RA, expression of IL-22 and IL-22R is increased on rheumatoid arthritis synovial fibroblasts. IL-22 has been showed to promote the proliferation of synovial fibroblasts through induction of chemokine CCL2(43). In a model of inflammatory arthritis, IL-22-deficient mice demonstrated an increased production of type II collagen (CII)-specific, and yet showed less severe form of arthritis than wild-type mice(38). In the same study, IL-22 was found to promote osteoclastogenesis and this effect may be associated with the reduced severe arthritis in IL-22-deficient mice(38).The Kim group observed that IL-22 promoted osteoclastogenesis in RA by induction of receptor activator of nuclear factor kappa-B ligand (RANKL) in human synovial fibroblasts(44). These data suggest that IL-22 has a pathogenic role in RA. Similarly, using IL-22 neutralizing antibody, the Marijinissen group assessed the potential for IL-22 depletion in a model of spontaneous mice in IL-1R antagonist-deficient (IL-1Ra−/−) mice, and their results showed that administration of anti-IL-22 of IL-1Ra−/− mice significantly reduced the inflammation and bone erosion(45). However, a more recent study showed that IL-22 reduces the severity of collagen-induced arthritis, when administered prior to the onset of the disease, the mechanism of which is associated with increased levels of IL-10(46). These findings suggest that IL-22 has dual functions, i.e. protective or pathogenic, in inflammatory arthritis, depending on the different phases of the disease development.
5c. Hepatitis
Hepatocytes are important target cells of IL-22 IL-22 was able to induce mRNA expression of acute phase protein such as serum amyloid A (SAA), α1-antichymotrypsin, and haptoglobin in the HepG2 human hepatoma cell line and concordantly, an increase of SAA mRNA expression in the liver of IL-22 treated mice(1). The administration of anti-IL-22 antibody resulted in incipient liver necrosis during Salmonella enteritidis-infected p35-deficient mice(47). Although the molecular mechanisms of IL-22 action in different liver injury models remain to be elucidated, IL-22 is generally considered to be protective in liver diseases.
5d. Graft versus Host Disease (GVHD)
GVHD is the result of alloreactive donor T cells attacking host tissues, including the skin, liver and gastrointestinal (GI) track. The role of IL-22 in GVHD has not been extensively addressed. The Hanash group demonstrated that recipient IL-22 deficiency led to increased crypt apoptosis, depletion of intestinal stem cells (ISCs), and loss of epithelial integrity(34).The elimination of IL-22 with an IL-22-neutralizimg antibodies led to a significantly increased GHVD mortality(34). which suggests IL-22 as a critical regulator of tissue sensitivity to GVHD and a protective factor for ISCs during inflammatory intestinal damage.
5e. Allergic diseases
Allergic diseases such as atopic dermatitis (AD) and allergic asthma are chronic inflammatory diseases, characterized by infiltration and accumulation of eosinophil, T cells and mast cells. Classically, allergic inflammation is induced by an initial Th2-driven phase, which precedes Th1-dominated phase. However, recent studies suggest that IL-17 and IL-22 play a role in sustained inflammation in allergic diseases. Upregulated expression of IL-22 was present in skin from AD patients(48). Also, IL-22 is detected at the site of allergic airway inflammation(49). In a mouse model of asthma, The Schnydr group found enhanced eosinophil recruitment and increased eosinophil peroxidase activity in the lungs of mice that had received neutralizing anti-IL-22 antibodies during the antigen challenge(50). Furthermore, Kentaro group have recently showed that anti–IL-22 antibody enhanced antigen-induced IL-25 production in the airways, which is known to enhance Th2-type immune responses in the airways, and enhanced the eosinophil recruitment into the airways(51). These results suggest that IL-22 attenuates antigen-induced airway inflammation in part by inhibiting the expression of IL-25 in lung epithelial cells.
6. Conclusions
Since the discovery of IL-22, many studies focus on its cellular sources, receptor expression, signaling transduction pathway, transcriptional regulation and function. It is well-known that IL-22 is a critical cytokine in a number of immune processes and plays an important role in immune responses. Recently, the role of IL-22 in autoimmune diseases is coming out more prominently both in animal studies as well as in human studies. Administration with recombinant or antagonistic cytokine or gene therapy delivery of IL-22 has been showed to alleviate tissue destruction during inflammation. IL-22 may be a new therapeutic weapon within the armamentarium of autoimmune diseases treatment. However, large studies are needed to provide information on the therapeutic effect, adverse events of any anti-cytokine or recombinant cytokine therapy in the treatment of autoimmune diseases.
Take-home message.
Interleukin-22(IL-22) –a member of the IL-10 cytokine family – is secreted by a broad variety of lymphocytes
IL-22 completes its biological effects by the IL-22-IL-22R pathway
IL-22 plays an important role in host defense against Gram-negative bacterial organisms, and has been implicated in autoimmune diseases
IL-22 knockout and experimental delivery of anti-IL-22 antibody has confirmed a critical role of IL-22 in several autoimmune diseases, such as psoriasis, rheumatoid arthritis (RA), hepatitis, graft versus host disease (GHVD) and allergic diseases
Acknowledgments
This work was partly by Supported by the NIH (grants AR-059103 and AI-084359), the Rheumatology Research Foundation of the American College of Rheumatology (Within Our Reach program grant), and Science Foundation of Science and Technology Department of Zhejiang Province (No.2007C3305).
Footnotes
Competing interests The authors declare no competing interests.
Author contributions X. Y. Yang and S. G. Zheng contributed equally to researching the data for the article, discussing the content, writing the article and reviewing and/or editing of the manuscript before submission.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Xuyan Yang, Department of Rheumatology, Second Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou 310009, P.R. China.
Song Guo Zheng, Division of Rheumatology, Department of Medicine at Penn State University Hershey College of Medicine, Hershey, 17033, USA.
References
- 1.Dumoutier L, Louahed J, Renauld JC. Cloning and characterization of IL-10-related Tcell-derived inducible factor (IL-TIF), a novel cytokine structurally related to IL-10 and inducible by IL-9. J Immunol. 2000;164:1814–1819. doi: 10.4049/jimmunol.164.4.1814. [DOI] [PubMed] [Google Scholar]
- 2.Dumoutier L, Van Roost E, Colau D, Renauld JC. Human interleukin-10-related T cell-derived inducible factor: molecular cloning and functional characterization as an hepatocyte-stimulating factor. Proc Natl Acad Sci U S A. 2000;29:97. doi: 10.1073/pnas.170291697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dumoutier L, Van Roost E, Ameye G, Michaux L, Renauld JC. IL-TIF/IL-22: genomic organization and mapping of the human and mouse genes. Genes Immun. 2000;1(8):488–94. doi: 10.1038/sj.gene.6363716. [DOI] [PubMed] [Google Scholar]
- 4.Wolk K, Sabat R. Interleukin-22: a novel T- and NK-cell derived cytokine that regulates the biology of tissue cells. Cytokine Growth Factor Rev. 2006;17(5):367–80. doi: 10.1016/j.cytogfr.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10(8):857–63. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- 6.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203(10):2271–9. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol. 2009;10(8):864–71. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- 8.Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, et al. Generation of pathogenic T(H)17 cells in the absence of TGF-β signalling. Nature. 2010;467(7318):967–71. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28(1):29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453(7191):106–9. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 11.Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest. 2009;119(12):3573–85. doi: 10.1172/JCI40202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nograles KE, Zaba LC, Shemer A, Fuentes-Duculan J, Cardinale I, Kikuchi T, et al. IL-22-producing “T22” T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J Allergy Clin Immunol. 2009;123(6):1244–52. doi: 10.1016/j.jaci.2009.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 14.Simonian PL, Wehrmann F, Roark CL, Born WK, O’Brien RL, Fontenot AP. γδ T cells protect against lung fibrosis via IL-22. J Exp Med. 2010;207(10):2239–53. doi: 10.1084/jem.20100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457(7230):722–5. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luci C, Reynders A, Ivanov II, Cognet C, Chiche L, Chasson L, et al. Influence of the transcription factor RORgammat on the development of NKp46+ cell populations in gut and skin. Nat Immunol. 2009;10(1):75–82. doi: 10.1038/ni.1681. [DOI] [PubMed] [Google Scholar]
- 17.Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29(6):958–70. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Marchesi F, Martin AP, Thirunarayanan N, Devany E, Mayer L, Grisotto MG, et al. CXCL13 expression in the gut promotes accumulation of IL-22-producing lymphoid tissue-inducer cells, and formation of isolated lymphoid follicles. Mucosal Immunol. 2009;2(6):486–94. doi: 10.1038/mi.2009.113. [DOI] [PubMed] [Google Scholar]
- 19.Sanos SL, Bui VL, Mortha A, Oberle K, Heners C, Johner C, et al. RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat Immunol. 2009;10(1):83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Satoh-Takayama N, Dumoutier L, Lesjean-Pottier S, Ribeiro VS, Mandelboim O, Renauld JC, et al. The natural cytotoxicity receptor NKp46 is dispensable for IL-22-mediated innate intestinal immune defense against Citrobacter rodentium. J Immunol. 2009;183(10):6579–87. doi: 10.4049/jimmunol.0901935. [DOI] [PubMed] [Google Scholar]
- 21.Buonocore S, Ahern PP, Uhlig HH, Ivanov II, Littman DR, Maloy KJ, et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464(7293):1371–5. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tachiiri A, Imamura R, Wang Y, Fukui M, Umemura M, Suda T. Genomic structure and inducible expression of the IL-22 receptor alpha chain in mice. Genes Immun. 2003;4(2):153–9. doi: 10.1038/sj.gene.6363934. [DOI] [PubMed] [Google Scholar]
- 23.Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21(2):241–54. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Wolk K, Haugen HS, Xu W, Witte E, Waggie K, Anderson M, et al. IL-22 and IL-20 are key mediators of the epidermal alterations in psoriasis while IL-17 and IFN-gamma are not. J Mol Med (Berl) 2009;87(5):523–36. doi: 10.1007/s00109-009-0457-0. [DOI] [PubMed] [Google Scholar]
- 25.Xu W, Presnell SR, Parrish-Novak J, Kindsvogel W, Jaspers S, Chen Z, et al. A soluble class II cytokine receptor, IL-22RA2, is a naturally occurring IL-22 antagonist. Proc Natl Acad Sci USA. 2001:9511–9516. doi: 10.1073/pnas.171303198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sugimoto K, Ogawa A, Mizoguchi E, Shimomura Y, Andoh A, Bhan AK, et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest. 2008;118(2):534–44. doi: 10.1172/JCI33194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson MS, Feng CG, Barber DL, Yarovinsky F, Cheever AW, Sher A, et al. Redundant and pathogenic roles for IL-22 in mycobacterial, protozoan, and helminth infections. J Immunol. 2010;184(8):4378–90. doi: 10.4049/jimmunol.0903416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sonnenberg GF, Fouser LA, Artis D. Functional biology of the IL-22-IL-22R pathway in regulating immunity and inflammation at barrier surfaces. Adv Immunol. 2010;107:1–29. doi: 10.1016/B978-0-12-381300-8.00001-0. [DOI] [PubMed] [Google Scholar]
- 29.Pickert G, Neufert C, Leppkes M, Zheng Y, Wittkopf N, Warntjen M, et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med. 2009;206(7):1465–72. doi: 10.1084/jem.20082683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sonnenberg Gregory F, Fouser Lynette A, Artis David. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. 2011;12(5):383–90. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- 31.Kreymborg K, Etzensperger R, Dumoutier L, Haak S, Rebollo A, Buch T, et al. IL-22 is expressed by Th17 cells in an IL-23-dependent fashion, but not required for the development of autoimmune encephalomyelitis. J Immunol. 2007;179(12):8098–104. doi: 10.4049/jimmunol.179.12.8098. [DOI] [PubMed] [Google Scholar]
- 32.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Flavell RA. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity. 2007;27(4):647–59. doi: 10.1016/j.immuni.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29(6):947–57. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanash AM, Dudakov JA, Hua G, O’Connor MH, Young LF, Singer NV, et al. Interleukin-22 protects intestinal stem cells from immune-mediated tissue damage and regulates sensitivity to graft versus host disease. Immunity. 2012;37(2):339–50. doi: 10.1016/j.immuni.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Couturier M, Lamarthée B, Arbez J, Renauld JC, Bossard C, Malard F, et al. IL-22 deficiency in donor T cells attenuates murine acute graft-versus-host disease mortality while sparing the graft-versus-leukemia effect. Leukemia. 2013;27(7):1527–37. doi: 10.1038/leu.2013.39. [DOI] [PubMed] [Google Scholar]
- 36.Shin HJ, Baker J, Leveson-Gower DB, Smith AT, Sega EI, Negrin RS. Rapamycin and IL-2 reduce lethal acute graft-versus-host disease associated with increased expansion of donor type CD4+CD25+Foxp3+ regulatory T cells. Blood. 2011;118(8):2342–50. doi: 10.1182/blood-2010-10-313684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lan Q, Zhou X, Fan H, Chen M, Wang J, Ryffel B, et al. Polyclonal cd4+foxp3+ treg cells induce tgfβ-dependent tolerogenic dendritic cells that suppress the murine lupus-like syndrome. J Mol Cell Biol. 2012;4:409–419. doi: 10.1093/jmcb/mjs040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geboes L, Dumoutier L, Kelchtermans H, Schurgers E, Mitera T, Renauld JC, et al. Proinflammatory role of the Th17 cytokine interleukin-22 in collagen-induced arthritis in C57BL/6 mice. Arthritis Rheum. 2009;60(2):390–5. doi: 10.1002/art.24220. [DOI] [PubMed] [Google Scholar]
- 39.Van Belle AB, de Heusch M, Lemaire MM, Hendrickx E, Warnier G, Dunussi-Joannopoulos K, et al. IL-22 is required for imiquimod-induced psoriasiform skin inflammation in mice. J Immunol. 2012;188(1):462–9. doi: 10.4049/jimmunol.1102224. [DOI] [PubMed] [Google Scholar]
- 40.Wolk K, Witte E, Wallace E, Döcke WD, Kunz S, Asadullah K, et al. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol. 2006;36(5):1309–23. doi: 10.1002/eji.200535503. [DOI] [PubMed] [Google Scholar]
- 41.Sabat R, Wolk K. Research in practice: IL-22 and IL-20: significance for epithelial homeostasis and psoriasis pathogenesis. J Dtsch Dermatol Ges. 2011;9(7):518–23. doi: 10.1111/j.1610-0387.2011.07611.x. [DOI] [PubMed] [Google Scholar]
- 42.Eyerich S, Wagener J, Wenzel V, Scarponi C, Pennino D, Albanesi C, et al. IL-22 and TNF-α represent a key cytokine combination for epidermal integrity during infection with Candida albicans. Eur J Immunol. 2011;41(7):1894–901. doi: 10.1002/eji.201041197. [DOI] [PubMed] [Google Scholar]
- 43.Ikeuchi H, Kuroiwa T, Hiramatsu N, Kaneko Y, Hiromura K, Ueki K, et al. Expression of interleukin-22 in rheumatoid arthritis: potential role as a proinflammatory cytokine. Arthritis Rheum. 2005;52(4):1037–46. doi: 10.1002/art.20965. [DOI] [PubMed] [Google Scholar]
- 44.Kim KW, Kim HR, Park JY, Park JS, Oh HJ, Woo YJ, et al. Interleukin-22 promotes osteoclastogenesis in rheumatoid arthritis through induction of RANKL in human synovial fibroblasts. Arthritis Rheum. 2012;64(4):1015–23. doi: 10.1002/art.33446. [DOI] [PubMed] [Google Scholar]
- 45.Marijnissen RJ, Koenders MI, Smeets RL, Stappers MH, Nickerson-Nutter C, Joosten LA, et al. Increased expression of interleukin-22 by synovial Th17 cells during late stages of murine experimental arthritis is controlled by interleukin-1 and enhances bone degradation. Arthritis Rheum. 2011;63(10):2939–48. doi: 10.1002/art.30469. [DOI] [PubMed] [Google Scholar]
- 46.Sarkar S, Zhou X, Justa S, Bommireddy SR. Interleukin-22 reduces the severity of collagen-induced arthritis in association with increased levels of interleukin-10. Arthritis Rheum. 2013;65(4):960–71. doi: 10.1002/art.37849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schulz SM, Köhler G, Schütze N, Knauer J, Straubinger RK, Chackerian AA, et al. Protective immunity to systemic infection with attenuated Salmonella enterica serovar enteritidis in the absence of IL-12 is associated with IL-23-dependent IL-22, but not IL-17. J Immunol. 2008;181(11):7891–901. doi: 10.4049/jimmunol.181.11.7891. [DOI] [PubMed] [Google Scholar]
- 48.Nograles KE, Zaba LC, Shemer A, Fuentes-Duculan J, Cardinale I, Kikuchi T, et al. IL-22-producing “T22” T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J Allergy Clin Immunol. 2009;123(6):1244–52. doi: 10.1016/j.jaci.2009.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schnyder B, Lima C, Schnyder-Candrian S. Interleukin-22 is a negative regulator of the allergic. doi: 10.1016/j.cyto.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 50.Schnyder B, Lima C, Schnyder-Candrian S. Interleukin-22 is a negative regulator of the allergic response. Cytokine. 2010;50(2):220–7. doi: 10.1016/j.cyto.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 51.Takahashi K, Hirose K, Kawashima S, Niwa Y, Wakashin H, Iwata A, et al. IL-22 attenuates IL-25 production by lung epithelial cells and inhibits antigen-induced eosinophilic airway inflammation. J Allergy Clin Immunol. 2011;128(5):1067–76. doi: 10.1016/j.jaci.2011.06.018. [DOI] [PubMed] [Google Scholar]